Abstract
Purpose
A small-scale randomized controlled trial conducted by our group found that four of seven retinitis pigmentosa (RP) subjects who received six weekly Transcorneal Electrical Stimulation (TES) sessions developed significant improvements in visual acuity (VA), quick contrast sensitivity function (qCSF), and/or Goldmann visual fields (GVF). We longitudinally monitored three of these participants for declining visual function due to natural RP progression to determine the duration of their responses and administered retreatments.
Methods
Over a period of 29–35 months, repeated ETDRS VA, qCSF and/or GVF tests and three to six TES treatment courses consisting of six weekly sessions were administered in each eye of three RP participants every four to 16 months in an unmasked, prospective case series study.
Results
For two participants, there were significant VA improvements of 44–52 letters (0.88–1.04 logMAR) and 15–23 letters (0.3–0.46 logMAR) in the worse eye at baseline after each of three or four treatment courses of TES compared to initial baseline. They had no significant decreases from baseline for VA or qCSF over 29 to 35 months, The third participant had a significant mean improvement in VA in the eye with better baseline vision (p = 0.004) and binocularly (p < 0.001) following six treatment courses over the 29-month period. For the first two participants, mean annual rates of GVF change for each eye ranged from −5% to 0% with the V4e stimulus, and −26% to +33% the III4e stimulus. The third participant’s mean annual GVF changes were +14 to +35%, with a statistically significant improvement across 29 months for both the V4e and III4e stimuli in the right eye (p = 0.045; p = 0.015) and the V4e stimulus in the left eye (p = 0.047).
Conclusion
Following encouraging visual improvements after TES that lasted for several months, it appears it may be possible to restore and prevent slowly diminishing vision over time with retreatments, which requires confirmation in a large-scale randomized controlled trial.
Keywords: Contrast sensitivity, Goldmann visual field, Retinitis pigmentosa, Transcorneal electrical stimulation, Visual acuity
Introduction
Transcorneal Electrical Stimulation (TES) is a minimally invasive, readily applicable intervention that may have the potential to slow the progression of retinitis pigmentosa (RP) or improve visual function as per findings in animal or in vitro models of RP [1, 2] and human studies [3–6]. Several previous basic science studies support the hypothesis that TES might induce a beneficial effect via several mechanisms: neurotrophic, anti-apoptotic, anti-glutamate, and anti-inflammatory [1, 2], which are believed to influence visual function improvements and help to reduce the progression rate of RP. It has been proposed that the neuroprotection mediated by electro-stimulation occurs via several counteracting trophic factors that mediate microglial activation and suppression to create homeostatic balance and a nurturing microenvironment suitable for the rescue of apoptotic photoreceptor cells [1, 2].
A case study of a patient with Best vitelliform macular dystrophy reported significantly improved visual acuity (VA) from 1.0 to 0.2 logMAR a month after receiving two TES sessions. Then after two and a half years without TES treatments, this patient’s VA regressed to 0.55 logMAR, at which time two additional TES sessions were administered; the patient’s VA improved again to 0.2 logMAR and was maintained for at least 17 months [7]. Two groups previously documented significantly improved visual or retinal function in RP patients who received 1-year of weekly TES treatments during randomized controlled trials [4, 5]. A short-term, small-scale randomized controlled trial conducted by our group found that four of seven RP subjects who received six weekly TES sessions developed significant improvements in VA, quick contrast sensitivity function (qCSF), and/or Goldmann visual fields (GVF) after the six sessions and sustained a month later, whereas none of the sham intervention control subjects had a significant visual improvement [6]. Longer-term studies of RP patients treated with TES for more than a year have not yet been published. Our current goal was to conduct a longitudinal monitoring study to determine the longevity of visual improvements among three participants with RP who developed improved VA, qCSF and/or GVFs during our previous randomized controlled trial after six weekly sessions of TES [6]. In addition, we administered retreatments with TES when their visual function declined due to the natural progression of RP. We hypothesized that TES would be helpful to restore diminishing vision over time; on the other hand, we were uncertain whether TES would lose efficacy at some point longitudinally due to the inherent retinal degenerative processes in RP.
