Abstract
Porcine circovirus type 2 (PCV2), an immunosuppression pathogen, is often found to increase the risk of other pathogenic infection. Yet, the relative immune mechanisms determining the susceptibility of PCV2-infected animals remain unclear. Herein, we confirmed that PCV2 infection suppressed IL-12p40 expression and host Th1 immune response, leading to a weakened pathogenic clearance upon PRRSV or H. parasuis infection. PCV2 infection suppressed pathogens, LPS/IFN-γ or LPS/R848-induced IL-12p40 expression in porcine alveolar macrophages. PCV2 Cap was the major component to suppress IL-12p40 induction by LPS/IFN-γ, LPS/R848, PRRSV or H. parasuis. Either wild-type PCV2 or mutants PCV2-Rep1 and PCV1-Cap2 which contained PCV2 Cap significantly decreased IL-12p40 levels and increased the replication of PRRSV and H. parasuis in the lung tissues relative to mock or PCV1 infection. gC1qR, a Cap binding protein, was not involved in IL-12p40 induction, but mediated the inhibitory effect of PCV2 Cap on IL-12p40 induction. PCV2 also activated PI3K/Akt1 and p38 MAPK signalings to inhibit IL-12p40 expression via inhibition of NF-kB p65 binding to il12B promoter and upregulation of miR-23a and miR-29b. Knockdown of Akt1 and p38 MAPK down-regulated miR-23a and miR-29b and increased IL-12p40 expression. Inhibition of miR-23a and miR-29b attenuated the inhibitory effect of PCV2 on IL-12p40 induction, resulting in an increased IL-12p40 expression and Th1 cells population and reduced susceptibility to PRRSV or H. parasuis. Taken together, these results demonstrate that PCV2 infection suppresses IL-12p40 expression to lower host Th1 immunity to increase the risk of other pathogenic infection via gC1qR-meidated PI3K/Akt1 and p38 MAPK signalings activation.
Keywords: Piglets, Porcine alveolar macrophages, PCV2, IL-12p40, Immunity suppression
Introduction
Porcine circovirus (PCV) is a nonenveloped single-stranded DNA virus, contains the non-pathogenic PCV1 and pathogenic PCV2 (1, 2). PCV have two major open reading frames (ORF), ORF1 encoding the replicase of virus (Rep) and ORF2 encoding the only capsid (Cap) (1). Rep is the most conserved protein between PCV1 and PCV2, while Cap is significantly divergent (1). PCV2 is the primary causative pathogen of porcine circovirus associated diseases (PCVAD), which is among the most economically significant diseases wasting the global swine industry today (2). PCVAD is a multifactorial disease that is usually observed co-infection of PCV2 with other pathogens, such as porcine reproductive respiratory syndrome virus (PRRSV), porcine parvovirus (PPV), or haemophilus parasuis (H. parasuis), et. al (3, 4). PCV2 infection is required for the occurrence of PCVAD, but PCV2 infection alone rarely produces the full spectrum or severity of clinical disease (5, 6). Thus, PCV2 infection is considered to affect the host immune system, which leads to increased susceptibility in PCV2-infected animals (7).
In response to microbial pathogens attack, interleukin-12 (IL-12), as a key pro-inflammatory cytokine, plays a pivotal role in the generation of Th1 immune for combating of pathogens infection (8, 9). IL-12 is a 70 kDa heterodimeric cytokine composed of p35 and p40 subunits, and produced by antigen presenting cells (APCs), including monocytes/macrophages, dendritic cells and B cells (10). IL-12p40 regulation is considered to be more critical for IL-12 production, comparing to IL-12p35 that can’t be secreted without binding to IL-12p40 (11, 12). Thus IL-12p40 seems to play a more dominant role in the promoting of Th1 cell development (13, 14). Several studies have proved that PCV2 infection inhibits the IL-12p40 expression both in vivo and in vitro (15–18). However, the molecular mechanisms underlying PCV2 inhibition of IL-12p40 expression are remaining to be determined.
In this study, we firstly determined the Th1 immune response and IL-12p40 production of PCV2-infected piglets challenged with PRRSV or H. parasuis, and confirmed that PCV2 infection suppressed the host Th1 immune response to PRRSV or H. parasuis through the inhibition of IL-12p40 induction in macrophages. Then we further analyzed and determined the correlation of IL-12p40 induction, Th1 cell percentage and pathogenic clearance, and identified the roles of PCV2 Rep and Cap in its suppression of IL-12p40 expression. Results showed that PCV2 Cap play a predominant role in inhibition of IL-12p40 expression induced by other pathogens or Toll-like receptor agonists in PCV2-infected PAMs. PCV2 Cap-binding protein gC1qR and PCV2-activated PI3K/Akt1 and p38 MAPK signalings, as well as upregulated miR-23a and miR-29b, are the key regulators in PCV2 inhibition of IL-12p40. These results would be helpful to explain how PCV2 infection suppresses IL-12 production to increase the risk of other infection.
Materials and methods
Ethics statement
All animal experiments were approved by the Institutional Animal Care and Use Committee (IACUC) of Northwest A&F University (permit number: 20161013 & 20170924), and were performed according to the Animal Ethics Procedures and Guidelines of the People’s Republic of China. No other specific permissions were required for these activities. This study did not involve endangered or protected species.
Cells, viruses and reagents
PK-15 cells, Marc-145 cells, and 3D4/21 cells (CRL-2843) were purchased from ATCC and maintained in our laboratory. The primary porcine alveolar macrophages (PAMs) were obtained from the lungs of piglets as previously described (19). Peripheral blood mononuclear cells (PBMCs) were prepared by centrifugation over Lymphocyte Separation Medium (P8610, Solarbio, China) as manufacturer’s instruction. PK-15 cells and Marc-145 were maintained in Dulbecco’s minimum essential medium (DMEM) (12100046, Invitrogen) supplemented with 10% heat-inactivated fetal bovine serum (FBS) (13011-8611, Tianhang Biotechnology, China). 3D4/21 and primary PAMs were cultured in RPMI 1640 medium (31800022, Invitrogen) with 10% heat-inactivated FBS, sodium pyruvate (11360070, Gibco), nonessential amino acids (11140050, Gibco), 100 U/ml penicillin and 0.1 mg/ml streptomycin. PCV1 (AY193712) and PCV2 (EU366323) were stocked in our laboratory and propagated in PK-15 cells. PRRSV (FJ548855.1) previously isolated by our team was propagated in Marc-145 cells (3). The copy numbers of the viruses were quantified by real time PCR and the 50% tissue culture infective dose (TCID50) were measured by the Reed-Muench method. Haemophilus parasuis was isolated from the native herd, and cultured with Tryptone soya agar (TSA, 22091, Sigma-Aldrich) and Tryptone soya broth (TSB, 51228, Sigma-Aldrich), supplemented with 2% beta-Nicotinamide adenine dinucleotide trihydrate (NAD, N7004, Sigma-Aldrich) and 5% FBS.
The toll-like receptor agonist LPS (L2880) and R848 (tlrl-r848) was purchased from Sigma and InvivoGen, respectively. The IFN-γ (985-PI) was purchased from R&D. The flow cytometry buffers, monensin and APC-conjugated anti-IFN-γ (502511, 4S.B3), FITC-conjugated anti-CD4 (317408, OKT4), APC-conjugated anti-CD68 (333810, Y1/82A), and PE-conjugated anti-IL-12p40 (501807, C11.5) were purchased from BioLegend.
Animal, housing and experiment design
Six-weeks-old cross-bred piglets were purchased from a native herd free of PCV2, PRRSV, PPV, haemophilus parasuis and other major swine pathogens as determined by PCR and ELISA. All piglets were housed under the same conditions and treated in a similar way. For the first experiment, thirty piglets were randomly divided into 3 groups and inoculated with PCV1 (4×105 TCID50), PCV2 (4×105 TCID50), or mock (same volume DMEM) for 1 week, respectively. Then the pigs were further challenged with PRRSV (105 TCID50) or H. parasuis (108 CFU) for another 24 h. For the second experiment, another seventy piglets were divided into 7 groups to inoculate PCV1 (4×105 TCID50), PCV2 (4×105 TCID50), PCV1 mutants (4×105 TCID50), PCV2 mutants (4×105 TCID50), or mock (same volume DMEM), respectively, and further challenged with PRRSV (105 TCID50) or H. parasuis (108 CFU). The PAMs and PBMCs of the piglets were prepared for further analysis.
Infection and stimulation of porcine alveolar macrophages
PAMs were seeded into 6-well plates at 1×106 cells/well, and were mock-infection, PCV1 mutants-infection (MOI of 1), PCV2 mutants-infection (MOI of 1), PCV1-infection (MOI of 1), or PCV2-infection (MOI of 1). At 24 hours post infection (h.p.i.), the media were refreshed, and then the cells were challenged with PRRSV (MOI of 1) or H. parasuis (MOI of 1), or stimulated with LPS (1000 ng/ml) plus IFN-γ (100 ng/ml) or LPS (1000 ng/ml) plus R848 (5 μg/ml). The supernatants of the cells were measured by ELISA, and IL-12p40 protein and mRNA levels of the cells were measured by flow cytometry and quantitative PCR, respectively.
For siRNA transfection, PAMs seeded in 6-well plates were transfected with 100 nM Akt1, p38, or ERK1 specific siRNAs for 24 h. Then the cells were infected by PCV2 and followed by LPS/IFN-γ stimulation. The siRNA used in this work were targeted to Akt1 (NM_001159776.1), p38 (XM_001929490.5), and ERK1 (NM_001198922.1), respectively. The Akt1 siRNA (si-Akt1) sequence was AACGAGGCGAGTACATCAAGA. The p38 siRNA (si-p38) sequence was AAGCTATCCAGACCATTTCAA. And the ERK1 siRNA (s-ERK1) sequence was AAGCTCTTGAAGACGCAGCAC.
ELISA
The supernatants of treated cells were harvested and the levels of IL-12p40 and IFN-γ secretion were measured by commercial enzyme-linked immunosorbent assay (ELISA) kit (P1240, R&D, and 430101, BioLegend) according to the manufacturer’s instructions.
Flow cytometry
The PBMCs and PAMs were harvested and washed by PBS, then the surface and intracellular proteins of the cells were stained according to the manufacturers illustrate. Briefly, the washed cells were resuspended with staining buffer and conjugated antibodies were added to stain the surface proteins. Then the cells were fixed and permeabilized to stain the intracellular proteins with the conjugated antibodies. After wash process, the stained cells were analyzed using BD Accuri™ C6 Flow Cytometer, and the gating strategies were showed in supplementary figure S1.
Quantitative PCR
The quantitative PCR (qPCR) were performed as previously described (19). Briefly, the total RNA of the cells was isolated by TRIzol (Invitrogen), the reverse transcriptions were performed with Random primers or microRNA’s specific primers. The expression levels were measured by SYBR-green based real-time PCR using a Bio-Rad IQ5 Real-Time PCR System. The primer sequences for PCV1 were TCTTTCGGCGCCATCTGTAA and CAGCAGCGCACTTCTTTCAC. The primers for PCV2 were ATAACCCAGCCCTTCTCCTACC and GGCCTACGTGGTCTACA TTTCC. And the primers for IL-12p40 (NM_214013.1) were TGTTCAAGTTC AGGGCAAGA and CAGGAGGAGCTGTAGTAGCG. The miRNAs primers were present in supplement table 1.
