Abstract
This study examined biological sex differences in the development of mild cognitive impairment (MCI) and probable Alzheimer’s disease (AD) development as predicted by changes in the hippocampus or white matter hyperintensities. A secondary data analysis of the National Alzheimer’s Coordinating Center Uniform Data Set was conducted. We selected samples of participants with normal cognition at baseline who progressed to MCI (n = 483) and those who progressed to probable AD (n = 211) to determine if hippocampal volume or white matter hyperintensities (WMH) at baseline predicted progression to probable AD or MCI and whether the rate of progression differed between men and women. The survival analyses indicated that changes in hippocampal volumes affected the progression to probable AD (HR = 0.535, 95% CI [0.300–0.953]) only among women. White men had an increased rate of progression to AD (HR = 4.396, CI [1.012–19.08]; HR = 4.665, 95% CI [1.072–20.29]) compared to men in other race and ethnic groups. Among women, increases in hippocampal volume ratio led to decreased rates of progressing to MCI (HR = 0.386, 95% CI [0.166–0.901]). Increased WMH among men led to faster progression to MCI (HR = 1.048. 95% CI [1.011–1.086]). Women and men who were older at baseline were more likely to progress to MCI. In addition, results from longitudinal analyses showed that women with a higher CDR global score, older age at baseline, or more disinhibition symptoms experienced higher odds of MCI development. Changes in hippocampal volumes affect the progression to or odds of probable AD (and MCI) more so among women than men, while changes in WMH affected the progression to MCI only among men.
Keywords: Alzheimer disease, brain, disease progression, women, hippocampus, magnetic resonance imaging, men, sex characteristics, white matter hyperintensitie
Introduction
Currently 5.3 million people over the age of 65 are diagnosed with Alzheimer’s disease dementia in the United States (Alzheimer’s Association, 2017), though this number likely underestimates true prevalence. Of those living with Alzheimer’s disease today, two-thirds are women (Snyder et al., 2016). This recognition has led to emergent research that seeks to understand the causes and risk across the spectrum of dementia development (Katz et al., 2012), in part to account for these sex differences. Despite the fact that calls for including women in clinical research and the study of sex differences in disease development have been ongoing (Mazure & Swendsen, 2016), there are surprisingly few studies addressing these questions by examining biological sex differences. In fact, some researchers maintain that sex differences in dementia development do not exist, that the risk is equal between sexes within age cohorts, and that the difference in prevalence is only attributable to life expectancy rather than sex differences (Hebert, Scherr, McCann, Beckett, & Evans, 2001). Others attribute the difference in prevalence to survival bias, citing better cardiac health for men living past the age of 65, thereby decreasing their risk of dementia development (Chêne et al., 2015). We hypothesize that there are biological sex differences in the development of and progression to mild cognitive impairment (MCI) and probable Alzheimer’s disease (AD) development when using white matter hyperintensities (WMH) or hippocampal volume as predictors of progression.
Research regarding sex differences in dementia development typically points to the longer life expectancy generally experienced by women as the rationale for a higher occurrence in women (Hebert et al., 2001; Snyder et al., 2016). Even still, current research suggests that there are significant sex differences in advanced aging populations. For instance, in one study men diagnosed with MCI were more likely to have diabetes, history of a stroke, and a higher body mass index, while women reported increased instances of sleep disturbance, social isolation, comorbid disabilities, and rated their health poorly. As such, men and women displayed differing risk profiles (Artero et al., 2008) Another study focused on the projected risk of AD development in men and women once they reached the age of 85 (Andersen et al., 1999), using a relative and cumulative risk model. Findings indicated that 81.7% of women and 24% of men, 90 years of age, were diagnosed with AD, while sex differences were not found with regard to vascular dementia. Further, 65-year-old women had a cumulative risk for developing dementia that was twice that of men studied (Andersen et al., 1999).
Sex and gender are both important considerations when exploring differences in chronic disease development. Sex differences are biological variances between men and women, including chromosomal differences (XX versus XY chromosomes), gonadal differences, and hormonal differences. This article explores sex differences solely, though gender differences that develop through cultural customs, rearing, roles, and access to educational and employment opportunities may also influence the etiology and progression of chronic diseases (Mielke, Vemuri, & Rocca, 2014). Furthermore, biological factors influence other aspects of development over the life course, such as neurological development and degeneration. Accordingly, neurological differences between women and men must be fully explored to understand these differences, though a complete explanatory theoretical framework for sex differences in dementia development remains to be seen. Sex-determining genes along with fetal hormones contribute to sex-based differences in brain development, composition, and function (Mazure & Swendsen, 2016). These differences inform variances in the development, onset, and the prevalence of neurological diseases (Mazure & Swendsen, 2016).
Levels of sex steroids naturally change and decrease with age (Bahl et al., 2009). For instance, decreased testosterone and altered dehydroepiandrosterone sulphate (DHEAS) levels were found to be correlated in men patients with AD (Bahl et al., 2009), suggesting that higher DHEAS levels may be linked to better cognition (Hildreth et al., 2013). Other researchers, focusing on women’s twofold risk of developing a number of affective disorders, as well as dementia (but not Parkinson’s disease), identify estradiol fluctuations as a potential culprit (Epperson, Kim, & Bale, 2014). These hormonal changes are thought to lead to deteriorating cognition in women with declining estrogen levels, though introducing transdermal estradiol or unopposed conjugated estrogen have not demonstrated a statistically significant ability to improve memory scores nor stop or slow cognitive decline (Henderson, 2008). Research in this area is ongoing.
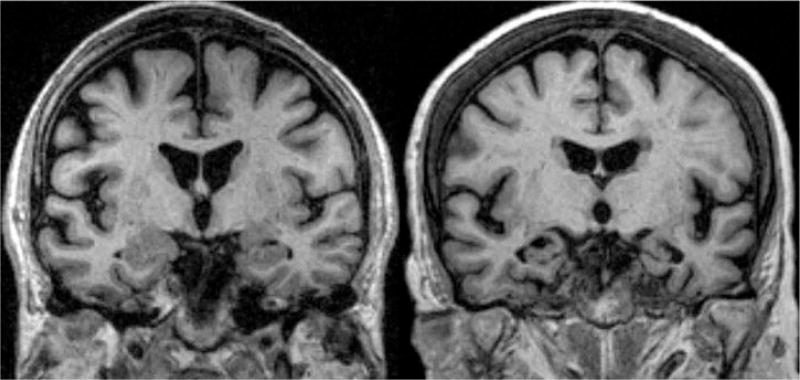
Loss of hippocampal volume is often correlated with neurodegeneration and memory loss, ultimately resulting in dementia (see Figure 1 for a visualization). The hippocampus is a brain structure in the medial temporal lobe that plays an important role in memory binding, encoding, and retrieval (Eichenbaum, 2017). Hippocampal atrophy has also been found in individuals who have been diagnosed with posttraumatic stress disorder (PTSD), with volume differences up to 10% reported between those with and without PTSD (Gilbertson et al., 2002). Glucocorticoids, which are adrenal steroids that are released during times of stress, have also been implicated in hippocampal atrophy in rodent models (Sapolsky, 2000). In older adults, neurofibrillary-associated degeneration starts in the hippocampus and entorhinal cortex and proceeds throughout the brain (Braak & Braak, 1991; Serrano-Pozo, Frosch, Masliah, & Hyman, 2011). Atrophy of the hippocampus early in the disease manifestation makes this brain structure an important biomarker of neurodegeneration. Brickman et al. (2015) found that smaller hippocampal volume at baseline and decreases in hippocampal volume over time each predicted progression to AD, though the results were not differentiated by biological sex. A myriad of investigations target the role of this brain structure in subcortical ischemic vascular disease leading to dementia (Fein et al., 2000), Parkinson’s disease (Bouchard et al., 2008), dementia with Lewy Bodies (Barber, Ballard, McKeith, Gholkar, & O’Brien, 2000), and other dementia and age-related diseases. Studies have confirmed that the hippocampus displays volume loss, which correlates with cognitive status, such that individuals with AD experienced the highest annual rates of atrophy, followed by individuals with MCI transiting to AD, individuals with MCI but not declining, individuals with normal cognition but declining to MCI, and healthy controls (Jack et al., 2000). A study examining patients with amnestic MCI (aMCI) (Bai et al., 2009), found that hippocampal volumes were larger in men. Hippocampal volumes were significantly reduced among those with aMCI, but the sex differences were not related to degree of atrophy. Even though there was equivalent atrophy among men and women with aMCI, women demonstrated an increase in impaired cognitive function as compared to men, suggesting that women were more vulnerable to impairment at an earlier stage in the disease spectrum (Bai et al., 2009).
Figure 1.
Coronal T1-weighted MRI scans of control (left) and patient with AD (right). The patient with AD shows atrophy of the hippocampus. Both subjects are 75 years old. The patient with AD shows clear atrophy of the hippocampus. Modified from Dialogues in Clinical Neuroscience with permission of the publisher (Les Laboratories Servier©, Suresnes, France). Original source: Scheltens, P. (2009). Imaging in Alzheimer’s disease. Dialogues in Clinical Neuroscience, 11, 191–199.
