Abstract
Protection against microbial infection by the induction of inflammation is a key function of the interleukin-1 superfamily, including both classical IL-1 and the new IL-36 cytokine families. Candida albicans is a frequent human fungal pathogen causing mucosal infections. Although the initiators and effectors important in protective host responses to C. albicans are well described, the key players in driving these responses remain poorly defined. Recent work has identified a central role played by IL-1 in inducing innate type 17 immune responses to clear C. albicans infections. Despite this, lack of IL-1 signaling does not result in complete loss of immunity, indicating that there are other factors involved in mediating protection to this fungus. Here, we identify IL-36 cytokines as a new player in these responses. We show that C. albicans infection of the oral mucosa induces the production of IL-36. In common with IL-1α/β, induction of epithelial IL-36 depends on the hypha-associated peptide toxin, Candidalysin. Epithelial IL-36 gene expression requires p38-MAPK/c-Fos, NF-κB and PI3K signaling, and is regulated by the MAPK phosphatase MKP1. Oral candidiasis in IL-36R−/− mice shows increased fungal burdens and reduced IL-23 gene expression, indicating a key role played by IL-36 and IL-23 in innate protective responses to this fungus. Strikingly, we observed no impact on gene expression of IL-17 or IL-17-dependent genes, indicating that this protection occurs via an alternative pathway to IL-1-driven immunity. Thus, IL-1 and IL-36 represent parallel epithelial cell-driven protective pathways in immunity to oral C. albicans infection.
Keywords: IL-36, Candida albicans, IL-23, fungal immunity, epithelial cell
Introduction
Mucosal barrier surfaces are equipped with multiple rapid-acting innate immune strategies to counter pathogenic, often invasive microbes. The oral mucosa is particularly vulnerable to such pathogens, yet our understanding of the host immune arsenal in this tissue remains surprisingly limited (1). In settings of weakened immunity or immunosuppression, the oral cavity becomes permissive to the growth of the opportunistic fungal pathogen, Candida albicans, causing a painful condition termed oropharyngeal candidiasis (OPC, thrush) that can be associated with nutritional deficiency, failure to thrive and an increased risk of esophageal cancer (2). To date there are no licensed vaccines to C. albicans, or indeed to any fungi (3, 4).
Oral epithelial cells (OECs) lining the palate, gingiva, buccal mucosa, and tongue are key ‘first-responders’ during C. albicans infection (5). In addition to providing structural integrity to the mucosal barrier, OECs are critical orchestrators of innate defenses in the mouth. OECs respond to C. albicans-mediated tissue injury by activating damage-associated immune effectors including IL-1 family cytokines, S100A8/9 (calprotectin), and β-defensins (6, 7). The transition of commensal C. albicans cells on mucosal surfaces to invasive pathogenic cells requires hyphal formation. These hyphae secrete a pore-forming peptide toxin called Candidalysin that damages the oral epithelium during tissue invasion, but also induces protective host responses including inflammatory cytokines (6). In particular, Candidalysin-induced IL-1α/β triggers innate Type-17 immunity, which is vital for resolution of C. albicans oral infections in mice and humans (7–9). Additionally, IL-1R signaling in the oral mucosa is an important trigger for the mobilization of neutrophils to limit growth of pathogenic C. albicans (10). Thus, OEC-driven IL-1 production is a crucial host defense component of immunity to C. albicans oral infections.
Emerging data indicate that IL-36 is also an important mediator of innate immunity during microbial infections (11–15). IL-36 is part of the IL-1 superfamily and includes three agonistic cytokines (IL-36α, IL-36β, and IL-36γ) and a receptor antagonist (IL-36RA) (16). IL-36 cytokines signal through a receptor composed of the IL-36R and IL-1R accessory protein (encoded by Il1rl2 and Il1rap, respectively), which signal through the NF-κB and MAPK pathways (17). Like IL-1, IL-36 induces chemokines such as CCL2, CCL20, CXCL1, and CXCL5 in epithelial cells, which subsequently promote recruitment of neutrophils and lymphocytes (18–20). IL-36 also triggers expression of β-defensins which exert direct antimicrobial activity (21). Recent studies showed that the Staphylococcus aureus pore-forming toxin phenol-soluble modulin alpha (PSMα) induces IL-36 in keratinocytes, which in turn activates a Type 17 response (14, 15). Thus, IL-36 is an integral component of mucosal and dermal host defense, but to date little is known about the influence of IL-36 family cytokines in oral fungal infections such as oral candidiasis.
Here, we report that the production of IL-36 cytokines by oral epithelial cells is markedly induced following C. albicans infection, and that IL-36R-deficient mice are highly susceptible to OPC. Susceptibility is associated with dampened antifungal innate immune responses and reduced expression of IL-23. Expression of IL-36 was not induced by hyphae per se, but required the hypha-derived peptide toxin Candidalysin in both mice and human cells. Thus, our data reveal regulatory mechanisms that control IL-36 in OECs and illuminate its importance in antifungal defenses.
Materials and Methods
Mice
All mice were derived from a C57BL/6 background. Experiments were performed on both sexes, with age- and sex-matched controls. Act1−/− mice were from U. Siebenlist (NIH), IL-36R−/− mice were provided by Amgen Inc. IL-23R−/− mice were a kind gift from Dr. Vijay Kuchroo (Harvard Medical School and Brigham and Women’s Hospital, Boston MA 0211). IL-1R1−/− mice were purchased from JAX and expanded at the University of Pittsburgh. For experiments involving IL-36R−/− mice, WT control mice were from Taconic. For other studies, WT mice were from JAX. OPC was performed by sublingual inoculation with a 2.5 mg cotton ball saturated in C. albicans for 75 min (22). Tongue homogenates were prepared on a GentleMACS (Miltenyi) and CFU determined by plating on YPD/chloramphenicol agar. Protocols were approved by the University of Pittsburgh IACUC. All efforts were made to minimize suffering, in accordance with recommendations in the Guide for the Care and Use of Laboratory Animals of the NIH.
Cell lines, Reagent and Candida strains
Human buccal epithelial carcinoma TR146 cell monolayers were grown in DMEM-F12/10% FBS, and experiments were performed in serum-free DMEM-F12. Inhibitors of p38-MAPK (SB203580;10μM), NF-κB (BAY11-7082; 2μM) and PI3K (LY294002; 50μM) were from Merck. Antibodies to phospho-c-Jun, -c-Fos and -MKP-1 were from Cell Signalling Technologies. Anti-rabbit secondary antibodies were from Jackson Immunoresearch. Fungal strains: C. albicans reference strain SC5314 (23), parental strain BWP17 (24), yeast-locked flo8Δ/Δ (25), eed1Δ/Δ and eed1Δ/Δ+EED1 (26), efg1/cph1Δ/Δ (27), ece1Δ/Δ, ece1Δ/Δ +ECE1, ece1Δ/Δ +ECE1Δ184-279 (6).