Luciferase reporter assays
The PAMs were transfected with the reporter vector containing NF-κB response elements based on pGL4 (pGL4.32[luc2P/NF-κB-RE/Hygro]) and the normalizing control vector pRL Renilla Luciferase (Promega) together with specific siRNAs for Akt1, p38, ERK1, or negative control, and then infected by mock or PCV2 for another 24 h, followed by LPS/IFN-γ stimulation. At 24 h post stimulation, the luciferase activities were determined with Dual-Luciferase reporter systems (Promega) according to the manufactory’s instruction.
Macrophage and PBMC cocultures
The PAMs were seeded into 6-well plates (1×106 cells per well), transfected with miRNA specific inhibitors, and then the cells were infected with PCV2 or mock for 24 h. Then PAMs were incubated with PBMC isolated from healthy piglets in a 1:1 ratio and further challenged with PRRSV or H. parasuis for 24 h. PBMC and the PAMs were harvested from the coculture by gentle pipetting, resuspended in 1 × PBS. The IL-12p40 expression by PAMs and IFN-γ expression by CD4+ T cells were analyzed by flow cytometry, and the replication of PRRSV and H. parasuis were measured.
Statistical analysis
The results are representative of three independent experiments. And the data are presented as mean ± SEM (SD). Comparisons between 2 groups were performed by unpaired Student’s t test, while multiple group data were analyzed by ANOVA followed by Bonferroni post-hoc test. Statistical significance and very significant was defined as P < 0.05 and P < 0.01.
Results
PCV2 infection suppresses IL-12p40 expression and Th1 immune response to promote PRRSV and H. parasuis infection in the lung of piglets
Th1 immune response derived by IL-12 plays a critical role in intracellular pathogen clearance (20, 21), and several studies have revealed that PCV2 infection affects IL-12 expression (15–18). To figure out how PCV2 infection influence IL-12 expression and the Th1 immune response of piglets, the piglets infected by PCV1 or PCV2 were challenged by PRRSV or H. parasuis to test the effects of PCV2 infection on the expression of IL-12p40 and IFN-γ and the activation of Th1 cells subsets. Upon PRRSV or H. parasuis challenge, serum IL-12p40 and IFN-γ were more significant upregulation in mock- and PCV1-infected pigs than that in PCV2-infected pigs (Fig. 1A); more CD4+INF-γ+ Th1 cells were produced in PBMCs isolated from mock- and PCV1-infected piglets than PCV2-infected piglets (Fig. 1B). Likewise, in lung and pulmonary lymph nodes, the levels of IL-12p40 and IFN-γ and the percentage of CD4+INF-γ+ Th1 cells were higher in mock- and PCV1-infected pigs than that in PCV2-infected pigs after PRRSV or H. parasuis challenge (Fig. 1C, D), These results suggest that PCV2 infection suppresses IL-12p40 expression and the host Th1 immune response to the secondary infection in the piglets.
Figure 1. PCV2 infection suppresses IL-12p40 induction and Th1 immune response of piglets.
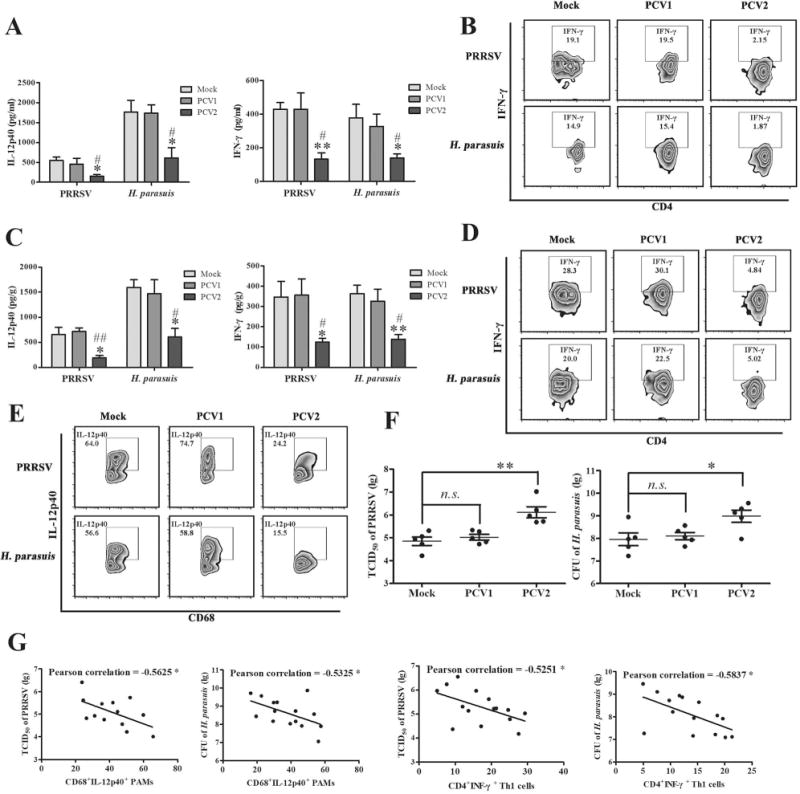
The piglets were infected by PCV1 (4×105 TCID50), PCV2 (4×105 TCID50), or Mock (same volume of medium) for 1 weeks, respectively, and then been challenged with 105 TCID50 PRRSV or 108 CFU H. parasuis for another 24 h. (A) The serum IL-12p40 and IFN-γ of the infected piglets were measured by ELISA. (B) The PBMC of the infected piglets were isolated and strained with CD4 and IFN-γ monoclonal antibodies to measure the CD4+IFN-γ+ Th1 cells. (C) The expression of IL-12p40 and IFN-γ were measured in lung by ELISA. (D) The CD4+IFN-γ+ Th1 cells in pulmonary lymph nodes were measured by flow cytometry. (E) The IL-12p40 expression of CD68 positive porcine alveolar macrophages were analyzed by flow cytometry in the PAMs from mock-, PCV1-, or PCV2-infected piglets upon PRRSV and H. parasuis challenges. (F) The replication of PRRSV and H. parasuis in the lungs were measured. (G) The correlation of the replication of PRRSV and H. parasuis with the percentages of IL-12p40 or IFN-γ positive cells in different dosage of PCV2-infected piglets were analyzed. The results are mean ± SEM of 3 independent experiments, or mean ± SD representative of 3 independent experiments. *P < 0.05, **P < 0.01 versus Mock infection cells; #P < 0.05, ##P < 0.01 versus PCV1-infected cells in A and C.
Next, we tested and compared the IL-12p40 expression of porcine alveolar macrophages isolated from mock-, PCV1-, PCV2-infected piglets. Results showed that PRRSV and H. parasuis challenge induced more IL-12p40-positive cells production (64.0%, 56.6%) in the CD68+ macrophages from mock-infected pigs than that induced in the CD68+ macrophages from PCV2-infected piglets (24.2%, 15.5%), while PCV1-infected piglets showed a similar change as mock-infected pigs in the percentages of CD68+IL-12p40+ PAMs upon PRRSV and H. parasuis challenges (74.7%, 58.8%) (Fig. 1E), suggesting that PCV2 infection significantly suppresses the IL-12p40 expression of PAMs upon secondary various infections, but PCV1 does not. To further determine whether the PCV2 infection affect the replication of PRRSV or H. parasuis, the lungs of mock-, PCV1-, or PCV2-infected piglets were collected and the levels of PRRSV or H. parasuis were detected. Results showed that the TCID50 of PRRSV and CFU of H. parasuis were no significant difference between mock-infected and PCV1-infected pigs, while PCV2 infection markedly promoted the replication of both PRRSV and H. parasuis relative to mock and PCV1 infection (Fig. 1F). Furthermore, Pearson correlation analysis showed the percentages of CD68+IL-12p40+ PAMs and CD4+INF-γ+ Th1 cells were inversely associated with the replication levels of PRRSV and H. parasuis in piglets infected with different doses of PCV2 (Fig. 1G). Together, these results demonstrate that PCV2 infection suppresses IL-12p40 expression and Th1 immune response to promote PRRSV and H. parasuis infection in the lung of piglets.
PCV2 infection inhibits pathogens- or pathogens associated molecular pattern (PAMP)-induced IL-12p40 expression in porcine alveolar macrophages both ex vivo and in vitro
To further confirm that PCV2 infection could suppress IL-12p40 induction, the PAMs isolated from mock-, PCV1- and PCV2-infected pigs were cultured ex vivo for 24 h, and the cells were further infected by PRRSV or H. parasuis, or stimulated by pathogens associated molecular pattern (PAMP) such as bacterial lipopolysaccharide (LPS), TLR7/8 agonist (R848) and macrophage activating cytokine (IFN-γ) to mimic the effect of RNA viruses and bacteria infection. PRRSV and H. parasuis infection did significantly induce IL-12p40 expression in mock-, PCV1-, and PCV2-infected PAMs at both protein and mRNA levels, but the upregulation of IL-12p40 was remarkably lower in PCV2-infected PAMs (Fig. 2A, B). Meanwhile, LPS/IFN-γ or LPS/R848 stimulation also significantly induce IL-12p40 in mock-, PCV1-, and PCV2-infected PAMs at both protein and mRNA levels, and PCV2 infection also efficiently inhibited the upregulation of IL-12p40 (Fig 2C, D). Consistent with the ex vivo results, PCV2-infected fresh PAMs also showed a lower IL-12p40 expression relative to mock- or PCV1-infected fresh PAMs when these cells were further infected with PRRSV or H. parasuis, or stimulated with LPS/IFN-γ or LPS/R848 (Fig. 2E-H). These results further confirm that PCV2 infection suppresses pathogens-induced IL-12p40 expression in PAMs.
Figure 2. PCV2 infection suppresses the expression of IL-12p40 in both transcriptional and post-transcriptional levels in porcine alveolar macrophages.
