Patients with basilar artery stenosis (n=126; 66 symptomatic and 60 asymptomatic) underwent high-resolution MR imaging. The relationship between imaging findings (intraplaque hemorrhage, contrast enhancement, degree of stenosis, minimal lumen area, and plaque burden) and symptoms was analyzed. Intraplaque hemorrhage was identified in 22 patients (17.5%), including 21 (31.8%) symptomatic patients and 1 (1.7%) asymptomatic patient. Multivariate analysis showed that intraplaque hemorrhage was the strongest independent marker of symptomatic status. Contrast enhancement was also independently associated with symptomatic status. The authors conclude that intraplaque hemorrhage is present in both low- and high-grade stenotic basilar artery plaques and is independently associated with symptomatic stroke status. Intraplaque hemorrhage may identify high-risk plaque and provide new insight into the management of patients with stroke without significant stenosis.
Abstract
BACKGROUND AND PURPOSE:
Intraplaque hemorrhage within intracranial atherosclerotic plaques identified by high-resolution MR imaging has been studied as a potential marker of stroke risk. However, previous studies only examined intracranial arteries with high-grade stenosis (degree of stenosis, >50%). This study aimed to ascertain the clinical relevance of intraplaque hemorrhage in patients with low- and high-grade stenotic basilar artery plaques.
MATERIALS AND METHODS:
Patients with basilar artery stenosis (n = 126; mean age, 62 ± 10 years; 66 symptomatic and 60 asymptomatic) underwent high-resolution MR imaging. The relationship between imaging findings (intraplaque hemorrhage, contrast enhancement, degree of stenosis, minimal lumen area, and plaque burden) and symptoms was analyzed.
RESULTS:
Intraplaque hemorrhage was identified in 22 patients (17.5%), including 21 (31.8%) symptomatic patients and 1 (1.7%) asymptomatic patient. Multivariate analysis showed that intraplaque hemorrhage was the strongest independent marker of symptomatic status (odds ratio, 27.5; 95% CI, 3.4–221.5; P = .002). Contrast enhancement was also independently associated with symptomatic status (odds ratio, 9.9; 95% CI, 1.5–23.6; P = .016). Stenosis, minimal lumen area, and plaque burden were not correlated with symptoms (P > .05). Intraplaque hemorrhage was present in both low- and high-grade stenotic basilar arteries (11.3% versus 16.3%, P = .63). Diagnostic performance values of intraplaque hemorrhage for patients with acute/subacute symptomatic stroke were the following: specificity, 98.3%; sensitivity, 31.8%; positive predictive value, 95.5%; and negative predictive value, 56.7%.
CONCLUSIONS:
Intraplaque hemorrhage is present in both low- and high-grade stenotic basilar artery plaques and is independently associated with symptomatic stroke status. Intraplaque hemorrhage may identify high-risk plaque and provide new insight into the management of patient with stroke without significant stenosis.
Intracranial atherosclerotic disease (ICAD) is a major cause of stroke that has likely been underappreciated, in part due to challenges in detecting intracranial atherosclerotic plaque.1 ICAD may produce ischemia through multiple mechanisms, including the following: thrombotic occlusion, occlusion of small perforating arteries, plaque rupture leading to artery-to-artery embolization, and vessel luminal narrowing leading to hypoperfusion.2 Current practice guidelines rely solely on the degree of stenosis (often ≥50%) of major intracranial arteries in determining management strategies. However, many authors have questioned this practice, especially given the high prevalence at postmortem examination of mild and moderate intracranial arterial stenosis in fatal stroke.2–6 Branch occlusive disease, in particular, has been underestimated and appears to constitute a more common cause of stroke.5 Other factors, including plaque composition, arterial hemodynamic features, and collateral status, have been proposed as alternative variables to better predict recurrent stroke risk.4
ICAD with ischemia caused by artery-to-artery embolization from plaque rupture may require more aggressive antiplatelet therapy to mitigate clot progression, whereas stenotic disease resulting in hypoperfusion might benefit more from angioplasty.2 Given these important distinctions in treatment selection for patients with ICAD based on stroke mechanism, the assessment of culprit plaque location and morphology is clinically relevant.2 In the past decade, many advances have been made in visualizing large-vessel intracranial plaque morphology and composition using high-resolution MR imaging (HR-MRI).7,8 Expanded use of HR-MRI could result in the reclassification of many strokes previously termed cryptogenic and could improve clinical decision-making. A few studies have examined atherosclerotic plaque of the posterior circulation using HR-MRI with improved characterization of basilar artery ICAD.9–13
Intraplaque hemorrhage (IPH) occurs in atherosclerotic plaque and is attributed to fragile neovascularity with endothelial disruption that increases plaque wall stress, making plaque more vulnerable.14 IPH was first identified as a T1-weighted hyperintense signal in extracranial carotid plaque imaging,15 and subsequent articles have supported carotid IPH as a risk factor for recurrent stroke independent of stenosis.16,17 Preliminary work using intracranial HR-MRI showed a similar appearance of IPH within intracranial arteries in symptomatic ICAD.18 Xu et al19 observed T1-weighted hyperintense signal more often in symptomatic middle cerebral artery plaque than in asymptomatic plaques. More recently, in patients with basilar artery stenosis, HR-MRI has revealed IPH as more frequent in symptomatic patients than in asymptomatic patients (42.3% versus 21.4%), suggesting a risk for IPH with a relative risk of 1.64.12
These prior basilar artery HR-MRI studies have only imaged basilar artery plaque with high-grade stenosis. The primary aim of this study was to ascertain the presentation and clinical relevance of basilar artery IPH in both low- and high-grade stenotic basilar artery plaques. Given the frequency of nonstenotic ICAD in stroke and preliminary evidence supporting IPH as a risk factor, we hypothesize that IPH status might be useful in distinguishing acute/subacute symptomatic from chronic/asymptomatic basilar artery plaque regardless of the degree of stenosis.