RNA, qPCR and siRNA knockdown
TR146 cells: RNA was extracted using the Nucleospin II RNA isolation kit (Macherey-Nagel). Isolated RNA was treated with the Turbo DNAse free kit (Life Technologies) to remove genomic DNA contamination. cDNA was created using the Superscript IV reverse transcriptase kit (Thermo Scientific). Real time PCR was performed on cDNA using EvaGreen qPCR mastermix (Newmarket Scientific, UK) on a Rotorgene 6000 (Corbett Research) and normalized to YWHAZ. Primer sequences were from Primerbank (28); IL-36α forward, 5′-GATGTGTGCTAAAGTCGGGGA-3′, IL-36α reverse, 5′-ACAGACTCGAAGGTGGAGTTC-3′, IL-36γ forward, 5′-GAAACCCTTCCTTTTCTACCGTG-3′, IL-36γ reverse, 5′-GCTGGTCTCTCTTGGAGGAG-3′.
Mouse: Frozen tongue was homogenized in RLT buffer (RNAeasy kit; Qiagen) with a GentleMACS Dissociator (Miltenyi). cDNA was synthesized with a SuperScript III first-strand synthesis system (Invitrogen, Carlsbad, CA). Relative quantification was determined by real-time PCR with SYBR green (Quanta BioSciences, Gaithersburg, MD) normalized to Gapdh. Primers were from SA Biosciences (Qiagen). Results were analyzed on a 7300 real-time PCR system (Applied Biosystems).
RNA silencing by siRNA was performed with the HiPerFect reagent (Qiagen) as described: c-Jun (29), c-Fos (30), and MKP-1 (31). Validation by qPCR verified that targeted genes were reduced by at least 80% and maintained for at least 24 h (data not shown).
Western blotting
TR146 cells were lysed with modified RIPA lysis buffer (32) containing phosphatase (Sigma-Aldrich) and protease inhibitors (Perbio Science) and separated on 12% SDS-PAGE before being transferred to nitrocellulose membrane. Memrbanes were incubated with primary antibody overnight and developed using the Immobilon ECL reagent (Millipore) ebore being exposed to X-ray film.
Human IL-36γ ELISA
Monoclonal antibodies against human IL-36g were generated in C57B/6 mice or Sprague Dawley rats using recombinant IL-36γ Ser18-Asp169 as antigen. Splenocytes were fused with myeloma cell lines Y3-AG 1.2.3 (rat) or SP2/O-Ag14 (mouse) as appropriate. Characterization of mAbs identified a mouse hybridoma (B5A2) as a capture antibody, and a rat hybridoma (HCL17) as a detection antibody for sandwich ELISA. Antibodies were purified using protein A or protein G affinity chromatography. HCL17 was biotinylated using EZ link NHS-LC-biotin kits (Thermo Scientific). ELISA plates (Nunc. Life Technologies, UK) were coated with 2 μg/ml B5A2 capture antibody and ELISAs performed by standard methods. Detection of Cathepsin S and CCL20 was determined using ELISA kits from R&D systems.
Statistics
All data was analyzed by Student’s t-test or one-way ANOVA with post-hoc Dunnett’s multiple comparisons. A P value of <0.05 was considered significant.
Results
IL-36 is induced in oral candidiasis
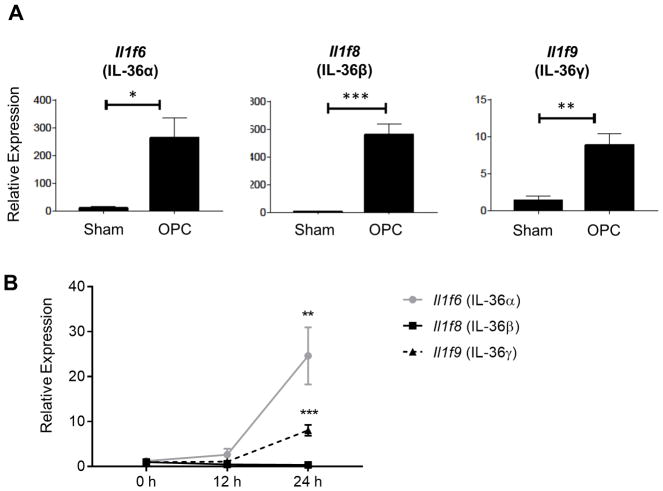
Like immunocompetent humans, wild type (WT) mice are resistant to OPC (33). Since most strains of mice do not carry C. albicans as a commensal fungus (34, 35), resistance is due to a potent innate immune response induced in the oral mucosa. This response clears the fungus within 1–2 days of infection without causing the overt symptoms of thrush (8, 36). To investigate the role of IL-36 in response to OPC, WT mice were infected with the SC5314 strain of C. albicans and gene expression in tongue tissue monitored. Among the genes induced during OPC in WT mice, we observed robust induction of IL-36 family cytokines at day 2 p.i. (Fig 1A). IL-36α and IL-36γ were transcribed as early as day 1 post-infection (p.i.), whereas IL-36β was detected at day 2 p.i. (Fig 1A and B). These results suggested the involvement of IL-36 in promoting oral immunity to C. albicans.
Figure 1. IL-36 cytokines are induced during acute oropharyngeal candidiasis.
WT mice were challenged sublingually with PBS (sham) or C. albicans (OPC). (A) Tongue homogenates were prepared 2 days post-infection (p.i.), and total mRNA was subjected to quantitative real-time polymerase chain reaction (qPCR) normalized to Gapdh. Graphs depict relative expression of indicated genes as mean + SEM, and normalized to sham. Data are compiled from 3–5 mice per group from two independent experiments. (B) Relative mRNA expression of indicated genes in the tongue at 0, 12, and 24 h p.i. Data are compiled from 3–5 WT mice per group from a single experiment. Data were analyzed by Student’s t test or one-way ANOVA. *P < 0.05, **P < 0.01, and ***P < 0.001.