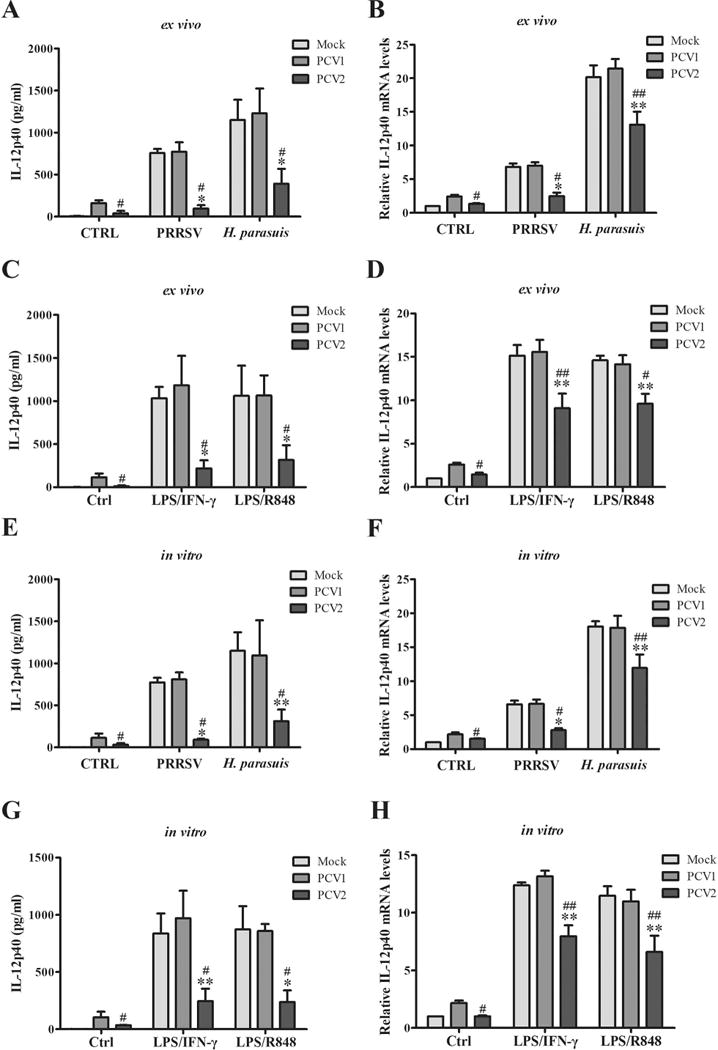
(A-D) The porcine alveolar macrophages were isolated from mock-, PCV1- or PCV2-infected piglets. Then the cells were seeded into 6-well plates and cultured for 24 h, further infected by 1 MOI PRRSV or 50 MOI H. parasuis for 6 h or 24 h, or stimulated by LPS/IFN-γ (1000 ng/ml, 100 ng/ml) or LPS/R848 (1000 ng/ml, 5 μg/ml) for 6 h or 24 h. The protein and mRNA levels of IL-12p40 were measured by ELISA and q-PCR, respectively. (E-H) The PAMs from health pigs were isolated and cultured for 24 h, then the cells were infected by PCV1, PCV2, or mock for 24 h and further challenged by PRRSV or H. parasuis, or stimulated by LPS/IFN-γ or LPS/R848. The protein and mRNA levels of IL-12p40 expression were measured by ELISA and qPCR, respectively. *P < 0.05, **P < 0.01 versus Mock infected cells with same secondary infection or same stimulation. #P < 0.05, ##P < 0.01 versus PCV1 infected cells with same secondary infection or same stimulation.
PCV2 Cap plays predominant role in the suppression of IL-12p40 expression and promoting the infection of other pathogens in vivo
To make clear which component of PCV2 plays the critical roles in the suppression of IL-12p40 expression, PAMs were infected with the recombinant adenoviruses expressing PCV2 Rep (rAd-Rep), PCV2 Cap (rAd-Cap), or blank control adenoviruses (rAd-Blank) for 24 h, then stimulated by LPS/IFN-γ or LPS/R848 to detect IL-12p40 expression. Rep and Cap protein were expressed in the PAMs infected with rAd-Rep and rAd-Cap, respectively (Fig. S2A), and markedly suppressed IL-12p40 expression induced by LPS/IFN-γ or LPS/R848 at both protein and mRNA levels (Fig. 3A, B). While the expression of IL-12p40 induced by LPS/IFN-γ or LPS/R848 was lower in rAd-Cap infected cells compared to that in rAd-Rep-infected cells (Fig. 3A, B). These results suggest that PCV2 Cap can more strongly inhibit IL-12p40 induction relative to Rep protein.
Figure 3. PCV2 Cap is the major component to suppress IL-12p40 expression in vivo.
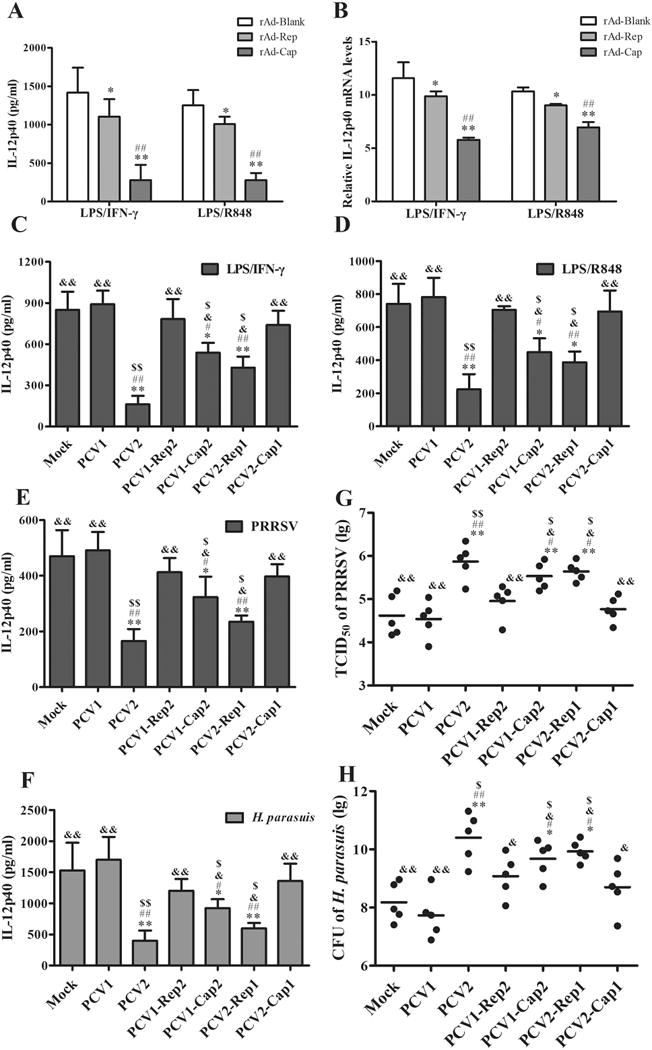
(A, B) The PAMs were infected by 100 MOI of empty adenovirus (rAd-Blank), recombinant adenovirus expressing PCV2 Rep (rAd-Rep), or recombinant adenovirus expressing PCV2 Cap (rAd-Cap) for 24 h, respectively. Then the cells were stimulated by LPS/IFN-γ or LPS/R848, and the protein and mRNA levels of IL-12p40 were measured by ELISA and q-PCR. (C, D) The PAMs were infected by mock, PCV1, PCV2, PCV1-Rep2, PCV1-Cap2, PCV2-Rep1, or PCV2-Cap1 at 1 MOI for 24 h, then the cells were stimulated by LPS/IFN-γ or LPS/R848 for another 24 h. The IL-12p40 secretion were measured by ELISA. (E-H) piglets were infected with Mock, PCV1, PCV2, PCV1-Rep2, PCV1-Cap2, PCV2-Rep1, or PCV2-Cap1 at 4×105 TCID50 for 1 week, then the pigs were further infected by PRRSV or H. parasuis for 24 h. The serum IL-12p40 levels of the infected pigs and replication of PRRSV or H. parasuis were measured. (A, B) *P < 0.05, **P < 0.01 versus rAd-Blank infected cells. #P < 0.05, ##P < 0.01 versus rAd-Rep infected cells. (C - H) *P < 0.05, **P < 0.01 versus mock infection cells. #P < 0.05, ##P < 0.01 versus PCV1-infected cells. &P < 0.05, &&P < 0.01 versus PCV2-infected cells. $P < 0.05 versus PCV1-Rep2- or PCV2-Cap1-infected cells.
To further confirm the roles of Cap and Rep in the inhibition of IL-12p40 expression, we constructed PCV1 mutants that replaced ORF1 or ORF2 in PCV1 backbone by PCV2 ORF1 or ORF2 (named PCV1-Rep2 and PCV1-Cap2), and PCV2 mutants that replaced ORF1 or ORF2 in PCV2 backbone by PCV1 ORF1 or ORF2 (named PCV2-Rep1 and PCV2-Cap1), respectively (Fig. S2B). When PAMs were infected with PCV1, PCV2, PCV1 mutants, or PCV2 mutants, IL-12p40 was more markedly induced by PCV1, PCV1-Rep2, or PCV2-Cap1 infection than PCV2, PCV1-Cap2, or PCV2-Rep1 infection (Fig. S2C). Upon LPS/IFN-γ or LPS/R848 stimulation, IL-12p40 production was also apparently lower in PCV2-, PCV1-Cap2-, and PCV2-Rep1-infected PAMs than that in PCV1- or mock-infected PAMs, whereas PCV1-Rep2 and PCV2-Cap1 infection did not show a significantly inhibitory effect on IL-12 induction relative to PCV1 or mock infection (Fig. 3C, D). Likewise, in the in vivo experiments, PRRSV or H. parasuis challenge induced a lower level of IL-12p40 in PCV2-, PCV1-Cap2-, or PCV2-Rep1-infected piglets, when compared with PCV1- or mock-infected piglets (Fig. 3E, F). Consistent with the difference of IL-12p40 in different PCV infected piglets, the TCID50 of PRRSV and CFU of H. parasuis in the lung tissues from PCV2-, PCV1-Cap2-, or PCV2-Rep1-infected piglets were significantly increased compared with PCV1- or mock-infected piglets (Fig. 3G, H). Of notes, in both PRRSV and H. parasuis challenged piglets, PCV2 infection more markedly inhibited IL-12p40 induction and promoted the infection of PRRSV and H. parasuis relative to PCV1 and all PCV1 and PCV2 mutants, while PCV1-Cap2 and PCV2-Rep1 mutants showed stronger inhibitory effects on IL-12p40 induction and stronger enhance effects on PRRSV and H. parasuis infection than PCV1-Rep2 and PCV2-Cap1 mutants (Fig. 3E-H). These results further confirm that Cap protein plays a predominant role in inhibition of IL-12p40 induction and promoting the infection of other pathogens relative to Rep protein in vivo, even though over-expression of Rep shows a certain inhibitory effect on IL-12p40 induction in vitro.
Host gC1qR protein is crucial for the suppression of IL-12p40 induction in PCV2-infected PAMs
In the previous work, we found that PCV2 infection induces IL-10 production in PAMs by Cap and gC1qR interaction (19). To determine whether gC1qR participates in the suppression of IL-12p40 production in PCV2-infected cells, we compared the induction of IL-12p40 in the wild-type (gC1qR+/+) and gC1qR deficient (gC1qR−/−) PAMs which were generated in our previous work. In mock infection cells, gC1qR deficience did not significantly affect LPS/IFN-γ or LPS/R848-induced IL-12p40 production, but in PCV2-infected cells, IL-12p40 levels was markedly higher in gC1qR−/− cells than in gC1qR+/+ cells upon LPS/IFN-γ stimulation (Fig. 4A). In another word, even though IL-12p40 levels were lower in PCV2-infected gC1qR+/+ cells than mock-infected gC1qR+/+ cells, but there was no significant difference between PCV2-infected gC1qR−/− cells and mock-infected gC1qR−/− cells (Fig. 4A). gC1qR deficience also showed similar effects on the PCV2-induced IL-12p40 inhibition in mRNA levels in PAMs (Fig. 4B), suggesting that gC1qR is involved in the PCV2-induced IL-12p40 inhibition. To further determine gC1qR is involved in Cap-mediated but not in Rep-mediated IL-12p40 suppression, gC1qR+/+ and gC1qR−/− PAMs were infected with recombinant adenovirus expressing PCV2 Rep or Cap. Following by LPS/IFN-γ stimulation, IL-12p40 protein and mRNA did not show significant difference between gC1qR+/+ and gC1qR−/− PAMs after rAd-blank or rAd-Rep infection, but gC1qR−/− PAMs were able to express more IL-12p40 than gC1qR+/+ PAMs after rAd-Cap infection (Fig. 4C, D), suggesting that gC1qR is only involved in Cap-mediated IL-12p40 suppression in PCV2-infected PAMs.
