Abstract
Reproductive lesions have been described in various non-human primate species including rhesus macaques (Macaca mulatta), cynomolgus macaques (Macaca fasicularis), baboons (Papio spp.), squirrel monkeys (Saimiri sciureus), and chimpanzees (Pan spp.), yet there are few publications describing reproductive disease and pathology in Japanese macaques (Macaca fuscata). A retrospective evaluation of post-mortem reports for two captive M. fuscata populations housed within zoos from 1982 through 2015 was completed comparing reproductive diseases diagnosed by gross pathology and histopathology. Disease prevalence, organs affected, and median age at death between the two institutions was also compared. Fifteen female captive M. fuscata ranging in age from 15 to 29 years were identified with reproductive tract lesions including endometriosis, endometritis, leiomyoma, leiomyosarcoma, and adenomyosis. No significant differences were identified in disease prevalence, organs affected, and median age of death between the two institutions. Endometriosis was the most common disease process identified, found in ten out of fifteen (66.7%) cases, followed by leiomyoma (26.7%). In four cases (26.7%), severe endometriosis and secondary hemorrhage was indicated as the cause of death or primary reason for humane euthanasia. These findings were compared to a separate population of Japanese macaques managed within a research facility in the U.S. with a prevalence of endometriosis of 7.6%. This study discusses possible risk factors and potential treatment options for the management of endometriosis in captive M. fuscata.
Keywords: Reproduction, Endometriosis, Japanese macaque, Leiomyoma, Macaca fuscata
INTRODUCTION
Nonhuman primates have been pivotal in the understanding of both animal and human female reproductive physiology. Primate use as a human model has improved human diagnostics and treatment regimens for reproductive disease processes. Various literature has been published on disease processes affecting the reproductive tract regarding multiple non-human primate species, including rhesus macaques (Macaca mulatta), cynomolgus macaques (Macaca fasicularis), baboons (Papio spp.), squirrel monkeys (Saimiri sciureus), chimpanzees (Pan spp.), and orangutans (Pongo spp.). Although information is available on the aforementioned species, reports of female reproductive pathology in Japanese macaques (Macaca fuscata) or captive Macaca spp. housed within zoological institutions is lacking.
A female Japanese macaque has an estimated lifespan of approximately 25 years of age, with a single report of a female living to 32 years old.28 Females become reproductively mature between 2–4 years of age with the majority of fecundity between 5–19 years of age, although parturition has been documented in fertile females up to 25 years of age.19, 31
This report evaluates and compares the occurrence of reproductive lesions in female M. fuscata housed within two zoological institutions and compares these parameters and the age of death between the two institutions.
MATERIALS AND METHODS
Necropsy reports were evaluated from the University of Minnesota’s Veterinary Diagnostic Laboratory for individual animals submitted from two zoological institutions, Blank Park Zoo (BPZ) in Des Moines, IA USA and Minnesota Zoological Garden (MZG) in Apple Valley, MN USA. All reports were from adult female Japanese macaques that died between the ages of 15 and 30 years of age. Necropsies were performed within 48 hours of death and representative sections of the reproductive tract were collected, fixed in 10% neutral buffered formalin, embedded in paraffin, sectioned, and stained with hematoxylin and eosin. A total of 17 individual necropsy reports were evaluated, eight animals from BPZ and nine from MZG.
MZG acquired nineteen individuals (6 male, 13 female) in 1978 from the Arashiyama West colony in Laredo, Texas. The Arashiyama West colony was founded in 1972 by the transfer of 150 individuals from Kyoto, Japan. The MZG colony has historically been comprised of approximately 26 macaques, approximately one third male and two thirds female. Breeding and the production of offspring was frequent until 1992 when males were surgically castrated or vasectomized to inhibit reproduction. In 2002, a reproductively intact male was introduced and breeding resumed at that time. No contraceptive measures were instituted within the female population during the period covered by this survey.
BPZ acquired the first nine individuals of the colony from MZG in 1987. The BPZ colony has been historically comprised of approximately 15 macaques at any given time, approximately one quarter male and three quarters female. In the 1990’s, males within BPZ were surgically castrated or vasectomized to inhibit the production of offspring. Female macaques remained intact with no contraceptives administrated and were allowed to cycle naturally. In 2009, breeding with natural mating resumed with the introduction of a reproductively mature intact male.
