Paraneoplastic neurologic disorder (PND) is a remote manifestation triggered by altered immune response against neoplasms. Emerging discovery of antineuronal antibodies and diagnostic tool development, such as PET-CT, allow us the early recognition and treatment of these disorders.1 Neuromyelitis optica spectrum disorder (NMOSDs) is an inflammatory disease affecting the optic nerve and spinal cord, where autoantibodies against aquaporin 4 (AQP4-IgG) supposed to play a pathogenic role.2 Several tumors, such as breast cancer or carcinoid, were reported to be associated with paraneoplastic NMOSD based on immunoreactivity to de novo AQP4 in the tumor.3 One may have thought because AQP4 is expressed not only on CNS but also on muscle, kidney, and stomach,4 alteration of tumor associated self-antigens expression can cause paraneoplastic NMOSD in the tumors of these organs. However, AQP4 expression is known to decline during carcinogenesis in the gastric cancer.5 Contrary, programmed cell death ligand 1 (PD-L1) on tumor that couples with PD-1, an inhibitory receptor on both CD4+ and CD8+ T cells, to play an important role in the ability of tumor cells to escape from the host immune system.6 Indeed, 40% of patients with advanced gastric cancer express PD-L1 and proved clinical benefit by an immune check-point therapy targeting PD-1/PD-L1 axis.7 Here, we report a paraneoplastic NMOSD case with adenocarcinoma of the esophagogastric (EG) junction that exhibited immunoreactivity to AQP4 and negative for PD-L1 expression.
Case report
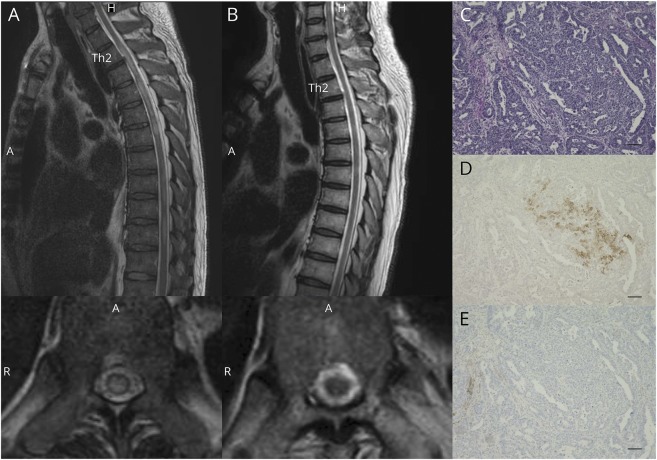
A 61-year-old man with a history of hypertension and reflux esophagitis visited the emergency department with severe nausea and epigastric pain. He was diagnosed with unstable angina pectoris and admitted to the hospital 1 month before his neurologic symptoms. Coronary artery bypass surgery was performed for triple-vessel coronary atherosclerosis. Two days after surgery, he experienced numbness of 4 extremities. Neurologic examination revealed mild muscle weakness of both legs with pyramidal signs and loss of sensation below thoracic (Th) 6 level. His symptoms exacerbated to complete paraplegia within a week. Cervical and thoracic spinal MRI showed transverse T2-weighted high-intensity signals in Th2-6, 7–8, and 9–10 (figure, A). Vision assessment was normal. CSF analysis was 6 cellular/μL with normal total protein and glucose, elevated myelin basic protein 1,460 ng/mL (normal <102 ng/mL), IgG index was 0.38 (normal), and no CSF specific oligoclonal bands were detected. Positive serology results included antinuclear antibodies (1:40, cytoplasmic pattern) and elevated AQP4-IgG levels (ELISA, >75 units/mL; normal, <5 units/mL). High-dose IV methylprednisolone therapy was followed by plasma exchanges and high-dose IV immunoglobulin, and then, oral prednisolone treatment was started. PET-CT detected a significant uptake at EG junction tumor, which was confirmed by esophagogastroduodenoscopy. Pathologic examination of a biopsy specimen revealed adenocarcinoma that was clinically diagnosed as Siewert type I, with the epicenter located 1–5 cm above the EG junction. Thoracoscopic subtotal esophagectomy was performed after those immunotherapies. The patient exhibited gradual relief of sensory disturbance and paraplegia. Two months after surgery, he was able to ambulate with assistance. MRI on 1 year later showed improvement in T2-weighted high-intensity lesions (figure, B) and elevated serum AQP4-IgG levels became undetectable. Hematoxylin-eosin (figure, C) and anti-AQP4 antibody (figure, D) (Millipore, AB3594) staining of formalin-fixed paraffin-embedded sections (5 μm) of the adenocarcinoma revealed clustered AQP4-positive tumor cells (brown cytoplasm). Of note, these tumor cells were negative for PD-L1 staining (figure, E) (Agilent, 22c3 pharmDx).