IL-36 induction requires the hypha-associated fungal toxin Candidalysin
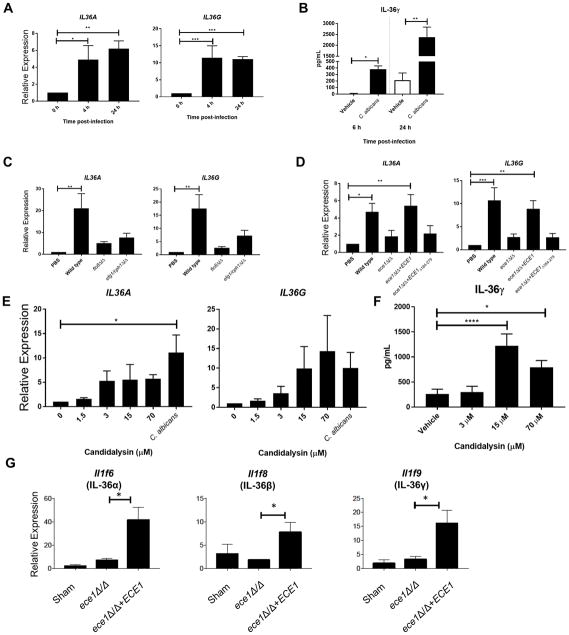
Oral epithelial cells (OECs) are the first cells to encounter C. albicans during OPC and regulate important early innate inflammatory responses to pathogenic invading hyphae. To determine whether C. albicans induced expression of IL-36 directly in OECs, we infected monolayers of the human buccal epithelial cell line TR146 with C. albicans (6, 32). Expression of IL36A and IL36G but not IL36B mRNA (Fig 2A) were induced within 4–6 hours following C. albicans in vitro infection. Likewise, secretion of IL-36γ protein was induced as early as 6 h post-infection (Fig 2B). IL-36 has been reported to require Cathepsin S-dependent processing for full efficacy (37), but while OECs naturally secreted this enzyme, C. albicans infection did not alter its release (Supp Fig 1A).
Figure 2. IL-36 cytokines are induced during C. albicans infection of human oral epithelial cells.
TR146 human oral epithelial cells were challenged with PBS or C. albicans. (A) IL-36 gene expression from RNA prepared at 0, 4 and 24 h post-infection (p.i.) normalized to YWHAZ. Graphs depict relative expression of indicated genes as mean + SEM, normalized to 0 h time point. (B) IL-36γ protein secretion at varying times p.i. with C. albicans determined by ELISA. (C) IL-36 gene expression from RNA prepared at 24 h p.i. of epithelial cells with wild-type or yeast-locked (flo8Δ/Δ, efg1/cph1Δ/Δ) C. albicans strains normalized to YWHAZ. Graphs depict relative expression of indicated genes as mean + SEM, normalized to PBS-treated cells. (D) IL-36 gene expression from RNA prepared at 24 h p.i. of epithelial cells with wild-type or ECE1 null (ece1Δ/Δ), Candidalysin null (ece1Δ/Δ+ECE1Δ184-279) or ECE1 re-integrant (ece1Δ/Δ+ECE1) C. albicans strains normalized to YWHAZ. Graphs depict relative expression of indicated genes as mean + SEM, normalized to PBS-treated cells. (E) IL-36 gene expression from RNA prepared at 24 h post-treatment. of epithelial cells with different concentrations of Candidalysin normalized to YWHAZ. Graphs depict relative expression of indicated genes as mean + SEM, normalized to PBS-treated cells. (F) IL-36γ protein secretion at varying times post-treatment with different concentrations of Candidalysin determined by ELISA. (G) WT mice infected with indicated strain of C. albicans and gene expression was measured at day 2 p.i. Data are mean + SEM of (A–F) 3 independent experiments or (G) normalized to sham, from 3–5 mice per group in two independent experiments. Data were analyzed by Student’s t test or one-way ANOVA. *P < 0.05, **P < 0.01, and ***P < 0.001.
As C. albicans morphology impacts pathogenicity and epithelial cell responses (32, 38, 39), we hypothesized that hypha formation would be required to induce IL-36. Indeed, infection of TR146 OECs with C. albicans ‘yeast-locked’ mutants that cannot form hyphae (flo8Δ/Δ (25) and efg1/cph1Δ/Δ (27)) resulted in reduced expression of IL36A and IL36G compared to the parental wild type strain (BWP17) (Fig 2C). IL-1 induction during OPC requires signals from Candidalysin, a pore-forming peptide that is secreted by hyphae and generated by processing of the Ece1p protein (6, 7, 40). To determine whether IL-36 production is regulated by Candidalysin, TR146 OECs were infected with mutants lacking the entire ECE1 gene (ece1Δ/Δ), just the Candidalysin peptide encoding region (ece1Δ/Δ+ECE1Δ184-279), or an ECE1 control reintegrant strain (ece1Δ/Δ+ECE1) (6). Infection of OECs with the BWP17 or the ece1Δ/Δ+ECE1 reintegrant strains stimulated IL36A and IL36G gene expression, whereas neither the ece1Δ/Δ nor ece1Δ/Δ+ECE1Δ184-279 mutants induced these cytokines appreciably (Fig 2D). To determine whether Candidalysin alone was sufficient for induction of IL-36, TR146 cells were treated with lytic (> 15 μM) and sub-lytic doses (< 15 μM) of synthetic Candidalysin peptide. Both IL36A and IL36G gene expression (Fig 2E) and IL-36γ protein (Fig 2F) were induced by Candidalysin concentrations as low as 3 μM. Thus, Candidalysin induces production of IL-36 family cytokines in OECs.
To determine whether IL-36 is regulated by Candidalysin during OPC in vivo, WT mice were challenged with a C. albicans strain lacking the Candidalysin encoding gene ECE1 (ece1Δ/Δ) or the reintegrant control (ece1Δ/Δ+ECE1), and correlates of infection were assessed. Consistent with the findings in TR146 cells, expression of all three IL-36 cytokines was markedly reduced following infection with an ece1Δ/Δ mutant (Fig 2G). Together, these data demonstrate that IL-36 is induced in OECs following infection with invasive hyphae in a Candidalysin-dependent manner.
Functional role of NF-κB, MAPK and PI3K signalling pathways in IL-36 family gene expression
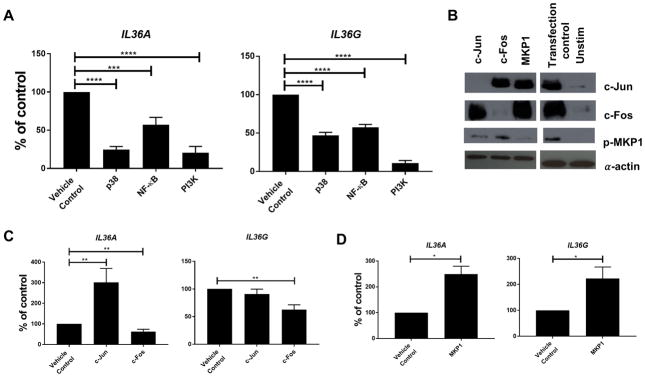
We next sought to define the C. albicans-induced epithelial signalling pathways that regulate IL-36 cytokine production. We first focused on the p38-MAPK pathway, since p38-MAPK-induced c-Fos is a key driver of the epithelial innate danger response to Candidalysin and is required to induce other innate cytokines such as IL-1β and CCL20 (6, 32). To that end, TR146 cells were infected with C. albicans in conjunction with pharmacological inhibitors of MAPK. Blockade of p38-MAPK with SB203580 impaired IL36A (48%) and IL36G (40%) gene expression in infected cells (Fig 3A). In contrast, neither JNK-MAPK nor ERK1/2-MAPK inhibition affected gene expression of IL-36 genes (data not shown). We next interrogated the role of the p38-MAPK-induced transcription factors c-Jun and c-Fos (AP-1) using siRNA knockdown (32) (Fig 3B). Knockdown of c-Fos lead to a modest but statistically significant reduction in IL36A (20%) and IL36G (32%) gene expression (Fig 3C). In contrast, knockdown of c-Jun led to a significant increase in IL36A gene expression (365%), but had no effect on IL36G expression (Fig 3C). The MAPK phosphatase MKP1 (DUSP1) is a negative regulator of the p38- and JNK-MAPK pathways, and both production and stabilisation of this phosphatase are triggered by C. albicans hyphae. Knockdown of MKP1 caused a significant increase in IL36A (229%) and IL36G (155%) expression (Fig 3D), consistent with our prior findings for G-CSF and GM-CSF (32). Together, these data support a positive role for p38-MAPK/c-Fos in triggering IL-36 gene induction and MKP-1 as a negative feedback regulator of MAPK-induced signalling.