Methods
Institutional Review Board approval was obtained from the Nova Southeastern University and this research followed the tenets of the Declaration of Helsinki. Written informed consent was obtained from the subjects after explanation of the nature and possible consequences of the study.
The characteristics of the three participants are listed in Table 1. They participated in a double-masked, randomized controlled trial of TES at our center from Fall 2014 to Summer 2015 (i.e., clinicaltrials.gov NCT02086890) and were subsequently recruited to join the present study in which their visual status was monitored longitudinally and retreatments with TES were administered once declines in visual function were noted.
Table 1.
Participants’ characteristics, including age in years at start of TES treatments, self-reported onset age of initial symptoms of nyctalopia or night vision loss in years, self-reported onset age of initial indications of visual field (VF) loss in years, female (F) or male (M) gender, and presumed genetic mutation inheritance type based on family history of RP (i.e., AD: autosomal dominant, AR: autosomal recessive)
| ID | Age | Onset Age Nyctalopia |
Onset VF loss |
Gender | Race | Genetic | Baseline visual function
|
||
|---|---|---|---|---|---|---|---|---|---|
| VA OD | VA OS | qCSF AULCSF | |||||||
| 1 | 44 | 13 | 8 | F | Caucasian | AD | 20/252 | 20/675 | 0.05 OU |
| 2 | 47 | 16 | 24 | M | Black | AR | 20/50 | 20/275 | 0.33 OD |
| 3 | 34 | birth | birth | F | Asian Indian | AR (Bardet-Biedl) | 20/252 | 20/318 | 0.03 OU |
All vision tests were administered by an unmasked, experienced examiner (AKB). Best-corrected VA was measured in each eye using the Early Treatment of Diabetic Retinopathy Study (ETDRS; Lighthouse International, New York, NY, USA) three-chart series at three meters for subject 2, which was modified to 1 m for severely reduced acuities if fewer than ten letters were initially identified, as was the case for subjects 1 and 3. The quick Contrast Sensitivity Function (qCSF) letter identification test (Adaptive Sensory Technology; San Diego, CA, USA) with 25 trials for each eye and binocular tests performed at four meters for subject 2, or at two meters for subjects 1 and 3 with severely reduced VA. The qCSF test provides results for the area under the log CSF (AULCSF) and sensitivity at 1.5 cycles per degree (cpd). Binocular testing was not completed for subject 2 since he was unable to maintain a single fused image at baseline (confusion). Subject 1 did not complete the qCSF test with her worse seeing eye (OS) since it was unable to see the test stimuli. GVF kinetic perimetry was obtained in each eye using V4e and III4e test targets according to previously published methodology, [8] which was later digitized to calculate the total seeing log retinal area [9]. Optical Coherence Tomography (OCT) using the Cirrus 4000 (Zeiss; San Diego, CA, USA) was obtained to detect any presence of cystoid changes within the foveal region pre-TES and at the follow-up visit that occurred at approximately 1-month after each TES treatment course. At 1.5 to 2.5 years post-TES, OCT images were obtained with a newer instrument at our center, the Cirrus 5000 (Zeiss; San Diego, CA, USA).
Based on previous studies of TES in RP patients [3–6], we hypothesized that the greatest visual function changes would occur approximately four to 7 weeks after completing six sessions of TES. Therefore, each subject was seen for testing at that time point, as well as within a week of completing six TES sessions and within a week prior to starting a new treatment course with TES. In between retreatments with TES, participants returned for follow-up visits to assess their visual function every 1 to 12 months. Retreatments with TES were given every four to 16 months, dependent on when visual function declines occurred and the ability of participants to attend visits at our center, which was limited for subject 2 due to transportation and subject 1 who resided in a different state. This was an exploratory study in which we did not set a visit schedule since we did not know the course of visual function changes a priori and anticipated that they might be different across participants. Each visit for visual function testing lasted approximately three to 4 h. Subjects were offered a lunch voucher to take a break during the visual function testing. At each visit, tests were obtained in the same order and time of day to attempt to minimize fluctuations or potential effects of fatigue. Data collection occurred from September 2014 through August 2017.