Figure 4. gC1qR is critical for IL-12p40 suppression induced by PCV2 Cap protein.
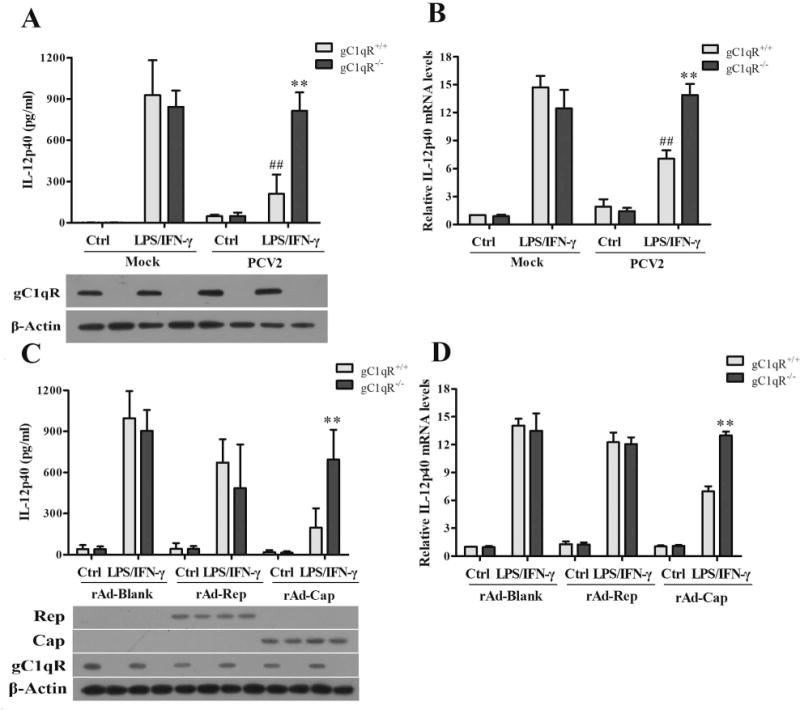
(A, B) PCV2 infected wild-type (gC1qR+/+) or gC1qR knockout (gC1qR−/−) PAMs for 24 h, then the cells were stimulated by LPS/IFN-γ for 6 h or 24 h. The expression of IL-12p40 were measured by ELISA and qPCR. And the expression of gC1qR were analyzed by western blotting as the bottom panel showed. (C, D) The gC1qR+/+ and gC1qR−/− PAMs were infected by rAd-Blank, rAd-Rep, or rAd-Cap for 24 h, respectively. Then the cells were further stimulated by LPS/IFN-γ for 6 h or 24 h, and the expression of IL-12p40 were measured by ELISA and qPCR. The expression of PCV2 Rep, Cap and host gC1qR were analyzed as the bottom panel by western blotting. **P < 0.01 versus PCV2 or rAd-Cap infected gC1qR+/+ PAMs. ##P < 0.01 versus mock infection gC1qR+/+ PAMs.
Akt1 and p38 MAPK are involved in the suppression of IL-12p40 expression in PCV2- infected PAMs
PI3K/Akt1, ERK, and p38 MAPK pathways have been identified to be activated by Cap in PCV2-infected PAMs previously (19), and these pathways might be also critical for the regulation of IL-12p40 expression in porcine macrophages (22). To determine the roles of these signalings in PCV2 inhibition of IL-12p40 expression, the specific siRNAs of Akt1, p38 MAPK, and ERK1 were used to downregulate these proteins expression (Fig. S2D), IL-12p40 expression were examined in these cells after PCV2 infection. Results showed that Akt1 siRNA (si-Akt1) treatment seemed not only remarkably improved the IL-12p40 expression in mock-infection PAMs in both protein and mRNA levels, but also could markedly decrease the inhibitory effects of PCV2 on IL-12p40 expression upon LPS/IFN-γ stimulation (Fig. 5A, B). Same as the treatment of si-Akt1, the p38 MAPK specific siRNA could also increase LPS/IFN-γ-induced IL-12p40 production in PCV2-infected PAMs in both protein and mRNA levels, whereas the ERK1 specific siRNA did not affect the IL-12p40 expression no matter in mock-infected cells or in PCV2-infected cells (Fig. 5A, B). Furthermore, Akt1 and p38 MAPK specific siRNAs treatment could efficiently increase the binding of NF-κB p65 to the il12B (IL-12p40) promoter, whereas ERK1 specific siRNA did not (Fig. 5C). And the down-regulation of Akt1 and p38 MAPK significantly raised the NF-κB p65 activity in PCV2-infected cells (Fig. 5D). These results indicate that Akt1 and p38 MAPK participate in the suppression of IL-12p40 expression in PCV2-infected PAMs, at least in transcriptional level.
Figure 5. PCV2 infection activates PI3K/Akt and p38 MAPK signaling pathways to suppress IL-12p40 expression in transcriptional level.
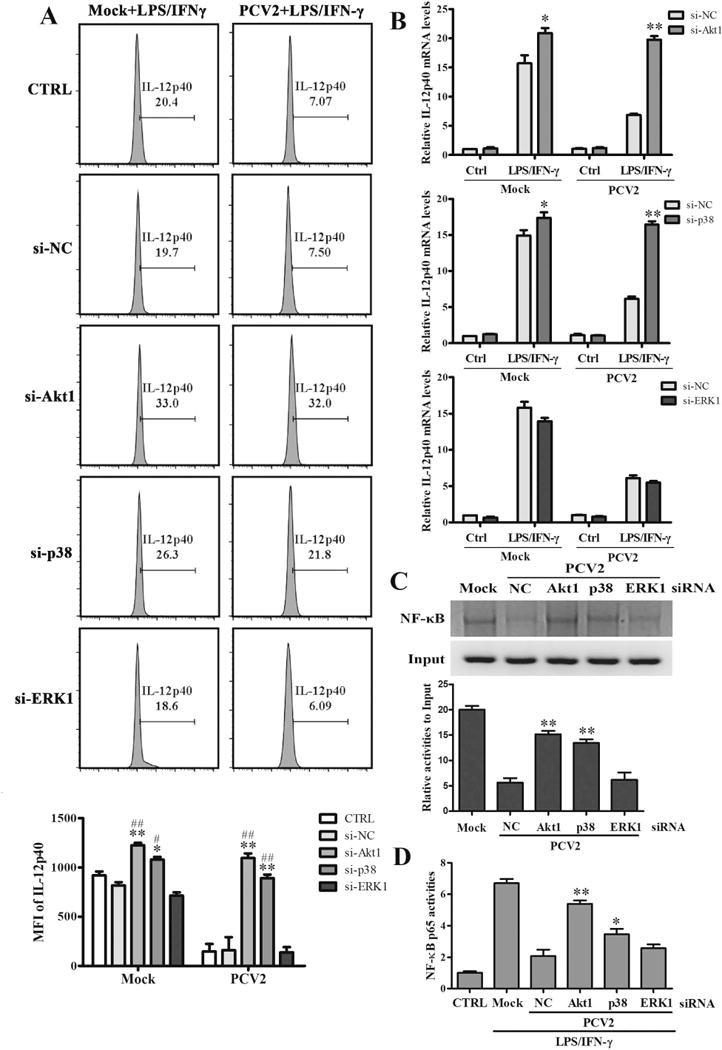
The specific siRNAs of Akt1, p38 MAPK, ERK1, or negative control siRNA were transfected into PAMs for 24 h. Then the cells were infected by mock or PCV2 and followed by LPS/IFN-γ stimulation. The IL-12p40 expression were detected by flow cytometry (A) and qPCR (B). And the binding activities of NF-κB p65 to il12p40 promoter were measured using ChIP assay (C). (D) The NF-κB p65 activity was measured by Dual-Luciferase reporter assays. *P < 0.05, **P < 0.01 versus negative control siRNA transfected PAMs (si-NC) in same infection or Mock. #P < 0.05, ##P < 0.01 versus control PAMs (CTRL) in same infection or Mock.
PCV2 Cap upregulates miR-23a, miR-23b, miR-29a and miR-29b expression in PAMs
To further figure out whether PCV2 infection regulate IL-12p40 expression in post-transcriptional levels, the 12 miRNAs that were predicted to regulate porcine IL-12p40 were analyzed in PCV2-infected PAMs by qPCR. The results showed that the expression levels of miR-23a, miR-23b, miR-29a, and miR-29b were significantly upregulated in PCV2-infected PAMs compared to mock infection PAMs, especially miR-23a and miR-29b (Fig. 6A). To find out which component of PCV2 regulated these miRNAs expression, the expression of these miRNAs were detected in the PCV2-, PCV2-Cap1-, or PCV2-Rep1-infected PAMs. Compared to mock infection, PCV2 and PCV2-Rep1 infection significantly up-regulated miR-23a, miR-23b, miR-29a, and miR-29b expression in PAMs, yet PCV2-Cap1 infection did not significantly induce these miRNAs (Fig. 6B). Consistently, the recombinant adenoviruses that expressed PCV2 Rep did not significantly upregulated miR-23a, miR-23b, miR-29a, and miR-29b expression in PAMs, whereas recombinant adenoviruses rAd-Cap could induce these miRNA expression (Fig. 6C). These results demonstrate that PCV2 infection can upregulate miR-23a, miR-23b, miR-29a, and miR-29b expression in PAMs, and PCV2 Cap is the key compound for upregulation of these miRNAs expression.
Figure 6. PCV2 Cap upregulates miR-23a, miR-23b, miR-29a, and miR-29b expression in PAMs.