Figure 3. IL-36 gene expression is regulated by NF-κB, MAPK and particularly PI3 kinase signaling.
(A) IL-36 gene expression from RNA prepared at 0, 4 and 24 h post-infection (p.i.) with C. albicans of TR146 epithelial cells pre-treated with signaling pathway inhibitors normalized to YWHAZ. Graphs depict percentage inhibition of indicated normalized gene expression relative to the DMSO vehicle control. (B) Effect of siRNA knockdown in TR146 epithelial cells on protein expression 2 h p.i. with C. albicans. (C) Impact of siRNA knockdown of c-Jun or c-Fos on gene expression of IL-36 genes 24 h p.i. with C. albicans. Graphs depict percentage inhibition of indicated normalized gene expression relative to sham transfected cells. (D) Impact of siRNA knockdown of MKP1 on gene expression of IL-36 genes 24 h p.i. with C. albicans. Graphs depict percentage inhibition of indicated normalized gene expression relative to sham transfected cells. Data are (B) representative or (A, C, D) mean + SEM of 3 independent experiments and analyzed by one-way ANOVA. *P < 0.05, **P < 0.01, and ***P < 0.001.
Since the p38-MAPK/c-Fos pathway did not fully account for induction of IL-36, we also evaluated the role of NF-κB and PI3K pathways, which are implicated in the OEC response to C. albicans (32, 41). Inhibition of NF-κB signalling with BAY11-7082 resulted in a reduction in both IL36A (43%) and IL36G (43%) gene expression (Fig 3A). Strikingly, inhibition of PI3K signalling using LY294002 resulted in almost complete inhibition of IL-36 gene expression, with expression levels of both IL36A (87%) and IL36G (89%) reduced to resting levels (Fig 3A). Collectively, these data suggest that whilst p38-MAPK and NF-κB contribute to induction of IL-36 expression, PI3K plays the predominant role.
IL-36γ synergises with C. albicans to induce CCL20
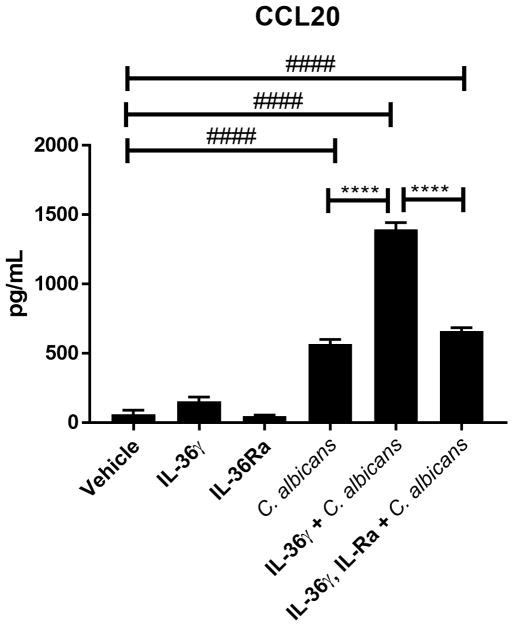
IL-36γ has previously been found to induce IL-17 and Type-17 related cytokines in response to both Aspergillus fumigatus and S. aureus infections (13–15), as well as antimicrobial peptides (AMPs) from keratinocytes (42, 43). Thus, we investigated whether IL-36γ induced the production of AMPs [human β-defensin 2 (BD2) and LL-37], or the Type-17-recruiting chemokine CCL20 (a ligand of CCR6) from OECs upon C. albicans infections. TR146 epithelial cells were stimulated with IL-36γ ± the IL-36 receptor antagonist (IL-36Ra) in the presence of C. albicans, and supernatants evaluated for BD2, LL-37and CCL20 release. IL-36γ did not induce secretion of the antimicrobial peptides HBD2 and LL-37 either with or without C. albicans infection at 6 or 24 h p.i. (data not shown), and IL-36γ alone did not induce CCL20 secretion (Fig 4). However, levels of CCL20 were markedly higher in supernatants from OECs infected with C. albicans in the presence of IL-36γ than in supernatants from OECs infected with C. albicans without IL-36γ at 6 h p.i. (Fig 4). This effect was reversed by addition of IL-36Ra (Fig 4). We further tested the ability of IL-36γ to synergize with IL-17 and synthetic Candidalysin. No discernible additive or synergistic induction of downstream genes was observed when TR146 cells were simultaneously incubated with IL-36γ and IL-17A, or IL-36γ with Candidalysin (Supp Fig 1B). Thus, in contrast to previous reports for IL-1 (44), IL-36γ triggers the release of CCL20 from epithelial cells in the presence of C. albicans, but not with Candidalysin alone or IL-17.
Figure 4. IL-36 synergises with C. albicans to induce CCL20 production.
Induction of CCL20 release in response to IL-36γ (100 ng/ml) stimulation with and without C. albicans infection after 6 h treatment and/or infection. IL-36γ stimulation is reversed by co-treatment with IL-36RA (1 μg/ml). Data are the mean + SEM of 3 independent experiments and analyzed by one-way ANOVA. ****P < 0.0001 relative to C. albicans + IL-36γ. #### P < 0.0001 relative to PBS control.
