Summary
Consciousness is determined both by level (e.g., being awake vs. being anesthetized) and content (i.e., the qualitative aspects of experience). Subcortical areas are known to play a causal role in regulating the level of consciousness [1–9] but the role of the cortex is less well understood. Clinical and correlative data have been used both to support and refute a role for prefrontal and posterior cortices in the level of consciousness [10–22]. Prefrontal cortex has extensive reciprocal connections to wake-promoting centers in the brainstem and diencephalon [23,24], and hence is in a unique position to modulate level of consciousness. Furthermore, a recent study suggested that the prefrontal cortex might be important in regulating level of consciousness [25] but causal evidence, and a comparison with more posterior cortical sites, is lacking. Therefore, to test the hypothesis that prefrontal cortex plays a role in regulating level of consciousness, we attempted to reverse sevoflurane anesthesia by cholinergic or noradrenergic stimulation of the prefrontal prelimbic cortex and two areas of parietal cortex in rat. General anesthesia was defined by loss of the righting reflex, a widely used surrogate measure in rodents. We demonstrate that cholinergic stimulation of prefrontal cortex, but not parietal cortex, restored wake-like behavior, despite continuous exposure to clinically relevant concentrations of sevoflurane anesthesia. Noradrenergic stimulation of the prefrontal and parietal areas resulted in electroencephalographic activation but failed to produce any signs of wake-like behavior. We conclude that cholinergic mechanisms in prefrontal cortex can regulate the level of consciousness.
Keywords: Consciousness, prefrontal cortex, parietal cortex, acetylcholine, noradrenaline, carbachol, sevoflurane anesthesia, electroencephalogram, rat, microdialysis
The eTOC blurb
Pal et al. show that cholinergic, but not noradrenergic, stimulation of prefrontal cortex can restore level of consciousness despite continuous anesthetic administration. Cholinergic or noradrenergic stimulation in the parietal region did not alter the behavioral state. Prefrontal cortex is a potential target for modulating level of consciousness.
Results
Cholinergic stimulation of prefrontal prelimbic cortex in anesthetized rat is sufficient to restore level of consciousness and wake-like behavior
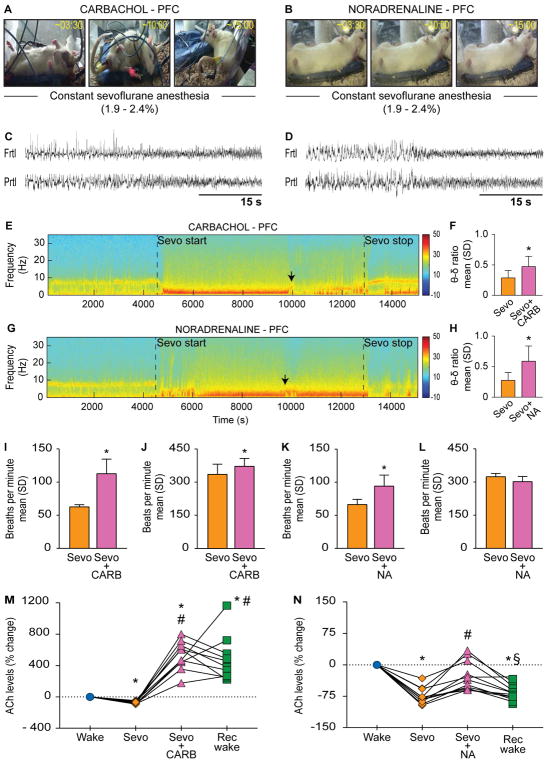
Reverse dialysis delivery of 5 mM carbachol (a mixed cholinergic agonist) into prefrontal prelimbic cortex (n=11) during sevoflurane anesthesia induced signs consistent with wakefulness in all 11 rats, and 4 out of the 11 rats regained complete mobility while continuously breathing clinically relevant concentrations of sevoflurane anesthesia (1.9–2.4%) (Figure 1A, see Video S1). The change in behavior was preceded by low-voltage fast activity in the electroencephalogram (Figure 1C), a characteristic feature of wakefulness. Additionally, power spectral density analysis demonstrated that the theta/delta frequency ratio, a standard metric for electroencephalographic activation, increased significantly during carbachol delivery [p=0.002, t(9)=−4.35, as compared to sevoflurane anesthesia] (Figure 1E–F). The electroencephalographic activation was also accompanied by increase in respiration rate [p=0.002, t(5)=−6.27] (Figure 1I) and a small but significant elevation of heart rate [p=0.04, t(5)=−2.82] (Figure 1J). Of note, carbachol-induced wake-like behavior was not a discrete event but persisted despite continuous anesthetic exposure. As opposed to cholinergic stimulation, reverse dialysis delivery of 20 mM noradrenaline (n=11) into prefrontal cortex of sevoflurane-anesthetized rats did not produce any change in the level of consciousness (Figure 1B, see Video S2) but did change the electroencephalographic pattern from high-voltage slow waves to low-voltage fast activity (Figure 1D). The low-voltage fast electroencephalogram showed increased theta/delta ratio [p=0.02, t(7)=−3.06, as compared to sevoflurane anesthesia], thereby confirming activation (Figure 1G–H). Noradrenaline also produced an increase in respiration rate [p=0.001, t(7)=−5.30] (Figure 1K) but there was no significant change in the heart rate [p=0.2, t(7)=1.57] (Figure 1L). These data demonstrate that cholinergic stimulation of prefrontal prelimbic cortex in the anesthetized rat is sufficient to restore the level of consciousness and wake-like behavior.
Figure 1. Cholinergic stimulation of prefrontal prelimbic cortex in anesthetized rat is sufficient to restore level of consciousness and wake-like behavior.
Panels in (A) and (B) show behavior after dialysis delivery of 5 mM carbachol (CARB) or 20 mM noradrenaline (NA) into prefrontal prelimbic cortex (PFC) of rats receiving continuous sevoflurane anesthesia (1.9–2.4%); numbers on the top right corner show the time elapsed (min:s) after CARB/NA reached the target site. C) and (D) demonstrate CARB- and NA-induced electroencephalographic activation, respectively. E) and (G) show representative spectrograms across the experimental timeline for CARB and NA groups, respectively; vertical color bar is the log scale for power spectral density. Black arrows indicate the approximate time of CARB- and NA-induced electroencephalographic activation quantified as theta/delta ratio in (F) and (H), respectively. I) and (J) show the effect of CARB on respiration and heart rate, respectively. K) and (L) show the effect of NA on respiration and heart rate, respectively. M) and (N) show changes in acetylcholine levels for each rat during sevoflurane anesthesia (Sevo), CARB/NA delivery into PFC during Sevo, and post-Sevo recovery wake (Rec wake) epochs as percent change from pre-anesthesia wake state (Wake). Significance symbols (p<0.05) show group level comparisons using Student’s two-tailed paired t-test: *compared to Wake, #compared to Sevo, §compared to CARB/NA. Multiple comparisons were Bonferroni corrected. Actual p values are reported in the results section. Frtl - frontal, δ - delta, Prtl - parietal, SD - standard deviation, θ - theta. See also Figure S1, Tables S1–S3, and Video S1.