For TES, a single use, sterile DTL plus electrode was placed on the surface of each eye with corneal anesthetic drops (Proparacaine) and gold-cup ground electrode on the temple, as described in a previous trial [3]. TES was administered to both eyes by an optometrist (KS) using an FDA approved, commercially available neurostimulator (TrioStim; Mettler Electronics Corp., Anaheim, CA, USA) for this off-label indication. The microcurrent settling of this instrument was set to deliver rectangular biphasic current pulses (5-ms positive, directly followed by 5-ms negative) with amplitudes up to 750 μA (the instrument’s maximum level) at a frequency of 20 Hz, for 30 min during six weekly sessions for all treatment courses, with the exceptions of the third treatment course in subject 2 and the fourth treatment course for subject 3, at which TES was applied for 45 min per session. None of the three participants had a measurable electrical phosphene threshold at the maximum setting of 750 μA in either eye. The absence of an electrical phosphene threshold was likely due to advanced or severe retinal degeneration since thresholds are elevated in RP compared to normals and with more advanced vision loss [10, 11]. All three participants received stimulation in each eye at 750 uA, which was maintained at a constant level for all sessions.
We compared changes in the visual function measures to previously published [6] 95% coefficients of repeatability (CR.95) values for test-retest variability in RP for VA (0.20 log units), qCSF (0.19 and 0.14 for monocular and binocular AULCSF, respectively; 0.17 and 0.16 for monocular and binocular sensitivity at 1.5 cpd; calculated from unpublished baseline data from our previous clinical trial [6]), and GVF (65% for V4e; 109% for III4e) to help determine significant changes in our participants that exceeded these CR.95 s. Longitudinal changes were analyzed using linear regression models or non-linear, two parameter exponential growth curve functions, with p < 0.05 defined as statistically significant, using Stata/IC version 13.1 (Stata Corp., College Station, TX).
Results
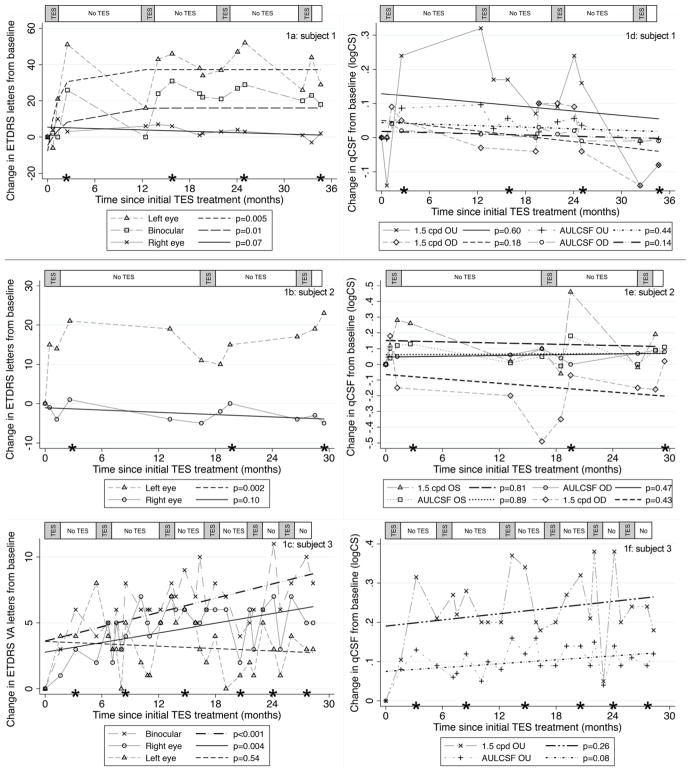
Fig. 1 displays the results for VA and/or qCSF tests over time in each of our three participants; these aspects of central visual function improved in one eye of each participant after every retreatment with TES, while in-between treatments, vision regressed back toward baseline due to diminishing treatment effects and/or typical RP disease progression. For subjects 1 and 2 in Figs. 1a and b, the VA for the worse eye at