Fifteen out of seventeen necropsy reports were included in statistical analysis. Criteria for inclusion required full necropsy reports with both gross evaluation and histopathology. Two cases did not meet the criteria for inclusion but are included in the discussion. Age at death was assessed for normality through assessment of skewness, kurtosis, normality plots and Shapiro-Wilk test for normality. This variable was presented as median and range given a non-normal distribution. Categorical variables including presence of disease in different locations in the urogenital tract, and different specific lesions detected, were summarized by frequencies and percentages. Fisher’s exact tests were used to compare these categorical variables between the two collections. Age at death was compared between the two collections using a Mann-U Whitney test. The statistical analysis was performed using commercially available software. A statistically significant difference was declared if P < or equal to 0.05.
RESULTS
Necropsies were performed on 15 female Japanese macaques ranging from 15 to 29 years of age. A summary of necropsy findings is shown in Table 1. Lesions of the reproductive tract are described in Table 2. In total, 14 of 15 individuals had reproductive tract lesions of varying types. The youngest individual was the only animal in which no reproductive lesions were identified. Of the lesions identified, the uterus was affected in 13/15 (86.7%), ovaries 2/15 (13.3%), vagina 2/15 (13.3%), urinary tract 2/15 (13.3%), gastrointestinal tract 5/15 (33.3%), and omentum/abdominal cavity 6/15 (40.0%). The uterine lesions were macroscopically characterized as either individual firm beige delineated or infiltrative masses in the uterine wall or firm beige poorly defined and frequently confluent nodules of the uterine wall and adjacent mesometrial adipose tissue leading to an enlargement and distortion of the uterus and mesometrium (Fig. 1).
Table 1.
Locations of lesions associated with reproductive disease in individuals of two captive populations of Japanese macaques (Macaca fuscata)
| Case number | Age (years) at death | Number of offspring | Uterus | Ovary | Vagina | Urinary tract | Gastrointestinal tract | Omentum/Abdominal Cavity |
|---|---|---|---|---|---|---|---|---|
| 1 | 15 | 0 | − | − | − | − | − | − |
| 2 | 18 | 1 | + | − | − | − | − | − |
| 3 | 19 | 0 | − | − | + | − | − | − |
| 4 | 19 | 0 | + | − | − | − | + | − |
| *5 | 20 | 6 | + | − | − | − | − | − |
| 6 | 22 | 2 | + | − | − | + | + | − |
| 7 | 23 | 0 | + | − | − | − | − | + |
| 8 | 23 | 5 | + | + | − | − | − | + |
| *9 | 23 | 0 | + | − | − | − | − | − |
| 10 | 24 | 0 | + | + | − | − | − | + |
| 11 | 24 | 1 | + | − | + | − | + | − |
| 12 | 25 | 0 | + | − | − | + | + | + |
| 13 | 25 | 6 | + | − | − | − | − | − |
| 14 | 27 | 1 | + | − | − | − | + | − |
| 15 | 27 | 0 | + | − | − | − | − | + |
| 16 | 28 | 0 | + | − | − | − | − | + |
| 17 | 29 | 4 | + | − | − | − | − | − |
designates cases not included in the statistical analysis
Table 2.
Incidence of reproductive lesions described in 15 female Japanese macaques (Macaca fuscata).
| Pathology Described | n |
|---|---|
| Endometriosis | 10 |
| Endometrial hyperplasia | 4 |
| Leiomyoma | 4 |
| Endometritis | 1 |
| Leiomyosarcoma | 1 |
| Adenomyosis | 1 |
| Granulosa cell tumor | 1 |
| Dystrophic mineralization | 1 |
| Uterine rupture | 1 |
Figure 1.
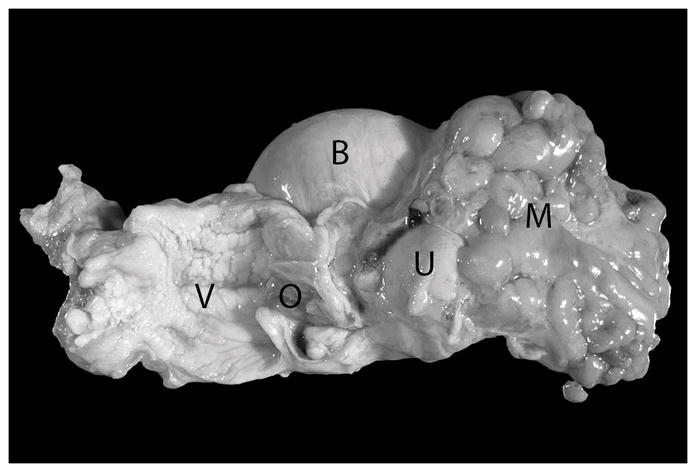
Vagina (opened – V), uterine isthmus with external orifice (opened – O), uterine body (U), and urinary bladder of a 20 year old Japanese macaque (Macaca fuscata). An approximately 8 cm in diameter firm mass encompassing the uterine horns and ovaries and partially incorporating the uterine body is present (M). Mesometrial and serosal adipose tissue is broadly adherent to this mass.
