Abstract
Objective
The current investigation evaluated a novel extended release delivery system for treating inner ear diseases. The platform technology consists of a film forming agent (FFA) and microsphere component to localize and extend drug delivery within the ear.
Study Design
Studies evaluated dissolution kinetics of microspheres with multiple encapsulates, testing of a variety of FFAs, and ability to localize to the round window membrane in mice in vivo.
Setting
Studies were completed at Orbis Biosciences and The University of Kansas Medical Center
Subjects
In conjunction with in vitro characterization, an infrared dye-containing microsphere formulation was evaluated for round window membrane (RWM) localization and general tolerability in C57/BL6 Mus musculus for 35 days.
Methods
In vitro characterization was performed using upright diffusion cells on cellulose acetate membranes, with drug content quantified by high performance liquid chromatography. Mus musculus dosing of infrared dye-containing microspheres was performed under anesthesia with a 27 GA needle and 2.0 uL injection volume.
Results
In vitro dissolution demonstrates the ability of the FFA with microsphere platform to release steroids, proteins, peptides, and nucleic acids for at least one month, while necroscopy shows the ability of the FFA with dye-loaded microspheres to remain localized to Mus musculus RWM for the same period of time, with favorable tolerability.
Conclusions
Combining FFA and microsphere for localized drug delivery may enable cost-effective, extended release local delivery to the inner ear of new and existing small molecules, proteins, peptides, and nucleic acids.
Keywords: Sudden hearing loss, microspheres, steroid, transtympanic, round window membrane
Graphical Abstract
1. INTRODUCTION
New treatment options are needed for inner ear disorders including Meniere’s disease, sensorineural hearing loss, autoimmune inner ear disease, and tinnitus. While both systemic (via oral dosages) and local (via transtympanic injection) delivery of drugs to the inner ear have been a common practice in the otolaryngological medical community for some time, there has yet to be definitive, clinical-based evidence as to the utility of this approach to improve patient outcomes.1–6 With a paucity of FDA-approved drugs for use in the inner ear, physicians use improvised treatments, including the administration of off-label steroids, which lack safety and efficacy data. These ad hoc approaches often fail to achieve the desired outcomes; a result potentially attributable to insufficient and variable drug exposure in the inner ear.
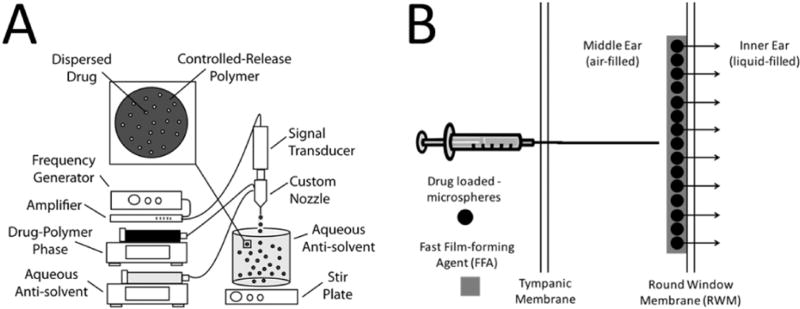
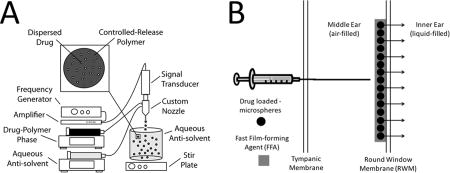
The current investigation describes an extended-release inner ear drug delivery platform with the potential to significantly improve treatment for a wide-range of otic disorders by maintaining precise and therapeutic drug levels in the inner ear for more than thirty (30) days after a single, transtympanic injection. The delivery platform is a composite of: (1) drug-loaded microspheres – produced using a precision particle fabrication technology (Fig. 1a) – that allow for precise control of drug release, and (2) a novel Fast Film-forming Agent (FFA) that serves as both a diluent for microsphere injection and a film that holds the microspheres to the round window membrane (RWM).The current investigation describes pilot in vitro evaluation of steroids, proteins, peptides, and nucleic acids and in vivo evaluation of the longevity of the film in the inner ear utilizing infrared dye-loaded microsphers and visualization of the inner ear space. Multiple FFA excipient and microsphere active ingredients were tested to determine the flexibility and optimization of the platform.