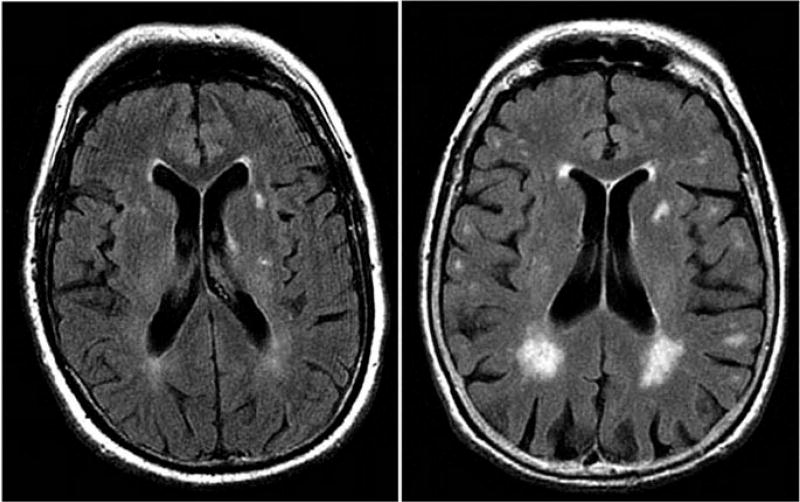
White matter hyperintensities (WMH) are the consequences of cerebral disease impacting small vessels (Prins & Scheltens, 2015). These changes in brain white matter are studied via neuroimaging and neuropathological testing and are indicative of dementia development in patients who have cerebrovascular diseases (Burton, McKeith, Burn, Williams, & O’Brien, 2004). These brain lesions, observed during magnetic resonance imaging (MRI), are found to be more severe and in larger quantity in older subjects and can be associated with poor medication response in patients (Taylor et al., 2008; see Figure 2 for an example). Studies have indicated that there is an association between WMH and executive dysfunction (Burton et al., 2004), as WMH has also been shown to be related to the speed of cognitive processing and executive performance in patients with certain types of dementia (subcortical ischemic vascular dementia and heterogeneous vascular dementia respectively) (Prins & Scheltens, 2015). Previous studies have found equivalent coherence of WMH in the brains of men and women and found the effects of WMH coherence to be equally distributed between the sexes with respect to measures of gait and balance (Sullivan et al., 2001). Other researchers have found that women accumulated twice the amount of deep WMH than men, though the progression of periventricular WMH was equal between the sexes in a 3-year follow-up period (Van Den Heuvel, Admiraal-Behloul, Ten Dam, Olofsen, & Bollen, 2004).
Figure 2.
White matter hyperintensities on magnetic resonance imaging (axial fluid attenuated inversion recovery sequence) in two 80-year-old patients: (left) minor white matter hyperintensities; (right) extensive white matter hyperintensities predominating in periventricular region. White matter lesions are considered present if hyperintense on T2 weighted, fluid attenuated inversion recovery, and proton density images, without prominent hypointensity on T1 weighted images. Reprinted with permission from: Debette and Markus (2010).
Although information regarding the impact of hippocampal volume, WMH, and dementia onset and development is available in the literature, sparse information exists regarding sex differences in this process. This study seeks to examine sex differences in the development of and progression to MCI and AD using hippocampal volume and WMH as predictors.
Methods
A secondary data analysis was conducted of data obtained from September 2005 to March 2017 from the National Alzheimer’s Coordinating Center (NACC) Uniform Data Set (UDS). Specifically, a subset of participants with MRI information, specifically white matter hyperintensities and hippocampal volume, was utilized for the current study. UDS data was based on 35 past and present Alzheimer’s Disease Centers (ADCs) and includes data collected at a baseline visit and subsequent annual evaluations. At each visit, clinicians obtain information regarding a variety of demographic and social history data, as well as medical history and use of medications by the participant. At the discretion of the individual ADCs, laboratory and imaging tests are conducted with participants to aid in diagnostic determinations and provide information about a medical illness when completing UDS forms. Some participants also agree to donate their brain tissue for analysis postmortem, and data obtained from autopsied brain samples are included in the data set. In accordance with the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease (McKhann et al., 2011), a diagnosis regarding cognitive status is determined by either a single clinician or by a group of two or more clinicians, neuropsychologists, or the examining physician (Beekly et al., 2004).
Researchers utilized version 2 of the Uniform Data Set from 2005 to 2015, and version 3 of the UDS, which corresponds to the McKhann et al. (2011) guidelines, from 2015–2017. Using the version 3 forms, neuropsychologists assess participants using the following tests: Montreal Cognitive Assessment (Nasreddine et al., 2005), Craft Story 21 (Craft et al., 1996), the Benson Complex Figure Copy, Number Span Test Forward and Backward, category fluency, Trail Making Test, Multilingual Naming Test (Ivanova, Salmon, & Gollan, 2013), and verbal fluency (F-words and L-words). The CDR (Morris, 1993) is a scale that assesses global staging in the development and severity of dementia, with a sensitivity of 95%, and specificity is 94% in distinguishing participants with and without dementia (Juva et al., 1995). The Clinical Dementia Rating (CDR) has been shown to be able to distinguish between participants with AD and those with normal cognition when comparing English and Spanish speakers. Even in the presence of education, age, and cultural differences, the CDR performed as intended in similar cohorts (Sano et al., 1997). The clinician’s diagnosis (either a single clinician or a consensus) is based upon the participant’s cognitive and behavioral status, biomarkers, imaging, genetics, and etiological diagnoses. Normal cognition is defined as a CDR (Morris, 1993) score of zero and results from neuropsychological testing in the normal range. All-cause dementia requires all of the following criteria to be met: cognitive or behavioral symptoms that interfere with the ability to function at work or with usual activities; a decline from previous levels of functioning that is not due to, or better explained by, cognitive impairment or a major psychiatric disorder; and the detection of cognitive impairment through history taking and objective cognitive assessment. In addition, the participant has to be impaired in one of the following domains: the ability to acquire and remember new information, impaired reasoning and handling of complex tasks and/or poor judgment, impaired visuospatial abilities, impaired language functions, or changes in personality or behavior. If the participant does not have dementia nor normal cognition, MCI is considered based on a change in cognition from a previous level, impairment in one or more cognitive domains (these include memory, language, executive function, attention, and visuospatial skills), and the preservation of independence in functional abilities.
Radiologists and other imaging specialists at 13 distinct ADCs voluntarily provide data from MRI scans, which NACC stores in a repository. MRIs at NACC are best characterized as a convenience sample of images. Imaging data collection and acquisition protocols vary by ADC. As a result, the MRI files analyzed herein may include T1-weighted, fluid attenuation inversion recovery (FLAIR), diffusion tensor imaging (DTI), T2-weighted, or other magnetic resonance series (and any combination thereof). Hippocampal (range: 1.00000–12.00000 cm3) and WMH (range: 0.00000–300.00000 cm3) volume was calculated by the IDEA lab at UC Davis following Alzheimer’s Disease Neuroimaging Initiative (ADNI) protocols. The specific protocol for WMH quantification has been described in detail previously (DeCarli, Maillard, & Fletcher, 2013). Hippocampal volumetric quantification was conducted using a semiautomated approach utilizing a commercially available high-dimensional brain mapping tool (Medtronic Surgical Navigation Technologies, Louisville, CO), which has been validated through the manual tracing of the hippocampus (Hsu et al., 2002). This method of hippocampal volumetric quantification demonstrates an intraclass coefficient of at least .94, which is comparable to manual tracing, which has a coefficient of .99 (Hsu et al., 2002).
Participants eligible for this study were those with complete hippocampal volume and WMH data (n = 1174). We further selected those who have had MCI diagnoses at a visit (treating that visit as the baseline) for the analysis of progression to probable AD and those who are cognitively normal at the baseline for the analysis of progression to MCI. After excluding participants with missing race/ethnicity, CDR global score, and/or neuropsychiatric symptom data, the number of participants included in the analytic sample was 483 for the analysis of progression to MCI and 211 for the analysis of progression to probable AD.
Univariate analysis was conducted to determine the frequencies and distributions of all possible demographic and cognitive variables, including age, education level (0–12 years, 12–16 years, and beyond 17 years), race and ethnicity (White non-Hispanic, Black non-Hispanic, Hispanic, and other race non-Hispanic group), APOE ε4 allele type (no ε4 allele, 1 ε 4 allele, two ε 4 alleles), CDR global score, all neuropsychiatric symptoms, comorbidities (diabetes, hypertension, and hypercholesterolemia), hippocampal volume, WMH, number of participants, and number of follow-up days between men and women. The measures of WMH and hippocampal volume were standardized by dividing by the total brain volume then multiplying by 100 in all analyses (O’Brien et al., 2011).