Materials and Methods
The authors declare that all supporting data are available within the article.
Study Population
This study was approved by the Changhai Hospital institutional review board with all patients providing written informed consent.
This was a prospective study. Patients with basilar artery atherosclerotic disease were recruited for this study between September 2013 and October 2016. The inclusion criteria were the following: 1) ischemic stroke or transient ischemic attacks in the basilar artery territory, and/or basilar artery stenosis of >30% on DSA, CTA, or MRA; and 2) >1 atherosclerotic risk factor, including hypercholesterolemia, hypertension, smoking, and diabetes mellitus. Exclusion criteria included the following: 1) coexistent unilateral or bilateral vertebral artery stenosis of >50% on MRA, 2) complete basilar artery occlusion, 3) dissection, 4) intracranial dolichoectasia, 5) nonatherosclerotic intracranial arterial disease (eg, inflammatory arteritis and congenital agenesis), 6) the presence of atrial fibrillation on 24-hour monitoring, and 7) clinical contraindications to MR imaging.
Symptomatic plaque was defined when conventional neuroimaging (FLAIR and diffusion-weighted images) demonstrated infarct within the basilar artery territory. Patients were classified into 2 groups based on their symptom presentation: 1) symptomatic ischemic stroke/TIA symptoms presenting <12 weeks before imaging; and 2) patients with asymptomatic basilar artery plaque without neuroimaging evidence of infarct.
Patients' clinical information including age, sex, diabetes, hypertension, smoking, hyperlipidemia, preadmission statin and aspirin use, ischemic coronary heart disease, and National Institutes of Health Stroke Scale score was collected.
MR Imaging Protocol
MR imaging was performed on a 3T whole-body MR imaging scanner (Skyra; Siemens, Erlangen, Germany) with a 20-channel phased array head and neck coil.
3D time-of-flight images were acquired in the axial plane (TR/TE = 21/3.43 ms, FOV = 181 × 200 mm, thickness = 0.7 mm, matrix = 331 × 384). 3D TOF images were reformatted using multiplanar reconstruction. HR-MRI was then performed in planes perpendicular to the basilar artery. The HR-MRI protocol included 3 sequences with 1 sequence repeated postcontrast (12 slices with 2-mm slice thickness; in-plane resolution = 0.4 × 0.3 mm, FOV = 100 × 100 mm, matrix = 256 × 320); precontrast T1-weighted fast spin-echo (TR/TE = 581/18 ms, echo-train length = 4, NEX = 4); T2-weighted FSE (TR/TE = 2890/46 ms, echo-train length = 20, NEX = 3); and postcontrast T1-weighted FSE. Inflow saturation bands were placed below the imaging slab for blood suppression. In addition, 12 coronal slices were scanned with similar scan parameters to help exclude the potential IPH-mimicking flow artifacts. Clinical DWI and FLAIR imaging were used for the identification of infarct.
Image Analysis
Stenosis value was measured independently on HR-MRI by 2 experienced radiologists (X.T. and Q.L., with 7 and 15 years' experience in neuroradiology) who were blinded to the patients' clinical information.20 The stenosis value was calculated as (1 − DStenosis / DNormal) × 100%, where DStenosis is the minimal lumen diameter at the site of maximal stenosis, and DNormal is the lumen diameter at the site of the normal basilar artery (either distal or proximal to the stenosis site). The presence of fresh IPH was identified as >150% signal relative to nearby medial pterygoid muscles on precontrast T1-weighted images by the 2 radiologists independently, blinded to the patient's clinical information.12 Because intraluminal thrombus/hematoma in intracranial dolichoectasia or dissection also exhibits hyperintense signal on precontrast T1-weighted images, we carefully excluded these conditions on the basis of their imaging features.21,22 Basilar dolichoectasia was identified if the basilar diameter was enlarged >4 mm and demonstrated a tortuous appearance on MRA.21 Dissection might appear with a dissection flap or double lumen or as a tapering vessel; intraluminal thrombus in dissection also usually involves a long segment.22 Acute or subacute thrombus demonstrates different morphology compared with IPH. Thrombus is long and close to the lumen, while IPH is focal within plaque and is often eccentric to the lumen.