Consistent with our recent report [26], sevoflurane anesthesia produced a significant decrease in acetylcholine levels in prefrontal cortex in both carbachol [p<0.0001, t(9)=13.86] and noradrenaline [p<0.0001, t(10)=12.96] groups, which was reversed by local delivery of carbachol or noradrenaline (Figure 1M–N). Carbachol in prefrontal cortex produced a substantial increase (~600%) in local acetylcholine levels as compared to both waking [p<0.0001, t(9)=−9.09] and sevoflurane anesthesia [p<0.0001, t(9)=−10.74] (Figure 1M). Following noradrenaline delivery, the acetylcholine levels showed a modest (~50%) but statistically significant increase as compared to sevoflurane anesthesia [p=0.001, t(10)=−5.70] that was not significantly different from the baseline waking state [p=0.2, t(10)=2.54] (Figure 1N). The acetylcholine levels in the carbachol group stayed significantly high during the post-sevoflurane recovery epoch as compared to both waking [p=0.003, t(9)=−5.41] and sevoflurane anesthesia [p=0.0008, t(9)=−6.34] epochs (Figure 1M); there was no significant difference in acetylcholine levels between carbachol and recovery wake epochs [p=1, t(9)=0.43]. In contrast, the acetylcholine levels during the post-sevoflurane recovery wake epoch in the noradrenaline group were significantly lower as compared to both waking [p<0.0001, t(10)=11.76] and noradrenaline [p=0.005, t(10)=4.67] epochs (Figure 1N).
Cholinergic stimulation of the parietal region (posterior parietal cortex and medial parietal association cortex) in anesthetized rat does not affect the level of consciousness
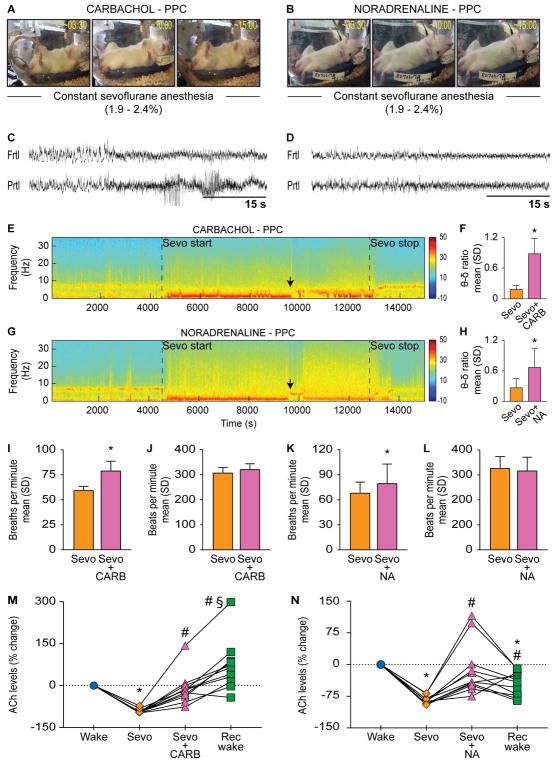
In order to confirm the site specificity of the effects observed in prefrontal cortex—and to test the alternate hypothesis [11,16,17,20] that posterior cortical areas, including parietal cortex, play a critical role in consciousness—we conducted similar cholinergic stimulation (5 mM carbachol) in two distinct regions within the parietal cortex of two separate groups of rats: 1) posterior parietal cortex (n=11) which is primarily a somatosensory area [27], and 2) medial parietal association cortex (n=8), which is linked to cognition and attention [28]. Medial parietal association cortex receives afferents from dorsal retrosplenial cortex, homologous to the human posterior cingulate cortex [29], and anterior thalamus rather than from sensorimotor areas [30]. An additional group of rats was similarly prepared for dialysis delivery of noradrenaline into posterior parietal cortex (n=11). Carbachol delivery into posterior parietal cortex during sevoflurane anesthesia did not induce wake-like behavior and none of the rats made any attempts at righting; some of the rats did display uncoordinated spastic muscle twitches and occasional movements in whiskers, limb, and tail (Figure 2A, see Video S1). Noradrenaline delivery into posterior parietal cortex during sevoflurane anesthesia also failed to produce any change in the level of consciousness such as wake-like behavior (Figure 2B, see Video S2). Similar to cholinergic and noradrenergic stimulation of prefrontal cortex, carbachol and noradrenaline delivery into posterior parietal cortex produced electroencephalographic pattern consistent with activation, i.e., the appearance of low-voltage fast electroencephalogram (Figure 2C–D) and increase in the theta/delta ratio [for carbachol group: p<0.001, t(10)=−8.72; for noradrenaline group: p=0.008, t(10)=−3.32, compared to sevoflurane anesthesia] (Figure 2E–H). Both carbachol [p=0.0008, t(8)=−5.24] and noradrenaline [p=0.03, t(8)=−2.6] increased the respiration rate (Figure 2I and K) but there was no significant change in the heart rate [p=0.06, t(8)=−2.24 for carbachol group; p=0.7, t(8)=0.48 for noradrenaline group] (Figure 2J and L). Sevoflurane anesthesia produced a decrease in acetylcholine levels in posterior parietal cortex in both carbachol [p<0.0001, t(10)=39.41] (Figure 2M) and noradrenaline [p<0.0001, t(10)=30.33] (Figure 2N) groups. As compared to sevoflurane epoch, dialysis delivery of carbachol and noradrenaline produced a modest but significant increase in local acetylcholine levels [p=0.002, t(10)=−5.27 for carbachol group; p=0.008, (10)=−4.43 for noradrenaline group], which reached the wake levels [p=1, t(10)=0.66 for carbachol group; p=1, t(10)=0.64 for noradrenaline group] (Figure 2M–N). In a separate group of 3 rats, we delivered a higher concentration of carbachol (15 mM) into posterior parietal cortex to ensure that the concentration of carbachol was not a limiting factor for the lack of behavioral effects. The higher concentration of carbachol did not produce any significantly different behavior than that observed after 5 mM carbachol (data not included in this study).
Figure 2. Cholinergic stimulation of posterior parietal cortex in anesthetized rat does not affect the level of consciousness.
Panels in (A) and (B) show behavior after dialysis delivery of 5 mM carbachol (CARB) or 20 mM noradrenaline (NA) into posterior parietal cortex (PPC) of rats receiving continuous sevoflurane anesthesia (1.9–2.4%); numbers on the top right corner show the time elapsed (min:s) after CARB/NA reached the target site. C) and (D) demonstrate CARB- and NA-induced electroencephalographic activation, respectively. E) and (G) show representative spectrograms across the experimental timeline for CARB and NA groups, respectively; vertical color bar is the log scale for power spectral density. Black arrows indicate the approximate time of CARB- and NA-induced electroencephalographic activation quantified as theta/delta ratio in (F) and (H), respectively. I) and (J) show the effect of CARB on respiration and heart rate, respectively. K) and (L) show the effect of NA on respiration and heart rate, respectively. M) and (N) show changes in acetylcholine levels for each rat during sevoflurane anesthesia (Sevo), CARB/NA delivery into PFC during Sevo, and post-Sevo recovery wake (Rec wake) epochs as percent change from pre-anesthesia wake state (Wake). Significance symbols (p<0.05) show group level comparisons using Student’s two-tailed paired t-test: *compared to Wake, #compared to Sevo, §compared to CARB/NA. Multiple comparisons were Bonferroni corrected. Actual p values are reported in the results section. Frtl - frontal, δ - delta, Prtl - parietal, SD - standard deviation, θ - theta. See also Figure S1, Tables S1–S3, and Video S2.