Endometriosis was diagnosed by histopathology in 10 individuals (Table 2). Endometriosis was histologically characterized by the presence of well differentiated endometrial glands supported by stromal cells in uterine serosal and mesometrial adipose tissue (Fig. 2). In two cases, endometriosis was the only lesion encountered. Five cases had endometriosis-associated hemorrhage of varying degrees. Four of these five cases had gross hemorrhage within body cavities implicated as the cause of death or contributed to clinical signs resulting in humane euthanasia. Three of these five cases had concurrent hemoabdomen, and one case had both hemoabdomen and hemothorax. One case had an endometriosis-associated uterine rupture. Six cases also noted endometriosis associated with extra-reproductive, intra-abdominal organs. In those cases, endometriosis was located on the serosal surface of the pelvic cavity, associated with the omentum, the serosal surface of the colon, and as free-floating grayish smooth masses within the abdominal cavity. Two cases had endometriosis contained within a single intrapelvic mass causing compression of the colon, one of which caused displacement of the bladder and colon, the other causing compression with secondary obstruction and impaction of the colon. One individual had dystrophic mineralization associated with endometriosis and three had concurrent endometrial hyperplasia. In addition, case 9 had gross lesions diagnosed as endometriosis, however this case was not included in the statistical analysis because histopathology was not performed. Adenomyosis, characterized by the presence of endometrial glands and stroma within the myometrium, was diagnosed in one individual with concurrent severe endometriosis and hemoabdomen.
Figure 2.
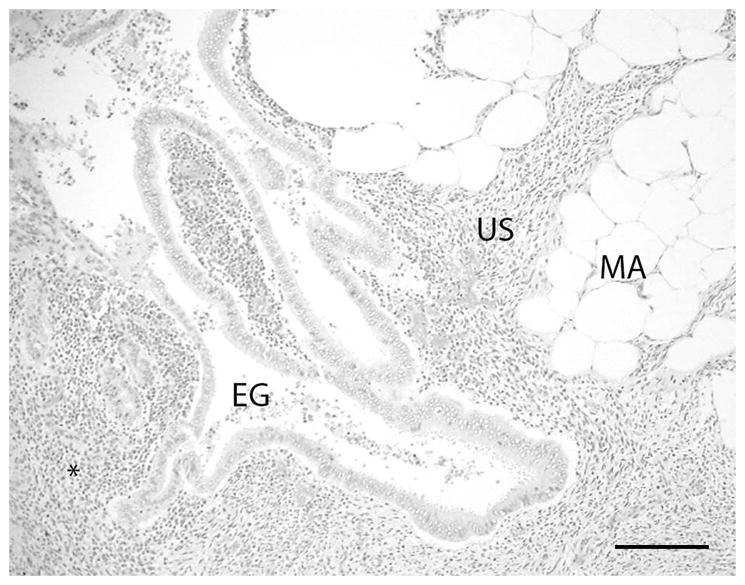
The mass consisted histologically of coalescing nodules composed of well differentiated endometrial glands (EG) with adjacent uterine stromal tissue (US) infiltrating into the serosal and mesometrial adipose tissue (MA). Lymphocytes and plasma cells and occasional neutrophilic granulocytes are present in the tissue surrounding the ectopic uterine glands (asterisk). A mild cellular exudate composed of neutrophilic granulocytes is present within the lumen of the glands. (H&E stain; bar = 200 microns)
Six reproductive lesions identified were neoplastic conditions: four uterine leiomyomas, one uterine leiomyosarcoma and one ovarian granulosa cell tumor. In case 13, leiomyoma was the only reproductive lesion described. In case 14, the leiomyoma compressed the left ureter resulting in megaureter and hydronephrosis. This case was also diagnosed with concurrent endometritis and endometrial hyperplasia. Leiomyoma was also diagnosed in case 12, with concurrent endometrial hyperplasia and the previously mentioned endometriosis-associated uterine rupture. A subserosal uterine leiomyoma was identified in case 8, concurrent with a granulosa cell tumor of the left ovary, and endometriosis. In case 5, a leiomyoma was diagnosed antemortem, in the uterus of a reproductive tract submitted for histopathology following an ovariohysterectomy performed as part of a caesarean section. This case was not included in statistical analysis. Case 7 was diagnosed with a leiomyosarcoma with tissue calcifications associated within the uterus.