Figure. Long cord lesions in spine MRI and aquaporin 4 (AQP4) immunoreactivity in adenocarcinoma cells.
(A) Sagittal and axial (Th10 level) T2-weighted images of the spine at disease onset. T2-increased signals were observed in the thoracic spinal cord. (B) Sagittal and axial (Th10 level) T2-weighted images of the spine 1 year after the onset. (C–E) Pathologic analysis of the adenocarcinoma at the esophagogastric junction (C: hematoxylin and eosin, D: AQP-4 immunoreactivity [brown], and E: programmed cell death ligand 1 immunoreactivity [brown]). Scale bars indicate 100 μm.
Discussion
The immunohistologic staining of antineuronal antibodies in the tumor supports a paraneoplastic etiology for some PND cases.1 Although we do not know whether the neurologic symptoms of the patient developed progressively if the tumor had remained untreated, AQP4-IgG has been proven to often cause recurrence of CNS attacks.2 In this case, the patient recovered from complete paraplegia, and serum AQP4-IgG became negative after the resection of its antigen source in the EG junction adenocarcinoma, further suggesting the paraneoplastic etiology.
Gastric cancer attenuates AQP4 expression and expresses PD-L1 during development.5,7 In this case, because PD-L1 was negative in tumor cells, immune surveillance might detect ectopic “unprotected” AQP4 expressed on EG junction epithelial cells as a neoantigen under carcinogenesis following metaplasia and produced AQP4-IgG cross-reacted with CNS targets relevant to NMOSD. Neurologic symptoms facilitated us further examination to detect tumor before expressing PD-L1 resulting in curative treatment. In advanced adenocarcinoma of the EG junction, PD-L1 expression may contribute evading such immune response yielding to intractable disease stage. The mechanisms how PNDs settle for small portion of patients with cancer still need further investigations; however, this case could provide an insight underlining PND establishment.
Acknowledgment
The authors thank people in the Department of Diagnostic Pathology at Kobe University Graduate School of Medicine for their help in preparing histology sections.
Author contributions
Study design and conceptualization: A. Sudo and N. Chihara. Clinical analysis: A. Sudo, N. Chihara, Y. Takenaka, T. Nakamura, T. Ueda, K. Sekiguchi, and T. Toda. Drafting the manuscript for intellectual content: A. Sudo and N. Chihara. Supervision of the entire study: T. Toda. All authors read and approved submission of the final version of the manuscript.
Study funding
This work was supported by a Grant-in-Aid for Young Scientists from the Japan Society for the Promotion of Science.
Disclosure
A. Sudo reports no disclosures. N. Chihara received speaker honoraria from Novartis, Eisai, Biogen Japan, and Takeda and received research support from the Japan Society for the Promotion of Science. Y. Takenaka, T. Nakamura, K. Sekiguchi, and T. Toda report no disclosures. Full disclosure form information provided by the authors is available with the full text of this article at Neurology.org/NN.
References
- 1.Dalmau J, Rosenfeld MR. Paraneoplastic syndromes of the CNS. Lancet Neurol 2008;7:327–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wingerchuk DM, Lennon VA, Lucchinetti CF, Pittock SJ, Weinshenker BG. The spectrum of neuromyelitis optica. Lancet Neurol 2007;6:805–815. [DOI] [PubMed] [Google Scholar]
- 3.Pittock SJ, Lennon VA. Aquaporin-4 autoantibodies in a paraneoplastic context. Arch Neurol 2008;65:629–632. [DOI] [PubMed] [Google Scholar]
- 4.Ratelade J, Bennett JL, Verkman AS. Intravenous neuromyelitis optica autoantibody in mice targets aquaporin-4 in peripheral organs and area postrema. PLoS One 2011;6:e27412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shen L, Zhu Z, Huang Y, et al. Expression profile of multiple aquaporins in human gastric carcinoma and its clinical significance. Biomed Pharmacother 2010;64:313–318. [DOI] [PubMed] [Google Scholar]
- 6.Dong H, Strome SE, Salomao DR, et al. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med 2002;8:793–800. [DOI] [PubMed] [Google Scholar]
- 7.Muro K, Chung HC, Shankaran V, et al. Pembrolizumab for patients with PD-L1-positive advanced gastric cancer (KEYNOTE-012): a multicentre, open-label, phase 1b trial. Lancet Oncol 2016;17:717–726. [DOI] [PubMed] [Google Scholar]