Survival analysis (Kleinbaum & Klein, 2012) was utilized to investigate whether women and men had different rates of progression to MCI or probable AD with an increase in hippocampal volumes or WMH. A failure event was defined as a participant’s progression to MCI or probable AD. These two diagnoses were treated as separate endpoints in separate analyses. Right censoring was used to account for participants whose diagnoses at the last visit had not progressed to probable AD (or MCI), though progression may occur in future visits but are unaccounted for at present. Time zero was equal to the participant’s baseline visit, and time was measured in days since the first visit. Outcomes are displayed as hazard ratios. Survival analyses were conducted using the Cox proportional hazards model (Cox, 1972). The variable of interest was WMH or hippocampal volume in separate analyses. The hypotheses were that women would experience a greater hazard of progression to probable AD (or MCI) compared to men given the same level of increase in the WMH or hippocampal volume, while adjusting for other covariates. The Cox proportional hazard method allows observations to be made based on the risk of any given event—in this case, the rate of progression to probable AD (or MCI) from baseline levels of covariates. Effects of each risk factor on progression to probable AD (or MCI) was followed for a 10-year period of time to draw conclusions on individual effects and additive effects. Previous studies have used this method in the determination of the effects of sex or APOE-related risk of the development of AD (Altmann, Tian, Henderson, & Greicius, 2014; Burke, Maramaldi, Cadet, & Kukull, 2016a, 2016b). We adjusted for variables that were significant in the log rank test for the progression to MCI or probable AD. For probable AD, the covariates included White non-Hispanic race and CDR global score. For MCI, the covariates were age, CDR global score, and neuropsychiatric symptoms (disinhibition, irritability, night-time behavior, and appetite).
Additionally, we used the generalized linear mixed model (GLMM; Breslow & Clayton, 1993) to examine whether the odds of probable AD (or MCI) changed differently over time (per 30 days since baseline) between women and men with different levels of hippocampal volumes or WMH. We performed the GLMM for women and men separately with hippocampal volumes or WMH as a covariate in the model. We also included other covariates used in the Cox model. Results are displayed as odds ratios. The statistical programs STATA (StataCorp, 2015) and SAS 9.4 (SAS Institute, Cary NC) were utilized for the analyses, and a P value < .05 was considered statistically significant.
Results
Baseline characteristics are summarized separately for women and men for the progression to probable AD (Table 1; Columns 1 to 3) and MCI (Table 1; Columns 4–6). There were 106 women and 105 men for the analysis of progression to probable AD. Among those participants, 26 women and 23 men progressed to probable AD by the end of the observation period. The average follow-up days were not statistically significantly different between women and men (1108.76 vs. 1021.10, P = .39). However, women were less likely to have a postgraduate degree (13.21% vs. 32.38%, P = .00), be White (55.66% vs. 73.33%, P = .01), and have diabetes (16.98% vs. 33.77%, P = .02) compared to men at the baseline. Women also had greater percentage of hippocampal volume relative to the total brain volume (0.60% vs. 0.57%, P = .00) compared to men.
Table 1.
Baseline characteristics of the participants overall and by sex.
| Progression to probable AD | Progression to MCI | |||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Variables | Women | Men |
P value of t or X2 |
Women | Men |
P value of t test or X2 |
| Age | 77.75 | 76.76 | .39 | 70.44 | 72.98 | .02 |
| Std deviation of age | [9.44] | [7.12] | [11.77] | [10.31] | ||
| Education: 0–12 yrs | 38.68% | 29.52% | .16 | 23.73% | 25.15% | .73 |
| Education: 12–16 yrs | 48.11% | 38.10% | .14 | 47.15% | 35.33% | .01 |
| Education: ≥17 yrs | 13.21% | 32.38% | .00 | 29.11% | 39.52% | .02 |
| Race: White | 55.66% | 73.33% | .01 | 69.94% | 76.05% | .16 |
| Race: Black/African American | 22.64% | 14.29% | .12 | 16.77% | 8.98% | .02 |
| Hispanic | 17.92% | 10.48% | .12 | 11.71% | 11.98% | .93 |
| Other racial group | 3.77% | 1.90% | .41 | 1.58% | 2.99% | .30 |
| No APOE ε4 | 59.43% | 56.19% | .63 | 66.46% | 61.68% | .30 |
| 1 APOE ε4 allele | 33.96% | 35.24% | .85 | 29.43% | 35.93% | .14 |
| 2 APOE ε4 alleles | 6.60% | 8.57% | .59 | 4.11% | 2.40% | .33 |
| CDRGLOB = 0 | 13.21% | 14.29% | .82 | 87.97% | 77.25% | .00 |
| CDRGLOB = 0.5 | 82.08% | 80.95% | .83 | 12.03% | 22.75% | .00 |
| CDRGLOB = 1 | 4.72% | 4.76% | .99 | 0.00% | 0.00% | NA |
| Delusion | 3.13% | 5.88% | .35 | 0.00% | 1.20% | .05 |
| Hallucination | 1.04% | 0.98% | .97 | 0.32% | 0.00% | .47 |
| Agitation | 14.58% | 17.65% | .56 | 6.01% | 8.98% | .23 |
| Depression | 34.38% | 29.41% | .45 | 14.56% | 20.96% | .07 |
| Anxiety | 16.67% | 20.59% | .48 | 9.49% | 12.57% | .30 |
| Elation | 2.08% | 3.92% | .45 | 0.32% | 0.60% | .65 |
| Apathy | 16.67% | 17.65% | .86 | 3.48% | 7.19% | .07 |
| Disinhibition | 8.33% | 11.76% | .42 | 4.43% | 4.19% | .90 |
| Irritability | 26.04% | 35.29% | .16 | 9.49% | 19.16% | .00 |
| Motor disturbance | 2.08% | 3.92% | .45 | 1.58% | 0.60% | .35 |
| Nighttime behavior | 15.63% | 22.55% | .22 | 10.76% | 15.57% | .13 |
| Appetite disturbance | 11.46% | 10.78% | .88 | 5.38% | 5.99% | .78 |
| Hypertension | 74.53% | 73.33% | .84 | 52.85% | 56.29% | .47 |
| Hypercholesterolemia | 66.04% | 67.62% | .81 | 53.80% | 59.28% | .25 |
| Diabetes | 16.98% | 30.77% | .02 | 19.94% | 21.56% | .68 |
| Hippocampal volume | 0.60% | 0.57% | .00 | 0.64% | 0.61% | .00 |
| Std deviation | [0.00] | [0.00] | [0.00] | [0.00] | ||
| White matter hyperintensities | 1.10% | 1.00% | .64 | 0.66% | 0.58% | .44 |
| Std deviation | [0.01] | [0.02] | [0.01] | [0.01] | ||
| Number of participants | 106 | 105 | 316 | 167 | ||
| Number of follow-up days | 1108.76 | 1021.10 | .39 | 1268.46 | 1170.3 | .22 |
| Std deviation | [786.45] | [702.00] | [885.21] | [780.99] | ||
There were 316 women and 167 men in the analysis of progression to MCI. Among those participants, 21 women and 24 men progressed to MCI at the end of the observation period. The average follow-up days were not statistically significantly different between women and men (1268.46 vs. 1170.30 days, P = .22). However, women were more likely to be younger (70.44 vs. 72.98, P = .02), college educated (47.15% vs. 35.33%, P = .01), Black non-Hispanic (16.77% vs. 8.98%, P = .02), and have a CDR global score of 0 (87.97% vs. 77.25%, P = .00) compared to men at the baseline. The proportion of women with more than 17 years of education (comparable to undergraduate education) was significantly lower than the proportion of men (29.11% vs. 39.52%, P = .02). There was also a lower percentage of women who had a CDR global score of 0.5 (12.03% vs. 22.75%, P = .00), delusions (0.00% vs. 1.20%, P = .05), and irritability (9.49% vs. 20.96%, P = .00) compared to men. Women also had a significantly greater percentage of hippocampal volume relative to total brain volume (0.64% vs. 0.61%, P = .00) at baseline.
Survival analyses were performed to identify main effects of MRI-derived indicators. Only covariates that were significant in the log-rank test were included to predict the hazard ratio of progression to probable AD (or MCI). Results are summarized in Table 2. Findings related to progression to probable AD are in columns 1 through 4, and progression to MCI are in columns 5 through 8. Among women, a 1% increase in hippocampal volume ratio (relative to total brain volume), resulted in a 46.5% reduction in the rate of progressing to AD. We did not find significant impact of hippocampal volume on the progression to AD among men. White men had an increased rate of progression to AD (HR = 4.396, 95% CI [1.012–19.08]).
Table 2.
Cox regression analyses examining the hazard of progression to Alzheimer’s disease or mild cognitive impairment given hippocampal volume or white matter hyperintensities.
| Progression to AD | Progression to MCI | |||||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| Hippocampal volume | WMH | Hippocampal volume | WMH | |||||
|
|
|
|
|
|||||
| Women | Men | Women | Men | Women | Men | Women | Men | |
| Hippocampal volume | 0.535** (0.300–0.953) | 0.548 (0.297–1.011) | 0.386** (0.166–0.901) | 0.975 (0.389–2.445) | ||||
| White matter hyperintensities | 0.991 (0.956–1.028) | 1.018 (0.996–1.040) | 1.010 (0.984–1.037) | 1.048** (1.011–1.086) | ||||
| White | 1.085 (0.467–2.521) | 4.396** (1.012–19.08) | 1.454 (0.656–3.223) | 4.665** (1.072–20.29) | ||||
| Age | 1.092*** (1.035–1.151) | 1.101*** (1.037–1.169) | 1.084*** (1.028–1.143) | 1.075** (1.008–1.146) | ||||
| CDRGLOB = 0.5 | 3.954 (0.526–29.71) | 0.679 (0.222–2.076) | 4.552 (0.613–33.81) | 0.807 (0.250–2.609) | 1.808 (0.605–5.406) | 1.165 (0.392–3.459) | 1.533 (0.525–4.478) | 1.110 (0.377–3.266) |
| CDRGLOB = 1 | 5.260 (0.465–59.52) | 7.562 (0.639–89.48) | ||||||
| Disinhibition | 3.014 (0.527–17.25) | 1.796 (0.360–8.967) | 1.979 (0.352–11.12) | 1.987 (0.394–10.03) | ||||
| Irritability | 1.486 (0.314–7.028) | 2.865 (0.985–8.331) | 1.570 (0.324–7.610) | 2.779 (0.934–8.266) | ||||
| Nighttime behavior | 1.832 (0.587–5.719) | 1.044 (0.263–4.135) | 2.048 (0.656–6.396) | 1.154 (0.312–4.263) | ||||
| Appetite disturbance | 1.127 (0.236–5.369) | 1.565 (0.280–8.745) | 1.425 (0.290–6.986) | 1.341 (0.233–7.721) | ||||
| Observations | 106 | 105 | 106 | 105 | 316 | 167 | 316 | 167 |
95% CI in parentheses.
