Abstract
Einsteck procedure refers to a method whereby the experimenter inserts material into the blastocoel cavity of an early amphibian embryo. This procedure is simpler to perform than other types of grafts, such as Spemann–Mangold, and with practice yields a sizable amount of data suitable for statistical analysis. This protocol for Einsteck transplantation in Xenopus describes the insertion of the gastrula-stage blastopore lip into the blastocoel cavity of a host embryo.
MATERIALS
It is essential that you consult the appropriate Material Safety Data Sheets and your institution’s Environmental Health and Safety Office for proper handling of equipment and hazardous materials used in this protocol.
RECIPES: Please see the end of this protocol for recipes indicated by <R>. Additional recipes can be found online at http://cshprotocols.cshlp.org/site/recipes.
Reagents
Ethanol (70%)
H2O (reverse osmosis [RODI] or distilled [dH2O])
-
Modified Barth’s saline (MBS) (1×) <R>
For grafting medium, use 1× MBS containing 50 μg/mL of gentamycin. For recovery medium, use 0.1× MBS containing 50 μg/mL of gentamycin. -
Xenopus laevis embryos (stages 8, 9, 10, or 10+; see Step 3)
Embryos are staged according to Nieuwkoop and Faber (1967).
Equipment
Dissecting microscope equipped with a gooseneck lighting system
-
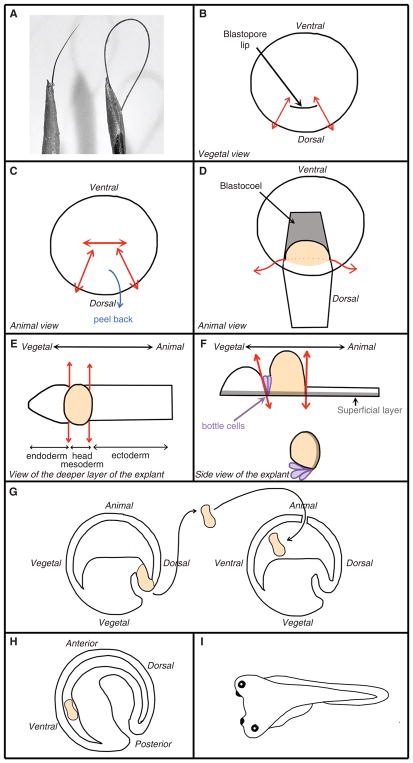
Eyelash knife
Select a human eyelash with the desired thickness and curvature (Fig. 1A) and thread it through a 23-gauge needle fitted on a 1-cc syringe. For safety purposes, the tip of the needle can be cut off with scissors before the threading. Fix the eyelash with a drop of nail polish or cyanoacrylate glue. Forceps (fine, to remove vitelline envelope)
-
Glass bead tool
Thin out the end of a Pasteur pipette under a benzene burner and melt the end into a ball roughly the size of gastrula-stage embryo (about 2 mm). -
Hair loop
Cut a human hair into 3-inch sections. Thread both ends of a section into a 23-gauge needle fitted on a 1-cc syringe. Push the loop into the needle until the desired stiffness is reached (Fig. 1A). Fix the hair with a drop of nail polish or cyanoacrylate glue. Petri dishes (60-mm, plastic), coated with a 4-mm layer of 1% agarose
-
Petri dishes (60-mm, plastic), coated with plasticine
Roll 2 tsp of plasticine (nondrying, toxin free, appropriate for young children) into a ball. Flatten it out into a plastic Petri dish by hand. Transfer pipette (disposable plastic or glass) with an opening of ≥2 mm, for transferring embryos
FIGURE 1.
Key tools and steps for Einsteck transplantation. (A) Eyelash knife (left) and hair loop (right). (B–I) Diagram representing the key steps of the grafting procedure.
METHOD
The Einsteck technique has been used to transplant various kinds of material, including archenteron roof, dorsal blastopore lip (Marx 1925), neurula-stage neural plate (Mangold 1933), or a bead coated with signaling molecules (Slack and Isaacs 1994); see Discussion. The technique was heavily used in the 1930s in the rush to discover the neural inducer (Mangold 1933; Needham 1942) and again in the 1980s and 1990s by scientists investigating the specification of the antero–posterior axis (Ruiz i Altaba and Melton 1989; Cho and De Robertis 1990; Blum et al. 1992; Slack and Isaacs 1994). This protocol is based on the method by Saxén and Toivonen (1962).
The grafting procedure should be conducted between 15°C and 19°C.
Sterilize a plasticine-coated dish with 70% ethanol for 10 min. Rinse for 30 sec with RODI H2O and then fill with grafting medium.
Using the glass bead tool, make two depressions in the plasticine as deep as one-third or one-half the diameter of an embryo (or about 1-mm).