The lumen and outer wall boundary were manually segmented using CMRtools software (Cardiovascular Imaging Solutions, London, UK) on T2-weighted images. The reproducibility of this area measurement method was previously reported (measurement error for plaque area: 7.5%).23
The contrast-enhancement percentage was measured at the slice of greatest enhancement, using adjacent gray matter (in a region of ∼15 mm2) to normalize signal intensity. The contrast-enhancement percentage was calculated as {[Signal of Plaque (Postcontrast) / Signal of Gray Matter (Postcontrast)] / [Signal of Plaque (Precontrast) / Signal of Gray Matter (Precontrast)] − 1} × 100%. Plaque burden was measured on the maximal stenosis site and was defined as (1 − Lumen Area / Outer Area) × 100%.
Statistical Analysis
Normality assumptions were formally assessed using a Shapiro-Wilk test. Distributions were summarized using the mean ± SD or median (interquartile range). Categoric data were expressed as counts or percentages. Continuous data were compared using either a Mann-Whitney U test or a Student t test. Categoric variables were analyzed using the Fisher exact test. Multivariate logistic regression analysis was used to determine the independent factors associated with acute/subacute symptoms. The intraclass correlation coefficient and Cohen κ coefficient were used to evaluate the agreement between 2 reviewers for the measurement of the degree of stenosis and the identification of IPH. A P value of < .05 was considered statistically significant. All P values were 2-sided. GraphPad Prism 5 software (GraphPad Software, San Diego, California) and R statistics (Version 3.1.3; www.r-project.org) were used for data analysis.
Results
Patients, Demographics, and Imaging Findings
A total of 175 patients met the inclusion criteria. Forty-nine patients were excluded due to intracranial aneurysms (n = 28), Moyamoya disease (n = 3), vasculitis (n = 1), dissection (n = 6), coexistent vertebral artery stenosis >50% (n = 8), and bad image quality (n = 3). As a result, 126 patients were included in the final analysis (mean age, 61.5 ± 10.0 years; 82 males; mean degree of stenosis of 52.6% ± 15.8%). A total of 66 patients were symptomatic, and 60 were asymptomatic. Forty-six patients had a degree of stenosis of ≤50% (low-grade stenosis; range, 13.4%–48.7%), while 80 patients had a degree of stenosis of >50% (high-grade stenosis; range, 50.1%–80.3%). Demographic information and imaging findings are summarized in Tables 1 and 2 on the basis of the patients' degree of stenosis and symptom status.
Table 1:
Clinical characteristics and imaging findings of patients with low- and high-grade stenotic basilar artery plaque
| All (n = 126) | Low-Grade Stenosis (<50%, n = 46) | High-Grade Stenosis (≥50%, n = 80) | P Value | |
|---|---|---|---|---|
| Age (mean) (yr) | 61.5 ± 10.0 | 63.70 ± 9.4 | 60.3 ± 10.1 | .07 |
| Sex (male) (No.) (%) | 82 (65.1%) | 33 (71.7%) | 49 (61.3%) | .23 |
| Diabetes (No.) (%) | 42 (33.3%) | 15 (32.6%) | 27 (33.8%) | .90 |
| Smoking (No.) (%) | 35 (27.8%) | 13 (28.3%) | 22 (27.5%) | .93 |
| Hypertension (No.) (%) | 101 (80.2%) | 37 (80.4%) | 64 (80.0%) | .96 |
| Hyperlipidemia (No.) (%) | 65 (51.6%) | 18 (39.1%) | 47 (58.8%) | .03a |
| Coronary artery disease (No.) (%) | 5 (4.0%) | 3 (6.5%) | 2 (2.5%) | .27 |
| Preadmission aspirin use (No.) (%) | 31 (24.6%) | 9 (19.6%) | 22 (27.5%) | .32 |
| Preadmission statin use (No.) (%) | 17 (13.5%) | 4 (8.7%) | 13 (16.3%) | .23 |
| NIHSS score (median) (range) | 1 (0–11) | 2 (0–11) | 0 (0–6) | <.001a |
| Degree of stenosis (mean) (%) | 52.6 ± 15.8 | 35.2 ± 9.7 | 62.6 ± 7.9 | <.001a |
| Enhancement percentage (mean) | 17.4 ± 28.4 | 21.1 ± 33.1 | 15.3 ± 25.3 | .27 |
| Intraplaque hemorrhage (No.) (%) | 22 (17.5%) | 9 (19.6%) | 13 (16.3%) | .63 |
| Minimum lumen area (mean) (mm2) | 3.2 ± 2.7 | 5.1 ± 3.4 | 2.1 ± 1.3 | <.001a |
| Plaque burden (mean) (%) | 84.0 ± 9.1 | 75.9 ± 8.2 | 88.6 ± 5.7 | <.001a |
| Symptomatic (No.) (%) | 66 (52.4%) | 28 (60.9%) | 38 (47.5%) | .15 |
Statistically significant.