P < .01,
P < .05.
We did not find a significant impact of WMH ratio (relative to the total brain volume) on progression to probable AD among either men or women (column 3–4 of Table 2). However, we again found that White men had an increased rate of progression to AD (HR = 4.665, 95% CI [1.072–20.29]) compared to men in other racial groups.
For the progression to MCI, among women, a 1% increase in hippocampal volume ratio (relative to total brain volume), decreased the rate of progressing to MCI by 61.4% (Column 5 of Table 2). We did not find significant impact of hippocampal volume on the progression to MCI among men (Column 6 of Table 2). Women (HR = 1.092, 95% CI [1.035–1.151]) and men (HR = 1.101, 95% CI [1.037–1.169]) who were older at the baseline visit were also more likely to progress to MCI.
For the progression to MCI, among women, we did not find the impact of WMH to be significant (Column 7 of Table 2). However, among men, the rate of progressing to MCI increased by 4.8% (Column 8 of Table 2) with every 1% increase in WMH ratio (relative to total brain volume). Women (HR = 1.084, 95% CI [1.028–1.143]) and men (HR = 1.075, 95% CI [1.008– 1.146]) who were older at the baseline visit were also more likely to progress to MCI.
Results from GLMM analyses (Table 3) showed that the odds of probable AD and MCI development increased over time for both women and men; however, the progression was slightly slower for women (see Row 3 in Table 3). Higher hippocampal volume (or less hippocampal atrophy) also significantly decreased the odds of AD development for women (OR = 0.403, 95% CI [0.231–0.701]) more than men (OR = 0.468, 95% CI [0.263–0.832]). Higher hippocampal volume also significantly decreased the odds of MCI development for women (OR < 0.01, 95% CI [0–0.026]), but not significantly for men. White men showed higher odds of probable AD development than men from other race and ethnicity groups when adjusting by hippocampal volume (OR = 5.297, 95% CI [1.485–18.893]) or WMH (OR = 5.278, 95% CI [1.462–19.054]). Women with a higher CDR global score, older age at baseline, and/or higher levels of disinhibition symptoms showed significantly higher odds of MCI development, but there was no significant difference for men.
Table 3.
Generalized linear mixed model analyses examining the odds of Alzheimer’s disease or mild cognitive impairment given hippocampal volume or white matter hyperintensities.
| Probable AD | MCI | |||||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| Hippocampal volume | WMH | Hippocampal volume | WMH | |||||
|
|
|
|
|
|||||
| Women | Men | Women | Men | Women | Men | Women | Men | |
| Hippocampal volume | 0.403 (0.231–0.701)*** | 0.468 (0.263–0.832)*** | <0.01*** (0–0.026) | 0.22 (0–3081.895) | ||||
| White matter hyperintensities | 0.984 (0.943–1.028) | 1.009 (0.981 –1.039) | 0.931 (0.678–1.278) | 1.541 (0.992–2.395) | ||||
| Time (per 30 days) | 1.027*** (1.013–1.041) | 1.032*** (1.017–1.047) | 1.024*** (1.011 –1.037) | 1.033*** (1.018–1.048) | 1.027*** (1.014–1.04) | 1.045*** (1.03–1.059) | 1.025*** (1.012–1.038) | 1.046*** (1.031 –1.061) |
| White | 1.166 (0.503–2.707) | 5.297** (1.485–18.893) | 1.417 (0.596–3.368) | 5.278*** (1.462–19.054) | ||||
| CDRGLOB = 0.5 | 4.475 (0.824–24.291) | 1.028 (0.277–3.813) | 5.294 (0.903–31.05) | 0.841 (0.233–3.03) | 4.442*** (1.62–12.174) | 1.729 (0.464–6.447) | 3.581** (1.306–9.821) | 1.741 (0.465–6.519) |
| CDRGLOB = 1 | 5.306 (0.528–53.341) | 10.634 (0.905–124.986) | ||||||
| Age | 1.096*** (1.042–1.152) | 1.051 (0.986–1.121) | 1.086*** (1.032–1.142) | 1.029 (0.962–1.1) | ||||
| Disinhibition | 6.01** (1.052–34.322) | 1.423 (0.14–14.41) | 3.126 (0.555–17.593) | 1.563 (0.157–15.588) | ||||
| Irritability | 1.408 (0.346–5.733) | 3.117 (0.817–11.894) | 1.566 (0.381 –6.43) | 3.015 (0.779–11.66) | ||||
| Nighttime behaviour | 1.708 (0.507–5.757) | 0.53 (0.104–2.689) | 1.929 (0.556–6.693) | 0.586 (0.122–2.804) | ||||
| Appetite | 0.425 (0.073–2.463) | 0.939 (0.107–8.236) | 0.655 (0.116–3.691) | 0.664 (0.068–6.446) | ||||
95% CI in parentheses.
P < .01,
P < .05.
Discussion
Our results indicate that there are sex differences in the development of probable AD and MCI as predicted by MRI indicators. These findings add to the mixed literature on sex differences in AD development. While previous researchers have found that women may be more susceptible to AD development, and that women are overrepresented among AD patients, these results indicate that women experienced a slightly slower odds of progressing to probable AD (or MCI) given higher levels of hippocampal volume. We did not observe similar impact of hippocampal on progression to AD or MCI among men. Women also experienced a lower hazard of progressing to MCI given a higher level of hippocampal volume compared to men. These results are in line with that from previous studies in terms of the role of hippocampal volume as a predictive tool, despite the fact that previous studies may not have analyzed differences by sex. The results were similar between the two statistical techniques utilized. The change in the significance for some variables is probably due to the different outcomes used in the different models. The survival analyses were performed using the Cox proportional hazard model and the sample was constructed using (a) the progression time from normal cognition to the diagnoses of MCI or censored, or (b) the progression time from the diagnoses of MCI to the diagnoses of probable AD or censored as the outcome. The longitudinal analyses were performed using GLMM, which analyzed the trajectory change of the odds of probable AD or MCI. The outcomes were the status of probable AD or MCI (yes/no) at each visit.
Participants with older age at baseline were more likely to progress to MCI, which is consistent with current knowledge regarding neuropathological processes of AD, in that advancing age is one of the greatest risk factors (Hebert, Weuve, Scherr, & Evans, 2013). Despite the fact that AD is not a normative part of brain aging, age is a risk factor nonetheless, as prevalence estimates indicate that 3% of 65–74-year-olds, 17% of 75–84-year-olds, and 32% of individuals over the age of 85 are diagnosed with AD dementia (Hebert et al., 2013).
One of the main findings of the current study is that changes in hippocampal volumes affected the progression to or odds of probable AD and MCI further among women rather than men. Increased hippocampal volume (or less hippocampal atrophy) decreased the odds of AD development for women (OR = 0.403, 95% CI [0.231–0.701]) compared to men (OR = 0.468, 95% CI [0.263–0.832]), and it also decreased the odds of MCI development for women (OR < 0.01, 95% CI [0–0.026]), but not significantly for men. Previous findings identify women age 65 or older to have a 38% higher risk of AD development than men of the same age cohort (Hurd, Martorell, Delavande, Mullen, & Langa, 2013), though our results indicate that, while the odds of probable AD and MCI development increased over time for both women and men, this process was slightly slower for women. This is in line with previous research in which hippocampal volumes predicted differences in “novel associative memory” for women, though the same was not true for men (Zheng et al., 2017). In addition, lower standardized hippocampal volume (< 0.005) has been associated with poorer performance on verbal memory assessments in women under the age of 70 (but not men) compared to those with greater hippocampal volumes, confirming the importance of hippocampal volume in predicting functional memory in women (Ystad et al., 2009).