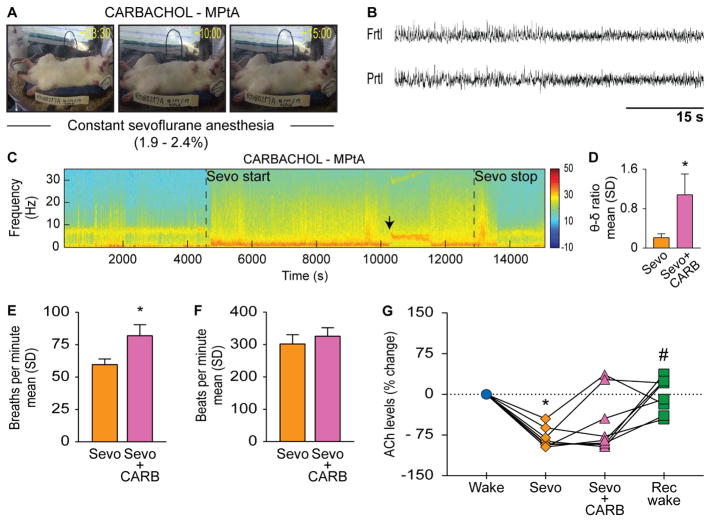
Dialysis delivery of 5 mM carbachol into medial parietal association cortex did not produce any signs of wake-like behavior (Figure 3A) but, similar to the effects observed in prefrontal and posterior parietal cortices, produced electroencephalographic activation as evidenced by the transition to low-voltage fast electroencephalogram (Figure 3B) and increase in the theta/delta ratio [p<0.001, t(6)= −6.36, compared to sevoflurane anesthesia] (Figure 3C–D). Electroencephalographic activation was accompanied by increase in respiration rate [p=0.004, t(5)=−4.94] (Figure 3E); there was no statistical change in heart rate [p=0.05, t(5)=−2.57] (Figure 3F). Sevoflurane produced a significant decrease in acetylcholine levels [p<0.0001, t(7)=12.25] in medial parietal association cortex, which was not reversed by the local carbachol delivery (Figure 3G). There was no difference in the acetylcholine levels between wake and carbachol [p=0.2, t(7)=2.73] or carbachol and recovery [p=0.3, t(7)=−2.27] epochs. As compared to sevoflurane epoch, the acetylcholine levels increased during the post-sevoflurane recovery epoch [p=0.007, t(7)=−5.27] and reached baseline wake levels (Figure 3G).
Figure 3. Cholinergic stimulation of medial parietal association cortex in anesthetized rat does not affect the level of consciousness.
A) shows effect on behavior after dialysis delivery of 5 mM carbachol (CARB) into medial parietal association cortex (MPtA) of rats receiving continuous sevoflurane anesthesia (1.9–2.4%). B) demonstrates CARB-induced electroencephalographic activation. C) representative spectrograms across the experimental timeline; vertical color bar is the log scale for power spectral density. Black arrows indicate the approximate time of CARB-induced electroencephalographic activation quantified as theta/delta ratio in (D). E) and (F) show the effect of CARB delivery on respiration and heart rate, respectively. G) show changes in acetylcholine levels for each rat during sevoflurane anesthesia (Sevo), CARB/NA delivery into PFC during Sevo, and post-Sevo recovery wake (Rec wake) epochs as percent change from pre-anesthesia wake state (Wake). Significance symbols (p<0.05) show group level comparisons using Student’s two-tailed paired t-test: *compared to Wake, #compared to Sevo, §compared to CARB/NA. Multiple comparisons were Bonferroni corrected. Actual p values are reported in the results section. Frtl - frontal, δ – delta, Prtl - parietal, SD - standard deviation, θ - theta. See also Figure S1 and Tables S1 and S2.
The post-sevoflurane recovery period in the rats that received carbachol in the prefrontal prelimbic cortex was characterized by generalized behavioral seizures and an epileptiform electroencephalographic pattern. In contrast, carbachol delivery into either posterior parietal cortex or medial parietal association cortex did not produce any signs of behavioral seizures or abnormal electroencephalographic patterns during the post-sevoflurane recovery epoch. However, 4/11 rats in the posterior parietal group showed intermittent spike-waveform pattern in the electroencephalogram immediately after carbachol delivery (Figure 2C). Noradrenaline delivery into either prefrontal or posterior parietal cortex did not produce any abnormal electroencephalographic patterns or behavioral seizures in any of the epochs.
Discussion
These data demonstrate that, while cholinergic and noradrenergic stimulation of prefrontal and parietal cortices can activate the cortex, only cholinergic stimulation of the prefrontal cortex restored level of consciousness and reversed the anesthetized state. These findings are consistent with recent work in humans demonstrating a positive effect of prefrontal stimulation on arousal in patients with pathologic disorders of consciousness [10,21,22], and a longstanding hypothesis that acetylcholine is a neurochemical correlate of the capacity for consciousness [31,32]. Furthermore, a recent electrophysiological study postulated that the prefrontal prelimbic cortex in rat, as was targeted in the current study, is a neural node in thalamocortical (and likely corticocortical) interactions that modulate both the induction and emergence from propofol anesthesia [25]. Sevoflurane was selected for this study because it is commonly used during human surgical procedures. Given the wide molecular and pharmacological diversity of general anesthetics, it is difficult to predict the outcome of similar experiments with another anesthetic agent from a different class. However, previously conducted studies have been successful in reversing propofol and isoflurane anesthesia by activating the ventral tegmental area [7,8], which suggests that targeting wake-promoting nodes is a generalized strategy for reversing general anesthesia that is independent of the anesthetic drug used.
It was unexpected that noradrenaline failed to induce any behavioral change despite its known role in wakefulness [2,3,6,9]. There can be several reasons for this unlikely finding. First, this could simply relate to the pharmacokinetics of noradrenaline and carbachol. Second, a recent study in rat barrel cortex showed that cholinergic stimulation through carbachol infusion during urethane anesthesia produced a tonic increase in firing rates whereas infusion of noradrenaline suppressed the overall firing rate [33]. Third, a more fundamental reason could be that noradrenaline activates the electroencephalogram and supports wakefulness but is neither necessary nor sufficient for wakefulness. This assertion is supported by a previous study showing that the ablation of noradrenergic neurons did not have a significant effect on the time spent in the wake state [34].
Our study also demonstrated that cortical dynamics can be dissociated from behavior, i.e., there can be an activated electroencephalogram in the absence of wakefulness. Such dissociation between electroencephalogram and behavior is also known to occur after systemic administration of atropine in rats, which show slow wave electroencephalogram in freely moving and apparently awake condition [35,36], and during rapid eye movement sleep, which is characterized by wake-like activations of the electroencephalogram. Similar dissociations between delta power—an electroencephalographic correlate of quiescence—and behavior has also been reported during sleep restriction, immune challenges, dietary changes, aging, and anesthesia [36,37].