The extent of reproductive lesions did not correlate with numbers of offspring produced within this population set as lesions were found in individuals ranging from zero to six births per individual (Table 1.). Of the 10 individuals diagnosed with endometriosis, five never gave birth to offspring, two had given birth once, and single cases gave birth to two, four, and five offspring respectively. Of the four individuals diagnosed with endometrial hyperplasia, two cases never produced offspring, and single cases gave birth to one, and four offspring. Of the four individuals diagnosed with a leiomyoma, one case never produced offspring, and single cases gave birth to one, five, and six offspring.
There were no differences in the age at death between the two zoological institutions (median age 24 years). No significant difference was found regarding the number of individuals with disease associated with the uterus, ovary, vagina, urinary tract, GI tract, or omentum/abdominal cavity between the two institutions. Finally, no significant difference was found with respect to histologically diagnosed lesions between the two institutions.
DISCUSSION
Endometriosis is a chronic gynecological disease in which hormonally responsive endometrial glands and stroma are located ectopically outside the uterine cavity, most commonly in the pelvic peritoneum, ovaries and rectovaginal septum.6 The most supported theory, the Sampson Hypothesis, proposes that endometriosis arises from the retrograde menstruation of endometrium through the Fallopian tubes into the peritoneal cavity.12, 24 As a result, decreased fertility, ectopic tissue formation, abdominal discomfort, defective ovarian function, menstrual irregularity, embryo implantation failure, and excessive uncontrollable hemorrhagic conditions can occur.17 Endometriosis is the most commonly recognized gynecological disease of menstruating non-human primates and was identified as the most common histological finding (66%) within this evaluation of 15 female M. fuscata post-mortem reports.30 The prevalence of the disease in zoological populations of Japanese macaques has not been previously reported. Although the prevalence varies considerably by species, the prevalence of endometriosis is much higher in these two M. fuscata populations compared to published reports in other non-human primate species.6, 22, 30
In an intraspecies comparison, the findings in the populations maintained within two zoological institutes differ considerably from a separate population of Japanese macaques managed within a biomedical research facility in the U.S. In that population, a review of necropsy findings of 52 female Japanese macaque 15 years and older, which were examined between 2000 and 2016, 23 animals had lesions of the reproductive tract. The most common diagnoses were endometriosis (4/52) and uterine leiomyoma (5/52). (Lewis, unpubl. data) That population was derived from a troop imported directly from the Hiroshima prefecture in 1965 and is genetically distinct from the zoo populations reported here.2 Although differences in management and medical history precluded incorporating this population into the present study, it is interesting to note that the incidence of endometriosis (7.6%) is significantly less than the population analyzed in this evaluation.
Factors influencing the development of endometriosis have been well documented in women and in non-human primates. Identified risk factors include advanced age, parity, past surgical interventions, long periods of time of uninterrupted menstrual cycles, exposure to toxins and radiation, and genetic predisposition.5, 7, 8, 9, 14, 15, 21, 23, 24, 31 Surgical interventions, toxin exposure, and radiation (outside of periodic radiographic diagnostics) were not documented in this study population. Due to the range of births present within the populations (0–6), the contribution of fecundity for the development of disease in these populations is uncertain. The factors most likely associated with the high prevalence of endometriosis within these two zoo populations are long periods of uninterrupted cycling and genetic variables. Both zoological institutions went through a period of reproductive management in which reproduction was inhibited for ≥10 years, but allowed normal menstrual cycling before the reintroduction of a fertile male. Genetic predisposition of endometriosis in both women and non-human primates is well documented.8, 14, 24, 31 All individuals within this study have familial lines originated from the Arashiyama West colony, making genetic similarities highly likely between the two groups in contrast to the research population derived from another region in Japan. In the two zoo colonies, small troop sizes and nonselective breeding greatly increases the chances of closely related breeding. Although kinship coefficients for the two zoo populations would be expected to support the level of genetic similarities, due to the high level of sire uncertainty these factors were unable to be determined.