IL-36R-deficient mice are susceptible to acute oral candidiasis
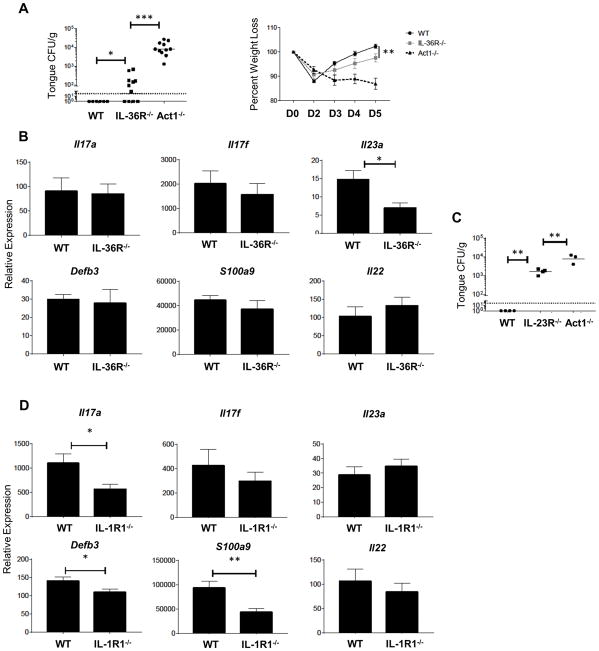
To determine whether IL-36 receptor signalling is required for immunity to OPC, we challenged IL-36R−/− mice with C. albicans and assessed oral fungal burdens 5 days p.i.. IL-36R−/− mice exhibited higher fungal loads and greater weight loss than WT controls (Fig 5A). Notably, the fungal burden in IL-36R−/− mice was similar to that seen previously in Il1r1 −/− mice (Fig 5A) (7), but was not as severe as in mice with defective IL-17 signaling (Act1−/−) (45). Thus, there is a non-redundant requirement for IL-36 in driving immunity to oral mucosal candidiasis.
Figure 5. IL-36R−/− mice are susceptible to acute oral candidiasis.
(A) Indicated mice were orally infected with C. albicans. Oral fungal load (top) was assessed at day 5 p.i., bars represent geometric mean CFU, dashed line is the limit of detection. Weight loss (bottom) measured throughout the course of oral infection. Each group contains 3–5 mice from at least two independent experiments. (B) Relative mRNA expression of indicated genes in the tongue measured at 2 days p.i. Data are compiled from 3–5 mice per group from three independent experiments. Bars are mean + SEM, normalized to WT sham. (C) Oral fungal burden of indicated mice at day 5 p.i. Data for WT group compiled from historical controls. Bars represent the geometric mean, data from one experiment. (D) Relative mRNA expression of indicated genes analyzed at 2 days p.i. in WT and IL-1R1−/− mice. Data are compiled from 10 mice per group from two independent experiments. Bars are mean + SEM, normalized to WT sham. Data were analyzed by Student’s t test or ANOVA. *P < 0.05, **P < 0.01, and ***P < 0.001.
To identify immune responses that are dysregulated in IL-36R−/− mice during OPC, we evaluated the expression of a panel of known antifungal genes at the peak of the immune response (day 2 p.i.) (8, 36). Surprisingly, the expression of IL-17 cytokines (Il17a, Il17f), multiple IL-17-dependent genes (Defb3, S100a9), the Type 17 cytokine IL-22, and IFNγ were comparable between WT and IL-36R−/− mice (Fig 5B and Supp Fig 1C). Despite this, the expression of Il23a was significantly and consistently impaired in IL-36R−/− mice (Fig 5B), and Il23r−/− mice failed to clear C. albicans from the oral cavity (Fig 5C). In sharp contrast to IL-36R−/− mice, IL-1R1−/− mice displayed reduced expression of IL-17 and IL-17-dependent genes but not IL-23 (Fig 5D). Thus, C. albicans-driven IL-36 responses protect against oral candidiasis in a parallel but independent fashion to the classical IL-1 cytokines by inducing IL-23, which is essential for controlling oral infection, but not IL-17 or IL-17-driven genes – the canonical innate anti-Candida response previously described (44, 46).
Discussion
The ability of the mucosal epithelium to discriminate between the commensal and pathogenic states of microbes is a crucial component of host barrier defenses. Emerging data indicate that the IL-36 cytokine family bolsters epithelial immunity at multiple mucosal sites (47). In addition to shaping adaptive immune responses (13, 16), IL-36 cytokines are important mediators of innate antimicrobial immunity in the epithelium (21). However, little is known about the role of IL-36 in the oral mucosa. The current study was prompted by our discovery of a signaling circuit in OPC, whereby the fungal toxin Candidalysin drives IL-1α/β release, which in turn triggers innate antifungal immune responses (7). Here, we show an analogous response also occurs via IL-36 family cytokines, which contribute non-redundantly to host defense in OPC, thus oral candidiasis responses form part of a wider spectrum of IL-36 involvement in antimicrobial immunity.
Even though IL-36 is crucial for controlling oral C. albicans infections, it was unexpected that IL-36-driven immunity appears to be independent of IL-17 and IL-17-dependent genes. This contrasts with the role of IL-1 in OPC which promotes proliferation of innate IL-17-producing T cells, and induction of IL-17 and IL-17 dependent genes (7). Instead, IL-36 deficiency led to reduced gene expression of IL23a, which is vital for defense against C. albicans (8). IL-36 is known to be a strong inducer of IL-23 in macrophages isolated from psoriasis patients (48), where IL-36 is known to be a key driver of disease so it is probable that the same mechanism is in effect in this study. Specifically, that epithelial-produced IL-36 drives IL-23 secretion from macrophages and other myeloid cells, leading to protection against C. albicans in an IL-17- and IFNγ-independent mechanism. Both IL-17 signaling and IL-23 are required for immunity to OPC, but there is evidence that in some circumstances their activities may be uncoupled. For example, IL-23 was shown to regulate gut epithelial integrity differently from IL-17 (49, 50). As such, blockade of IL-17 exacerbates inflammatory bowel disease (IBD) in humans, whereas anti-IL-23 therapy is beneficial (51). Hence, we speculate that in OPC the host may use IL-23-driven responses to complement Type-17 immunity in the oral mucosa, and that these two arms are regulated independently by IL-36 and IL-1 cytokines respectively (Supp Fig 2).
We recently described an epithelial signaling circuit that senses invasive fungal hyphae via Candidalysin activity to induce local innate immune responses, especially IL-1 (7). This study reveals that a Candidalysin activated danger response pathway also triggers the release of IL-36 from OECs. A similar scenario occurs in the skin, as described in two recent studies showing an antimicrobial defense mechanism that is activated by a secreted S. aureus toxin, PSMα (14, 15). Just as Candidalysin-deficient C. albicans strains fail to trigger Type-17 responses in the mouth, PSMα-deficient S. aureus bacteria do not induce dermal IL-17. Together, these findings indicate that the host barrier surfaces are programmed to discriminate between benign and pathogenic forms of commensal and opportunistic microbes by sensing tissue-destructive microbial toxins.