Sevoflurane is known to inhibit both nicotinic and muscarinic receptors [38,39] but based on our study we cannot distinguish between the relative contributions of muscarinic and nicotinic receptors in mediating carbachol-induced reversal of sevoflurane anesthesia. In addition, even though carbachol-induced increases in prefrontal acetylcholine and the simultaneous induction of wake-like behavior suggests a link between the levels of cortical acetylcholine and wakefulness, our study cannot rule out the possibility that the increase in prefrontal acetylcholine levels was an epiphenomenon of the wake state. Finally, we can only interpret these data in terms of the objectively observable level of consciousness (e.g., signs of wakefulness or anesthesia) and cannot comment on how prefrontal versus posterior cortical areas contribute to the phenomenal contents of consciousness.
Our study establishes the sufficiency of cholinergic stimulation in prefrontal cortex for restoring the level of consciousness in anesthetized rats. However, it does not exclude the possibility that the recruitment of additional brain areas, such as the posterior cortical “hot zone” proposed to be important for conscious contents [11,16,17,20], could be required to restore the complete spectrum of consciousness. Although these posterior cortical areas overwhelmingly represent sensory modalities and are likely candidates for the phenomenological component of conscious experience, previous reports have demonstrated a correlation between activity in these posterior areas and improvement in the signs of consciousness in patients with disorders of consciousness [12,15]. Nevertheless, it is becoming more widely appreciated that levels and contents of consciousness cannot be completely dissociated [40] so these findings might ultimately be relevant to the ongoing debate regarding the cortical sites that are critical for conscious experience. Furthermore, the prefrontal cortex could be a hub that regulates both level and content of consciousness, since activity in the dorsolateral prefrontal cortex of the nonhuman primate brain has recently been shown to be critical for reportable contents of visual consciousness [41,42].
In conclusion, these findings suggest that the prefrontal cortex is capable of regulating level of consciousness and reversing the anesthetized state. The homology between rodent and human prefrontal cortex, and the well-characterized tripartite circuitry between cholinergic basal forebrain and the prefrontal and parietal cortices [23,24,27], encourages further work to develop a more precise understanding of the mechanism by which the prefrontal cortex modulates level of consciousness.
STAR Methods
CONTACT FOR REAGENT AND RESOURCE SHARING
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, George A. Mashour (gmashour@umich.edu).
EXPERIMENTAL MODEL AND SUBJECT DETAILS
Rats
The study was approved by the Institutional Animal Care and Use Committee (University of Michigan, Ann Arbor, Michigan, USA), and was performed in accordance with the Guide for the Care and Use of Laboratory Animals (8th Edition, The National Academies Press, Washington D.C.) and the ARRIVE guidelines [43]. Male Sprague-Dawley rats (n=60, 300–350 g, Charles River Laboratories Inc., MA) were used in the study. The rats were housed in a temperature and light (12 h light: 12 h dark cycle, lights on at 6:00 am) controlled facility with ad libitum food and water.
METHOD DETAILS
Surgical Procedures
After anesthetic induction with 4–5% isoflurane in 100% oxygen, the rats were positioned to breathe through a rat anesthesia mask (Kopf Model 906) and immobilized in a stereotaxic frame using blunt ear bars (Model 963, David Kopf Instruments, Tujunga, CA). Inhaled isoflurane concentration was titrated to effect - absence of pedal and palpebral reflex - during the surgical procedure and was monitored using an anesthetic agent analyzer (Datex Medical Instrumentation, Inc., Tewksbury, MA). A rectal probe (Model 7001H, Physitemp Instruments, Inc., Clifton, New Jersey) was positioned to monitor the core body temperature and a small animal far-infrared heating pad (Kent Scientific Co., Torrington, Connecticut) was used to maintain the body temperature at 37.0 ± 1 °C. Subcutaneous buprenorphine (Buprenex ®, Reckitt Benckiser Pharmaceuticals Inc., Richmond, VA) was used for pre- (0.01 mg kg−1) and post-surgical (0.03 mg kg−1, every 8–12 hours for 48 hours) analgesia. In addition, a pre-surgical subcutaneous dose of carprofen (5 mg kg−1) was administered to potentiate the surgical analgesia. A single pre-surgical dose (20 mg kg−1, s.c.) of cefazolin (West-Ward-Pharmaceutical Corp., Eatontown, NJ) was used as a prophylactic antibiotic. Stainless steel screw electrodes were implanted through burr holes to record electroencephalogram from frontal (anterior-posterior: + 3.0 mm, medial-lateral: ± 2.5 mm), parietal (anterior-posterior: - 4.0 mm, medial-lateral: ± 2.5 mm), and occipital (anterior-posterior: - 8.0 mm, medial-lateral: ± 2.5 mm) cortices. A screw electrode was implanted over the nasal sinus to serve as the reference electrode. In addition, a CMA 11 microdialysis guide cannula (Harvard Apparatus, Holliston, MA) was implanted 1.0 mm above the prefrontal prelimbic cortex (anterior-posterior: + 3.0 mm, medial-lateral: 0.5 mm, ventral: 4.0 mm) or 2.0 mm above posterior parietal cortex (anterior-posterior: - 3.60 mm, medial-lateral: 2.6 mm, ventral: 2.0 mm, angle of 40 degrees) or 1.0 mm above medial parietal association cortex (anterior-posterior: - 3.72 mm, medial-lateral: 2.2 mm, ventral: 1.5 mm). The rat brain atlas by Paxinos and Watson [44] was used for the determination of stereotaxic coordinates and bregma was used as the stereotactic reference point. The free ends of the screw electrodes were mated with a six-pin pedestal (MS363, Plastics One, Roanoke, VA), which along with the microdialysis guide cannula was secured to the cranium using dental cement (Cat No. 51459, Stoelting Co, Woodlake, IL). At the completion of the surgery, the rats were returned to their home cages and kept under a heating lamp until they recovered from anesthesia and were ambulatory.
Electroencephalographic Recordings
Electroencephalographic signals from frontal, parietal, and occipital cortices were referenced to a stainless-steel screw electrode over the nasal sinus, amplified 5000x, and bandpass filtered between 0.1–300 Hz using a Grass Model 15 LT bipolar physiodata amplifier system (15A54 Quad Amplifier, Natus Neurology Inc., Warwick, RI). A MP150 data acquisition unit (Acqknowledge software version 4.1.1, Biopac Systems, Inc, Goleta, CA) was used for digitization (1 kHz) and storage of the electroencephalographic data. Electroencephalographic signals from the ipsilateral frontal and parietal areas were digitally subtracted to generate a single bipolar signal and divided into non-overlapped 10-second epochs. For each epoch, the power spectrum was estimated via Welch’s method (pwelch.m function in Matlab signal processing toolbox) and the theta/delta ratio was calculated as the mean power in the theta (3.5–10 Hz) band divided by the mean power in the delta (1–3 Hz) band, which was averaged across the available epochs at each studied state in each rat.