baseline (OS) improved significantly (i.e., greater than the CR.95 of 0.2 logMAR or ten letters) by 44–52 letters (0.88–1.04 logMAR) and 15–23 letters (0.3–0.46 logMAR), respectively, after each of three or four treatment courses of TES compared to initial baseline. For subject 1, the worse eye at baseline (OS) became the better eye post-TES, thus improvements in binocular VA of 23–31 letters (0.46–0.62 logMAR) after each of the four treatment courses were also documented. At 19 months post-TES, subject 1 elected to try a previously published electro-acupuncture protocol [6]; however, she did not develop any subsequent significant changes in her VA at the 20–22 month follow-ups, qCSF or GVFs following electro-acupuncture, as shown in Figs. 1a, d and 2a.
Fig. 1.
Panels 1a, 1b, and 1c show the change in ETDRS VA letters from baseline over time for subjects 1, 2, and 3, respectively (five letters = 1 line = 0.1 logMAR). Panels 1d, 1e, and 1f show the changes in the qCSF test results from baseline over time for subjects 1, 2, and 3, respectively. The asterisks along the x-axis indicate the assessment that occurred four to 7 weeks after completion of each TES treatment course of six weekly sessions. The bar along the top of each figure panel indicates the periods during which TES was administered (gray shaded areas) and when no TES was administered (white areas)
Fig. 2.
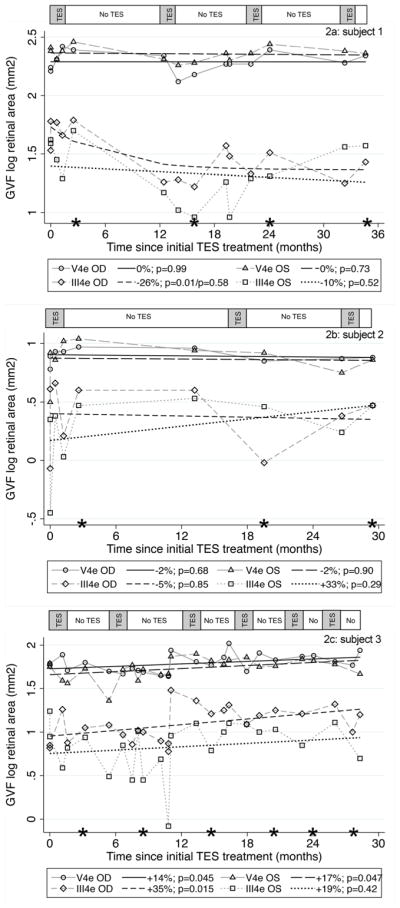
Panels 2a, 2b, and 2c show the GVF log retinal areas over time for subjects 1, 2, and 3, respectively. The asterisks along the x-axis indicate the assessment that occurred four to 7 weeks after completion of each TES treatment course of six weekly sessions. The bar along the top of each figure panel indicates the periods during which TES was administered (gray shaded areas) and when no TES was administered (white areas)
For subject 1 in Fig. 1d and subject 2 in Fig. 1e, the qCSF AULCSF did not improve or decline significantly over time (p > 0.05) and the results for each eye were within typical test-retest variability. For subject 1 in Fig. 1d, the binocular qCSF sensitivity at 1.5 cpd had a significant improvement outside test variability after the first three treatment courses. For subject 2 in Fig. 1e, the qCSF sensitivity at 1.5 cpd had a significant improvement outside test variability in the worse eye (OS) after each of the three treatment courses, and declined significantly in the better eye (OD) during the period between the first and second TES treatment courses, but then recovered in the better eye following the second and third treatment courses.