Figure 1.
(A) Manufacture of uniform drug loaded microspheres begins by dispersing drug in a solution of controlled release polymer and organic solvent. A uniform emulsion is made with acoustic excitation, after which the organic phase is extracted and microspheres lyophilized. Dried microsphere powders are then ready for resuspension in applicable film forming agent and transtympanic injection. (B) Once microspheres are suspended in an FFA component, they can be deposited on the round window membrane. Reversal of the procedure could be accomplished with water, as the FFA components are water soluble.
The intent of these studies is to successfully developed a prototype of an extended-release steroid-containing microsphere for use in FFA. This product, ORB-202, could replace the clinical practice of multiple transtympanic injections of steroid suspensions spaced over the course of several weeks, a treatment that is painful and inconvenient.1–6 The goal of ORB-202 for human use is to deliver 10 mg of steroid in a 200 µL injection volume through an 88.9 mm 26 GA needle, with less than 50% of the dose free for immediate therapeutic relief, and at least 50% of the dose available for sustained release. The current investigation describes development of the platform fostering ORB-202 and the associated in vitro and in vivo characterization of the platform.
2. MATERIALS AND METHODS
2.1 Materials
Poly(D,L-lactide-co-glycolide) (PLGA) copolymer (50:50 lactic acid : glycolic acid, acid end group, MW ~38,000 Da) of intrinsic viscosity (i.v.) 0.34 dL/g was purchased from Lakeshore Biomaterials (Birmingham, AL). Poly (vinyl alcohol) (PVA; 88% hydrolyzed, 25,000 Da) was obtained from Polysciences, Inc. (Warrington, PA). Betamethasone, betamethasone acetate, and betamethasone valerate were acquired from Sigma Aldrich (St. Louis, MO), and TEVA API (Woodcliff Lake, NJ), respectively. Dexamethasone, penicillin G sodium salt, albumin (from bovine serum), siRNA (unconjugated GAPDH positive control), and DNA (from salmon testes) were obtained from Sigma Aldrich (St. Louis, MO). C57/BL6 mice were acquired from Charles River Laboratories (Wilmington, MA). All animal work was approved by The University of Kansas Medical Center (Kansas City, KS) Institutional Animal Care and Use Committee (IACUC) under approval number 2015–2281, and was performed in the labs of Dr. Hinrich Staecker and The University of Kansas Medical Center’s Core Animal Facility.
2.2 Preparation of Microspheres
Three types of steroid-loaded microsphere formulations were prepared for in vitro evaluation: betamethasone, betamethasone acetate, and betamethasone valerate. Briefly, the betamethasone salt form of choice was co-dissolved with PLGA in dichloromethane (Sigma Aldrich, St. Louis, MO) such that the betamethasone content ranged from 1% w/w - 50% w/w on a dry particle bases. The solids concentration in each solution was adjusted such that the viscosity allowed ease of processing. Using PLGA-steroid solutions, uniform microspheres were prepared using technology described in previous reports.7–14 Using different frequencies of acoustic excitation produced by an ultrasonic transducer, regular jet instabilities were created in the polymer stream that produced uniform polymer droplets of ~60, 50, 40, 30, or 20µm in diameter. An annular carrier non-solvent stream, 0.5% w/v PVA in deionized (DI) H2O, surrounding the droplets was produced using a coaxial nozzle. The emanated polymer/carrier streams flowed into a beaker containing the non-solvent at 0.5% w/v in DI H2O to prevent aggregation of the particles. Incipient polymer droplets were stirred for 3–4 hours to allow solvent evaporation, which were then filtered and rinsed with DI H2O to remove residual PVA, and stored at −80 °C (Fig. 1a). Following 48 hours of lyophilization (Labconco, Kansas City, MO), the microspheres were stored at −80 °C until further use.