The epithelial signaling pathways by which C. albicans induces IL-36 expression share both common and distinct features from those pathways previously shown to be induced by Candidalysin. We have previously shown that C. albicans hyphae acting through Candidalysin induce activation of the p38/c-Fos and ERK1/2/MKP1 signaling pathways, whereas NF-κB is not activated directly by Candidalysin (5, 7, 32, 38). However, while IL-36 induction does partially involve p38-MAPk, it also involves NF-κB, and particularly PI3K, with the later playing a dominant role. Both p38-MAPK and NF-κB have been identified as key signaling pathways in eliciting IL-36 (52), but a role for PI3K has not so far been described. In contrast to a prior study that implicated both c-Jun and c-Fos in regulating IL-36 (52), we observed that c-Fos is the only AP-1 family member that induces its transcription in OECs. Interestingly, c-Jun appears to inhibit the production of IL-36α by OECs, although the significance of this in OPC remains unclear. Thus, c-Fos joins IRF6, IRAK1 and C/EBPβ as key factors regulating IL-36 gene expression. Future studies will focus on understanding the interplay and cell-type specific activities of these molecules controlling IL-1 family cytokines.
In conclusion, our findings provide fresh insights into the protective role of IL-36 in oral mucosal antifungal immunity. The complexity of the IL-1 family in shaping acute immune responses is highlighted by the unexpected differences in how IL-1 and IL-36 appear to act in this context. Both of these cytokines are current or potential targets of biologic therapies and understanding their activities with respect to microbial opportunistic infection will be important for predicting adverse side effects of such treatments.
Supplementary Material
Acknowledgments
We thank Amgen for kindly providing the IL-36R−/− mice. We also thank Dr. Duncan Wilson (University of Aberdeen) for constructing and providing WT (BWP17), ece1Δ/Δ, ece1Δ/Δ+ECE1, and ece1Δ/Δ+ECE1Δ184-279 strains.
This work was supported by NIH grants DE022550 and DE023815 (to SLG), the Medical Research Council (MR/M011372/1), Biotechnology & Biological Sciences Research Council (BB/N014677/1), the Rosetrees Trust (M680), and the National Institute for Health Research at Guys and St Thomas’s NHS Foundation Trust and King’s College London Biomedical Research Centre (IS-BRC-1215-20006) (to JRN).
Footnotes
AHV, HZ SLG and DLM designed the experiments and wrote the manuscript. AHV, HZ, NP, OWH, DS, JH, FEYA, BMC and JPR performed all experiments and data analysis. JSA and MS performed and analyzed IL36 ELISA data. MJM, JRN and BH provided key reagents and strains.
Disclosures
The authors declare no conflict of interests.
References
- 1.Moutsopoulos NM, Konkel JE. Tissue-Specific Immunity at the Oral Mucosal Barrier. Trends in immunology. 2017 doi: 10.1016/j.it.2017.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Koo S, Kejariwal D, Al-Shehri T, Dhar A, Lilic D. Oesophageal candidiasis and squamous cell cancer in patients with gain-of-function STAT1 gene mutation. United European Gastroenterol J. 2017;5:625–631. doi: 10.1177/2050640616684404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brown GD, Denning DW, Gow NA, Levitz SM, Netea MG, White TC. Hidden killers: human fungal infections. Sci Transl Med. 2012;4:165rv113. doi: 10.1126/scitranslmed.3004404. [DOI] [PubMed] [Google Scholar]
- 4.Fidel PL, Jr, Cutler JE. Prospects for development of a vaccine to prevent and control vaginal candidiasis. Curr Infect Dis Rep. 2011;13:102–107. doi: 10.1007/s11908-010-0143-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moyes DL, Richardson JP, Naglik JR. Candida albicans-epithelial interactions and pathogenicity mechanisms: scratching the surface. Virulence. 2015;6:338–346. doi: 10.1080/21505594.2015.1012981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moyes D, Wilson D, Richardson J, MS, Tang S, WJ, Hofs S, Gratacap R, Robbins J, Manohursingh R, Murciano C, Blagojevic M, Thavaraj S, Forster T, Hebecker B, Kaser L, Vizcay G, Iancu S, Kichik N, Hader A, Kurzai O, Luo T, Kruger T, Kniemeyer O, Cota E, Bader O, Wheeler R, Gutsmann T, Hube B, Naglik J. Candidalysin: A fungal peptide toxin critical for mucosal infection. Nature. 2016;532:64–68. doi: 10.1038/nature17625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Verma A, Richardson J, Zhou C, Coleman BM, Moyes D, Ho J, Huppler AR, Ramani K, McGeachy MJ, Mufazalov IA, Waisman A, Kane LP, Biswas P, Hube B, Naglik J, Gaffen SL. Oral epithelial cells orchestrate innate Type 17 responses to Candida albicans through the virulence factor Candidalysin. Sci Immunol. 2017;2:eeam8834. doi: 10.1126/sciimmunol.aam8834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Conti H, Shen F, Nayyar N, Stocum E, SJN, Lindemann M, Ho A, Hai J, Yu J, Jung J, Filler S, Masso-Welch P, Edgerton M, Gaffen S. Th17 cells and IL-17 receptor signaling are essential for mucosal host defense against oral candidiasis. The Journal of experimental medicine. 2009;206:299–311. doi: 10.1084/jem.20081463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li J, Vinh DC, Casanova JL, Puel A. Inborn errors of immunity underlying fungal diseases in otherwise healthy individuals. Curr Opin Microbiol. 2017;40:46–57. doi: 10.1016/j.mib.2017.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Altmeier S, Toska A, Sparber F, Teijeira A, Halin C, LeibundGut-Landmann S. IL-1 Coordinates the Neutrophil Response to C. albicans in the Oral Mucosa. PLoS Pathog. 2016;12:e1005882. doi: 10.1371/journal.ppat.1005882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aoyagi T, Newstead MW, Zeng X, Kunkel SL, Kaku M, Standiford TJ. IL-36 receptor deletion attenuates lung injury and decreases mortality in murine influenza pneumonia. Mucosal Immunol. 2017;10:1043–1055. doi: 10.1038/mi.2016.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ahsan F, Moura-Alves P, Guhlich-Bornhof U, Klemm M, Kaufmann SH, Maertzdorf J. Role of Interleukin 36gamma in Host Defense Against Tuberculosis. The Journal of infectious diseases. 2016;214:464–474. doi: 10.1093/infdis/jiw152. [DOI] [PubMed] [Google Scholar]