Subject 3 in Fig. 1c had a statistically significant mean improvement in VA in the right eye with better baseline vision by 3.4 letters on average (p = 0.004) and binocularly by 5.1 letters on average (p < 0.001) over the 29-month period. In addition, Fig. 1c shows there were significant, peak improvements in binocular VA of 10–11 letters after the third, fifth and sixth treatment courses for subject 3. Fig. 1f demonstrates that while subject 3 had increases on average across visits for the binocular qCSFAULCSF and sensitivity at 1.5 cpd, they were not statistically significant (p = 0.08; p = 0.26); however, the post-treatment improvements in sensitivity at 1.5 cpd exceed typical test variability (i.e., CR.95 = 0.16).
For subjects 1 and 2 shown in Fig. 2a and b, there were no significant changes in GVF log retinal area over 29 to 35 months (all p > 0.05), with the exception of the III4e stimulus in the right eye of subject 1, in which case the isopter located in the far periphery had a significant decrease between the first and second treatment courses (p = 0.01), then remained relatively stable between 12 and 35 months (p = 0.58) when retreatments were given more frequently (i.e., every 10 months). The GVF mean annual rates of change for each eye using V4e and III4e test stimuli ranged from −26% to 0% for subject 1 and −5% to +33% for subject 2. For subject 3 shown in Fig. 2c, the annual rates of change for GVF log retinal area were +14 to +35%, with a statistically significant improvement across 29 months for both the V4e and III4e stimuli in the right eye (p = 0.045; p = 0.015) and the V4e stimulus in the left eye (p = 0.047). Fig. 3 shows the GVF map results for the locations of vision that were plotted for each subject at baseline and the last follow-up visit post-TES (i.e., 29 to 35 months after initial TES).
Fig. 3.
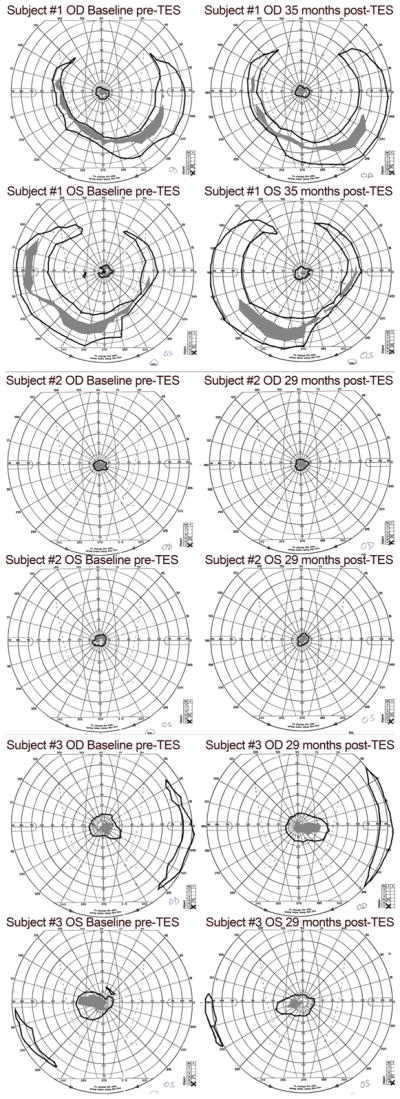
GVF maps of the plotted locations of vision in each eye of the three subjects at baseline (pre-TES)(shown in the left panels) and the last follow-up visit, 29–35 months post-TES (shown in the right panels). The V4e isopter is drawn with a black line and the III4e isopter is indicated by gray filled areas
Subjective improvements in functional vision included: (1) improved ability to detect colors, read package labels when shopping, and see food on a fork while eating for subject 1, (2) better fusion of the images between eyes, less subjective effort to focus and less affected by glare outdoors for subject 2, and (3) reduction in the computer screen magnification level for work, as well as improved mobility and independence for subject 3. In addition to these improvements, all three subjectively noted they did not perceive any deterioration of their vision since initiating TES, which they indicated was a departure from their usual trajectory and even more important than improvements.