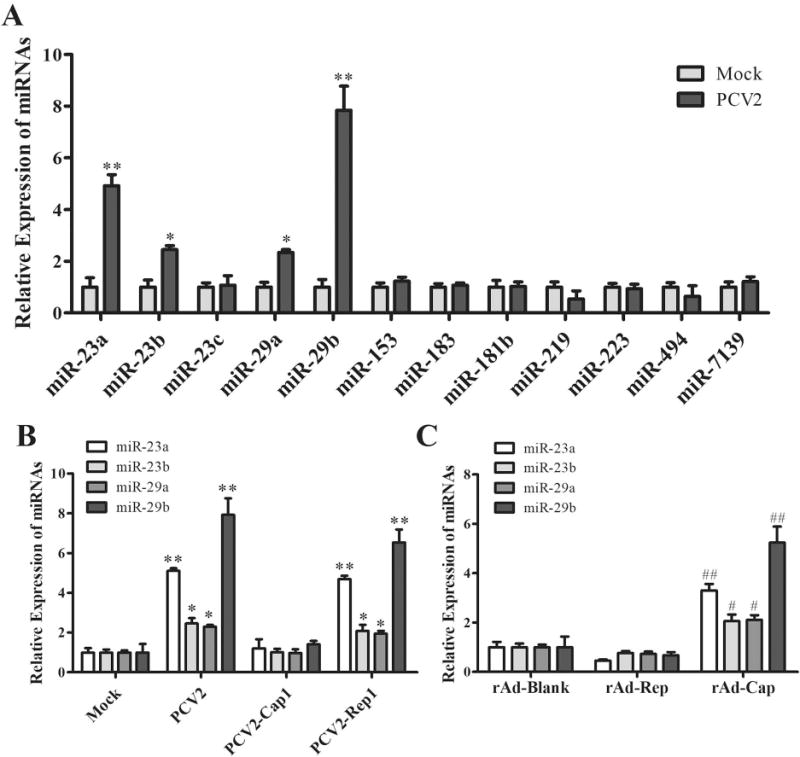
(A) The PAMs were infected by PCV2 or mock for 24 h, then the miRNAs that were predicted to regulate porcine IL-12p40 were analyzed by qPCR. (B) PCV2, PCV2-Cap1, PCV2-Rep1, or mock infected PAMs at 1 MOI for 24 h, the expression of miR-23a, miR-23b, miR-29a, and miR-29b were analyzed by qPCR. (C) The empty recombinant adenovirus (rAd-Blank), recombinant adenovirus expressing PCV2 Rep (rAd-Rep), and recombinant adenovirus expressing PCV2 Cap (rAd-Cap) infected PAMs at 100 MOI for 24 h, the expression of miR-23a, miR-23b, miR-29a, and miR-29b were analyzed by qPCR. *P < 0.05, **P < 0.01 versus Mock infection cells (A, B). #P < 0.05, ##P < 0.01 versus rAd-Blank-infected cells (C).
gC1qR, Akt1 and p38 MAPK participate in the regulation of miR-23a, miR-23b, miR-29a and miR-29b in PCV2-infected PAMs
Since PCV2 Cap could bind with gC1qR to activate PI3K/Akt1, ERK and p38 MAPK signalings, and Cap was the key component of PCV2 to upregulate miR-23a, miR-23b, miR-29a, and miR-29b expression in PAMs. We checked out whether gC1qR, Akt1, ERK1, and p38 MAPK participated in the regulation of miR-23a, miR-23b, miR-29a, and miR-29b in PCV2-infected PAMs. As the results shown, PCV2-induced miR-23a, miR-23b, miR-29a, and miR-29b upregulation were almost abolished in the gC1qR knockout PAMs (Fig. 7A), suggesting gC1qR is involved in mediating the up-regulation of 4 miRNAs in PCV2-infected cells. In the Akt1 specific siRNA-pretreated PAMs, PCV2-induced miR-23a, miR-23b, and miR-29b expression were inhibited, while miR-29a was not altered (Fig. 7B), suggesting Akt1 mainly mediate the up-regulation of miR-23a, miR-23b, and miR-29b in PCV2-infected cells. In the p38 specific siRNA-pretreated PAMs, PCV2-induced miR-29a and miR-29b expression reduced, while miR-23a and miR-23b expression did not change (Fig. 7C), suggesting p38 MAPK mainly mediate the up-regulation of miR-23a and miR-23b in PCV2-infected cells. In the ERK1 specific siRNA-pretreated PAMs, all of 4 miRNAs levels were not largely altered when compared with non-specific siRNA-pretreated PAMs (Fig.7D), suggesting ERK signaling is not involved in the regulation of these miRNAs. These results demonstrate that PCV2 binds with host gC1qR to regulate miR-23a and miR-23b expression through activation of PI3K/Akt1 signaling, to regulate miR-29a expression via p38 MPAK signaling, and to regulate miR-29b expression via PI3K/Akt1 and p38 MAPK signalings.
Figure 7. PCV2 Cap and gC1qR interaction activates PI3K/Akt1 and p38 MAPK signalings to regulate miR-23a, miR-23b, miR-29a and miR-29b expression.
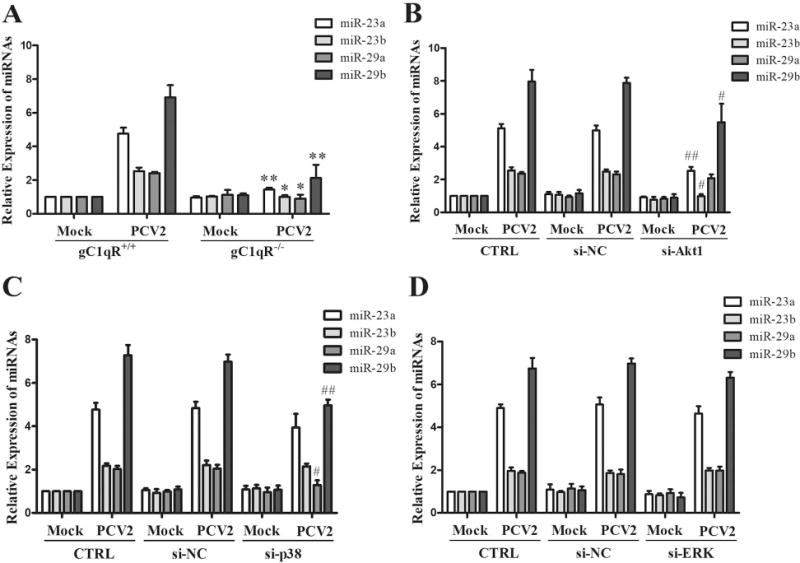
(A) PCV2 infected gC1qR+/+ and gC1qR−/− PAMs at 1 MOI for 24 h, the expression of miR-23a, miR-23b, miR-29a, and miR-29b were measured. (B-D) The specific siRNAs of Akt1 (B), p38 MAPK (C), and ERK1 (D) were transfected into wild-type PAMs for 24 h, then the cells were infected with 1 MOI of PCV2 for another 24 h. The expression of miR-23a, miR-23b, miR-29a, and miR-29b were analyzed. *P < 0.05, **P < 0.01 versus PCV2-infected gC1qR+/+ PAMs for same detected miRNA (A). #P < 0.05, ##P < 0.01 versus PCV2-infected negative control siRNA transfected cells for same miRNA detected (B - D).
PCV2 infection suppression of IL-12p40 induction mainly depends on miR-23a and miR-29b in post-transcriptional level
Since the miR-23a, miR-23b, miR-29a, and miR-29b were up-regulated in PCV2-infected PAMs and might be related to the IL-12p40 expression, we employed the mimics of these miRNAs to pretreat the PAMs, and to detect the IL-12p40 expression upon LPS/IFN-γ or LPS/R848 stimulation. Results showed the miR-23a, miR-23b, miR-29a, and miR-29b mimics treatment significantly repressed the LPS/IFN-γ or LPS/R848-induced IL-12p40 production in protein level (Fig. 8A). Meanwhile, miR-29a and -29b markedly reduce IL-12p40 mRNA levels when compared to the negative mimic treatment but miR-23a and -23b did not (Fig. 8B). These results suggest that miR-23a and -23b inhibit IL-12p40 expression at the post-transcriptional level, while miR-29a and -29b likely inhibit IL-12p40 expression at both transcriptional and post-transcriptional levels.
Figure 8. miR-23a and miR-29b play critical roles in post-transcriptional suppression of IL-12p40 expression in PCV2 infected cells.
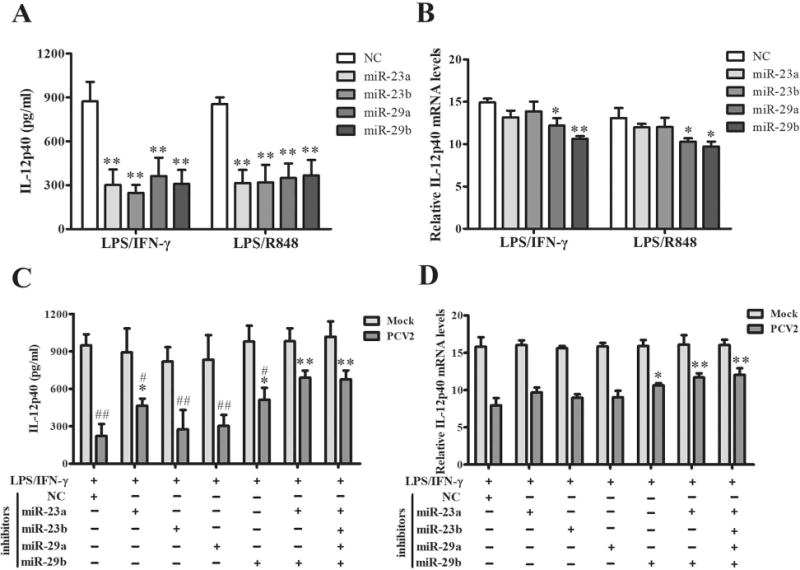
(A, B) The miR-23a, miR-23b, miR-29a, and miR-29b mimics were transfected into PAMs for 24 h, then the cells were stimulated by LPS/IFN-γ or LPS/R848 for another 6 h or 24 h. The expression of IL-12p40 were measured by ELISA and qPCR. (C, D) The specific inhibitors of miR-23a, miR-23b, miR-29a, and miR-29b were transfected into PAMs separately or combined, then the cells were infected by 1 MOI PCV2 for 24 h. And the cells were further stimulated by LPS/IFN-γ for 6 h or 24 h. The expression of IL-12p40 were measured by ELISA and qPCR. (A, B) *P < 0.05, **P < 0.01 versus negative control mimic transfected cells. (C, D) *P < 0.05, **P < 0.01 versus negative control inhibitor transfected PCV2-infected cells. #P < 0.05, ##P < 0.01 versus the PCV2-infcted cells transfected with the mix of all 4 miRNA inhibitors.
To determine whether these miRNAs regulate IL-12p40 expression at post-transcriptional level, we constructed reporter plasmids encoding the 3’UTR of wild-type porcine il12B mRNA downstream of the firefly luciferase gene, and parallel construct including mismatches in the predicted binding sites (miR-23a, -23b, -29a, or -29b) of the il12B 3’UTR (Fig. S3A). Reporter assays showed that miR-23a, -23b, -29a, and -29b mimics could decrease the levels of relative luciferase activities from porcine il12B WT-3’UTR compared to miRNA mimics control, but these miRNAs mimics transfection did not affect the luciferase activities of the cells transfected with the respective mutated-type reporters (Fig. S3B). These data confirm miR-23a, -23b, -29a, or -29b can transcriptionally regulate the IL-12p40 expression via targeting its 3’UTR.
Furthermore, to determine the regulatory roles of these miRNAs in PCV2 inhibition of IL-12p40 expression, cells were transfected with the specific inhibitors of miR-23a, miR-23b, miR-29a, and miR-29b or inhibitor control, and then infected with PCV2 or mock and stimulated by LPS/IFN-γ. The inhibitors reduced the respective miRNA over 3.5-fold (Fig. S3C). The specific inhibitors of miR-23a and miR-29b could significantly reverse the PCV2-induced IL-12p40 suppression in protein levels and miR-29b could promote IL-12p40 transcription, whereas miR-23b and miR-29a specific inhibitors did not significantly improve IL-12p40 expression no matter in either protein or mRNA level (Fig. 8C, D). Notably, treatment with both miR-23a and miR-29b specific inhibitors could more significantly increase the LPS/IFN-γ induced IL-12p40 secretion in PCV2 infected PAMs than miR-23a or miR-29b inhibitors treatment alone, and show a same effect as the inhibitors mix of 4 miRNAs (miR-23a, miR-23b, miR-29a, and miR-29b) (Fig. 8C). These results suggest that miR-23a and miR-29b play a predominant role in PCV2 suppression of IL-12p40 expression.