Reverse Dialysis Delivery of Carbachol and Noradrenaline
CMA 11 microdialysis probes (Cuprophane membrane, 0.24 mm diameter, 6 kD cut-off, 1 mm length for prefrontal and medial parietal association cortex, 2 mm for posterior parietal cortex; Harvard Apparatus, Holliston, MA) were continuously perfused with Ringer’s solution (147 mM NaCl, 2.4 mM CaCl2, 4.0 mM KCl, 10 μM neostigmine; pH 6.0 ± 0.2) at a flow rate of 2.0 μL min−1. A liquid switch (CMA 110, Harvard Apparatus, Holliston, MA) was used to switch between Ringer’s only and Ringer’s with either carbachol or noradrenaline solutions. To prevent auto-oxidation of noradrenaline, the Ringer’s solution for noradrenaline also contained 10 μM ascorbic acid and the solution was protected from light.
Acetylcholine Quantification
The changes in local acetylcholine levels after carbachol delivery were quantified using a high-performance liquid chromatography (HPLC) coupled with an electrochemical detector. From each microdialysis sample, 22 μL was injected into a HPLC paired with an electrochemical detector (Bioanalytical Systems, West Lafayette, IN). An ion-exchange (mobile phase: 50 mM Na2HPO4, pH 8.5) analytical column (MF-6150, Bioanalytical Systems, West Lafayette, IN) separated acetylcholine and choline in the dialysis samples, which were proportionately catalyzed into hydrogen peroxide by an immobilized enzyme reactor column (Shodex AFpak ACH-494, Showa Denko America, Inc., NY). Hydrogen peroxide was detected by reduction (applied potential: 100 mV, Ag+/AgCl reference electrode) at a dual glassy carbon (3 mm) working electrode coated (MF-1000, Bioanalytical Systems, West Lafayette, IN) with a surfactant (CF-1075, Bioanalytical Systems, West Lafayette, IN) and peroxidase polymer (CF-1070, Bioanalytical Systems, West Lafayette, IN). A seven-level acetylcholine-choline standard curve (0.05–1.0 pmol) was generated prior to each experiment and was used as a reference for the quantification of acetylcholine levels using LC Solutions software (Shimadzu Inc., USA). The microdialysis samples from the noradrenaline experiments were analyzed using a Thermo Finnigan TSQ Quantum Ultra AM triple quadrupole mass spectrometer equipped with an ion-max electrospray ionization source and coupled with a HPLC system (Waters Corporation). The HPLC system contained a quaternary low-pressure mixing pump with vacuum degassing, an autosampler with temperature-controlled tray, a column oven, and was equipped with a small-bore reverse phase HPLC column (Phenomenex Synergi™ 4 μm Hydro-RP 2×54mm). The mobile phase ‘A’ was 10 mM ammonium formate with 0.15% formic acid and mobile ‘B’ was 100% acetonitrile. A six-level calibration curve for acetylcholine was generated based on analyte to internal standard area ratios versus nanomolar concentration. The microdialysis samples were derivatized—prior to loading onto autosampler—using calcium carbonate buffer solution (pH 9.2) followed by 2% benzoyl chloride with the final addition of 1% sulfuric acid solution containing 13C-labeled internal standard. Xcalibur software was used to control the HPLC and mass spectrometer as well as to generate quantitation reports.
Histological Confirmation of the Microdialysis Sites
After 3–7 days of completion of the recording session, the rats were deeply anesthetized with a combination of ketamine and xylazine (80 mg kg−1 and 10 mg kg−1 body wt, i.p.). The rats were transcardially perfused with 100 mL of wash solution (phosphate buffered saline: 0.1 M, pH 7.2; 1219SK, EM Sciences, Hatfield, PA) and 250 mL of a fixative solution (4% paraformaldehyde and 4% sucrose in 0.1 M phosphate buffer, pH 7.2; 1224SK, EM Sciences, Hatfield, PA). The brains were removed and stored in fixative solution for at least 24 h at 4 degrees Celsius, and then equilibrated in 30% sucrose in phosphate buffer at the room temperature. Coronal sections (30 μm) were cut through the target brain regions - prefrontal prelimbic cortex, posterior parietal cortex, and medial parietal association cortex - on a cryostat (Leica Microsystems Nussloch GmbH, Nussloch, Germany). The sections were mounted on slides and stained with cresyl violet to confirm the location of microdialysis probe. Figure S1A–C shows the site of microdialysis in representative rat brain sections for all three anatomical sites.
Experimental Design
After 7–10 days of post-surgical recovery period and habituation to the experimental set-up, the rats were connected to the electroencephalogram recording system at least 30 minutes prior to the start of experimental session (9:30 am–10:00 am). A microdialysis probe being continuously infused with Ringer’s solution at 2.0 μL min−1 was lowered into the target areas for reverse dialysis delivery of carbachol or noradrenaline while simultaneously collecting local microdialysis samples for acetylcholine estimation: 1) prefrontal prelimbic cortex - 11 rats each for carbachol and noradrenaline groups, 2) posterior parietal cortex - 11 rats each for carbachol and noradrenaline groups, or 3) medial parietal association cortex - 8 rats for carbachol delivery. The concentration of carbachol (5 mM) was based on a previous study [27] and dose response experiments (n=5 rats) in which we titrated carbachol (2.5–5 mM) delivery into prefrontal prelimbic cortex to obtain the minimum concentration that produced electroencephalographic activation and wake behavior; the data from these experiments were not included in the analyses. The same carbachol concentration was used for posterior parietal cortex group. In addition, in a separate group of 3 rats, we delivered a higher concentration of carbachol (15 mM) into posterior parietal cortex to ensure that the concentration of carbachol is not a limiting factor for the lack of behavioral effects. The concentration of noradrenaline used in this study (20 mM) was informed by a previous report in which infusion of similar concentration of noradrenaline into basal forebrain produced microarousals in rats under desflurane anesthesia [6].