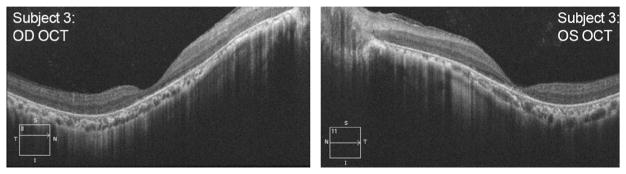
Figure 1 shows that remarkably, after 2.4 to 3 years of retreatments with TES, none of the three participants experienced a measurable deterioration of central visual function (i.e., VA or qCSF) that was worse than their initial baseline (i.e., outside of typical test-retest variability). Fundus photography showed no qualitative or visible changes in the macular pigmentation or atrophy in the three participants during the 2.4 to 3 year follow-up period, shown in Fig. 4. Macular hypopigmentation or atrophic changes were noted in all three subjects in Fig. 4, and the more subtle macular changes in subject 3 were confirmed with OCTas disruption and thinning of the photoreceptor outer segment and retinal pigment epithelium complex. Figure 5 shows the OCT images for each eye of subject 3 that were collected at 29 months post-TES. OCT revealed no cystoid macular edema or changes at any visit for the three subjects.
Fig. 4.
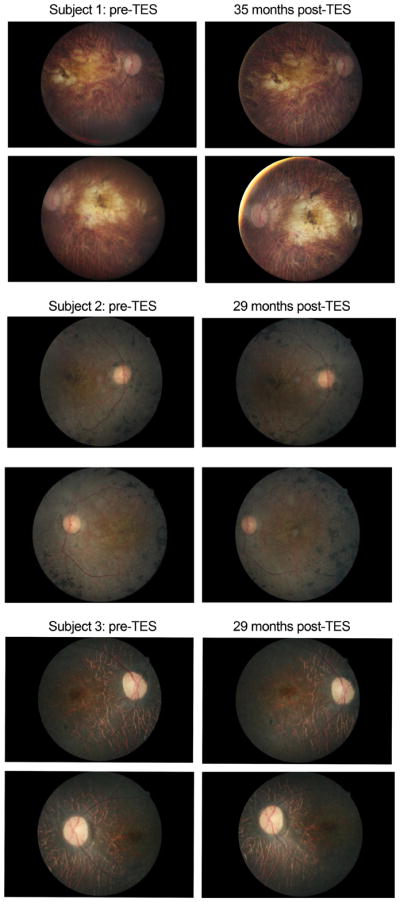
Fundus photos of the macula in each eye taken at baseline (pre-treatment) and the last follow-up assessment at 29 months for subjects 2 and 3, or 35 months for subject 1
Fig. 5.
OCT images of the macula obtained in each eye (OD in left panel and OS in right panel) of subject #3 at 29 months post-TES
Discussion
The present case study documented that three adults with RP who received three to six treatment courses of TES over 2.4 to 3 years developed repeated improvements in central visual function about four to 7 weeks post-treatment, with regression back toward baseline in between treatment courses, but no significant declines in vision beyond initial baseline. The VA results for subjects 1 and 2 in our study are similar to those reported for a patient with Best vitelliform macular dystrophy who received retreatments with TES over a similar period [7]. There was an absence of funduscopic macular changes for the three individuals in our study; however, we were unable to quantify regional macular thickness or photoreceptor changes over time, since SD-OCT was not available at our center at the start of the treatments.