- 13.Gresnigt MS, Rosler B, Jacobs CW, Becker KL, Joosten LA, van der Meer JW, Netea MG, Dinarello CA, van de Veerdonk FL. The IL-36 receptor pathway regulates Aspergillus fumigatus-induced Th1 and Th17 responses. Eur J Immunol. 2013;43:416–426. doi: 10.1002/eji.201242711. [DOI] [PubMed] [Google Scholar]
- 14.Liu H, Archer NK, Dillen CA, Wang Y, Ashbaugh AG, Ortines RV, Kao T, Lee SK, Cai SS, Miller RJ, Marchitto MC, Zhang E, Riggins DP, Plaut RD, Stibitz S, Geha RS, Miller LS. Staphylococcus aureus Epicutaneous Exposure Drives Skin Inflammation via IL-36-Mediated T Cell Responses. Cell Host Microbe. 2017;22:653–666e655. doi: 10.1016/j.chom.2017.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nakagawa S, Matsumoto M, Katayama Y, Oguma R, Wakabayashi S, Nygaard T, Saijo S, Inohara N, Otto M, Matsue H, Nunez G, Nakamura Y. Staphylococcus aureus Virulent PSMalpha Peptides Induce Keratinocyte Alarmin Release to Orchestrate IL-17-Dependent Skin Inflammation. Cell Host Microbe. 2017;22:667–677e665. doi: 10.1016/j.chom.2017.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jensen LE. Interleukin-36 cytokines may overcome microbial immune evasion strategies that inhibit interleukin-1 family signaling. Sci Signal. 2017:10. doi: 10.1126/scisignal.aan3589. [DOI] [PubMed] [Google Scholar]
- 17.Gresnigt MS, van de Veerdonk FL. Biology of IL-36 cytokines and their role in disease. Semin Immunol. 2013 doi: 10.1016/j.smim.2013.11.003. [DOI] [PubMed] [Google Scholar]
- 18.Chustz RT, Nagarkar DR, Poposki JA, Favoreto S, Jr, Avila PC, Schleimer RP, Kato A. Regulation and function of the IL-1 family cytokine IL-1F9 in human bronchial epithelial cells. Am J Respir Cell Mol Biol. 2011;45:145–153. doi: 10.1165/rcmb.2010-0075OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Foster AM, Baliwag J, Chen CS, Guzman AM, Stoll SW, Gudjonsson JE, Ward NL, Johnston A. IL-36 promotes myeloid cell infiltration, activation, and inflammatory activity in skin. J Immunol. 2014;192:6053–6061. doi: 10.4049/jimmunol.1301481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huynh J, Scholz GM, Aw J, Kwa MQ, Achuthan A, Hamilton JA, Reynolds EC. IRF6 Regulates the Expression of IL-36gamma by Human Oral Epithelial Cells in Response to Porphyromonas gingivalis. J Immunol. 2016;196:2230–2238. doi: 10.4049/jimmunol.1501263. [DOI] [PubMed] [Google Scholar]
- 21.Winkle SM, Throop AL, Herbst-Kralovetz MM. IL-36gamma Augments Host Defense and Immune Responses in Human Female Reproductive Tract Epithelial Cells. Frontiers in microbiology. 2016;7:955. doi: 10.3389/fmicb.2016.00955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Solis NV, Filler SG. Mouse model of oropharyngeal candidiasis. Nat Protoc. 2012;7:637–642. doi: 10.1038/nprot.2012.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gillum AM, Tsay EY, Kirsch DR. Isolation of the Candida albicans gene for orotidine-5′-phosphate decarboxylase by complementation of S. cerevisiae ura3 and E. coli pyrF mutations. Mol Gen Genet. 1984;198:179–182. doi: 10.1007/BF00328721. [DOI] [PubMed] [Google Scholar]
- 24.Wilson RB, Davis D, Mitchell AP. Rapid hypothesis testing with Candida albicans through gene disruption with short homology regions. Journal of bacteriology. 1999;181:1868–1874. doi: 10.1128/jb.181.6.1868-1874.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cao F, Lane S, Raniga PP, Lu Y, Zhou Z, Ramon K, Chen J, Liu H. The Flo8 transcription factor is essential for hyphal development and virulence in Candida albicans. Mol Biol Cell. 2006;17:295–307. doi: 10.1091/mbc.E05-06-0502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zakikhany K, Naglik JR, Schmidt-Westhausen A, Holland G, Schaller M, Hube B. In vivo transcript profiling of Candida albicans identifies a gene essential for interepithelial dissemination. Cellular microbiology. 2007;9:2938–2954. doi: 10.1111/j.1462-5822.2007.01009.x. [DOI] [PubMed] [Google Scholar]
- 27.Lo HJ, Kohler JR, DiDomenico B, Loebenberg D, Cacciapuoti A, Fink GR. Nonfilamentous C. albicans mutants are avirulent. Cell. 1997;90:939–949. doi: 10.1016/s0092-8674(00)80358-x. [DOI] [PubMed] [Google Scholar]
- 28.Spandidos A, Wang X, Wang H, Seed B. PrimerBank: a resource of human and mouse PCR primer pairs for gene expression detection and quantification. Nucleic Acids Res. 2010;38:D792–799. doi: 10.1093/nar/gkp1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang E, Feng X, Liu F, Zhang P, Liang J, Tang X. Roles of PI3K/Akt and c-Jun signaling pathways in human papillomavirus type 16 oncoprotein-induced HIF-1alpha, VEGF, and IL-8 expression and in vitro angiogenesis in non-small cell lung cancer cells. PloS one. 2014;9:e103440. doi: 10.1371/journal.pone.0103440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chambellan A, Leahy R, Xu W, Cruickshank PJ, Janocha A, Szabo K, Cannady SB, Comhair SA, Erzurum SC. Pivotal role of c-Fos in nitric oxide synthase 2 expression in airway epithelial cells. Nitric Oxide. 2009;20:143–149. doi: 10.1016/j.niox.2008.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kinney CM, Chandrasekharan UM, Mavrakis L, DiCorleto PE. VEGF and thrombin induce MKP-1 through distinct signaling pathways: role for MKP-1 in endothelial cell migration. Am J Physiol Cell Physiol. 2008;294:C241–250. doi: 10.1152/ajpcell.00187.2007. [DOI] [PubMed] [Google Scholar]
- 32.Moyes DL, Runglall M, Murciano C, Shen C, Nayar D, Thavaraj S, Kohli A, Islam A, Mora-Montes H, Challacombe SJ, Naglik JR. A biphasic innate immune MAPK response discriminates between the yeast and hyphal forms of Candida albicans in epithelial cells. Cell Host Microbe. 2010;8:225–235. doi: 10.1016/j.chom.2010.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kamai Y, Kubota M, Kamai Y, Hosokawa T, Fukuoka T, Filler S. New model of oropharyngeal candidiasis in mice. Anti-microb Agents Chemo. 2001;45:3195–3197. doi: 10.1128/AAC.45.11.3195-3197.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bar E, Gladiator A, Bastidas S, Roschitzki B, Acha-Orbea H, Oxenius A, LeibundGut-Landmann S. A novel Th cell epitope of Candida albicans mediates protection from fungal infection. J Immunol. 2012;188:5636–5643. doi: 10.4049/jimmunol.1200594. [DOI] [PubMed] [Google Scholar]