Endometriosis-associated hemorrhage was present in 50% of all identified endometriosis cases and a cause for mortality in four cases. Hemorrhage resulted in sequelae such as hypovolemia leading to circulatory collapse, cardiac arrest, and hepatic necrosis.
The most common neoplasia reported in female non-human primate reproductive organs is uterine leiomyomas, benign neoplasms arising from the smooth muscle within the uterine wall. Although all malignant neoplastic conditions are generally considered rare, the second most commonly reported reproductive neoplasm is endometrial adenocarcinoma.4, 11, 13, 30 Tumors of the female reproductive tract comprise approximately 15% of all tumors in non-human primates and 5–10% of all malignancies.6 Six individuals within this study had reproductive neoplasia identified by histopathology. The uterus was the most commonly identified organ affected by neoplasia within the two colonies (83.3%). Four had leiomyomas, one had leiomyosarcoma. The incidence of ovarian neoplasia was 16.6% represented by a single granulosa cell tumor. In the two cases of neoplastic disease with metastatic potential, the granulosa cell tumor and the leiomyosarcoma, metastases were not identified at necropsy.
The lack of differences in the types of reproductive lesions and the similarities of age at death found between BPZ and MZG were not unexpected. Both troops have been managed similarly over the last 30 years and both troops are largely derived from the same wild population.
Due to the high prevalence of endometriosis found within the two sample populations, and the potential life threatening consequences, discussion of treatment modalities is warranted. Both hormonal and surgical treatment of endometriosis is well documented in both women and non-human primates.10, 14, 16, 20 Surgical management can be further categorized as conservative or definitive. Conservative surgical treatment includes the preservation of fertility or the corrective management to infertility by resecting all ectopic endometrial tissues, removal of adhesions, and reconstruction of pelvic anatomy. Definitive surgical treatment involves complete hysterectomy and/or bilateral oophorectomy, along with resection of all ectopic endometrial tissues making treatment curative and preventing reoccurrence. Due to the goal of preserving fertility, conservative surgical treatment is much more common in women than primates.10 The recurrence rate of symptoms and lesions vary from 10 – 55% in women within 12 months post conservative surgical intervention.16
Hormonal alteration is a less invasive and lower cost treatment option. This treatment alters ovarian hormone levels throughout the body by reducing function of the ovary itself. A decrease in ovarian hormones allows other tissues to stop normal hormonally induced processes from occurring, including menstruation. Both oral and non-oral hormone contraceptives are currently used in both women and non-human primates for the treatment of endometriosis. Types of treatments include gonadotropin-based options such as GnRH agonists and progestin-based treatments.14, 16, 20 Although hormone-based treatments can induce a menopause-like state, they do not come without risks. Selective steroid regulation contributes to an altered metabolic endocrine status and potential subsequent secondary complications such as vasomotor symptoms, bone mineral density changes, and cardiovascular risks.25
Of the previously mentioned treatment options, prophylactic age-associated oophorectomy is the recommended treatment of choice to stop the potential formation of endometriosis and/or progression of disease. If surgical intervention is not an option, non-oral contraceptive methods using GnRH are recommended. Further research is recommended to explore the on-going prevalence of endometriosis and other reproductive diseases once prophylactic treatment is initiated in aged female M. fuscata.
Acknowledgments
The authors would like to thank the staff at the Blank Park Zoo and the Minnesota Zoo and Gardens for assisting in acquiring historical data and records. Special thanks to Dr. Anibal Armien, Veterinary Pathologist at University of Minnesota Veterinary Diagnostic Laboratory, Myrna Booth, Registrar at Blank Park Zoo, Tom Ness, Japanese macaque SSP studbook keeper, and Dr. David Hummel.