A previous study involving a retrospective review of records found that RP patients with either a bull’s-eye or geographic atrophic lesion had a predicted decrease in VA of 3–4 lines over a 5-year period [12]. Thus, RP patients with macular lesions, as was the case for our three participants, are likely to lose a significant amount of VA (~1.5 to 2 lines) over a 2.4 to 3 year period, which was not observed for the individuals who received TES in our case study. The mean annual rate of decline for GVF area in RP has been previously reported as ~10% across studies [13], and only one isopter in one eye of one of the three participants treated with TES in this longitudinal study developed a significant loss of GVF >10% in the first year (i.e., subject 1 OD III4e), which stabilized thereafter following more frequent retreatments.
The typical progression rates for the three RP patients in this study are unknown since they were not previously monitored using a standard protocol and validated visual function tests prior to joining this study and receiving TES. Since the progression rate is different for each individual with RP, we do not know yet whether TES was successful in reducing the rate of vision loss. However, the current study documented repeated significant improvements following each retreatment and prevention of significant losses in visual function. An interesting finding in the present study is that both eyes of an individual were treated with TES but both eyes did not respond similarly. In subjects 1 and 2, the eye with more advanced vision loss at baseline was the one that had a significant improvement, yet the underlying mechanism for this finding is not well understood. Future studies with a larger cohort of participants treated with TES and additional outcome measures will need to elucidate why one eye might have a greater tendency for improvement.
Neither of the previous clinical trials of TES for RP conducted by other groups [3–5] measured contrast sensitivity, which is an important aspect of visual functioning. The present study found qCSF improvements that correlated with improved VA, but in subject 2, contrast sensitivity with the qCSF test declined slightly prior to VA in the worse eye, and was more sensitive than VA for detecting declines in the better eye between the first and second treatment courses. The qCSF test may be a valuable outcome measure to include in future clinical trials. The mean rate of contrast sensitivity loss in RP patients has not been previously documented, which is another important area to pursue in order to help understand longitudinal changes.
Previous studies administered TES to RP subjects on a weekly basis continuously for a year instead of in six-week courses as in the present study. Future studies should determine if there are different outcomes that may be dependent on the frequency of administration of TES. It would be more convenient and less expensive if TES would be effective when administered periodically in 6 week courses instead of weekly on a continuous basis; however, it may be psychologically difficult for patients to lose vision, even temporarily, that they may gain after treatment. Therefore, it would be valuable to determine an ideal frequency of treatment courses with TES in which a minimal number of treatment sessions are provided to achieve efficacy, such that the visual improvements are maintained and effects do not diminish over time. We believe it is quite likely that longitudinally, over a period greater than 3 years, the efficacy of TES may be diminished due to the inherent RP disease processes, at which time visual function loss would occur and should be documented in future studies.
Although this small case series study did not involve masking to treatment and a placebo control, the encouraging findings of improved visual function with repeated treatment courses of TES and no evidence of vision loss over 2.4 to 3 years provide support for the conduct of a larger-scale, longitudinal randomized controlled trial of TES for RP. The current study also indicates that RP patients with measurable vision worse than 0.7 logMAR may benefit from TES, although the proportion of such patients who may develop a positive response is unknown, especially given the heterogeneity of RP. Another possible future investigative application of TES might be to determine if it can help serve as an adjunctive therapy to potentiate the effects of stem cell treatments for RP.
Acknowledgments
The authors wish to thank Marsha Zaman for her assistance with the study.
Funding This study was funded by the National Institutes of Health (NIH) R21 award EY023720 to AKB, and the Nova Southeastern University President’s Faculty Research and Development Grant award.
Footnotes
Compliance with ethical standards
Conflict of interest The authors declare that they have no conflict of interest.
Ethical approval All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent Informed consent was obtained from all individual participants included in the study.