Lastly, other variants or classes of chemical species – dexamethasone, penicillin, albumin, siRNA, DNA, and IR820 dye (all from Sigma Aldrich, St. Louis, MO) – were encapsulated with the same process described above to demonstrate platform flexibility. Active content for betamethasone, dexamethasone and penicillin was quantified using high performance liquid chromatography (HPLC) using an Agilent 1100 series equipped with a diode array detector (DAD) using previously-reported isocratic methods.15–17 Albumin content was determined with a micro-bicinchoninic acid (BCA) assay (ThermoFisher, Waltham, MA). Nucleic acid content was quantified with a pigogreen assay (Molecular Probes, Eugene, OR). IR dye content was quantified with intensity standardization using and IVIS Spectrum In Vivo Imaging System (see In Vivo Imaging) using and 800 ± 10 nm filter.
2.3 Film Forming Agent (FFA) Evaluation
Various aqueous and organic-soluble species known to be generally regarded as safe were solubilized in DI H2O or United States Pharmacopeia (USP) Class III solvents in varying concentrations. The polymers used for the FFAs (Table 2) were from the classes of vinyl alcohols (A), polysorbates (B), poloxamers (C), polyvidones (D), cellulose derivatives (E), and polyethers (F). The solutions were evaluated under four criteria: (1) initial contact angle, (2) drying time, (3) final droplet area in proportion to RWM area, and (4) substantiality of film forming behavior through an in-use application. Briefly, solutions were placed on grid paper coated with a hydrophobic layer comprised of polyethylene sheets (McMaster Carr, Elmhurst, IL). Droplet dimensions (such as height, contact radius, and cross sectional area) were measured empirically, after which a contact angle was estimated using the Young’s relation simplified to planar geometry, known as the “θ/2 method.” Drying time and final FFA radius was recorded after the measurement process. Descriptions on film consistency were also noted. Leading candidates were tested for droplet travel distance prior to hardening on 30° inclined uncoated glass slide measuring 75 mm × 25 mm (VWR, Radnor, PA).
Table 2.
Film forming agents evaluated for wettability, droplet size, and drying time.
| Material Class |
Formulation ID |
Initial Droplet Radius (mm) |
Final Film Radius (mm) |
Film Drying Time (Minutes) |
Area RWM/ Area Droplet (%) |
Film Appearance (Dried FFA) |
|---|---|---|---|---|---|---|
| A | FFA-1 | 6.0 | 6.6 | 90 | 2.2 | Dry, Clear, Flexible, Flat |
| A + B | FFA-2 | 6.5 | 7.3 | 75 | 1.9 | Dry, Clear, Flexible, Flat |
| A + B | FFA-3 | 6.8 | 7.5 | 60 | 1.7 | Dry, Heterogeneous, Clear, Semi-flexible, Curved |
| A + B | FFA-4 | 6.4 | 7.6 | 60 | 1.9 | Dry, Clear, Flexible, Flat |
| A + B | FFA-5 | 7.3 | 7.4 | 60 | 1.5 | Dry, Clear, Flexible, Curved |
|
| ||||||
| C | FFA-6 | 7.1 | 7.8 | 85 | 1.6 | Dry, Opaque, Flaky, Flat |
| C + A | FFA-7 | 7.4 | 9.2 | 75 | 1.5 | Dry, Semi-transparent, Flexible, Flat |
| C + D | FFA-9 | 6.9 | 7.3 | 75 | 1.7 | Dry, Opaque, Flaky, Flat |
| C + D | FFA-10 | 7.2 | 8.7 | 85 | 1.5 | Dry, Semi-transparent, Flexible, Curved |
| C + E | FFA-8 | 7.3 | 8.6 | 75 | 1.5 | Dry, Semi-transparent, Flaky, Flat |
|
| ||||||
| D | FFA-12 | 5.2 | 4.8 | 210 | 2.9 | Dry, Clear, Semi-flexible, Flat |
| D | FFA-13 | 5.9 | 5.0 | 210 | 2.3 | Mostly Solid, Clear, Rigid, Flat |