Select a donor and a host embryo and transfer the embryos to the plasticine-coated dish. Select a donor embryo between stage 10 (the forming blastopore lip appears as a dotted line with a light gray color) and 10+ (the blastopore lip is now a line of a dark gray color). Select a host embryo at late blastula stage (stage 8 or 9) or early gastrula stage (10+).
-
Remove the vitelline envelope from each embryo using the fine forceps. Ensure that the embryos never breach the liquid surface once the vitelline envelope is removed.
See Troubleshooting. Move the donor and host embryos into the plasticine depressions. Turn a donor embryo onto its animal (pigmented) side with the blastopore lip and dorsal marginal zone (the area between the lip and the pigmentation edge) clearly visible (Fig. 1B).
-
On the donor embryo, insert the tip of the eyelash knife at the commissure (corner) of the blastopore lip and thread it all the way to the animal pole. Cut the tissue by pressing the hair loop along the length of the eyelash knife. Repeat the process from the other commissure (Fig. 1B). Cut perpendicular to the first two cuts to free the pigmented edge of the explant (Fig. 1C) and peel the piece of tissue toward the vegetal pole (Fig. 1D).
The peeled tissue is made of superficial pigmented ectoderm with underlying deep mesoderm (Fig. 1D, orange tissue) and some endoderm. -
To free the explant from the embryo, insert the knife under the mesoderm and cut perpendicularly (Fig. 1D). Lay the explant with its superficial side down (Fig. 1E).
-
Trim the explant into a tissue containing the head mesoderm and the bottle cells.
To remove the neural ectoderm, insert the length of the eyelash knife under the dorsal side of the head mesoderm mass (i.e., cleft of Brachet) and press firmly onto the bottom of the dish.
To cut the mass of endoderm cells attached to the head mesoderm, align the knife-edge perpendicular to the head mesoderm mass (Fig. 1E,F).
-
Position a host embryo with its animal hemisphere (pigmented area) up. Using the eyelash knife, make an incision slightly larger than the width of the explant in the animal hemisphere.
The blastocoel cavity should be visible. -
Insert the explant into the blastocoel cavity (Fig. 1G).
See Troubleshooting. -
Let the incision heal for 20 min and then transfer the grafted embryo to an agarose-coated dish filled with recovery medium.
The incision should heal itself without resorting to glass cover bridges as described in other transplantation protocols (see Protocol: Cranial Neural Crest Transplants [Cousin 2018]). Because the blastocoel fluid escapes when the experimenter performs the incision, the edges of the incision should come together naturally and heal.By the end of gastrulation, the explant is pushed into an antero–ventral position by the involuted mesoderm (Fig. 1H). A secondary axis is visible as soon as the neurula stage (the day after the graft). At the early tailbud stage, a secondary head should be clearly visible (Fig. 1I).See Troubleshooting.
TROUBLESHOOTING
Problem (Step 4, Step 11): The embryo disaggregates.
Solution: The medium is the culprit. Make fresh medium, add gentamycin and avoid using HCl to adjust the pH as excess Cl− ions may affect the healing of the embryo.
Problem (Steps 4–10): The embryos develop too fast and the experimenter does not have the time to perform the grafts.
Solution: Keep the embryos, media and dishes at 15°C at least a few hours before grafting is scheduled. Perform the grafts on cooling table set up at 15°C if available.
DISCUSSION
This particular protocol has been used successfully in a classroom environment. Students that failed to induce secondary anterior structures with the Spemann–Mangold organizer transplants were usually able to do so using the Einsteck technique. The nature and extent of the induced axis will vary depending on the type of tissue or amount of growth factor used. In the case of the gastrula blastopore lip, a stage 10+ explant will be able to induce a secondary embryo with a fully formed head while an explant taken later (stage 10.5 or 11) will induce more posterior structures such as trunk and tail (Saxén and Toivonen 1962). The quality of the secondary axis induced depends on two variables: the age of the host embryos at the time of transplantation and the position of the explant after the host gastrulation is complete. If the host is at blastula stage, the grafted tissue will be able to induce a full secondary embryo attached to the ventral or lateral side of the primary embryo. If the embryo is already at the gastrula stage, the secondary axis may only consist of trunk and tail tissues (although we obtained full head-twinning when grafting CNC at stage 10+). This difference is caused by a change in the host tissue’s competency to respond to the signals secreted by the Spemann–Mangold organizer. The results may also vary depending on where the grafted tissue ends up at the conclusion of gastrulation. Some explants may end up on the anterior-most part of the embryo and therefore result in a well-formed head in the secondary embryo. Others may end up on a more lateral side, resulting in a smaller secondary embryo or a secondary embryo lacking a head. If one maintains the host embryo with its ventral side down during gastrulation, gravity will help maintain the grafted tissue in the ventral part of the blastocoel cavity, resulting in more uniform results across transplanted embryos
Generally speaking, Einsteck procedures are performed to assess the capacity of a transplanted tissue to change either the fate or the patterning of surrounding tissues (phenomenon of induction). For example, Otto Mangold performed Einsteck procedures with archenteron (primitive gut lumen) roof taken from various locations along the antero–posterior axis of a neurula-stage donor (Mangold 1933). Others showed that the treatment of donor embryos with retinoid acid before transplanting the Spemann organizer changes its patterning capabilities (Sive and Cheng 1991). For such experiments, one must design the appropriate negative and positive controls. The former may consist of transplantation of an unrelated tissue such as blastula-stage ectoderm tissue (i.e., animal cap). The latter may consist of transplantation of an untreated Spemann–Mangold organizer.