- 35.Hernández-Santos N, Huppler AR, Peterson AC, Khader SA, MKC, Gaffen SL. Th17 cells confer long term adaptive immunity to oral mucosal Candida albicans infections. Mucosal Immunol. 2013;6:900–910. doi: 10.1038/mi.2012.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Conti H, Bruno V, Childs E, Daugherty S, Hunter J, Mengesha B, Saevig D, Hendricks M, Coleman BM, Brane L, Solis NV, Cruz JA, Verma A, Garg A, Hise AG, Naglik J, Naglik JR, Filler SG, Kolls JK, Sinha S, Gaffen SL. IL-17RA signaling in oral epithelium is critical for protection against oropharyngeal candidiasis. Cell Host Microbe. 2016;20:606–617. doi: 10.1016/j.chom.2016.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ainscough JS, Macleod T, McGonagle D, Brakefield R, Baron JM, Alase A, Wittmann M, Stacey M. Cathepsin S is the major activator of the psoriasis-associated proinflammatory cytokine IL-36gamma. Proceedings of the National Academy of Sciences of the United States of America. 2017;114:E2748–E2757. doi: 10.1073/pnas.1620954114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Moyes DL, Murciano C, Runglall M, Islam A, Thavaraj S, Naglik JR. Candida albicans yeast and hyphae are discriminated by MAPK signaling in vaginal epithelial cells. PloS one. 2011;6:e26580. doi: 10.1371/journal.pone.0026580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Moyes DL, Murciano C, Runglall M, Kohli A, Islam A, Naglik JR. Activation of MAPK/c-Fos induced responses in oral epithelial cells is specific to Candida albicans and Candida dubliniensis hyphae. Med Microbiol Immunol. 2012;201:93–101. doi: 10.1007/s00430-011-0209-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Richardson JP, Mogavero S, Moyes DL, Blagojevic M, Kruger T, Verma AH, Coleman BM, De La Cruz Diaz J, Schulz D, Ponde NO, Carrano G, Kniemeyer O, Wilson D, Bader O, Enoiu SI, Ho J, Kichik N, Gaffen SL, Hube B, Naglik JR. Processing of Candida albicans Ece1p Is Critical for Candidalysin Maturation and Fungal Virulence. MBio. 2018:9. doi: 10.1128/mBio.02178-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Moyes DL, Shen C, Murciano C, Runglall M, Richardson JP, Arno M, Aldecoa-Otalora E, Naglik JR. Protection Against Epithelial Damage During Candida albicans Infection Is Mediated by PI3K/Akt and Mammalian Target of Rapamycin Signaling. The Journal of infectious diseases. 2014;209:1816–1826. doi: 10.1093/infdis/jit824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li N, Yamasaki K, Saito R, Fukushi-Takahashi S, Shimada-Omori R, Asano M, Aiba S. Alarmin function of cathelicidin antimicrobial peptide LL37 through IL-36gamma induction in human epidermal keratinocytes. J Immunol. 2014;193:5140–5148. doi: 10.4049/jimmunol.1302574. [DOI] [PubMed] [Google Scholar]
- 43.Johnston A, Xing X, Guzman AM, Riblett M, Loyd CM, Ward NL, Wohn C, Prens EP, Wang F, Maier LE, Kang S, Voorhees JJ, Elder JT, Gudjonsson JE. IL-1F5, -F6, -F8, and -F9: a novel IL-1 family signaling system that is active in psoriasis and promotes keratinocyte antimicrobial peptide expression. J Immunol. 2011;186:2613–2622. doi: 10.4049/jimmunol.1003162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Verma AH, Richardson JP, Zhou C, Coleman BM, Moyes DL, Ho J, Huppler AR, Ramani K, McGeachy MJ, Mufazalov IA, Waisman A, Kane LP, Biswas PS, Hube B, Naglik JR, Gaffen SL. Oral epithelial cells orchestrate innate type 17 responses to Candida albicans through the virulence factor candidalysin. Sci Immunol. 2017:2. doi: 10.1126/sciimmunol.aam8834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ferreira MC, Whibley N, Mamo AJ, Siebenlist U, Chan YR, Gaffen SL. Interleukin-17-induced protein lipocalin 2 is dispensable for immunity to oral candidiasis. Infection and immunity. 2014;82:1030–1035. doi: 10.1128/IAI.01389-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Conti HR, V, Bruno M, Childs EE, Daugherty S, Hunter JP, Mengesha BG, Saevig DL, Hendricks MR, Coleman BM, Brane L, Solis N, Cruz JA, Verma AH, Garg AV, Hise AG, Richardson JP, Naglik JR, Filler SG, Kolls JK, Sinha S, Gaffen SL. IL-17 Receptor Signaling in Oral Epithelial Cells Is Critical for Protection against Oropharyngeal Candidiasis. Cell Host Microbe. 2016;20:606–617. doi: 10.1016/j.chom.2016.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Blumberg H, Dinh H, Trueblood ES, Pretorius J, Kugler D, Weng N, Kanaly ST, Towne JE, Willis CR, Kuechle MK, Sims JE, Peschon JJ. Opposing activities of two novel members of the IL-1 ligand family regulate skin inflammation. J Exp Med. 2007;204:2603–2614. doi: 10.1084/jem.20070157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bridgewood C, Fearnley GW, Berekmeri A, Laws P, Macleod T, Ponnambalam S, Stacey M, Graham A, Wittmann M. IL-36gamma Is a Strong Inducer of IL-23 in Psoriatic Cells and Activates Angiogenesis. Front Immunol. 2018;9:200. doi: 10.3389/fimmu.2018.00200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lee J, Tato C, Joyce-Shaikh B, Gulan F, Cayatte C, Chen Y, Blumenschein W, Judo M, Ayanoglu G, McClanahan T, Li X, Cua D. Interleukin-23-dependent IL-17 production regulates intestinal epithelial permeability. Immunity. 2015;43:727–738. doi: 10.1016/j.immuni.2015.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Maxwell J, Zhang Y, Brown W, Smith C, Byrne F, Florino M, Stevens E, Bigler J, Davis J, Rottman J, Budelsky A, Symons A, Towne J. Differential roles for interleukin-23 and interleukin-17 in intestinal immunoregulation. Immunity. 2015;43:739–750. doi: 10.1016/j.immuni.2015.08.019. [DOI] [PubMed] [Google Scholar]
- 51.Hueber W, Sands BE, Lewitzky S, Vandemeulebroecke M, Reinisch W, Higgins PD, Wehkamp J, Feagan BG, Yao MD, Karczewski M, Karczewski J, Pezous N, Bek S, Bruin G, Mellgard B, Berger C, Londei M, Bertolino AP, Tougas G, Travis SP Secukinumab in Crohn’s Disease Study G. Secukinumab, a human anti-IL-17A monoclonal antibody, for moderate to severe Crohn’s disease: unexpected results of a randomised, double-blind placebo-controlled trial. Gut. 2012;61:1693–1700. doi: 10.1136/gutjnl-2011-301668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Takahashi K, Nishida A, Shioya M, Imaeda H, Bamba S, Inatomi O, Shimizu T, Kitoh K, Andoh A. Interleukin (IL)-1beta Is a Strong Inducer of IL-36gamma Expression in Human Colonic Myofibroblasts. PloS one. 2015;10:e0138423. doi: 10.1371/journal.pone.0138423. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.