|
| ||||||
| E | FFA-14 | 6.1 | 6.3 | 180 | 2.1 | Dry, Clear, Flexible, Flat |
| E | FFA-15 | 5.6 | 5.2 | 210 | 2.5 | Dry, Clear, Rigid, Curved |
|
| ||||||
| F | FFA-16 | 5.2 | 4.6 | 210 | 2.9 | Partial Liquid, Opaque, Tacky |
2.4 Syringeability Studies
Microspheres of various sizes were re-suspended in the leading FFA candidate at concentrations of 25, 50, 75, and 100 mg/mL and qualitative syringeability was evaluated with needle gauges of 21, 26, 27, and 30 (Beckton Dickinson, Franklin Lakes, NJ). Upper limits of syringeability for each needle gauge were determined by increasing microsphere concentration to the point of needle clogging. Optimized formulations of ORB-202 were tested for syringability through a glass, gas-tight syringe with 88.9 mm 26 GA stainless steel needle (Hamilton Company, Reno, NV).
2.5 In Vitro Dissolution
Dissolution testing of drug-loaded microspheres with and without a free drug fraction and/or a FFA component were tested on a custom vertical diffusion cell apparatus with a receptor phase composed of 5% w/v Brij O20 (Croda, Edison, NJ), and circulating water maintained at 37 °C. The cell membranes, composed of cellulose acetate (Sterlitech, Kent, WA), were pre-wetted and warmed prior to formulation testing. Briefly, following dissolution apparatus equilibrium, a 1000 µL aliquot of suspension was placed on the film, after which sampling was performed over a period of up to 42 days, depending on the formulation. The the in vitro dissolution studies were designed with similar drug per area ratio in order to mimic the dose that would be allowed through the RWM. Specifically, the Franz cell membrane cross sectional area (388 mm2) is ~500 times larger than the mouse RWM cross sectional area (< 1.0 mm2). Moreover, a 2 µL FFA injection in the mouse translated to a~ 1000 µL FFA deposition on the Franz cell apparatus in vitro. Moreover, the drug concentration within the FFA solution and per cross sectional area, being the same in both situations. Release of betamethasone, dexamethasone and penicillin was quantified using high performance liquid chromatography (HPLC) using an Agilent 1100 series (Agilent, Santa Clara, CA) equipped with a diode array detector (DAD) using previously-reported isocratic methods.15–17 Albumin content was determined with a micro-bicinchoninic acid (BCA) assay (ThermoFisher, Waltham, MA). Nucleic acid content was quantified with a pigogreen assay (Molecular Probes, Eugene, OR).
2.6 Delivery to the mouse round window
Fluorescent dye-loaded microspheres (n=2) and low-dose betamethasone-loaded microspheres with (n=4) and without (n=4) the optimized FFA formulation were deposited on mouse RWMs to evaluate initial and long-term in vivo localization performance. Mice without the FFA had microspheres delivered with only sterile saline. Briefly, C57/BL6 mice were anesthetized with a Ketamine/Xylazine cocktail, laid on their side, and immobilized. Post-auricular incision was made, the skin and soft tissue were retracted, and an access hole to the tympanic cavity was created with a 28 GA needle. Approximately 2.0 µL injections of 50 mg/mL fluorescent dye-loaded microspheres were then delivered directly above the RWM with a 10 µL Hamilton syringe. The mice were kept in this position for 5 minutes before being sutured for subsequent tests.