We also found that men experienced an increased hazard for progressing to MCI from normal cognition given increases in the WMH; however, we did not observe a similar impact of WMH on progression to MCI among women. We did not find a significant impact of WMH ratio (relative to the total brain volume) on progression to probable AD among either men or women. Brickman et al. (2015) found that higher levels of parietal lobe WMH at baseline, and a WMH volume increase in the parietal lobe, each predicted progression to AD. Interestingly, neither WMH in other brain regions nor cortical thickness predicted progression to AD. The results presented by Brickman et al. (2015) were not differentiated by biological sex. The current study did not examine regional accumulations of WMH, so we are unable to determine the regional effects of WMH, which may account for differences in our results versus those of Brickman et al.. In other studies that examined sex differences in WMH, researchers hypothesized that sex differences in vascular risk factors and outcomes, wherein men experience a higher incidence of cardiovascular disease and hypertension, explain differences in WMH by sex (Albert et al., 2010). Other studies demonstrate results indicating that WMH were more common and severe in their sample of women, but there were not any consequent differences in cognitive functioning by sex (Sachdev, Parslow, Wen, Anstey, & Easteal, 2009). One recent study found there to be differences related to WMH between sexes. In the Clinical Research Center for Dementia of South Korea study, sex-specific risk factors were explored in relation to future AD development in patients with mild cognitive impairment. Kim et al. (2015) reported that approximately 25% of patients developed incident dementia, while 90% eventually developed AD. Men experienced significant risk factors that included severe periventricular WMH and poorer global cognitive function, while risk in women was associated with APOE e4 alleles and depressive symptoms at baseline (Kim et al., 2015). In both the Washington Heights-Inwood Columbia Aging Project and in the Etude Santé Psychologique Prévalence Risques et Traitement study, APOE ε4 carriers displayed higher WMH volume, and WMH was associated with an increased risk of dementia (Brickman et al., 2014).
Unexpectedly, APOE ε4 did not differentially play a role in increasing or hastening the progression to MCI or AD among men and women, though previous studies have also found that APOE was not correlated with hippocampal volume in relation to the progression to AD (Ystad et al., 2009). Previous literature has noted that the link between APOE and Alzheimer’s disease is purportedly stronger in women than in men (HR = 1.81 for women; HR = 1.27 for men) (Altmann et al., 2014), though other studies have not supported the role of APOE ε4 in sex differences in AD development (Yip, Brayne, Easton, & Rubinsztein, 2002). An examination of APOE ε4 in an ADNI cohort of 339 participants showed that ε4/ε4 carriers experienced WMH accumulation at a rate of 22.5% a year compared to a 10% per year accumulation for ε3/ε4 carriers (Sudre et al., 2017).
Some studies have suggested that estrogen therapy may lead to less risk of cognitive decline, while others have not supported the protective effect of estradiol but have noted an improvement in global cognition among estrogen replacement users post menopause (Imtiaz, Tolppanen, Solomon, Soininen, & Kivipelto, 2017). In one study, the use of estrogen replacement therapy in women without APOE ε4 resulted in less cognitive decline than women ε4 carriers (Yaffe, Haan, Byers, Tangen, & Kuller, 2000). Given that APOE is a lipoprotein, researchers also found that women ε4 carriers had increased carotid wall thickness than women without ε4, and while oral estrogen use was significantly associated with less carotid wall thickness in women without ε4, the same was not true for women ε4 carriers. Given that carotid atherosclerosis and APOE are associated with a greater risk of AD, these findings support a mechanism by which APOE and estrogen interact to produce a higher risk in women ε4 carriers (Yaffe et al., 2000). In contrast to the results from Yaffe et al. (2000), Kantarci et al. (2016) found that transdermal 17β-estradiol therapy in women, ages 52–65 years of age, who were 5 to 36 months past menopause, had lower Pittsburgh compound B (PiB) standard unit value ratio values than comparable controls. This suggests a reduction in the deposition of amyloid-β among women who used transdermal 17β-estradiol therapy within 3 years of the beginning of menopause. While not considered in the current study, estrogen may have an explanatory role in the sex differences found in the progression to and development of MCI and AD, though the literature is mixed and not conclusive.
Consideration of the interactions between environmental and biological factors stand to move the field forward and provide a more contextualized and complete understanding of the ways in which biological sex differences and gendered life experiences interact to form risk and protective factors relevant for healthy and pathological aging. Research has established that adversity in childhood is related to a plethora of negative health outcomes, including dementia (Radford et al., 2017), and that sex differences may exist in relation to one’s vulnerability to the negative impact of adversity in late life (Choi, DiNitto, Marti, & Choi, 2017). Yet it remains unclear how these psychosocial factors operate jointly with biological factors to influence the progression of AD.
Recent research suggests that environmental factors and life stressors across the life span, such as adversity and psychological reactions to events, can negatively affect one’s physical health, biological characteristics, especially hormones, and neurobiology (Anda et al., 2006). The landmark Adverse Childhood Experiences (ACE) Study was among the first to find this striking correlation between negative life events in childhood (e.g., abuse, neglect, parent with addiction, poverty) and a person’s physical and mental health later in adulthood (Anda et al., 1999, 2006; Felitti et al., 1998). Researchers found a graded relation between adversity and health outcomes; the more ACEs people experienced, the more likely they were to have serious disease in adulthood, including ischemic heart disease, cancer, chronic lung disease, and liver disease. In addition, individuals experiencing four or more ACEs had an increased risk of alcoholism, drug addiction, depression, and attempted suicide compared to individuals reporting no ACEs (Felitti et al., 1998), and individuals experiencing six or more ACEs were more likely to die about 20 years earlier than those reporting fewer ACEs (Brown et al., 2009; Felitti et al., 1998). Some recent research has highlighted specific association between ACEs and outcomes later in life. For example, one’s socioeconomic status as a child was found to be correlated to the burden of WMH in late life (Murray, McNeil, Salarirad, Whalley, & Staff, 2014). Although ACEs are common in the general population, there is some research to suggest sex differences in the prevalence of certain ACEs. However, evidence is equivocal with respect to sex differences related to the manifestation of ACEs (Fang, Chuang, & Lee, 2016).
Research has also evidenced that life stressors later in life can influence one’s health, including neuroendocrine functioning (Kudielkaa & Kirschbaumb, 2005; Ong, Fuller-Rowell, Bonanno, & Almeida, 2011). Additionally, these impacts can vary between women and men. For example, Richardson et al. (2015) found that men and women responded differently, as measured by urinary cortisol levels, when their spouse died. That is, while women in the control group (i.e., no bereavement) evidenced increasing levels of cortisol, it was men in the bereaved group who demonstrated increasing levels of cortisol. These researchers also found that the direction of these associations depended on context of the death (e.g., prolonged death, caregiving, sudden death), highlighting the importance of fully understanding psychosocial and biological factors. Furthermore, certain genes, such as APOE, are thought to increase the brain’s vulnerability to life stress, such that carriers may have a stronger response to stress (Park, Nam, Sim, & Hong, 2015). Psychosocial stress and cumulative childhood adversity have also been associated with shorter telomeres, which are protein complexes at the ends of chromosomes (Kananen et al., 2010; Price, Kao, Burgers, Carpenter, & Tyrka, 2013).
Our results bring to light neuropathological differences between men and women, which future researchers may investigate further and identify sex-specific treatment options. These results suggest the necessity of assessing ways to limit neurodegenerative progression and to consider different approaches for men and women. Nelson, Gard, and Tabet (2014) reiterate these implications and urge researchers to continue attempting to understand pathological interactions among AD risk factors, which may lead to better treatment options, since effective therapies, at present, are limited. Increased knowledge regarding sex-specific risk factor interaction effects will allow scientists and physicians to begin directing patients to proper preventive treatment, potentially decreasing the manifestation of AD. There remain additional sex-specific disparities that must be addressed in future research, which may impact the way in which pharmaceutical and other therapies are chosen for individuals affected by AD. For example, Artero et al. (2008) identified a difference in risk factors between men and women progressing from MCI to AD. Their results indicated that progression among women is best predicted by the loss of instrumental activities of daily living and the presence of depression, whereas the APOE ε4 allele and a history of stroke predicted progression among men. Another study found a higher incidence of WMH in women and noted that estrogen use and APOE ε4 did not account for this difference. In a meta-analysis of 15 studies (n = 828 men and 1,238 women with AD), Irvine, Laws, Gale, and Kondel (2012) found that men with AD consistently performed better on verbal and visuospatial tasks and tests of episodic and semantic memory. This phenomenon was not better explained by age, education, or severity of AD. These researchers speculated that this could be explained by “reduction of estrogen in postmenopausal women, sex differences in AD pathology, and greater cognitive reserve in men” (Irvine et al., 2012). Researchers have attempted to illustrate these differences in neuropsychological performance using connectivity studies and diffusion tensor imaging (DTI) (Ingalhalikar et al., 2014). While utilizing DTI, researchers have found that brains in men appear to be structured in such a way as to “facilitate connectivity between perception and coordinated action,” and brains in women were structured to “facilitate communication between analytical and intuitive processing modes” (Ingalhalikar et al., 2014). Future studies replicating these findings can provide information on tailoring preventative and responsive treatment to target sex-specific risk factors.
The strengths of this study include the examination of individuals with similar hippocampal volume and WMH at baseline followed longitudinally over 10 years, whereas many studies only examine data for 5-year follow-up. Our overall sample size is in line with other extant literature, though the NACC sample is not considered to be nationally representative. Additionally, this sample was predominately White non-Hispanic. A more culturally diverse sample may generate interesting results with more generalizable findings. In addition, given that this is a secondary data analysis, with virtually no exposure history except that which can be inferred from the one to 10 years of observation per participant, it is not possible to imply or assume causality. In addition, though causes of hippocampal atrophy and WMH may stem from a variety of endogenous and exogenous sources, a lack of exposure history prohibits an analysis including history prior to the participants’ first observation. Given that we were interested in examining differences in brain structures in men and women as these regions impacted progression from normal cognition to MCI to AD, each participant acted as his/her own control as “factors that vary between individuals remain constant within the same individual” (Datar et al., 2012). This article was limited to the exploration of sex differences through the examination of two brain regions of interest. Future studies should additionally explore the effect of gender differences in addition to biological sex differences. In addition, future studies should account for the use of estradiol therapy in postmenopausal participants given that this may influence cognitive decline and may also interact with APOE.