Reduction of miR-23a and miR-29b induction improves IL-12p40 expression of PAMs and Th1 immune and represses the replication of other pathogens
To confirm the roles of miR-23a and miR-29b during the IL-12p40 expression and host Th1 immune suppression by PCV2 infection, PAMs were transfected with miR-23a and miR-29b specific inhibitors (anti-miR-23a and anti-miR-29b) or inhibitor mix, then PAMs were infected by PCV2 and incubated with PBMC isolated from healthy piglets, and then challenged with PRRSV or H. parasuis. Upon PRRSV or H. parasuis challenge, IL-12p40 positive cell percentage and expression were increased in either anti-miR-23a- or anti-miR-29b-treated PAMs, particularly in PAMs treated with both anti-miR-23a and anti-miR-29b, when compared to the miR-inhibitor control-transfected cells (Fig. 9A, B), suggesting that reduction of miR-23a and miR-29b can improve the IL-12p40 expression induced by other pathogens in PCV2-infected PAMs. Upon PRRSV or H. parasuis infection, the percentage of CD4+IFN-γ+ Th1 cells were increased in the PBMC cocultured with anti-miR-23a- or anti-miR-29b-treated PAMs, particularly in PBMC cocultured with anti-miR-mix (both anti-miR-23a and anti-miR-29b)-treated PAMs (Fig. 9C). In line with the increase of CD4+IFN-γ+ Th1 cells, the levels of IFN-γ in coculture system were markedly increased in cocultured cells with anti-miR-mix-treated PAMs (Fig. 9D). Consequently, the PRRSV and H. parasuis replication were significantly decreased in the cocultured cells with anti-miR-mix-treated PAMs (Fig. 9E). These results further demonstrate the roles of miR-23a and miR-29b during PCV2 infection, that these two miRNAs are employed by PCV2 to suppress IL-12p40 expression in PAMs which results in a lower host Th1 immune response to other pathogens.
Figure 9. Reduction of miR-23a and miR-29b improves IL-12p40 expression and Th1 immune and repress the replication of other pathogens.
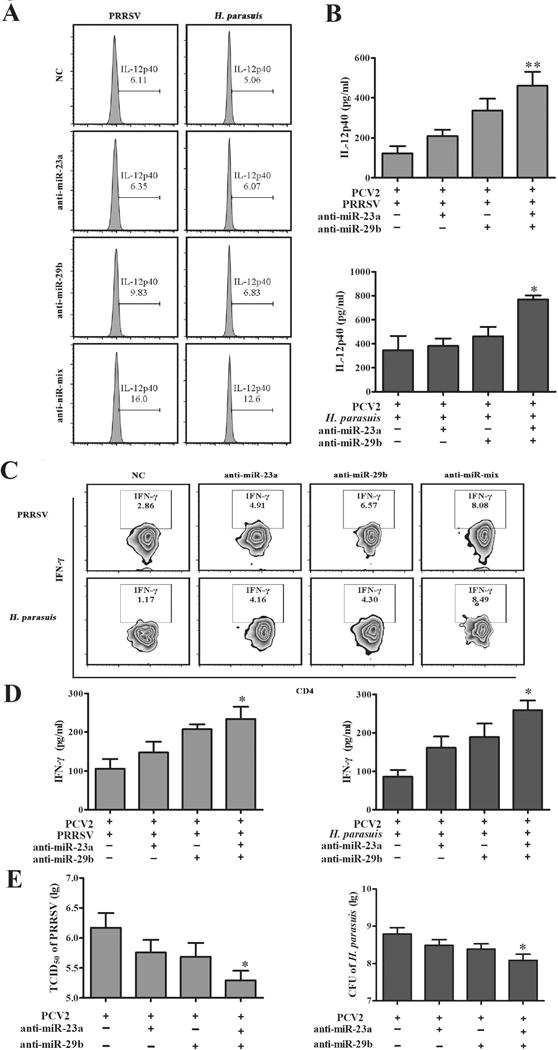
The PAMs were transfected with miR-23a and miR-29b specific inhibitors, then infected with PCV2 or mock at 1 MOI for 24 h. Then PAMs were incubated with PBMC isolated from healthy piglets in a 1:1 ratio and further challenged with PRRSV or H. parasuis for 24 h. (A, B) The IL-12p40 expression of the PAMs were analyzed by flow cytometry and ELISA. (C, D) The percentage of IFN-γ+CD4+ T cells were analyzed by flow cytometry and the levels of IFN-γ in coculture system were measured by ELISA. (E) The replication of PRRSV or H. parasuis were measured. *P < 0.05, **P < 0.01 versus PCV2 alone or PCV2 plus PRRSV or H. parasuis infection without specific miRNA inhibitor.
Discussion
Swine is one of the major animal species used in medical research, since they are very similar to humans in terms of genetics, anatomy, physiology, and immunology (23, 24). Besides the primate and murine immune system, swine immune system is probably the best characterized one (24). Like other mammals, pigs have a full set of innate and adaptive immune effectors, most of which share structural and functional similarities with their human counterparts (25). All these specialties make pigs to be an excellent animal model for studying the immunopathological mechanisms of various virus infectious diseases. Previous works have demonstrated that PCV2 infection can suppress the host immune system leading to the PCV2-infected pigs co-infection with other pathogens (2). In contrast, PCV1 infection does not induce pathological changes (26). In this study, we investigated how PCV2 infection interfere the immune response to other pathogens. The results demonstrate that PCV2 infection significantly suppresses other pathogens- or PAMP molecules-induced IL-12p40 expression of PAMs, leading to a relative lower Th1 immune response and a weakened pathogenic clearance upon other pathogens infection in PCV2 infection piglets than that in PCV1 infection piglets. In PCV2-infected PAMs, PCV2 Cap interact with gC1qR activates PI3K/Akt1 and p38 MAPK signalings to inhibit NF-kB transcriptional activity and upregulate miR-23a and miR-29b to suppresses IL-12p40 expression at both transcriptional and post-transcriptional levels (Fig.10).
Figure 10. Model of PCV2 infection inhibits IL-12p40 expression in porcine alveolar macrophages to further suppress host Th1 immune response.
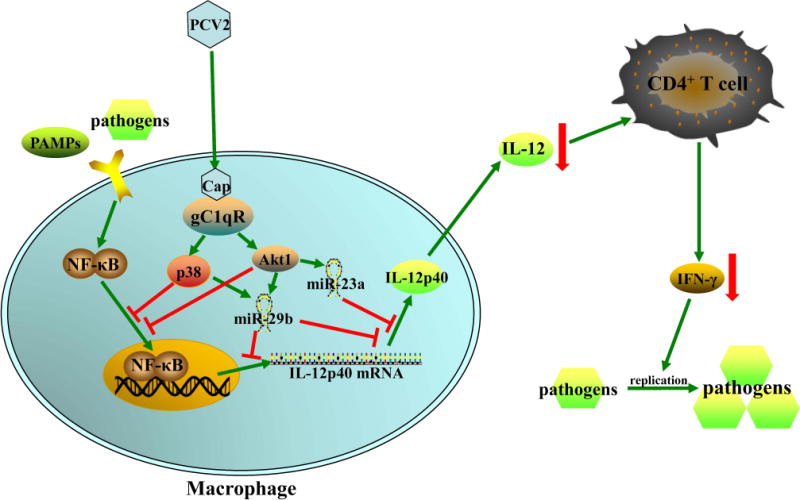
PCV2 infection activates PI3K/Akt1 and p38-MPAK signaling pathways via Cap and gC1qR interaction to suppress other pathogens- or PAMP molecules-induced IL-12p40 expression in transcriptional level. And the activated PI3K/Akt1 and p38 MAPK pathways also induce miR-23a and miR-29b up-regulation to suppress IL-12p40 expression at transcriptional and post-transcriptional level. The reduction of IL-12 secretion by PAMs results in a lower host Th1 immune response to pathogen infection. The green arrows mean induction, the red lines mean inhibition, and the red arrows mean down-regulation.
Macrophages are major innate immune cells involved in detecting infections, anti-infection response and inflammation aiming to pathogen elimination (20, 27). IL-12 is the key proinflammatory cytokine produced by activated macrophages in induction of host Th1 cell immune response (28). Several viruses have been shown to suppress host IL-12 expression. For example, human immunodeficiency virus type-1 (HIV-1) positive subjects show a significant decrease in levels of IL-12 (29). Chronic hepatitis B virus (HBV) infection is found selectively inhibiting TLR2 ligand-induced IL-12p40 mRNA expression in PMA-differentiated THP-1 macrophages (30). PRRSV infection reduces IL-12p40 secretion in monocytes-derived dendritic cells (31). Among the negative regulator of IL-12 expression, IL-10 play an important role as an anti-inflammatory cytokine (22). In chronic HCV patients, IL-10 is found suppressing TLR4 agonist induced IL-12 production (32). PCV2 infection has been shown to upregulate IL-10 production in PAMs during 24 h post PCV2 infection in previous study (19). Although IL-10 might be also involved in negative regulating IL-12 expression in PCV2-infected cells, IL-10 is not required for PCV2 suppression of IL-12p40 induction. In the IL-10 deficient macrophages, PCV2 infection also inhibited IL-12p40 expression (Fig. S3D), suggesting that PCV2 suppression of IL-12p40 induction is not completely dependent on IL-10. Not only in macrophages, PCV2 is also reported to suppress CpG- or R837-induced IL-12p40 expression in dendritic cells (33), but the mechanism is unknown until this work.
In previous study, Cap protein has been identified as a critical player in induction of IL-10 by PCV2 (19). In this work, recombinant adenovirus expressing PCV2 Rep and Cap showed that both Rep and Cap could inhibit the IL-12p40 expression induced by LPS/IFN-γ or LPS/R848 stimulation. However, PCV mutants containing PCV2 Rep without PCV2 Cap (PCV1-Rep2 and PCV2-Cap1) did not exhibit a significantly inhibitory effect on IL-12p40 expression no matter in vivo or in vitro. Since PCV2 replication is very slow or limited in macrophages, Rep expression is extremely low in the PCV1-Rep2 or PCV2-Cap1 infected PAMs. Thus, we speculated the difference of Rep in IL-12p40 suppression between rAd-Rep and PCV mutants might be due to the level of Rep protein in the cells. Massively exogenous overexpression of Rep is able to suppress IL-12p40 induction in PAMs infected with rAd-Rep, but PCV mutants contain PCV2 Rep is not able to suppress IL-12p40 induction due to a lower Rep level that is barely detected in cells. These results indicate that Cap is more critical compound in induction of IL-12p40 suppression relative to Rep during PCV2 infection.
Our previous study have also found that PCV2 Cap binds with gC1qR to regulate IL-10 production in PAMs (19). gC1qR is a major PRR that has been exploited by a wide range of bacterial and viral ligands to suppress the host’s immune response to promote their survival (34). To date, gC1qR has been reported interacting with the hepatitis C virus (HCV) core protein to suppress the IL-12 synthesis in macrophages and dendritic cells (35, 36). Herein, gC1qR was also been found to play a crucial role in the suppression of IL-12p40 expression, which was supported by the evidence that LPS/IFN-γ induced more IL-12p40 in the gC1qR knockout PAMs than in the wild-type PAMs, when the cells were infected by PCV2 or rAd-Cap. These data demonstrate that the interaction of Cap with gC1qR play important roles in both IL-10 and IL-12p40 regulation at different times post PCV2 infection.