Electroencephalographic and microdialysis data were collected simultaneously and continuously but the microdialysis samples were obtained in bins of 12.5 minutes as has been done in our recent publications [26,45]. The first three data samples were excluded from the analysis to avoid any possible confound associated with the insertion of the probe [26,45] after which the baseline data under pre-anesthesia wake condition were collected for 75 minutes. To keep the behavioral state constant during the pre-anesthesia baseline period, the rats were kept awake by the introduction of novel objects and gentle tapping on the outside of the recording chamber. After the completion of 75 minutes of baseline wake condition, sevoflurane exposure (1.9–2.4%) started and was titrated to produce unconsciousness as assessed through the loss of righting reflex along with complete behavioral immobility. Sevoflurane concentrations were continuously monitored using anesthesia monitors (Datex Medical Instrumentation, Inc., Tewksbury, MA) connected to the gas inlet and outlet ports on the recording chamber. At the onset of loss of righting reflex, a rectal probe connected to a far-infrared heating pad (Kent Scientific Co., Torrington, Connecticut) through a feedback temperature controller (Physitemp, Model RET-3) was positioned to monitor and maintain the body temperature at 37.0 ± 1 °C. In addition, a pulse oximetry sensor (MouseOx, Starr Life Science Corp., Oakmont, PA) was positioned on the foot or around the neck to record the changes in respiration rate, heart rate, and oxygen saturation levels. After 75 minutes of sevoflurane exposure, we reverse dialyzed either carbachol or noradrenaline for 12.5 minutes while the rats continued to breathe the same levels of sevoflurane anesthesia (1.9–2.4%). The sevoflurane exposure continued for an additional 50 minutes, after which the anesthetic exposure was stopped. Thereafter, the recovery wake data during the post-sevoflurane period was collected for 37.5 minutes. The pulse oximetry data were excluded in some of the rats because of the lack of consistently reliable signals and were analyzed in 1) 6/11 rats in carbachol-prefrontal cortex group, 2) 8/11 rats in the noradrenaline-prefrontal cortex group, 3) 9/11 rats in both the carbachol- and noradrenaline-posterior parietal cortex group, and 4) 6/8 rats in the carbachol-medial parietal association cortex group. We did not randomize carbachol and noradrenaline treatment because comparable behavioral response and cortical activation was expected. The investigators could not be blinded to the experimental groups because of the clear differences in the microdialysis guide cannula locations and the methodology for acetylcholine quantification: HPLC coupled with electrochemical detection was used for the acetylcholine quantification in the carbachol groups while mass spectrometry was used for the noradrenaline groups. Several of the experimental sessions across all experimental groups were videotaped and can be made available on request to the corresponding author. The experimental design is illustrated in supplementary figure S1D.
QUANTIFICATION AND STATISTICAL ANALYSIS
Statistical analyses were conducted in consultation with the Consulting for Statistics, Computing and Analytics Research unit at the University of Michigan, Ann Arbor. We designed the study to have 80% power (at alpha=0.05/6; Bonferroni correction for 6 pairwise tests) to detect an effect size (difference in means divided by standard deviation of difference) of 1.2 or larger. The effect size of 1.2 or larger was predicted based on a recently published, similar study from our laboratory [26]. Student’s two-tailed paired t-tests were used for all statistical comparisons using the programming and statistical language R v 3.3.1 (Studio Version 0.99.903 for Macintosh) [46]. A p value of <0.05 was considered statistically significant. Multiple comparisons between acetylcholine measurements were Bonferroni corrected. The respiration and heart rates were statistically compared 300 s before and 300 s after the visible change in electroencephalogram following carbachol or noradrenaline delivery into target brain areas. For theta/delta ratio, electroencephalogram segments (300s) from the last epoch during sevoflurane exposure immediately before carbachol or noradrenaline delivery was compared with the activated electroencephalogram segments (≤300s) immediately following carbachol/noradrenaline delivery. The effect of carbachol or noradrenaline on acetylcholine release was assessed by statistical comparison of the following epochs: 1) Wakefulness - last epoch from the baseline wake condition, 2) Sevoflurane anesthesia - last epoch during sevoflurane exposure and before carbachol or noradrenaline delivery, 3) Carbachol - epoch representing the perfusion of target regions with carbachol during sevoflurane anesthesia, 4) Noradrenaline - the presence of noradrenaline in the reverse dialysate interfered with the simultaneous estimation of acetylcholine because of which the first post-noradrenaline epoch (devoid of noradrenaline) during sevoflurane anesthesia was selected for acetylcholine estimation, 5) Recovery wake state - the emergence time from sevoflurane anesthesia was variable between rats but all rats recovered within 12 minutes of the cessation of sevoflurane exposure. Therefore, second post-sevoflurane epoch was selected for analysis. Acetylcholine quantification was conducted using two different techniques: HPLC combined with electrochemical detection for carbachol groups and mass spectrometry for noradrenaline groups. Therefore, to make the changes in acetylcholine levels in the carbachol and noradrenaline groups comparable, we have reported the data as percent change from baseline in the main manuscript. Raw data for respiration rate, heart rate, and acetylcholine levels, as well as the associated descriptive and inferential statistics are provided in the Tables S1–3.
Supplementary Material
Video shows the effect of dialysis delivery of 5 mM carbachol into prefrontal prelimbic cortex (left panel) and posterior parietal cortex (right panel) of rats receiving constant sevoflurane anesthesia at clinically relevant concentrations (1.9–2.4%). The panel on the left shows the restoration of wake-like behavior after carbachol delivery into prefrontal prelimbic cortex while the panel on the right shows the inability of carbachol in posterior parietal cortex to induce similar behavior. Please note that the restoration of wake-like behavior and mobility after carbachol delivery occurs under continuous sevoflurane exposure; the rat is breathing the same concentration of sevoflurane before, during, and after recovery of the righting reflex. The traces on top of the videos show the electroencephalographic activation in the presence (prefrontal prelimbic cortex) or absence (posterior parietal cortex) of carbachol-induced wake-like behavior. Topmost electroencephalographic trace (blue) is from frontal area while the electroencephalographic trace below it (green) is from the parietal area. Parietal electroencephalogram in the right panel - posterior parietal cortex - also shows the occurrence of spike-waveform discharge observed briefly in 4/11 rats in this cohort. Video in both panels are on similar time scales and start ~30 seconds before the carbachol-induced electroencephalographic activation, which in case of prefrontal cortex, precedes the behavioral change.
Video shows the effect of dialysis delivery of 20 mM noradrenaline into prefrontal prelimbic cortex (left panel) and posterior parietal cortex (right panel) of rats receiving constant sevoflurane anesthesia at clinically relevant concentrations (1.9–2.4%). Noradrenaline delivery into neither prefrontal prelimbic cortex (left panel) nor posterior parietal cortex (right panel) was effective in inducing any signs consistent with wakefulness. The traces on top of the videos show the electroencephalographic activation that occurred despite the absence of any signs of wakelike behavior. Topmost electroencephalographic trace (blue) is from frontal area while the electroencephalographic trace below it (green) is from the parietal area. Video in both panels are on similar time scales and start ~30 seconds before the noradrenaline-induced electroencephalographic activation.
Highlights.
Prefrontal cholinergic stimulation induces wake-like behavior in anesthetized rats
Cholinergic stimulation of parietal regions did not induce behavioral transitions
Prefrontal/parietal noradrenergic stimulation did not induce behavioral transitions
Electroencephalographic activation and level of consciousness are dissociable
Acknowledgments
The authors would like to thank Chris Andrews of the University of Michigan Center for Statistical Consultation and Research unit for help with statistical analysis, and Donald C. Fedrigon III for help with data collection. This work was funded by the National Institutes of Health (Bethesda, MD, USA) (R01GM098578 to GAM), and funding from the Department of Anesthesiology, University of Michigan Medical School, Ann Arbor.
Footnotes
Author Contributions
DP and GAM designed the study, interpreted the data, and prepared the manuscript; JD and AGH contributed to data interpretation and manuscript preparation; DP, JD, and TL conducted the experiments and collected the data; DP, JD, TL, DL, and CW analyzed the data. All authors read and approved the manuscript.