Table 2:
Clinical characteristics and imaging findings of patients with different symptom stages
| All (n = 126) | Symptomatic (n = 66) | Asymptomatic (n = 60) | P Value | |
|---|---|---|---|---|
| Age (mean) (yr) | 61.5 ± 10.0 | 61.7 ± 10.5 | 61.4 ± 9.4 | .86 |
| Sex (male) (No.) (%) | 82 (65.1%) | 50 (75.8%) | 32 (53.3%) | <.001a |
| Diabetes (No.) (%) | 42 (33.3%) | 23 (34.8%) | 19 (31.7%) | .71 |
| Smoking (No.) (%) | 35 (27.8%) | 25 (37.9%) | 10 (16.7%) | <.001a |
| Hypertension (No.) (%) | 101 (80.2%) | 52 (78.8%) | 49 (81.7%) | .69 |
| Hyperlipidemia (No.) (%) | 65 (51.6%) | 38 (57.6%) | 27 (45.0%) | .16 |
| Coronary artery disease (No.) (%) | 5 (4.0%) | 3 (4.5%) | 2 (3.3%) | .73 |
| Preadmission aspirin use (No.) (%) | 31 (24.6%) | 21 (31.8%) | 10 (16.7%) | .06 |
| Preadmission statin use (No.) (%) | 17 (13.5%) | 10 (15.2%) | 7 (11.7%) | .57 |
| NIHSS score (median) (range) | 1 (0–11) | 2 (0–11) | 0 (0–4) | <.001a |
| Degree of stenosis (mean) (%) | 52.6 ± 15.8 | 52.4 ± 15.5 | 52.8 ± 16.1 | .88 |
| Enhancement percentage (mean) | 17.4 ± 28.4 | 25.5 ± 26.1 | 8.5 ± 28.5 | <.001a |
| Intraplaque hemorrhage (No.) (%) | 22 (17.5%) | 21 (31.8%) | 1 (1.7%) | <.001a |
| Minimum lumen area (mean) (mm2) | 3.2 ± 2.7 | 3.7 ± 2.8 | 2.7 ± 2.6 | .04a |
| Plaque burden (mean) (%) | 84.0 ± 9.1 | 83.6 ± 9.8 | 84.4 ± 8.2 | .62 |
Statistically significant.
IPH was identified in 22 patients (17.5%), including 21 symptomatic patients (31.8%) and 1 (1.7%) asymptomatic patient (Table 2). IPH appeared over a range of stenosis values (Table 1), and there was no difference in the presentation rate of the basilar arteries in low- and high-grade stenosis (19.6% versus 16.3%, P = .63). The IPH-to-muscle signal ratio was 2.10 ± 0.54 (range, 1.50–3.19). In these 21 patients with stroke with IPH, 15 had paramedian pontine infarct stroke and 6 had lacunar infarction stroke. Univariate and multivariate analyses of the parameters associated with symptoms are shown in Table 3. IPH was the strongest independent indicator of symptomatic status (odds ratio, 27.5; 95% CI, 3.4–221.5; P = .002). Contrast enhancement was also independently associated with symptomatic status (odds ratio, 9.9; 95% CI, 1.5–23.6; P = .016). Degree of stenosis, minimal lumen area, and plaque burden were not significantly associated with symptoms (P > .05). The presence of IPH was not associated with stenosis (r = 0.16, P = .10) or plaque burden (r = 0.16, P = .11). Representative basilar artery plaque images with and without IPH are shown in Figs 1–3.
Table 3:
Univariate and multivariate analyses of the parameters associated with symptomatic status
| Variable | Univariate Analysis |
Multivariate Analysis |
||
|---|---|---|---|---|
| Odds Ratio (95% CI) | P | Odds Ratio (95% CI) | P | |
| Sex (male) | 2.7 (1.2–5.8) | <.001 | ||
| Smoking | 3.0 (1.3-.7.1) | <.001 | ||
| Enhancement percentage | 13.0 (2.6–25.0) | <.001 | 9.9 (1.5–23.6) | .016 |
| Intraplaque hemorrhage | 27.5 (3.6–212.4) | .002 | 27.5 (3.4–221.5) | .002 |
| Minimum lumen area | 1.2 (1.0–1.4) | .04 | ||
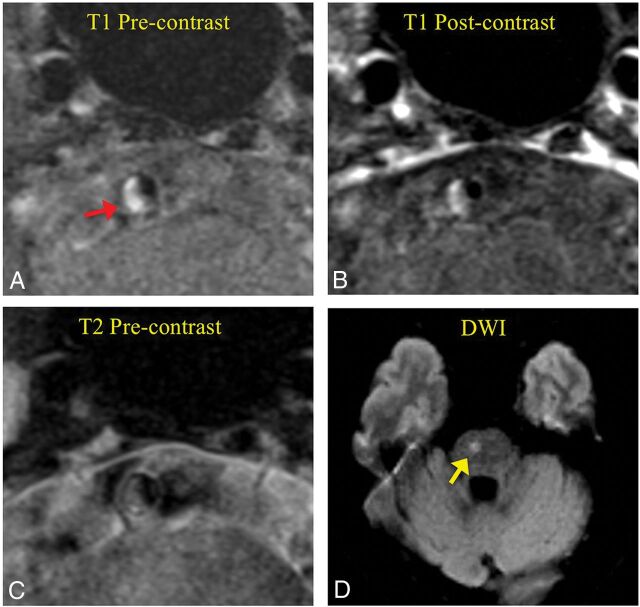
Fig 1.