IL-12p40 expression is regulated by multiple signaling pathways in macrophages (11, 28). Previous studies have shown PCV2 infection activates PI3K/Akt, p38 MAPK and ERK pathways through Cap and gC1qR interaction (19). All of these pathways have been reported to participate in the regulation of IL-12p40 expression (22). It has been reported that gC1qR ligation selectively inhibits TLR4-induced IL-12 production through activation of the PI3K pathway in human macrophages (37). Inhibition of Akt enhances IL-12 production in G. lamblia trophozoites stimulated mouse peritoneal macrophages (38). p38 MAPK mediates IL-12p40 inhibition by Nitric oxide in macrophages (39). ERK is activated to inhibit IL-12 production in macrophages treated with Leishmania lipophosphoglycans (40). Furthermore, research also found that PI3K/Akt is required for Neospora-induced p38 MAPK downregulation of IL-12 production (41). In this study, inhibition of either Akt1 or p38 MAPK by specific siRNAs reversed the PCV2-induced IL-12p40 suppression, while silence of ERK1 did not alter the IL-12p40 suppression. Inhibition of Akt1 or p38 MAPK enhanced themselves IL-12p40 expression in PAMs upon LPS/IFN-γ stimulation. Inhibition of Akt1 induced significant higher IL-12p40 expression than inhibition of p38 MAPK in PCV2-infected PAMs. Previously, PI3K/Akt and p38 MAPK pathways are considered as upstream signalings promote NF-κB activities (42–44). But, recently, other studies show that PI3K/Akt1 and p38 MAPK can inhibit NF-κB p65 activities in macrophages, that PI3K/Akt1 contributes to M2 polarization and negative regulates NF-κB p65 signaling to inhibit IL-12 expression (45–47), and p38 MAPK has been employed by some pathogens to suppress IL-12 production (48–50). These results suggest that the roles of Akt1 and p38 MAPK are alterative in the regulation of NF-κB p65 transcriptional activity during different infections, different stages of infection or different cells. In the present study, Akt1 and p38 MAPK signaling was also confirmed to inhibit the NF-kB p65 transcriptional activity and binding activities of NF-κB p65 to IL-12p40 promoter in PCV2-infected PAMs. However, more detailed information about how PI3K/Akt1 and p38 MAPK signal inhibits NF-kB transcriptional activity is ongoing studied. Interestingly, the data presented here suggest that p38 MAPK negatively regulates IL-12p40 expression in porcine alveolar macrophages (PAMs), particularly in the cells that AKT1 signaling are activated at the same time.
In addition, we observed the reduction of IL-12p40 mRNA levels were not as far as the protein levels. Base on that, we further screened and identified the miRNAs that could inhibit IL-12p40 expression of PAMs at post-transcriptional level. Several miRNAs have been reported to regulate human or mouse IL-12p40 expression in post-transcriptional level (28). miR-23 is the only broadly conserved miRNA family among vertebrates with conserved site in IL-12p40 3’-UTR based on TargetScan and miRWalk. Except that, several poorly conserved sites for other conserved miRNA family can be found on porcine IL-12p40 3’-UTR. In PCV2-infected PAMs, miR-23a, miR-23b, miR-29a and miR-29b were found to be upregulated. These miRNAs were confirmed directly binding to the 3’-UTR of IL-12p40 mRNA, and negatively regulated IL-12p40 expression. The up-regulation of all these four miRNAs were dependent on the presence of Cap binding protein gC1qR. In the signaling activated by PCV2, PI3K/Akt1 was involved in the regulation of miR-23a, miR-23b and miR-29b expression; p38 MAPK was involved in regulating miR-29a and miR-29b expression in PCV2-infected PAMs. Since the inhibition of Akt1 and p38 MAPK did not totally inhibited the miRNAs expression, we considered Akt1 and p38 participates in the regulation of miRNAs, but not the only regulators. Among 4 miRNAs, miR-23a and miR-29b were markedly upregulated by PCV2 and played a predominant role in regulation of IL-12p40 at post-transcriptional level. Inhibition of both miR-23a and miR-29b could reverse the IL-12p40 expression as well as all four miRNAs inhibitors work together. Because of the PCV2 infection also suppressed IL-12p40 expression in transcriptional level, the suppression of only post-transcriptional level could not completely reverse the IL-12p40 expression. Because it is hardness to confirm the action of miRNAs in vivo, we used a co-culture experiment to determine the action of these miRNAs in PCV2-induced IL-12p40 suppression. We found reduction of miR-23a and miR-29b induction in the PCV2-infected PAMs could improve IL-12p40 expression, and promoter co-cultured PBMCs to produce more IFN-γ positive T cells. Meanwhile, the replication of challenged PRRSV or H. parasuis was significantly inhibited. In this case, we figure out that PCV2 infection suppresses IL-12p40 expression in post-transcriptional level mainly via up-regulation of miR-23a and miR-29b in PAMs.
In summary, this study provides certain evidences that PCV2 infection suppress other pathogens-induced IL-12p40 expression at both transcriptional and post-transcriptional levels through the Cap and gC1qR interaction-mediated PI3K/Akt1 and p38 MAPK pathways activation and miR-23a and miR-29b upregulation, resulting in a lower Th1 immune response and a weakened pathogenic clearance. Inhibition of PI3K/Akt1 and p38 MAPK pathways activation and downregulation of miR-23a and miR-29b can decrease the risk of secondary infection in PCV2-infeced animals. These findings might help us to further understand the relative immune mechanisms determining the susceptibility of PCV2-infected animals.
Supplementary Material
Acknowledgments
We thank Dr. Hai Zhang of Fourth Military Medical University for guidance and help in animal experiments. We also thank to other members of Liu laboratory and Tong laboratory for their helps in this work.
FUNDING INFORMATION
This work was supported by the National Natural Science Foundation of China (31672535), and US NIH grants (1R01AI112381 and 1R21AI109464) for SLL. This work was also supported by the science and technology innovation project in Shaanxi province (2016KTCL02-13), the central project of major agricultural technology promotion funds (K3360217060), and the Fundamental Research Funds for the Central Universities (2452017023).
References
- 1.Rosario K, Breitbart M, Harrach B, Segales J, Delwart E, Biagini P, Varsani A. Revisiting the taxonomy of the family Circoviridae: establishment of the genus Cyclovirus and removal of the genus Gyrovirus. Arch Virol. 2017;162:1447–1463. doi: 10.1007/s00705-017-3247-y. [DOI] [PubMed] [Google Scholar]
- 2.Ren L, Chen X, Ouyang H. Interactions of porcine circovirus 2 with its hosts. Virus Genes. 2016;52:437–444. doi: 10.1007/s11262-016-1326-x. [DOI] [PubMed] [Google Scholar]
- 3.Xu XG, Chen GD, Huang Y, Ding L, Li ZC, Chang CD, Wang CY, Tong DW, Liu HJ. Development of multiplex PCR for simultaneous detection of six swine DNA and RNA viruses. J Virol Methods. 2012;183:69–74. doi: 10.1016/j.jviromet.2012.03.034. [DOI] [PubMed] [Google Scholar]
- 4.Liu S, Li W, Wang Y, Gu C, Liu X, Charreyre C, Fan S, He Q. Coinfection with Haemophilus parasuis serovar 4 increases the virulence of porcine circovirus type 2 in piglets. Virol J. 2017;14:227. doi: 10.1186/s12985-017-0890-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baro J, Segales J, Martinez J. Porcine circovirus type 2 (PCV2) enteric disease: An independent condition or part of the systemic disease? Vet Microbiol. 2015;176:83–87. doi: 10.1016/j.vetmic.2015.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Opriessnig T, Halbur PG. Concurrent infections are important for expression of porcine circovirus associated disease. Virus Res. 2012;164:20–32. doi: 10.1016/j.virusres.2011.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chae C. Porcine respiratory disease complex: Interaction of vaccination and porcine circovirus type 2, porcine reproductive and respiratory syndrome virus, and Mycoplasma hyopneumoniae. Vet J. 2016;212:1–6. doi: 10.1016/j.tvjl.2015.10.030. [DOI] [PubMed] [Google Scholar]
- 8.Schmitt N, Ueno H. Regulation of human helper T cell subset differentiation by cytokines. Curr Opin Immunol. 2015;34:130–136. doi: 10.1016/j.coi.2015.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Muraille E, Leo O, Moser M. TH1/TH2 paradigm extended: macrophage polarization as an unappreciated pathogen-driven escape mechanism? Front Immunol. 2014;5:603. doi: 10.3389/fimmu.2014.00603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wu Y, Bastian D, Schutt S, Nguyen H, Fu J, Heinrichs J, Xia C, Yu XZ. Essential Role of Interleukin-12/23p40 in the Development of Graft-versus-Host Disease in Mice. Biol Blood Marrow Transplant. 2015;21:1195–1204. doi: 10.1016/j.bbmt.2015.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Trinchieri G. Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nat Rev Immunol. 2003;3:133–146. doi: 10.1038/nri1001. [DOI] [PubMed] [Google Scholar]
- 12.Yu ZB, Huang C, Zhang Q, Feng WH. Porcine reproductive and respiratory syndrome virus (PRRSV) induces IL-12p40 production through JNK-AP-1 and NF-kappa B signaling pathways. Virus Res. 2016;225:73–81. doi: 10.1016/j.virusres.2016.09.009. [DOI] [PubMed] [Google Scholar]
- 13.Becher B, Durell BG, Noelle RJ. Experimental autoimmune encephalitis and inflammation in the absence of interleukin-12. J Clin Invest. 2002;110:493–497. doi: 10.1172/JCI15751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Piccotti JR, Li K, Chan SY, Ferrante J, Magram J, Eichwald EJ, Bishop DK. Alloantigen-reactive Th1 development in IL-12-deficient mice. J Immunol. 1998;160:1132–1138. [PubMed] [Google Scholar]
- 15.Wang X, Chen L, Yuan W, Li Y, Li L, Li T, Li H, Song Q. Effect of porcine circovirus type 2 (PCV2) on the function of splenic CD11c+ dendritic cells in mice. Arch Virol. 2017;162:1289–1298. doi: 10.1007/s00705-017-3221-8. [DOI] [PubMed] [Google Scholar]
- 16.Kekarainen T, Montoya M, Mateu E, Segales J. Porcine circovirus type 2-induced interleukin-10 modulates recall antigen responses. J Gen Virol. 2008;89:760–765. doi: 10.1099/vir.0.83354-0. [DOI] [PubMed] [Google Scholar]