The inductive capacity of a signaling molecule can also be assessed using this technique by implanting beads coated with the molecules of interest (Slack and Isaacs 1994). If molecules cannot be coated on beads, one can graft small animal cap from embryos injected with the mRNA encoding such proteins. For each of these cases, proper negative controls must be designed. For bead experiments, implanting an uncoated bead will be sufficient. If modified animal caps are used, the grafting of unmodified animal cap is appropriate. The experimenter will need to design positive controls appropriate for the inquiry. If one is assessing the ability of a compound or tissue to induce neural tissue, a positive control could consist of the graft of a Spemann–Mangold organizer or a bead coated with a well-known neural inducer.
Depending on the experimenter’s inquiry, the results can be analyzed by either qualitative methods such as gross morphology (i.e., apparition of a secondary head) and histology or quantitative methods such as in situ hybridization, western blot and Q-PCR.
RECIPE
Modified Barth’s Saline (MBS) (1X)
| CaCl2 | 0.41 mM |
| CaNO3 | 0.3 mM |
| HEPES-NaOH | 15 mM |
| KCl | 1 mM |
| MgSO4 | 0.82 mM |
| NaCl | 88 mM |
| NaHCO3 | 2.4 mM |
Adjust pH to 7.6. Store at room temperature for up to one month.
Acknowledgments
The author would like to thank Professor Ray Keller for his invaluable insight into Xenopus transplantations. H.C. is supported by NIH/NIDCR DE025691.
References
- Blum M, Gaunt SJ, Cho KW, Steinbeisser H, Blumberg B, Bittner D, De Robertis EM. Gastrulation in the mouse: the role of the homeo-box gene goosecoid. Cell. 1992;69:1097–1106. doi: 10.1016/0092-8674(92)90632-m. [DOI] [PubMed] [Google Scholar]
- Cho KW, De Robertis EM. Differential activation of Xenopus homeo box genes by mesoderm-inducing growth factors and retinoic acid. Genes Dev. 1990;4:1910–1916. doi: 10.1101/gad.4.11.1910. [DOI] [PubMed] [Google Scholar]
- Cousin H. Cranial neural crest transplants. Cold Spring Harb Protoc. 2018 doi: 10.1101/pdb.prot097402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangold O. Uber die Induktionsfähigkeit der verscheidenen Bezirke der Neurula von Urodelen. Naturwissenschaften. 1933;43:761–766. [Google Scholar]
- Marx A. Experimentelle Untersuchungen zur Frage der Determonation der Medullarplatte. Wilhelm Roux Arch Entwicklungsmeck Org. 1925;105:20–44. doi: 10.1007/BF02083724. [DOI] [PubMed] [Google Scholar]
- Needham J. Biochemistry and Morphogenesis. Cambridge Univ. Press; Cambridge: 1942. [Google Scholar]
- Nieuwkoop PD, Faber J. Normal table of Xenopus Laevis (Daudin) 2. North-Holland; Amsterdam: 1967. [Google Scholar]
- Ruiz i Altaba A, Melton DA. Interaction between peptide growth factors and homoeobox genes in the establishment of anteroposterior polarity in frog embryos. Nature. 1989;341:33–38. doi: 10.1038/341033a0. [DOI] [PubMed] [Google Scholar]
- Saxén L, Toivonen S. Primary embryonic induction. Acedemic Press; London: 1962. [DOI] [PubMed] [Google Scholar]
- Sive HL, Cheng PF. Retinoic acid perturbs the expression of Xhox.lab genes and alters mesodermal determination in Xenopus laevis. Genes Dev. 1991;5:1321–1332. doi: 10.1101/gad.5.8.1321. [DOI] [PubMed] [Google Scholar]
- Slack JM, Isaacs HV. The Einsteck-method: position and structure of projections formed by implants of a ventral character. Dev Biol. 1994;161:313–317. doi: 10.1006/dbio.1994.1031. [DOI] [PubMed] [Google Scholar]