Intraplaque hemorrhage presenting in a low-grade stenotic basilar artery plaque (43% degree of stenosis) in an acute symptomatic female patient (65 years of age). A, T1-weighted black-blood MR imaging shows high signal (fresh IPH, red arrow) in the plaque. B, Postcontrast T1-weighted image shows slight enhancement of the plaque. C, T2-weighted image shows isointense signal of the plaque. D, DWI shows infarct in the brain stem (yellow arrow).
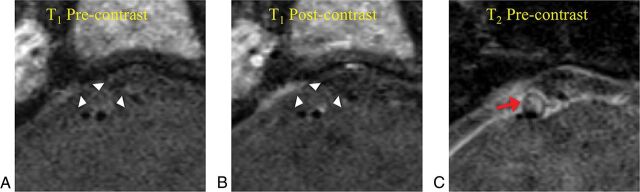
Fig 2.
A high-grade stenotic basilar artery plaque (degree of stenosis, 73%) without intraplaque hemorrhage in an asymptomatic female patient (56 years of age). A, T1-weighted black-blood MR imaging shows isointense signal in the plaque. B, Postcontrast T1-weighted image shows enhancement of the plaque surface. C, T2-weighted image shows high signal of the plaque (red arrow).
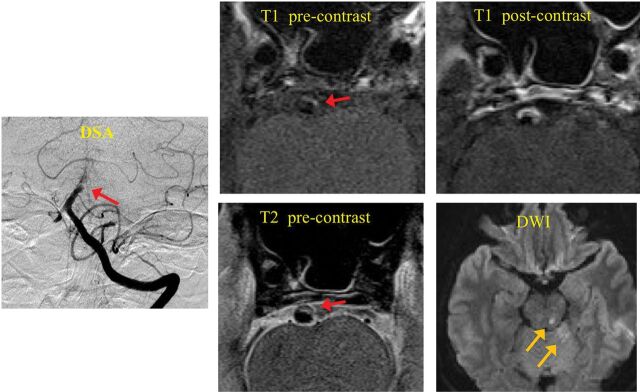
Fig 3.
A symptomatic patient with infarctions distal to the location of basilar artery plaque. Red arrows show the location of the plaque. Yellow arrows show the location of infarctions on DWI. T1-weighted black-blood MR imaging shows high signal in the plaque (IPH). Postcontrast T1-weighted image shows strong enhancement of the plaque.
The diagnostic performance of IPH for acute/subacute symptomatic patients with stroke is summarized in Table 4. IPH had a high specificity of 98.3% and a high positive predictive value of 95.5%; however, the sensitivity (31.8%) and negative predictive value (56.7%) were lower.
Table 4:
Diagnostic performance of intraplaque hemorrhage in identifying symptomatic/asymptomatic basilar artery plaque
| IPH-Positive (n = 22) | IPH-Negative (n = 104) | |
|---|---|---|
| Symptomatic | 21 | 45 |
| Asymptomatic | 1 | 59 |
| Diagnostic performance (%) (95% CI) | ||
| Specificity | 98.3 (91.1–100.0) | |
| Sensitivity | 31.8 (20.9–44.4) | |
| PPV | 95.5 (74.4–99.3) | |
| NPV | 56.7 (52.6–60.8) | |
Note:—PPV indicates positive predict value; NPV, negative predict value.
MR Imaging Measurements Reproducibility
There was excellent interreader agreement for measuring the degree of stenosis (intraclass correlation coefficient = 0.97; 95% CI, 0.94–0.98) and for identification of IPH (96% agreement, κ = 0.88; 95% CI, 0.76–1.00).
Discussion
This study adds to the growing literature that emphasizes the importance of intracranial plaque properties compared with the degree of luminal narrowing.2 In this study, IPH was the only finding associated with stroke-symptom status, whereas contrast enhancement, degree of stenosis, minimal lumen area, and plaque burden were not associated with symptom status. Because IPH was present in both low- and high-grade stenotic basilar artery plaque with a high positive predictive value (95.5%) for symptomatic stroke, it is evident that symptomatic ischemia may be explained by factors other than stenosis resulting in hypoperfusion. The presence of IPH likely indicates a high risk for artery-to-artery embolic occlusion following plaque rupture. In this conceptualization, future stroke risk for a smaller plaque with IPH is possibly greater than for a larger plaque with a stable fibrous cap.