- 17.Kekarainen T, Montoya M, Dominguez J, Mateu E, Segalés J. Porcine circovirus type 2 (PCV2) viral components immunomodulate recall antigen responses. Vet Immunol Immunopathol. 2008;124:41–49. doi: 10.1016/j.vetimm.2008.01.031. [DOI] [PubMed] [Google Scholar]
- 18.Darwich L, Pie S, Rovira A, Segales J, Domingo M, Oswald IP, Mateu E. Cytokine mRNA expression profiles in lymphoid tissues of pigs naturally affected by postweaning multisystemic wasting syndrome. J Gen Virol. 2003;84:2117–2125. doi: 10.1099/vir.0.19124-0. [DOI] [PubMed] [Google Scholar]
- 19.Du Q, Huang Y, Wang T, Zhang X, Chen Y, Cui B, Li D, Zhao X, Zhang W, Chang L, Tong D. Porcine circovirus type 2 activates PI3K/Akt and p38 MAPK pathways to promote interleukin-10 production in macrophages via Cap interaction of gC1qR. Oncotarget. 2016;7:17492–17507. doi: 10.18632/oncotarget.7362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weiss G, Schaible UE. Macrophage defense mechanisms against intracellular bacteria. Immunol Rev. 2015;264:182–203. doi: 10.1111/imr.12266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zuckermann FA, Martin S, Husmann RJ, Brandt J. Use of interleukin 12 to enhance the cellular immune response of swine to an inactivated herpesvirus vaccine. Adv Veter Med Ap. 1999;41:447–461. doi: 10.1016/s0065-3519(99)80034-2. [DOI] [PubMed] [Google Scholar]
- 22.Ma X, Yan W, Zheng H, Du Q, Zhang L, Ban Y, Li N, Wei F. Regulation of IL-10 and IL-12 production and function in macrophages and dendritic cells. F1000Research. 2015;4 doi: 10.12688/f1000research.7010.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Khatri M, Richardson LA, Meulia T. Mesenchymal stem cell-derived extracellular vesicles attenuate influenza virus-induced acute lung injury in a pig model. Stem Cell Res Ther. 2018;9:17. doi: 10.1186/s13287-018-0774-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mair KH, Sedlak C, Kaser T, Pasternak A, Levast B, Gerner W, Saalmuller A, Summerfield A, Gerdts V, Wilson HL, Meurens F. The porcine innate immune system: an update. Dev Comp Immunol. 2014;45:321–343. doi: 10.1016/j.dci.2014.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sang Y, Ruchala P, Lehrer RI, Ross CR, Rowland RR, Blecha F. Antimicrobial host defense peptides in an arteriviral infection: differential peptide expression and virus inactivation. Viral Immunol. 2009;22:235–242. doi: 10.1089/vim.2009.0005. [DOI] [PubMed] [Google Scholar]
- 26.Pineyro PE, Kenney SP, Gimenez-Lirola LG, Opriessnig T, Tian D, Heffron CL, Meng XJ. Evaluation of the use of non-pathogenic porcine circovirus type 1 as a vaccine delivery virus vector to express antigenic epitopes of porcine reproductive and respiratory syndrome virus. Virus Res. 2016;213:100–108. doi: 10.1016/j.virusres.2015.11.005. [DOI] [PubMed] [Google Scholar]
- 27.Nyman TA, Matikainen S. Proteomics to study macrophage response to viral infection. J Proteomics. 2017 doi: 10.1016/j.jprot.2017.06.018. In Press. [DOI] [PubMed] [Google Scholar]
- 28.Cui B, Liu W, Wang X, Chen Y, Du Q, Zhao X, Zhang H, Liu SL, Tong D, Huang Y. Brucella Omp25 Upregulates miR-155, miR-21-5p, and miR-23b to Inhibit Interleukin-12 Production via Modulation of Programmed Death-1 Signaling in Human Monocyte/Macrophages. Front Immunol. 2017;8:708. doi: 10.3389/fimmu.2017.00708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Valdivia A, Ly J, Gonzalez L, Hussain P, Saing T, Islamoglu H, Pearce D, Ochoa C, Venketaraman V. Restoring Cytokine Balance in HIV-Positive Individuals with Low CD4 T Cell Counts. AIDS Res Hum Retroviruses. 2017;33:905–918. doi: 10.1089/aid.2016.0303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang S, Chen Z, Hu C, Qian F, Cheng Y, Wu M, Shi B, Chen J, Hu Y, Yuan Z. Hepatitis B virus surface antigen selectively inhibits TLR2 ligand-induced IL-12 production in monocytes/macrophages by interfering with JNK activation. J Immunol. 2013;190:5142–5151. doi: 10.4049/jimmunol.1201625. [DOI] [PubMed] [Google Scholar]
- 31.Liu J, Wei S, Liu L, Shan F, Zhao Y, Shen G. The role of porcine reproductive and respiratory syndrome virus infection in immune phenotype and Th1/Th2 balance of dendritic cells. Dev Comp Immunol. 2016;65:245–252. doi: 10.1016/j.dci.2016.07.012. [DOI] [PubMed] [Google Scholar]
- 32.Liu BS, Groothuismink ZM, Janssen HL, Boonstra A. Role for IL-10 in inducing functional impairment of monocytes upon TLR4 ligation in patients with chronic HCV infections. J Leukoc Biol. 2011;89:981–988. doi: 10.1189/jlb.1210680. [DOI] [PubMed] [Google Scholar]
- 33.Vincent IE, Balmelli C, Meehan B, Allan G, Summerfield A, McCullough KC. Silencing of natural interferon producing cell activation by porcine circovirus type 2 DNA. Immunology. 2007;120:47–56. doi: 10.1111/j.1365-2567.2006.02476.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pednekar L, Valentino A, Ji Y, Tumma N, Valentino C, Kadoor A, Hosszu KK, Ramadass M, Kew RR, Kishore U, Peerschke EI, Ghebrehiwet B. Identification of the gC1qR sites for the HIV-1 viral envelope protein gp41 and the HCV core protein: Implications in viral-specific pathogenesis and therapy. Mol Immunol. 2016;74:18–26. doi: 10.1016/j.molimm.2016.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yi W, Zhang P, Liang Y, Zhou Y, Shen H, Fan C, Moorman JP, Yao ZQ, Jia Z, Zhang Y. T-bet-mediated Tim-3 expression dampens monocyte function during chronic hepatitis C virus infection. Immunology. 2016;150:301–311. doi: 10.1111/imm.12686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Waggoner SN, Hall CH, Hahn YS. HCV core protein interaction with gC1q receptor inhibits Th1 differentiation of CD4+ T cells via suppression of dendritic cell IL-12 production. J Leukoc Biol. 2007;82:1407–1419. doi: 10.1189/jlb.0507268. [DOI] [PubMed] [Google Scholar]
- 37.Waggoner SN, Cruise MW, Kassel R, Hahn YS. gC1q Receptor Ligation Selectively Down-Regulates Human IL-12 Production through Activation of the Phosphoinositide 3-Kinase Pathway. J Immunol. 2005;175:4706–4714. doi: 10.4049/jimmunol.175.7.4706. [DOI] [PubMed] [Google Scholar]
- 38.Li X, Zhang X, Gong P, Xia F, Li L, Yang Z, Li J. TLR2(−/−) Mice Display Decreased Severity of Giardiasis via Enhanced Proinflammatory Cytokines Production Dependent on AKT Signal Pathway. Front Microbiol. 2017;8:1186. doi: 10.3389/fimmu.2017.01186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Boddupalli CS, Ghosh S, Rahim SS, Nair S, Ehtesham NZ, Hasnain SE, Mukhopadhyay S. Nitric oxide inhibits interleukin-12 p40 through p38 MAPK-mediated regulation of calmodulin and c-rel. Free Radic Biol Med. 2007;42:686–697. doi: 10.1016/j.freeradbiomed.2006.12.014. [DOI] [PubMed] [Google Scholar]
- 40.Feng GJ, Goodridge HS, Harnett MM, Wei XQ, Nikolaev AV, Higson AP, Liew FY. Extracellular signal-related kinase (ERK) and p38 mitogen-activated protein (MAP) kinases differentially regulate the lipopolysaccharide-mediated induction of inducible nitric oxide synthase and IL-12 in macrophages: Leishmania phosphoglycans subvert macrophage IL-12 production by targeting ERK MAP kinase. J Immunol. 1999;163:6403–6412. [PubMed] [Google Scholar]
- 41.Mota CM, Oliveira AC, Davoli-Ferreira M, Silva MV, Santiago FM, Nadipuram SM, Vashisht AA, Wohlschlegel JA, Bradley PJ, Silva JS, Mineo JR, Mineo TW. Neospora caninum Activates p38 MAPK as an Evasion Mechanism against Innate Immunity. Front Microbiol. 2016;7:1456. doi: 10.3389/fmicb.2016.01456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ojaniemi M, Glumoff V, Harju K, Liljeroos M, Vuori K, Hallman M. Phosphatidylinositol 3-kinase is involved in Toll-like receptor 4-mediated cytokine expression in mouse macrophages. Eur J Immunol. 2003;33:597–605. doi: 10.1002/eji.200323376. [DOI] [PubMed] [Google Scholar]
- 43.Pasquali MAD, Gelain DP, Zeidan-Chulia F, Pires AS, Gasparotto J, Terra SR, Moreira JCF. Vitamin A (retinol) downregulates the receptor for advanced glycation endproducts (RAGE) by oxidant-dependent activation of p38 MAPK and NF-kB in human lung cancer A549 cells. Cell Signal. 2013;25:939–954. doi: 10.1016/j.cellsig.2013.01.013. [DOI] [PubMed] [Google Scholar]
- 44.Saha RN, Jana M, Pahan K. MAPK p38 regulates transcriptional activity of NF-kappaB in primary human astrocytes via acetylation of p65. J Immunol. 2007;179:7101–7109. doi: 10.4049/jimmunol.179.10.7101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Waggoner SN, Cruise MW, Kassel R, Hahn YS. gC1q receptor ligation selectively down-regulates human IL-12 production through activation of the phosphoinositide 3-kinase pathway. J Immunol. 2005;175:4706–4714. doi: 10.4049/jimmunol.175.7.4706. [DOI] [PubMed] [Google Scholar]
- 46.Vergadi E, Ieronymaki E, Lyroni K, Vaporidi K, Tsatsanis C. Akt Signaling Pathway in Macrophage Activation and M1/M2 Polarization. J Immunol. 2017;198:1006–1014. doi: 10.4049/jimmunol.1601515. [DOI] [PubMed] [Google Scholar]
- 47.Tongaonkar P, Trinh KK, Schaal JB, Tran D, Gulko PS, Ouellette AJ, Selsted ME. Rhesus macaque theta-defensin RTD-1 inhibits proinflammatory cytokine secretion and gene expression by inhibiting the activation of NF-kappaB and MAPK pathways. J Leukoc Biol. 2015;98:1061–1070. doi: 10.1189/jlb.3A0315-102R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lecocq M, Detry B, Guisset A, Pilette C. FcalphaRI-mediated inhibition of IL-12 production and priming by IFN-gamma of human monocytes and dendritic cells. J Immunol. 2013;190:2362–2371. doi: 10.4049/jimmunol.1201128. [DOI] [PubMed] [Google Scholar]
- 49.Yang Z, Zhang X, Darrah PA, Mosser DM. The regulation of Th1 responses by the p38 MAPK. J Immunol. 2010;185:6205–6213. doi: 10.4049/jimmunol.1000243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mota CM, Oliveira ACM, Davoli-Ferreira M, Silva MV, Santiago FM, Nadipuram SM, Vashisht AA, Wohlschlegel JA, Bradley PJ, Silva JS, Mineo JR, Mineo TWP. Neospora caninum Activates p38 MAPK as an Evasion Mechanism against Innate Immunity. Front Microbiol. 2016;7:1456. doi: 10.3389/fmicb.2016.01456. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