LITERATURE CITED
- 1.Alexander BK, Hall AS, Bowers JM. A primate corral. J Am Vet Med Assoc. 1969;155(7):1144–1150. [PubMed] [Google Scholar]
- 2.Barrier BF, Allison J, Hubbard GB, Dick EJ, Jr, Brasky KM, Schust DJ. Spontaneous adenomyosis in the chimpanzee (Pan troglodytes): a first report and review of the primate literature: case report. Hum Reprod. 2007;22(6):1714–1717. doi: 10.1093/humrep/dem038. [DOI] [PubMed] [Google Scholar]
- 3.Brown SL, Anderson DC, Dick EJ, Jr, Guardado-Mendoza R, Garcia AP, Hubbard GB. Neoplasia in the chimpanzee (Pan spp.) J Med Primatol. 2009;38(2):137–144. doi: 10.1111/j.1600-0684.2008.00321.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burney R, Giudice LC. Pathogenesis and pathophysiology of endometriosis. Fertil Steril. 2012;8(3):511–519. doi: 10.1016/j.fertnstert.2012.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coe CL, Lemieux AM, Rier SE, Uno H, Zimbric ML. Profile of endometriosis in the aging female rhesus monkey. J Gerontol A Biol Sci Med Sci. 1998;53(1):M3–7. doi: 10.1093/gerona/53a.1.m3. [DOI] [PubMed] [Google Scholar]
- 6.Cooper TK, Gabrielson KL. Spontaneous lesions in the reproductive tract and mammary gland of female non-human primates. Birth Defects Res B Dev Reprod Toxicol. 2007;80(2):149–170. doi: 10.1002/bdrb.20105. [DOI] [PubMed] [Google Scholar]
- 7.D’Hooghe TM, Kyama CM, Chai D, Fassbender A, Vodolaskaia A, Bokor A, Mwenda JM. Nonhuman primate models for translational research in endometriosis. Reprod Sci. 2009;16(2):152–161. doi: 10.1177/1933719108322430. [DOI] [PubMed] [Google Scholar]
- 8.Dick EJ, Jr, Hubbard GB, Martin LJ, Leland MM. Record review of baboons with histologically confirmed endometriosis in a large established colony. J Med Primatol. 2003;32(1):39–47. doi: 10.1034/j.1600-0684.2003.00008.x. [DOI] [PubMed] [Google Scholar]
- 9.Fanton JW, Golden JG. Radiation-induced endometriosis in Macaca mulatta. Radiat Res. 1991;126(2):141–146. [PubMed] [Google Scholar]
- 10.Fanton JW, Yochmowitz MG, Wood DH, Salmon YL. Surgical treatment of endometriosis in 50 rhesus monkeys. Am J Vet Res. 1986;47(7):1602–1604. [PubMed] [Google Scholar]
- 11.Fazleabas AT, Brudney A, Gurates B, Chai D, Bulun S. A modified baboon model for endometriosis. Ann N Y Acad Sci. 2002;995(1):308–317. doi: 10.1111/j.1749-6632.2002.tb02791.x. [DOI] [PubMed] [Google Scholar]
- 12.Graham KJ, Hulst FA, Vogelnest L, Fraser IS, Shilton CM. Uterine adenomyosis in an orangutan (Pongo abelii/pygmaeus) Aust Vet J. 2009;87(1–2):66–69. doi: 10.1111/j.1751-0813.2008.00370.x. [DOI] [PubMed] [Google Scholar]
- 13.Green AL, Tolwani RJ, Waggie KS, Otto GM. Endometriosis and a paraovarian cyst in a rhesus macaque. Vet Radiol Ultrasound. 1999;40(3):271–274. doi: 10.1111/j.1740-8261.1999.tb00361.x. [DOI] [PubMed] [Google Scholar]
- 14.Hadfield RM, Yudkin PL, Cow CL, Scheffler J, Uno H, Barlow DH, Kemnitz JW, Kennedy SH. Risk factors for endometriosis in the rhesus monkey (macaca mulatta): a case-control study. Hum Reprod. 1997;3(2):109–115. doi: 10.1093/humupd/3.2.109. [DOI] [PubMed] [Google Scholar]
- 15.Haghpeykar HS, Poindexter AN., III Epidemiology of endometriosis among parous women. Obstet Gynecol. 1995;85(6):983–992. doi: 10.1016/0029-7844(95)00074-2. [DOI] [PubMed] [Google Scholar]
- 16.Johnson NP, Hummelshoj L. Consensus on current management of endometriosis. Hum Reprod. 2013;28(6):1552–1568. doi: 10.1093/humrep/det050. [DOI] [PubMed] [Google Scholar]