A study of basilar artery ICAD in patients with at least 30% stenosis found T1-weighted hyperintense intraplaque signal in 8 of 38 cases (21%), which could indicate IPH, though the authors did not specifically term this finding IPH.9 The prevalence of basilar artery T1-weighted hyperintense signal reported by Huang et al9 (21%) was similar to that reported in the current study (19.6%). On the other hand, a study of 74 patients with >50% stenosis documented IPH in 42.3% of patients with an increased frequency in symptomatic patients.12 The reason for this higher incidence of IPH may be related to the larger plaque size in these patients with a mean stenosis of 72.9%, though we did not observe a significant difference between IPH frequency in stenotic-versus-nonstenotic plaque in our study.12 Alternatively, patients in our study were younger than those of Yu et al,12 with a difference in mean age of approximately 10 years between the populations in the 2 studies. IPH may occur more frequently with age, though this hypothesis is untested. The diagnostic performance of IPH in the aforementioned study was similar to that in the current study with relatively high specificity (79%) but low sensitivity (53%).12 A study of IPH in other intracranial arteries has observed similar findings. IPH was significantly more frequent in symptomatic middle cerebral artery plaque than in asymptomatic plaque (19.6% versus 3.2%).19
While current management guidelines focus on stenosis, our work and that of others supports the use of identification of plaque properties to better indicate future stroke risk. Our study emphasizes the highly specific (98.3%) nature of IPH as an indicator of symptomatic stroke, implying that this represents unstable basilar artery atherosclerotic plaque. Unfortunately, IPH is not particularly sensitive (31.8%) to the presence of a symptomatic basilar artery. These observations likely reflect the multiple stroke mechanisms encountered in ICAD, in which IPH could predispose to artery-to-artery embolization with small-vessel occlusion and could cause branch occlusive disease affecting perforating arteries. On the other hand, some symptomatic strokes in this study could also be caused by stenotic basilar artery plaque leading to hypoperfusion. IPH was the best overall marker of symptomatic plaque with an odds ratio of 27.5. The findings of this study support the use of IPH in basilar artery atherosclerotic plaque as a better indicator of symptomatic plaque rather than stenosis or plaque burden alone.
Other intracranial plaque characteristics including contrast enhancement and minimal lumen area have been associated with symptoms.24,25 We observed a similar relationship between contrast enhancement and symptom status in our study. A smaller study examining the basilar artery with contrast-enhanced HR-MRI observed wall enhancement in patients with recent infarction and also those who would go on to have ischemic events.26 Another study of intracranial atherosclerotic disease also concluded that contrast enhancement was associated with culprit plaques with a substantial odds ratio of approximately 35.25 Research suggests that the vasa vasorum evolves with age and demonstrates different distributions with less vasa vasorum in the intracranial vasculature and differing distributions within the cerebral vascular territories.27 Theoretically, enhancement of the basilar artery wall could reflect physiologic enhancement of the vasa vasorum rather than pathologic plaque enhancement, which could reduce the specificity of vessel wall enhancement. An improved understanding of the vasa vasorum may enhance the specificity of basilar artery enhancement.
Further work is needed to histologically validate HR-MRI assessment of IPH, with only 1 case of histologically verified intracranial IPH demonstrated as T1-weighted hyperintense intraplaque signal on postmortem HR-MRI.28 Due to the reliance on postmortem assessment of plaque, histologic validation of intracranial plaque imaging characteristics is more challenging than in the extracranial carotid artery plaque, which may be ascertained following endarterectomy. While a study of intracranial plaque characterization using ex vivo 3T HR-MRI in 53 postmortem specimens determined relaxation times for many plaque components, no IPH was encountered in these plaque specimens.29 Therefore, IPH detection on HR-MRI largely infers plaque characteristics from existing extracranial atherosclerosis imaging literature.
Our results cannot directly confer causality of IPH in symptomatic stroke, and this cross-sectional study design cannot provide an assessment of future stroke risk. Prospective assessment of IPH and subsequent stroke risk would extend the clinical relevance of the findings of this study, and such data could be used in risk-assessment calculations. HR-MRI can also determine IPH timing and duration. Potentially, signal characteristics of IPH could provide information on the onset of hemorrhage, and serial examination of patients with ICAD exhibiting IPH could provide insight into its natural history. Moreover, serial assessment of intracranial IPH evolution while patients were on different pharmacologic therapies might potentially provide objective information regarding treatment efficacy.
In addition, there are technical limitations to our study. Our study used a standard 2D T1-weighted black-blood fast spin-echo sequence. The use of 3D sequences with higher resolution,30 better T1-weighted contrast,31 and advanced blood-suppression techniques (such as diffusion preparation32 or variable flip angle train30) could potentially improve the identification of IPH. However, the 3D high-resolution sequences can increase scan time, the use of diffusion preparation can reduce the signal-to-noise ratio and induce T2 contrast,32 and variable flip angle trains can induce blurring with a wider point-spread function.30 All of these limitations will need to be accounted for in future clinical studies.
Conclusions
Evidence from this study suggests that IPH in the basilar artery found on HR-MRI identifies atherosclerotic lesions that are more likely to be symptomatic regardless of the degree of stenosis. This plaque property appears to be substantially associated with symptom status, whereas many other factors, including plaque size, contrast enhancement, and degree of stenosis, do not. In the future, the use of HR-MRI for the early detection of basilar artery IPH may allow clinicians to select individuals at greater risk of imminent stroke and help provide optimal therapeutic intervention.