The current study does not offer or recommend potential treatment for AD—it only addresses the issue that there are sex differences in health outcomes among women versus men. Future research must determine the proper courses of therapeutic treatment to better address these physiological differences. Currently, there is limited treatment available for AD. Therapies effective in delaying the progression of AD, and designed to treat the disease once it has manifested, must be discovered to fight the symptoms and causes of the disease, while also taking into account sex-related differences in disease development.
In conclusion, future neurobiological studies should identify the specific sex differences in neuropathology and the mechanism leading to sex differences in neurodegenerative decline. Conceptual frameworks exist that may inform research design, sample selection, and the theoretical underpinnings of the hypothesized sex differences. Joel and McCarthy (2017) outline four dimensions to structure such an examination of sex differences, including whether the variations are: “(1) persistent vs. transient across the lifespan; (2) context independent vs. dependent; (3) dimorphic vs. continuous; and (4) a direct vs. an indirect consequence of sex.” Data representing decades of exposure, neuropsychological assessments, and life history are necessary to provide a comprehensive understanding of how differences in neurodegeneration may occur by sex and to properly control for all relevant explanatory factors. Other contributing factors should be examined, such as the presence of anxiety and depression among women and how that may affect the disease process and progression to AD. It is known that anxiety and depression are more prevalent among women across the life span (Kessler, 2003). Additionally, psychiatric factors affect cognitive performance and decline. Identifying sex differences among psychiatric factors in MCI and AD patients, both premorbidly and after diagnosis, can help identify how the presence of these symptoms moderates disease progression. Careful consideration of one’s life experiences across the life span, but especially adverse childhood events, should also be considered in future research and included, even retrospectively, in assessments of older adults participating in both cross-sectional and longitudinal studies. The interaction between environmental and biological factors should not be overlooked, as it stands to significantly influence individuals’ health. Other factors such as sex differences in cardiovascular risk factors, quality of life, or caregiver burden should also be investigated in relation to progression from MCI to AD. Understanding sex differences will help aid in developing sex-specific treatments that may be more beneficial for women diagnosed with AD and/or in preventing the onset of AD.
Acknowledgments
Funding
The NACC database is funded by NIA/NIH Grant U01 AG016976. NACC data are contributed by the NIA-funded ADCs: P30 AG019610 (PI Eric Reiman, MD), P30 AG013846 (PI Neil Kowall, MD), P50 AG008702 (PI Scott Small, MD), P50 AG025688 (PI Allan Levey, MD, PhD), P50 AG047266 (PI Todd Golde, MD, PhD), P30 AG010133 (PI Andrew Saykin, PsyD), P50 AG005146 (PI Marilyn Albert, PhD), P50 AG005134 (PI Bradley Hyman, MD, PhD), P50 AG016574 (PI Ronald Petersen, MD, PhD), P50 AG005138 (PI Mary Sano, PhD), P30 AG008051 (PI Steven Ferris, PhD), P30 AG013854 (PI M. Marsel Mesulam, MD), P30 AG008017 (PI Jeffrey Kaye, MD), P30 AG010161 (PI David Bennett, MD), P50 AG047366 (PI Victor Henderson, MD, MS), P30 AG010129 (PI Charles DeCarli, MD), P50 AG016573 (PI Frank LaFerla, PhD), P50 AG016570 (PI Marie-Francoise Chesselet, MD, PhD), P50 AG005131 (PI Douglas Galasko, MD), P50 AG023501 (PI Bruce Miller, MD), P30 AG035982 (PI Russell Swerdlow, MD), P30 AG028383 (PI Linda Van Eldik, PhD), P30 AG010124 (PI John Trojanowski, MD, PhD), P50 AG005133 (PI Oscar Lopez, MD), P50 AG005142 (PI Helena Chui, MD), P30 AG012300 (PI Roger Rosenberg, MD), P50 AG005136 (PI Thomas Montine, MD, PhD), P50 AG033514 (PI Sanjay Asthana, MD, FRCP), P50 AG005681 (PI John Morris, MD), and P50 AG047270 (PI Stephen Strittmatter, MD, PhD).
Footnotes
Color versions of one or more of the figures in the article can be found online at www.tandfonline.com/wjwa.
References
- Albert M, Massaro J, DeCarli C, Beiser A, Seshadri S, Wolf PA, Au R. Profiles by sex of brain MRI and cognitive function in the Framingham Offspring study. Alzheimer Disease and Associated Disorders. 2010;24(2):190–193. doi: 10.1097/WAD.0b013e3181c1ed44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altmann A, Tian L, Henderson VW, Greicius MD. Sex modifies the APOE-related risk of developing Alzheimer’s disease. Annals of Neurology. 2014;75(4):563–573. doi: 10.1002/ana.24135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alzheimer’s Association. 2017 Alzheimer’s disease facts and figures. 2017 http://www.alz.org/documents_custom/2017-facts-and-figures.pdf.
- Anda RF, Croft JB, Felitti VJ, Nordenberg D, Giles WH, Williamson DF, Giovino GA. Adverse childhood experiences and smoking during adolescence and adulthood. JAMA. 1999;282(17):1652–1658. doi: 10.1001/jama.282.17.1652. [DOI] [PubMed] [Google Scholar]
- Anda RF, Felitti VJ, Bremner JD, Walker JD, Whitfield C, Perry BD, Giles WH. The enduring effects of abuse and related adverse experiences in childhood. European Archives of Psychiatry and Clinical Neuroscience. 2006;256(3):174–186. doi: 10.1007/s00406-005-0624-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen K, Launer LJ, Dewey ME, Letenneur L, Ott A, Copeland JRM The EURODEM Research Group. Gender differences in the incidence of AD and vascular dementia: The EURODEM studies. Neurology. 1999;1992;53(9) doi: 10.1212/WNL.53.9.1992. [DOI] [PubMed] [Google Scholar]
- Artero S, Ancelin M-L, Portet F, Dupuy A, Berr C, Dartigues J-F, Ritchie K. Risk profiles for mild cognitive impairment and progression to dementia are gender specific. Journal of Neurology, Neurosurgery & Psychiatry. 2008;79(9):979–984. doi: 10.1136/jnnp.2007.136903. [DOI] [PubMed] [Google Scholar]
- Bahl JM, Bennett P, Simonsen AH, Waldemar G, Hougaard DM, Heegaard NH. Comparative study of sex hormone serum levels and CSF markers in patients with Alzheimer’s disease. Alzheimer’s & Dementia: The Journal of the Alzheimer’s Association. 2009;5(4):P346–P347. [Google Scholar]
- Bai F, Zhang Z, Watson DR, Yu H, Shi Y, Zhu W, Qian Y. Absent gender differences of hippocampal atrophy in amnestic type mild cognitive impairment. Neuroscience Letters. 2009;450(2):85–89. doi: 10.1016/j.neulet.2008.11.055. [DOI] [PubMed] [Google Scholar]
- Barber R, Ballard C, McKeith IG, Gholkar A, O’Brien JT. MRI volumetric study of dementia with Lewy bodies: A comparison with AD and vascular dementia. Neurology. 2000;54(6):1304–1309. doi: 10.1212/wnl.54.6.1304. [DOI] [PubMed] [Google Scholar]
- Beekly DL, Ramos EM, Van Belle G, Deitrich W, Clark AD, Jacka ME, Kukull WA. The National Alzheimer’s Coordinating Center (NACC) database: An Alzheimer disease database. Alzheimer Disease & Associated Disorders. 2004;18(4):270–277. [PubMed] [Google Scholar]
- Bouchard TP, Malykhin N, Martin WRW, Hanstock CC, Emery DJ, Fisher NJ, Camicioli RM. Age and dementia-associated atrophy predominates in the hippocampal head and amygdala in Parkinson’s disease. Neurobiology of Aging. 2008;29(7):1027–1039. doi: 10.1016/j.neurobiolaging.2007.02.002. [DOI] [PubMed] [Google Scholar]
- Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathologica. 1991;82(4):239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- Breslow NE, Clayton DG. Approximate inference in generalized linear mixed models. Journal of the American Statistical Association. 1993;88(421):9–25. [Google Scholar]
- Brickman AM, Schupf N, Manly JJ, Stern Y, Luchsinger JA, Provenzano FA, Portet F. APOE ε4 and risk for Alzheimer’s disease: Do regionally distributed white matter hyperintensities play a role? Alzheimer’s & Dementia. 2014;10(6):619–629. doi: 10.1016/j.jalz.2014.07.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brickman AM, Zahodne LB, Guzman VA, Narkhede A, Meier IB, Griffith EY, Mayeux R. Reconsidering harbingers of dementia: Progression of parietal lobe white matter hyperintensities predicts Alzheimer’s disease incidence. Neurobiology of Aging. 2015;36(1):27–32. doi: 10.1016/j.neurobiolaging.2014.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown DW, Anda RF, Tiemeier H, Felitti VJ, Edwards VJ, Croft JB, Giles WH. Adverse childhood experiences and the risk of premature mortality. American Journal of Preventive Medicine. 2009;37(5):389–396. doi: 10.1016/j.amepre.2009.06.021. [DOI] [PubMed] [Google Scholar]