- 17.Kaspareit J, Friderichs-Gromoll S, Buse E, Habermann G. Spontaneous neoplasms observed in cynomolgus monkeys (Macaca fascicularis) during a 15-year period. Exp Toxicol Pathol. 2007;59(3):163–169. doi: 10.1016/j.etp.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 18.Koyama N, Takahata Y, Huffman MA, Norikoshi K, Suzuki H. Reproductive parameters of female Japanese macaques: thirty years data from the Arashiyama troops, Japan. Primates. 1992;33(1):33–47. [Google Scholar]
- 19.Marr-Belvin AK, Bailey CC, Knight HL, Klumpp SA, Westmoreland SV, Miller AD. Ovarian pathology in rhesus macaques: a 12-year retrospective. J Med Primatol. 2010;39(3):170–176. doi: 10.1111/j.1600-0684.2010.00409.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mattison JA, Ottinger MA, Powell D, Longo DL, Ingram DK. Endometriosis: clinical monitoring and treatment procedures in rhesus monkeys. J Med Primatol. 2007;36(6):391–398. doi: 10.1111/j.1600-0684.2006.00208.x. [DOI] [PubMed] [Google Scholar]
- 21.Rier SE, Martin DC, Bowman RE, Dmowski WP, Becker JL. Endometriosis in rhesus monkeys (Macaca mulatta) following chronic exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin. Fundam Appl Toxicol. 1993;21(4):433–441. doi: 10.1006/faat.1993.1119. [DOI] [PubMed] [Google Scholar]
- 22.Slayden OD. Induced endometriosis in nonhuman primates. Biol Reprod [Internet] 2013;88(2):43, 1–2. doi: 10.1095/biolreprod.113.107722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Splitter GA, Kirk JH, Mac Kenzie WF, Rawlings CA. Endometriosis in four irradiated rhesus monkeys. Vet Path. 1972;9(4):249–262. doi: 10.1177/030098587200900404. [DOI] [PubMed] [Google Scholar]
- 24.Stefansson H, Geirsson RT, Steinthorsdottir V, Jonsson H, Manolescu A, Kong A, Ingadottir G, Culcher J, Stefansson K. Genetic factors contribute to the risk of developing endometriosis. Hum Reprod. 2002;17(3):555–559. doi: 10.1093/humrep/17.3.555. [DOI] [PubMed] [Google Scholar]
- 25.Surrey ES. Steroidal and nonsteroidal “add back” therapy: extending safety and efficacy of gonadotropin-releasing hormone agonist in the gynecologic patient. Fertil Steril. 1995;64(4):673–685. doi: 10.1016/s0015-0282(16)57837-6. [DOI] [PubMed] [Google Scholar]
- 26.Tardif S, Carville A, Elmore D, Williams LE, Rice K. Reproduction and breeding of nonhuman primates. In: Abee CR, Mansfield K, Trardif S, Morris T, editors. Nonhuman primates in biomedical research: biology and management. 2. Vol. 1. San Diego (CA): Elsevier; 2012. pp. 197–237. [Google Scholar]
- 27.Uno H. Age-related pathology and biosenescent markers in captive rhesus macaque. Age. 1997;20(1):1–13. doi: 10.1007/s11357-997-0001-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Videan EN, Satterfield WC, Buchl S, Lammey ML. Diagnosis and prevalence of uterine leiomyomata in female chimpanzees (Pan troglodytes) Am J Primatol. 2011;73(7):665–670. doi: 10.1002/ajp.20947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wilkinson M, Walters A, Smith T, Wilkinson A. Reproductive abnormalities in aged female Macaca fascicularis. J Med Primatol. 2008;37(s1):88–93. doi: 10.1111/j.1600-0684.2007.00268.x. [DOI] [PubMed] [Google Scholar]
- 30.Wolfe LD, Sabra Noyes MJ. Reproductive senescence among female Japanese macaques (Macaca fuscata fuscata) J Mammal. 1981;62(4):698–705. [Google Scholar]
- 31.Zondervan KT, Weeks DE, Colman R, Cardon LR, Hadfield R, Schleffler J, Goudy Trainor A, Coe CL, Kemnitz JQ, Kennedhy SH. Familial aggregation of endometriosis in a large pedigree of rhesus macaques. Hum Reprod. 2004;19(2):448–455. doi: 10.1093/humrep/deh052. [DOI] [PubMed] [Google Scholar]