ABBREVIATIONS:
- HR-MRI
high-resolution MR imaging
- ICAD
intracranial atherosclerotic disease
- IPH
intraplaque hemorrhage
Footnotes
Disclosures: Chengcheng Zhu—RELATED: Grant: National Institutes of Health–National Heart, Lung, and Blood Institute, Comments: supported by National Heart, Lung, and Blood Institute grant K99HL136883. Jianping Lu—RELATED: Grant: National Nature Science Foundation of China (No. 31470910).
This work was supported by the National Natural Science Foundation of China (31470910) and National Institutes of Health grants R01HL114118, R01NS059944, and K99HL136883.
Paper previously presented, in part, at: Annual Meeting and Exhibition of the International Society for Magnetic Resonance in Medicine, May 7–13, 2016; Singapore.
References
- 1. Gorelick PB, Wong KS, Bae HJ, et al. Large artery intracranial occlusive disease: a large worldwide burden but a relatively neglected frontier. Stroke 2008;39:2396–99 10.1161/STROKEAHA.107.505776 [DOI] [PubMed] [Google Scholar]
- 2. Bodle JD, Feldmann E, Swartz RH, et al. High-resolution magnetic resonance imaging: an emerging tool for evaluating intracranial arterial disease. Stroke 2013;44:287–92 10.1161/STROKEAHA.112.664680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mazighi M, Labreuche J, Gongora-Rivera F, et al. Autopsy prevalence of intracranial atherosclerosis in patients with fatal stroke. Stroke 2008;39:1142–47 10.1161/STROKEAHA.107.496513 [DOI] [PubMed] [Google Scholar]
- 4. Leng X, Wong KS, Liebeskind DS. Evaluating intracranial atherosclerosis rather than intracranial stenosis. Stroke 2014;45:645–51 10.1161/STROKEAHA.113.002491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ryoo S, Park JH, Kim SJ, et al. Branch occlusive disease: clinical and magnetic resonance angiography findings. Neurology 2012;78:888–96 10.1212/WNL.0b013e31824c4699 [DOI] [PubMed] [Google Scholar]
- 6. Adams HP Jr, Bendixen BH, Kappelle LJ, et al. Classification of subtype of acute ischemic stroke: definitions for use in a multicenter clinical trial—TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke 1993;24:35–41 10.1161/01.STR.24.1.35 [DOI] [PubMed] [Google Scholar]
- 7. Degnan AJ, Gallagher G, Teng Z, et al. MR angiography and imaging for the evaluation of middle cerebral artery atherosclerotic disease. AJNR Am J Neuroradiol 2012;33:1427–35 10.3174/ajnr.A2697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dieleman N, van der Kolk AG, Zwanenburg JJ, et al. Imaging intracranial vessel wall pathology with magnetic resonance imaging: current prospects and future directions. Circulation 2014;130:192–201 10.1161/CIRCULATIONAHA.113.006919 [DOI] [PubMed] [Google Scholar]
- 9. Huang B, Yang WQ, Liu XT, et al. Basilar artery atherosclerotic plaques distribution in symptomatic patients: a 3.0T high-resolution MRI study. Eur J Radiol 2013;82:e199–203 10.1016/j.ejrad.2012.10.031 [DOI] [PubMed] [Google Scholar]
- 10. Klein IF, Lavallée PC, Mazighi M, et al. Basilar artery atherosclerotic plaques in paramedian and lacunar pontine infarctions: a high-resolution MRI study. Stroke 2010;41:1405–09 10.1161/STROKEAHA.110.583534 [DOI] [PubMed] [Google Scholar]
- 11. Kim YS, Lim SH, Oh KW, et al. The advantage of high-resolution MRI in evaluating basilar plaques: a comparison study with MRA. Atherosclerosis 2012;224:411–16 10.1016/j.atherosclerosis.2012.07.037 [DOI] [PubMed] [Google Scholar]
- 12. Yu JH, Kwak HS, Chung GH, et al. Association of intraplaque hemorrhage and acute infarction in patients with basilar artery plaque. Stroke 2015;46:2768–72 10.1161/STROKEAHA.115.009412 [DOI] [PubMed] [Google Scholar]
- 13. Chung JW, Kim BJ, Choi BS, et al. High-resolution magnetic resonance imaging reveals hidden etiologies of symptomatic vertebral arterial lesions. J Stroke Cerebrovasc Dis 2014;23:293–302 10.1016/j.jstrokecerebrovasdis.2013.02.021 [DOI] [PubMed] [Google Scholar]
- 14. Huang X, Teng Z, Canton G, et al. Intraplaque hemorrhage is associated with higher structural stresses in human atherosclerotic plaques: an in vivo MRI-based 3D fluid-structure interaction study. Biomed Eng Online 2010;9:86 10.1186/1475-925X-9-86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Moody AR, Murphy RE, Morgan PS, et al. Characterization of complicated carotid plaque with magnetic resonance direct thrombus imaging in patients with cerebral ischemia. Circulation 2003;107:3047–52 10.1161/01.CIR.0000074222.61572.44 [DOI] [PubMed] [Google Scholar]