Declaration of Interests
The authors declare no competing interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Alkire MT, McReynolds JR, Hahn EL, Trivedi AN. Thalamic microinjection of nicotine reverses sevoflurane-induced loss of righting reflex in the rat. Anesthesiology. 2007;107:264–272. doi: 10.1097/01.anes.0000270741.33766.24. [DOI] [PubMed] [Google Scholar]
- 2.Carter ME, Yizhar O, Chikahisa S, Nguyen H, Adamantidis A, Nishino S, Deisseroth K, de Lecea L. Tuning arousal with optogenetic modulation of locus coeruleus neurons. Nat Neurosci. 2010;13:1526–1533. doi: 10.1038/nn.2682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Franks NP. General anaesthesia: from molecular targets to neuronal pathways of sleep and arousal. Nat Rev Neurosci. 2008;9:370–86. doi: 10.1038/nrn2372. [DOI] [PubMed] [Google Scholar]
- 4.Han Y, Shi YF, Xi W, Zhou R, Tan ZB, Wang H, Li XM, Chen Z, Feng G, Luo M, et al. Selective activation of cholinergic basal forebrain neurons induces immediate sleep-wake transitions. Curr Biol. 2014;24:693–698. doi: 10.1016/j.cub.2014.02.011. [DOI] [PubMed] [Google Scholar]
- 5.Moruzzi G, Magoun HW. Brain stem reticular formation and activation of the EEG. Electroencephalogr Clin Neurophysiol. 1949;1:455–473. [PubMed] [Google Scholar]
- 6.Pillay S, Vizuete JA, McCallum JB, Hudetz AG. Norepinephrine infusion into nucleus basalis elicits microarousal in desflurane-anesthetized rats. Anesthesiology. 2011;115:733–742. doi: 10.1097/ALN.0b013e31822c5ee1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Solt K, Van Dort CJ, Chemali JJ, Taylor NE, Kenny JD, Brown EN. Electrical stimulation of the ventral tegmental area induces reanimation from general anesthesia. Anesthesiology. 2014;121:311–319. doi: 10.1097/ALN.0000000000000117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Taylor NE, Van Dort CJ, Kenny JD, Pei J, Guidera JA, Vlasov KY, Lee JT, Boyden ES, Brown EN, Solt K. Optogenetic activation of dopamine neurons in the ventral tegmental area induces reanimation from general anesthesia. Proc Natl Acad Sci U S A. 2016;113:12826–12831. doi: 10.1073/pnas.1614340113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vazey EM, Aston-Jones G. Designer receptor manipulations reveal a role of the locus coeruleus noradrenergic system in isoflurane general anesthesia. Proc Natl Acad Sci U S A. 2014;111:3859–3864. doi: 10.1073/pnas.1310025111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Angelakis E, Liouta E, Andreadis N, Korfias S, Ktonas P, Stranjalis G, Sakas DE. Transcranial direct current stimulation effects in disorders of consciousness. Arch Phys Med Rehabil. 2014;95:283–9. doi: 10.1016/j.apmr.2013.09.002. [DOI] [PubMed] [Google Scholar]
- 11.Boly M, Massimini M, Tsuchiya N, Postle BR, Koch C, Tononi G. Are the neural correlates of consciousness in the front or in the back of the cerebral cortex? Clinical and neuroimaging evidence. J Neurosci. 2017;37:9603–9613. doi: 10.1523/JNEUROSCI.3218-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Corazzol M, Lio G, Lefevre A, Deiana G, Tell L, André-Obadia N, Bourdillon P, Guenot M, Desmurget M, Luauté J, et al. Restoring consciousness with vagus nerve stimulation. Curr Biol. 2017;27:R994–R996. doi: 10.1016/j.cub.2017.07.060. [DOI] [PubMed] [Google Scholar]
- 13.Del Cul A, Dehaene S, Reyes P, Bravo E, Slachevsky A. Causal role of prefrontal cortex in the threshold for access to consciousness. Brain. 2009;132:2531–40. doi: 10.1093/brain/awp111. [DOI] [PubMed] [Google Scholar]
- 14.Dehaene S, Charles L, King JR, Marti S. Toward a computational theory of conscious processing. Curr Opin Neurobiol. 2014;25:76–84. doi: 10.1016/j.conb.2013.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.King JR, Sitt JD, Faugeras F, Rohaut B, El Karoui I, Cohen L, Naccache L, Dehaene S. Information sharing in the brain indexes consciousness in noncommunicative patients. Curr Biol. 2013;23:1914–9. doi: 10.1016/j.cub.2013.07.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Koch C, Massimini M, Boly M, Tononi G. Neural correlates of consciousness: progress and problems. Nat Rev Neurosci. 2016;17:307–321. doi: 10.1038/nrn.2016.22. [DOI] [PubMed] [Google Scholar]
- 17.Koch C, Massimini M, Boly M, Tononi G. Posterior and anterior cortex — where is the difference that makes the difference? Nat Rev Neurosci. 2016;17:666. doi: 10.1038/nrn.2016.105. [DOI] [PubMed] [Google Scholar]
- 18.Koukouli F, Rooy M, Changeux JP, Maskos U. Nicotinic receptors in mouse prefrontal cortex modulate ultraslow fluctuations related to conscious processing. Proc Natl Acad Sci. 2016;113:14823–14828. doi: 10.1073/pnas.1614417113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Odegaard B, Knight RT, Lau H. Should a few null findings falsify prefrontal theories of conscious perception? J Neurosci. 2017;37:9593–9602. doi: 10.1523/JNEUROSCI.3217-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Storm JF, Boly M, Casali AG, Massimini M, Olcese U, Wilke CMAM. Consciousness regained: disentangling mechanisms, brain systems, and behavioral responses. J Neurosci. 2017;37:10882–10893. doi: 10.1523/JNEUROSCI.1838-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thibaut A, Bruno MA, Ledoux D, Demertzi A, Laureys S. tDCS in patients with disorders of consciousness: sham-controlled randomized double-blind study. Neurology. 2014;82:1112–8. doi: 10.1212/WNL.0000000000000260. [DOI] [PubMed] [Google Scholar]
- 22.Thibaut A, Wannez S, Donneau AF, Chatelle C, Gosseries O, Bruno MA, Laureys S. Controlled clinical trial of repeated prefrontal tDCS in patients with chronic minimally conscious state. Brain Inj. 2017;31:466–474. doi: 10.1080/02699052.2016.1274776. [DOI] [PubMed] [Google Scholar]
- 23.Briand LA, Gritton H, Howe WM, Young DA, Sarter M. Modulators in concert for cognition: modulator interactions in the prefrontal cortex. Prog Neurobiol. 2007;83:69–91. doi: 10.1016/j.pneurobio.2007.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hoover WB, Vertes RP. Anatomical analysis of afferent projections to the medial prefrontal cortex in the rat. Brain Struct Funct. 2007;212:149–179. doi: 10.1007/s00429-007-0150-4. [DOI] [PubMed] [Google Scholar]
- 25.Flores FJ, Hartnack KE, Fath AB, Kim SE, Wilson MA, Brown EN, Purdon PL. Thalamocortical synchronization during induction and emergence from propofol-induced unconsciousness. Proc Natl Acad Sci U S A. 2017;114:E6660–E6668. doi: 10.1073/pnas.1700148114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pal D, Silverstein BH, Lee H, Mashour GA. Neural correlates of wakefulness, sleep, and general anesthesia: an experimental study in rat. Anesthesiology. 2016;125:929–942. doi: 10.1097/ALN.0000000000001342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nelson CL, Sarter M, Bruno JP. Prefrontal cortical modulation of acetylcholine release in posterior parietal cortex. Neuroscience. 2005;132:347–359. doi: 10.1016/j.neuroscience.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 28.Reep RL, Corwin JV. Posterior parietal cortex as part of a neural network for directed attention in rats. Neurobiol Learn Mem. 2009;91:104–113. doi: 10.1016/j.nlm.2008.08.010. [DOI] [PubMed] [Google Scholar]