References
- 1.Sehic A, Guo S, Cho KS, et al. Electrical stimulation as a means for improving vision. Am J Pathol. 2016;186(11):2783–2797. doi: 10.1016/j.ajpath.2016.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tao Y, Chen T, Liu ZY, et al. Topographic quantification of the Transcorneal electrical stimulation (TES)-induced protective effects on N-methyl-N-Nitrosourea-treated retinas. Invest Ophthalmol Vis Sci. 2016;57(11):4614–4624. doi: 10.1167/iovs.16-19305. [DOI] [PubMed] [Google Scholar]
- 3.Schatz A, Röck T, Naycheva L, et al. Transcorneal electrical stimulation for patients with retinitis pigmentosa: a prospective, randomized, sham-controlled exploratory study. Invest Ophthalmol Vis Sci. 2011;52(7):4485–4496. doi: 10.1167/iovs.10-6932. [DOI] [PubMed] [Google Scholar]
- 4.Schatz A, Pach J, Gosheva M, et al. Transcorneal electrical stimulation for patients with retinitis Pigmentosa: a prospective, randomized, sham-controlled follow-up study over 1 year. Invest Ophthalmol Vis Sci. 2017;58(1):257–269. doi: 10.1167/iovs.16-19906. [DOI] [PubMed] [Google Scholar]
- 5.Robles-Camarillo D, Niño-de-Rivera L, López-Miranda J, et al. The effect of transcorneal electrical stimulation in visual acuity: retinitis pigmentosa. J Biomed Sci Eng. 2013;6:1–7. [Google Scholar]
- 6.Bittner AK, Seger K, Salveson R, et al. Randomized controlled trial of electro-stimulation therapies to modulate retinal blood flow and visual function in retinitis Pigmentosa. Acta Ophthalmol. 2017 doi: 10.1111/aos.13581. [DOI] [PMC free article] [PubMed]
- 7.Ozeki N, Shinoda K, Ohde H, et al. Improvement of visual acuity after transcorneal electrical stimulation in case of best vitelliform macular dystrophy. Graefes Arch Clin Exp Ophthalmol. 2013;251(7):1867–1870. doi: 10.1007/s00417-013-2341-4. [DOI] [PubMed] [Google Scholar]
- 8.Bittner AK, Iftikhar MH, Dagnelie G. Test-retest, within-visit variability of Goldmann visual fields in retinitis pigmentosa. Invest Ophthalmol Vis Sci. 2011;52(11):8042–8046. doi: 10.1167/iovs.11-8321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barry MP, Bittner AK, Yang L, Marcus R, Iftikhar MH, Dagnelie G. Variability and errors of manually digitized Goldmann visual fields. Optom Vis Sci. 2016;93(7):720–730. doi: 10.1097/OPX.0000000000000869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Morimoto T, Fukui T, Matsushita K, et al. Evaluation of residual retinal function by pupillary constrictions and phosphenes using transcorneal electrical stimulation in patients with retinal degeneration. Graefes Arch Clin Exp Ophthalmol. 2006;244(10):1283–1292. doi: 10.1007/s00417-006-0260-3. [DOI] [PubMed] [Google Scholar]
- 11.Naycheva L, Schatz A, Röck T, et al. Phosphene thresholds elicited by transcorneal electrical stimulation in healthy subjects and patients with retinal diseases. Invest Ophthalmol Vis Sci. 2012;53(12):7440–7448. doi: 10.1167/iovs.12-9612. [DOI] [PubMed] [Google Scholar]
- 12.Flynn MF, Fishman GA, Anderson RJ, Roberts DK. Retrospective longitudinal study of visual acuity change in patients with retinitis Pigmentosa. Retina. 2001;21(6):639–646. doi: 10.1097/00006982-200112000-00012. [DOI] [PubMed] [Google Scholar]
- 13.Grover S, Fishman GA, Anderson RJ, Alexander KR, Derlacki DJ. Rate of visual field loss in retinitis pigmentosa. Ophthalmology. 1997;104(3):460–465. doi: 10.1016/s0161-6420(97)30291-7. [DOI] [PubMed] [Google Scholar]