- Burke SL, Maramaldi P, Cadet T, Kukull W. Associations between depression, sleep disturbance, and apolipoprotein E in the development of Alzheimer’s disease: Dementia. International Psychogeriatrics. 2016a;28(9):1409–1424. doi: 10.1017/S1041610216000405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke SL, Maramaldi P, Cadet T, Kukull W. Neuropsychiatric symptoms and apolipoprotein E: Associations with eventual Alzheimer’s disease development. Archives of Gerontology and Geriatrics. 2016b;65:231–238. doi: 10.1016/j.archger.2016.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton EJ, McKeith IG, Burn DJ, Williams ED, O’Brien JT. Cerebral atrophy in Parkinson’s disease with and without dementia: A comparison with Alzheimer’s disease, dementia with Lewy bodies and controls. Brain. 2004;127(4):791–800. doi: 10.1093/brain/awh088. [DOI] [PubMed] [Google Scholar]
- Chêne G, Beiser A, Au R, Preis SR, Wolf PA, Dufouil C, Seshadri S. Gender and incidence of dementia in the Framingham Heart Study from mid-adult life. Alzheimer’s & Dementia. 2015;11(3):310–320. doi: 10.1016/j.jalz.2013.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi NG, DiNitto DM, Marti CN, Choi BY. Association of adverse childhood experiences with lifetime mental and substance use disorders among men and women aged 50+ years. International Psychogeriatrics. 2017;29(3):359–372. doi: 10.1017/S1041610216001800. [DOI] [PubMed] [Google Scholar]
- Cox DR. Regression models and life-tables. Journal of the Royal Statistical Society, Series B (Methodological) 1972;34(2):187–220. [Google Scholar]
- Craft S, Newcomer J, Kanne S, Dagogo-Jack S, Cryer P, Sheline Y, Alderson A. Memory improvement following induced hyperinsulinemia in Alzheimer’s disease. Neurobiology of Aging. 1996;17(1):123–130. doi: 10.1016/0197-4580(95)02002-0. [DOI] [PubMed] [Google Scholar]
- Datar M, Muralidharan P, Kumar A, Gouttard S, Piven J, Gerig G, Fletcher PT. Mixed-effects shape models for estimating longitudinal changes in anatomy. In: Durrleman S, Fletcher T, Gerig G, Niethammer M, editors. Spatio-temporal image analysis for longitudinal and time-series image data. STIA 2012. Vol. 7570. Berlin/Heidelberg, Germany: Springer; 2012. pp. 76–87. Lecture notes in computer science. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debette S, Markus HS. The clinical importance of white matter hyperintensities on brain magnetic resonance imaging: Systematic review and meta-analysis. BMJ. 2010;341:c3666. doi: 10.1136/bmj.c3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeCarli C, Maillard P, Fletcher E. Alzheimer’s Disease Neuroimaging Initiative. Department of Neurology and Center for Neuroscience, University of California at Davis; 2013. Four tissue segmentation in ADNI II; pp. 1–6. Retrieved from: https://www.alz.washington.edu/WEB/adni_proto.pdf. [Google Scholar]
- Eichenbaum H. Memory: Organization and control. Annual Review of Psychology. 68(1):19–45. doi: 10.1146/annurev-psych-010416-044131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epperson CN, Kim DR, Bale TL. Estradiol modulation of monoamine metabolism: One possible mechanism underlying sex differences in risk for depression and dementia. JAMA Psychiatry. 2014;71(8):869–870. doi: 10.1001/jamapsychiatry.2014.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang L, Chuang D-M, Lee Y. Adverse childhood experiences, gender, and HIV risk behaviors: Results from a population-based sample. Preventive Medicine Reports. 2016;4:113–120. doi: 10.1016/j.pmedr.2016.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fein G, Sclafani VD, Tanabe J, Cardenas V, Weiner MW, Jagust WJ, Chui H. Hippocampal and cortical atrophy predict dementia in subcortical ischemic vascular disease. Neurology. 2000;55(11):1626–1635. doi: 10.1212/wnl.55.11.1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felitti V, Anda R, Nordenberg D, Williamson D, Spitz A, Edwards V, Marks J. Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults: The Adverse Childhood Experiences study. American Journal of Preventive Medicine. 1998;14:245–258. doi: 10.1016/s0749-3797(98)00017-8. [DOI] [PubMed] [Google Scholar]
- Gilbertson MW, Shenton ME, Ciszewski A, Kasai K, Lasko NB, Orr SP, Pitman RK. Smaller hippocampal volume predicts pathologic vulnerability to psychological trauma. Nature Neuroscience. 2002;5(11):1242–1247. doi: 10.1038/nn958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebert LE, Scherr PA, McCann JJ, Beckett LA, Evans DA. Is the risk of developing Alzheimer’s disease greater for women than for men? American Journal of Epidemiology. 2001;153(2):132–136. doi: 10.1093/aje/153.2.132. [DOI] [PubMed] [Google Scholar]
- Hebert LE, Weuve J, Scherr PA, Evans DA. Alzheimer disease in the United States (2010–2050) estimated using the 2010 census. Neurology. 2013;80(19):1778–1783. doi: 10.1212/WNL.0b013e31828726f5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson VW. Cognitive changes after menopause: Influence of estrogen. Clinical Obstetrics and Gynecology. 2008;51(3):618–626. doi: 10.1097/GRF.0b013e318180ba10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hildreth KL, Gozansky WS, Jankowski CM, Grigsby J, Wolfe P, Kohrt WM. Association of serum dehydroepiandrosterone sulfate and cognition in older adults: Sex steroid, inflammatory, and metabolic mechanisms. Neuropsychology. 2013;27(3):356–363. doi: 10.1037/a0032230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu Y-Y, Schuff N, Du A-T, Mark K, Zhu X, Hardin D, Weiner MW. Comparison of automated and manual MRI volumetry of hippocampus in normal aging and dementia. Journal of Magnetic Resonance Imaging. 2002;16(3):305–310. doi: 10.1002/jmri.10163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurd MD, Martorell P, Delavande A, Mullen KJ, Langa KM. Monetary costs of dementia in the United States. New England Journal of Medicine. 2013;368(14):1326–1334. doi: 10.1056/NEJMsa1204629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imtiaz B, Tolppanen AM, Solomon A, Soininen H, Kivipelto M. Estradiol and cognition in the Cardiovascular Risk Factors, Aging and Dementia (CAIDE) cohort study. Journal of Alzheimer’s Disease. 2017;56(2):453–458. doi: 10.3233/JAD-160643. [DOI] [PubMed] [Google Scholar]
- Ingalhalikar M, Smith A, Parker D, Satterthwaite TD, Elliott MA, Ruparel K, Verma R. Sex differences in the structural connectome of the human brain. Proceedings of the National Academy of Sciences. 2014;111(2):823–828. doi: 10.1073/pnas.1316909110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irvine K, Laws KR, Gale TM, Kondel TK. Greater cognitive deterioration in women than men with Alzheimer’s disease: A meta analysis. Journal of Clinical and Experimental Neuropsychology. 2012;34(9):989–998. doi: 10.1080/13803395.2012.712676. [DOI] [PubMed] [Google Scholar]
- Ivanova I, Salmon DP, Gollan TH. The multilingual naming test in Alzheimer’s disease: Clues to the origin of naming impairments. Journal of the International Neuropsychological Society. 2013;19(3):272–283. doi: 10.1017/S1355617712001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack CR, Petersen RC, Xu Y, O’Brien PC, Smith GE, Ivnik RJ, Kokmen E. Rates of hippocampal atrophy correlate with change in clinical status in aging and AD. Neurology. 2000;55(4):484–489. doi: 10.1212/wnl.55.4.484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joel D, McCarthy M. Incorporating sex as a biological variable in neuropsychiatric research: Where are we now and where should we be? Neuropsychopharmacology. 2017;42:379–385. doi: 10.1038/npp.2016.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juva K, Sulkava R, Erkinjuntti T, Ylikoski R, Valvanne J, Tilvis R. Usefulness of the Clinical Dementia Rating Scale in screening for dementia. International Psychogeriatrics. 1995;7(1):17–24. doi: 10.1017/s1041610295001815. [DOI] [PubMed] [Google Scholar]
- Kananen L, Surakka I, Pirkola S, Suvisaari J, Lönnqvist J, Peltonen L, Hovatta I. Childhood adversities are associated with shorter telomere length at adult age both in individuals with an anxiety disorder and controls. PloS One. 2010;5(5):e10826. doi: 10.1371/journal.pone.0010826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantarci K, Lowe VJ, Lesnick TG, Tosakulwong N, Bailey KR, Fields JA, Miller VM. Early postmenopausal transdermal 17β-estradiol therapy and amyloid-β deposition. Journal of Alzheimer’s Disease. 2016;53(2):547–556. doi: 10.3233/JAD-160258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz MJ, Lipton RB, Hall CB, Zimmerman ME, Sanders AE, Verghese J, Derby CA. Age and sex specific prevalence and incidence of mild cognitive impairment, dementia and Alzheimer’s dementia in blacks and whites: A report from the Einstein Aging Study. Alzheimer Disease and Associated Disorders. 2012;26(4):335. doi: 10.1097/WAD.0b013e31823dbcfc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC. Epidemiology of women and depression. Journal of Affective Disorders. 2003;74(1):5–13. doi: 10.1016/s0165-0327(02)00426-3. [DOI] [PubMed] [Google Scholar]