- 16. McNally JS, McLaughlin MS, Hinckley PJ, et al. Intraluminal thrombus, intraplaque hemorrhage, plaque thickness, and current smoking optimally predict carotid stroke. Stroke 2015;46:84–90 10.1161/STROKEAHA.114.006286 [DOI] [PubMed] [Google Scholar]
- 17. Altaf N, MacSweeney ST, Gladman J, et al. Carotid intraplaque hemorrhage predicts recurrent symptoms in patients with high-grade carotid stenosis. Stroke 2007;38:1633–35 10.1161/STROKEAHA.106.473066 [DOI] [PubMed] [Google Scholar]
- 18. Turan TN, Bonilha L, Morgan PS, et al. Intraplaque hemorrhage in symptomatic intracranial atherosclerotic disease. J Neuroimaging 2011;21:e159–61 10.1111/j.1552-6569.2009.00442.x [DOI] [PubMed] [Google Scholar]
- 19. Xu WH, Li ML, Gao S, et al. Middle cerebral artery intraplaque hemorrhage: prevalence and clinical relevance. Ann Neurol 2012;71:195–98 10.1002/ana.22626 [DOI] [PubMed] [Google Scholar]
- 20. Liu Q, Huang J, Degnan AJ, et al. Comparison of high-resolution MRI with CT angiography and digital subtraction angiography for the evaluation of middle cerebral artery atherosclerotic steno-occlusive disease. Int J Cardiovasc Imaging 2013;29:1491–98 10.1007/s10554-013-0237-3 [DOI] [PubMed] [Google Scholar]
- 21. Lou M, Caplan LR. Vertebrobasilar dilatative arteriopathy (dolichoectasia). Ann N Y Acad Sci 2010;1184:121–33 10.1111/j.1749-6632.2009.05114.x [DOI] [PubMed] [Google Scholar]
- 22. Choi YJ, Jung SC, Lee DH. Vessel wall imaging of the intracranial and cervical carotid arteries. J Stroke 2015;17:238–55 10.5853/jos.2015.17.3.238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zhang X, Zhu C, Peng W, et al. Scan-rescan reproducibility of high resolution magnetic resonance imaging of atherosclerotic plaque in the middle cerebral artery. PLoS One 2015;10:e0134913 10.1371/journal.pone.0134913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Teng Z, Peng W, Zhan Q, et al. An assessment on the incremental value of high-resolution magnetic resonance imaging to identify culprit plaques in atherosclerotic disease of the middle cerebral artery. Eur Radiol 2016;26:2206–14 10.1007/s00330-015-4008-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Qiao Y, Zeiler SR, Mirbagheri S, et al. Intracranial plaque enhancement in patients with cerebrovascular events on high-spatial-resolution MR images. Radiology 2014;271:534–42 10.1148/radiol.13122812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lou X, Ma N, Ma L, et al. Contrast-enhanced 3T high-resolution MR imaging in symptomatic atherosclerotic basilar artery stenosis. AJNR Am J Neuroradiol 2013;34:513–17 10.3174/ajnr.A3241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Portanova A, Hakakian N, Mikulis DJ, et al. Intracranial vasa vasorum: insights and implications for imaging. Radiology 2013;267:667–79 10.1148/radiol.13112310 [DOI] [PubMed] [Google Scholar]
- 28. Chen XY, Wong KS, Lam WW, et al. High signal on T1 sequence of magnetic resonance imaging confirmed to be intraplaque haemorrhage by histology in middle cerebral artery. Int J Stroke 2014;9:E19 10.1111/ijs.12277 [DOI] [PubMed] [Google Scholar]
- 29. Jiang Y, Zhu C, Peng W, et al. Ex-vivo imaging and plaque type classification of intracranial atherosclerotic plaque using high resolution MRI. Atherosclerosis 2016;249:10–16 10.1016/j.atherosclerosis.2016.03.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zhu C, Haraldsson H, Tian B, et al. High resolution imaging of the intracranial vessel wall at 3 and 7 T using 3D fast spin echo MRI. MAGMA 2016;29:559–70 10.1007/s10334-016-0531-x [DOI] [PubMed] [Google Scholar]
- 31. Zhu DC, Vu AT, Ota H, et al. An optimized 3D spoiled gradient recalled echo pulse sequence for hemorrhage assessment using inversion recovery and multiple echoes (3D SHINE) for carotid plaque imaging. Magn Reson Med 2010;64:1341–51 10.1002/mrm.22517 [DOI] [PubMed] [Google Scholar]
- 32. Zhu C, Graves MJ, Yuan J, et al. Optimization of improved motion-sensitized driven-equilibrium (iMSDE) blood suppression for carotid artery wall imaging. J Cardiovasc Magn Reson 2014;16:61 10.1186/s12968-014-0061-5 [DOI] [PMC free article] [PubMed] [Google Scholar]