- 29.Lu H, Zou Q, Gu H, Raichle ME, Stein EA, Yang Y. Rat brains also have a default mode network. Proc Natl Acad Sci U S A. 2011;109:3979–3984. doi: 10.1073/pnas.1200506109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wilber AA, Clark BJ, Demecha AJ, Mesina L, Vos JM, McNaughton BL. Cortical connectivity maps reveal anatomically distinct areas in the parietal cortex of the rat. Front Neural Circuits. 2015;8:1–15. doi: 10.3389/fncir.2014.00146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Perry E, Walker M, Grace J, Perry R. Acetylcholine in mind: a neurotransmitter correlate of consciousness? Trends Neurosci. 1999;22:273–280. doi: 10.1016/s0166-2236(98)01361-7. [DOI] [PubMed] [Google Scholar]
- 32.Woolf NJ, Butcher LL. Cholinergic systems mediate action from movement to higher consciousness. Behav Brain Res. 2011;221:488–498. doi: 10.1016/j.bbr.2009.12.046. [DOI] [PubMed] [Google Scholar]
- 33.Castro-Alamancos MA, Gulati T. Neuromodulators produce distinct activated states in neocortex. J Neurosci. 2014;34:12353–12367. doi: 10.1523/JNEUROSCI.1858-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Blanco-Centurion C, Gerashchenko D, Shiromani PJ. Effects of saporin- induced lesions of three arousal populations on daily levels of sleep and wake. J Neurosci. 2007;27:14041–8. doi: 10.1523/JNEUROSCI.3217-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Qiu MH, Chen MC, Lu J. Cortical neuronal activity does not regulate sleep homeostasis. Neuroscience. 2015;297:211–8. doi: 10.1016/j.neuroscience.2015.03.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Davis CJ, Clinton JM, Jewett KA, Zielinski MR, Krueger JM. Delta wave power: an independent sleep phenotype or epiphenomenon? J Clin Sleep Med. 2011;7:S16–18. doi: 10.5664/JCSM.1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gaskell AL, Hight DF, Winders J, Tran G, Defresne A, Bonhomme V, Raz A, Sleigh JW, Sanders RD. Frontal alpha-delta EEG does not preclude volitional response during anesthesia: prospective cohort study of the isolated forearm technique. Br J Anaesth. 2017;119:664–673. doi: 10.1093/bja/aex170. [DOI] [PubMed] [Google Scholar]
- 38.Nietgen GW, Hönemann CW, Chan CK, Kamatchi GL, Durieux ME. Volatile anesthetics have differential effects on recombinant m1 and m3 muscarinic acetylcholine receptor function. Br J Anaesth. 1998;81:569–577. doi: 10.1093/bja/81.4.569. [DOI] [PubMed] [Google Scholar]
- 39.Tassonyi E, Charpantier E, Muller D, Dumont L, Bertrand D. The role of nicotinic acetylcholine receptors in the mechanisms of anesthesia. Brain Res Bull. 2002;57:133–150. doi: 10.1016/s0361-9230(01)00740-7. [DOI] [PubMed] [Google Scholar]
- 40.Bachmann T, Hudetz AG. It is time to combine the two main traditions in the research on the neural correlates of consciousness: C = L × D. Front Psychol. 2014;5:940. doi: 10.3389/fpsyg.2014.00940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mashour GA. The controversial correlates of consciousness. Science. 2018;360:493–494. doi: 10.1126/science.aat5616. [DOI] [PubMed] [Google Scholar]
- 42.van Vugt B, Dagnino B, Vartak D, Safaai H, Panzeri S, Dehaene S, Roelfsema PR. The threshold for conscious report: Signal loss and response bias in visual and frontal cortex. Science. 2018;360:537–542. doi: 10.1126/science.aar7186. [DOI] [PubMed] [Google Scholar]
- 43.Kilkenny C, Browne WJ, Cuthill IC, Emerson M, Altman DG. Improving bioscience research reporting: the ARRIVE guidelines for reporting animal research. PLoS Biol. 2010;8:e1000412. doi: 10.1371/journal.pbio.1000412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 6. London: Academic Press; 2007. [Google Scholar]
- 45.Pal D, Hambrecht-Wiedbusch VS, Silverstein BH, Mashour GA. Electroencephalographic coherence and cortical acetylcholine during ketamine-induced unconsciousness. Br J Anaesth. 2015;114:979–989. doi: 10.1093/bja/aev095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing; Vienna, Austria: 2016. URL: https://www.R-project.org/ [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Video shows the effect of dialysis delivery of 5 mM carbachol into prefrontal prelimbic cortex (left panel) and posterior parietal cortex (right panel) of rats receiving constant sevoflurane anesthesia at clinically relevant concentrations (1.9–2.4%). The panel on the left shows the restoration of wake-like behavior after carbachol delivery into prefrontal prelimbic cortex while the panel on the right shows the inability of carbachol in posterior parietal cortex to induce similar behavior. Please note that the restoration of wake-like behavior and mobility after carbachol delivery occurs under continuous sevoflurane exposure; the rat is breathing the same concentration of sevoflurane before, during, and after recovery of the righting reflex. The traces on top of the videos show the electroencephalographic activation in the presence (prefrontal prelimbic cortex) or absence (posterior parietal cortex) of carbachol-induced wake-like behavior. Topmost electroencephalographic trace (blue) is from frontal area while the electroencephalographic trace below it (green) is from the parietal area. Parietal electroencephalogram in the right panel - posterior parietal cortex - also shows the occurrence of spike-waveform discharge observed briefly in 4/11 rats in this cohort. Video in both panels are on similar time scales and start ~30 seconds before the carbachol-induced electroencephalographic activation, which in case of prefrontal cortex, precedes the behavioral change.
Video shows the effect of dialysis delivery of 20 mM noradrenaline into prefrontal prelimbic cortex (left panel) and posterior parietal cortex (right panel) of rats receiving constant sevoflurane anesthesia at clinically relevant concentrations (1.9–2.4%). Noradrenaline delivery into neither prefrontal prelimbic cortex (left panel) nor posterior parietal cortex (right panel) was effective in inducing any signs consistent with wakefulness. The traces on top of the videos show the electroencephalographic activation that occurred despite the absence of any signs of wakelike behavior. Topmost electroencephalographic trace (blue) is from frontal area while the electroencephalographic trace below it (green) is from the parietal area. Video in both panels are on similar time scales and start ~30 seconds before the noradrenaline-induced electroencephalographic activation.