- Kim S, Kim MJ, Kim S, Kang HS, Lim SW, Myung W, Seo SW. Gender differences in risk factors for transition from mild cognitive impairment to Alzheimer’s disease: A CREDOS study. Comprehensive Psychiatry. 2015;62:114–122. doi: 10.1016/j.comppsych.2015.07.002. [DOI] [PubMed] [Google Scholar]
- Kleinbaum D, Klein M. Survival analysis—A self-learning text. 3. New York, NY: Springer; 2012. Retrieved from http://www.springer.com/us/book/9781441966452. [Google Scholar]
- Kudielkaa B, Kirschbaumb C. Sex differences in HPA axis responses to stress: A review. Biological Psychology. 2005;69:113–132. doi: 10.1016/j.biopsycho.2004.11.009. [DOI] [PubMed] [Google Scholar]
- Mazure CM, Swendsen J. Sex differences in Alzheimer’s disease and other dementias. The Lancet Neurology. 2016;15(5):451. doi: 10.1016/S1474-4422(16)00067-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR, Kawas CH, Phelps CH. The diagnosis of dementia due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s & Dementia. 2011;7(3):263–269. doi: 10.1016/j.jalz.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mielke MM, Vemuri P, Rocca WA. Clinical epidemiology of Alzheimer’s disease: Assessing sex and gender differences. Clinical Epidemiology. 2014;6:37–48. doi: 10.2147/CLEP.S37929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris JC. The Clinical Dementia Rating (CDR): Current version and scoring rules. Neurology. 1993;43(11):2412–2414. doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- Murray AD, McNeil CJ, Salarirad S, Whalley LJ, Staff RT. Early life socioeconomic circumstance and late life brain hyperintensities—A population based cohort study. PLOS One. 2014;9(2):e88969. doi: 10.1371/journal.pone.0088969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasreddine ZS, Phillips NA, Bédirian V, Charbonneau S, Whitehead V, Collin I, Chertkow H. The Montreal Cognitive Assessment, MoCA: A brief screening tool for mild cognitive impairment. Journal of the American Geriatrics Society. 2005;53(4):695–699. doi: 10.1111/j.1532-5415.2005.53221.x. [DOI] [PubMed] [Google Scholar]
- Nelson L, Gard P, Tabet N. Hypertension and inflammation in Alzheimer’s disease: Close partners in disease development and progression. Journal of Alzheimer’s Disease. 2014;41(2):331–343. doi: 10.3233/JAD-140024. [DOI] [PubMed] [Google Scholar]
- O’Brien LM, Ziegler DA, Deutsch CK, Frazier JA, Herbert MR, Locascio JJ. Statistical adjustments for brain size in volumetric neuroimaging studies: Some practical implications in methods. Psychiatry Research. 2011;193(2):113–122. doi: 10.1016/j.pscychresns.2011.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ong AD, Fuller-Rowell TE, Bonanno GA, Almeida DM. Spousal loss predicts alterations in diurnal cortisol activity through prospective changes in positive emotion. Health Psychology. 2011;30(2):220–227. doi: 10.1037/a0022262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S, Nam Y-Y, Sim Y, Hong JP. Interactions between the apolipoprotein E ε4 allele status and adverse childhood experiences on depressive symptoms in older adults. European Journal of Psychotraumatology. 2015;6(1):25178. doi: 10.3402/ejpt.v6.25178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price LH, Kao H-T, Burgers DE, Carpenter LL, Tyrka AR. Telomeres and early-life stress: An overview. Biological Psychiatry. 2013;73(1):15–23. doi: 10.1016/j.biopsych.2012.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prins ND, Scheltens P. White matter hyperintensities, cognitive impairment and dementia: An update. Nature Reviews Neurology. 2015;11(3):157–165. doi: 10.1038/nrneurol.2015.10. [DOI] [PubMed] [Google Scholar]
- Radford K, Delbaere K, Draper B, Mack HA, Daylight G, Cumming R, Broe GA. Childhood stress and adversity is associated with late-life dementia in Aboriginal Australians. The American Journal of Geriatric Psychiatry. 2017;25(10):1097–1106. doi: 10.1016/j.jagp.2017.05.008. [DOI] [PubMed] [Google Scholar]
- Richardson VE, Bennett KM, Carr D, Gallagher S, Kim J, Fields N. How does bereavement get under the skin? The effects of late-life spousal loss on cortisol levels. The Journals of Gerontology: Series B. 2015;70(3):341–347. doi: 10.1093/geronb/gbt116. [DOI] [PubMed] [Google Scholar]
- Sachdev PS, Parslow R, Wen W, Anstey KJ, Easteal S. Sex differences in the causes and consequences of white matter hyperintensities. Neurobiology of Aging. 2009;30(6):946–956. doi: 10.1016/j.neurobiolaging.2007.08.023. [DOI] [PubMed] [Google Scholar]
- Sano M, Mackell JA, Ponton M, Ferreira P, Wilson J, Pawluczyk S, Thal LJ. The Spanish Instrument Protocol: Design and implementation of a study to evaluate treatment efficacy instruments for Spanish-speaking patients with Alzheimer’s disease. The Alzheimer’s Disease Cooperative Study. Alzheimer Disease and Associated Disorders. 1997;11(Suppl 2):S57–64. [PubMed] [Google Scholar]
- Sapolsky RM. Glucocorticoids and hippocampal atrophy in neuropsychiatric disorders. Archives of General Psychiatry. 2000;57(10):925–935. doi: 10.1001/archpsyc.57.10.925. [DOI] [PubMed] [Google Scholar]
- Serrano-Pozo A, Frosch MP, Masliah E, Hyman BT. Neuropathological alterations in Alzheimer disease. Cold Spring Harbor Perspectives in Medicine. 2011;1(1):a006189. doi: 10.1101/cshperspect.a006189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder HM, Asthana S, Bain L, Brinton R, Craft S, Dubal DB, Carrillo MC. Sex biology contributions to vulnerability to Alzheimer’s disease: A think tank convened by the Women’s Alzheimer’s Research Initiative. Alzheimer’s & Dementia. 2016;12:1186–1196. doi: 10.1016/j.jalz.2016.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- StataCorp. Stata statistical software: Release 14. College Station, TX: Author; 2015. Retrieved from http://www.stata.com/new-in-stata/ [Google Scholar]
- Sudre CH, Cardoso MJ, Frost C, Barnes J, Barkhof F, Fox N, Ourselin S. APOE ε4 status is associated with white matter hyperintensities volume accumulation rate independent of AD diagnosis. Neurobiology of Aging. 2017;53(Supplement C):67–75. doi: 10.1016/j.neurobiolaging.2017.01.014. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Adalsteinsson E, Hedehus M, Ju C, Moseley M, Lim KO, Pfefferbaum A. Equivalent disruption of regional white matter microstructure in ageing healthy men and women. NeuroReport. 2001;12(1):99–104. doi: 10.1097/00001756-200101220-00027. [DOI] [PubMed] [Google Scholar]
- Taylor WD, Züchner S, McQuoid DR, Payne ME, MacFall JR, Steffens DC, Krishnan KRR. The brain-derived neurotrophic factor VAL66MET polymorphism and cerebral white matter hyperintensities in late-life depression. The American Journal of Geriatric Psychiatry. 2008;16(4):263–271. doi: 10.1097/JGP.0b013e3181591c30. [DOI] [PubMed] [Google Scholar]
- Van Den Heuvel DMJ, Admiraal-Behloul F, Ten Dam VH, Olofsen H, Bollen LEM PROPSER Study Group. Different progression rates for deep white matter hyperintensities in elderly men and women. Neurology. 2004;63(9):1699–1701. doi: 10.1212/01.WNL.0000143058.40388.44. [DOI] [PubMed] [Google Scholar]
- Yaffe K, Haan M, Byers A, Tangen C, Kuller L. Estrogen use, APOE, and cognitive decline: Evidence of gene–environment interaction. Neurology. 2000;54(10):1949–1954. doi: 10.1212/wnl.54.10.1949. [DOI] [PubMed] [Google Scholar]
- Yip AG, Brayne C, Easton D, Rubinsztein DC. Apolipoprotein E4 is only a weak predictor of dementia and cognitive decline in the general population. Journal of Medical Genetics. 2002;39(9):639–643. doi: 10.1136/jmg.39.9.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ystad MA, Lundervold AJ, Wehling E, Espeseth T, Rootwelt H, Westlye LT, Lundervold A. Hippocampal volumes are important predictors for memory function in elderly women. BMC Medical Imaging. 2009;9:17. doi: 10.1186/1471-2342-9-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Z, Li R, Xiao F, He R, Zhang S, Li J. Sex matters: Hippocampal volume predicts individual differences in associative memory in cognitively normal older women but not men. Frontiers in Human Neuroscience. 2017;11:93. doi: 10.3389/fnhum.2017.00093. [DOI] [PMC free article] [PubMed] [Google Scholar]