2.7 In Vivo Imaging
Following dosing, mice dosed with PLGA microspheres loaded with 1% w/w IR-820 dye were imaged on an IVIS Spectrum in vivo Imaging System (Perkin-Elmer, Waltham, MA) at the University of Kansas Medical Center to confirm localization of the formulation to the middle ear space. Negative control mice were injected with fluorescent microspheres without the FFA component (saline injection vehicle). Imaging using the IVIS Spectrum was performed every day after dosing until loss of ability to visualize florescence in the ear. Upon no longer visualizing in vivo evidence of florescence, mice were euthanized on a set schedule (Day 21, 28, and 35) and necroscopy performed.
2.8 Necroscopy
At 21, 28, and 35 days post drug delivery, mice were euthanized via inhalation of CO2 from a pressurized tank in an uncrowded cage for 5 minutes to ensure complete asphyxia. After sufficient CO2 exposure, as judged by a hind-paw pinch, cervical dislocation was performed. Following this, necropsy and histology were performed to evaluate microsphere localization and inflammatory responses.
2.9 Immunohistochemistry
Mice sacrificed at 28 days had the drug treated temporal bones removed, decalcified, and embedded in paraffin. Paraffin sections were cut (5µm) and dried overnight at 40°C. Sample slides were rehydrated and underwent antigen retrieval via enzymatic digestion using Digest-All 2® (Invitrogen®, Waltham, MA) for 10 minutes at 37°C. The sections were then incubated at room temperature with 5% BSA block. After washing in PBS, primary antibodies were applied at room temperature for 3 hours in a humid chamber. TNF-α (ab6671, Abcam, Cambridge, MA) and IL-6 (ab9324, Abcam, Cambridge, MA) were used to indicate pro-inflammatory cytokines. TNF-α was diluted to 1:100 and IL-6 was diluted to 0.5µg/ml. After washing with PBS, immunohistochemical detection was performed using anti-rabbit (1:1000; Alexa Fluor 555nm, Invitrogen®, Waltham, MA) for TNF-α and anti-mouse (1:1000; Alexa Fluor 488nm, Invitrogen®, Waltham, MA). The secondary incubation was for 60 minutes at room temperature. After a final set of PBS washes, the sections were mounted with Prolong Gold Antifade® reagent containing DAPI and coverslipped. Pictures were taken on TE 2000 Inverted Nikon Microscope (Nikon®, Kanagawa, Japan).
3. RESULTS
3.1 Preparation of Microspheres
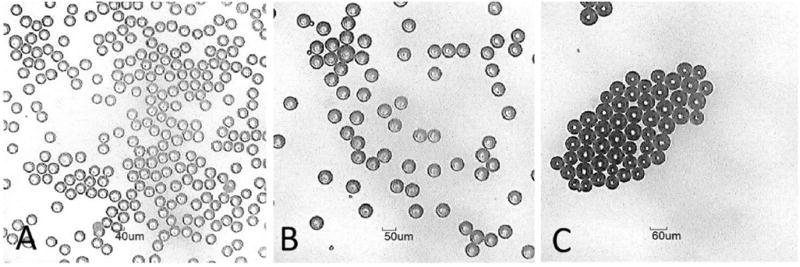
Microsphere formulations were manufactured with varying concentrations and types of chemical species (Table 1). Independent of drug content, microspheres exhibited uniform, spherical morphology with diameters within 5% of the mean (Fig. 2a–b). Steroid content at levels near 50% w/w were achieved with specific salt forms of betamethasone. Figure 2 (a–c) provides images of three sizes of microspheres: (a) 40 µm, (b) 50 µm, and (c) 60 µm. Microspheres prepared for dosing of mice utilized IR-820 dye for imaging purposes. The IR-820 dye was loaded at 1% w/w and the microsphere size was an average size of 30 µm.
Table 1.
Microsphere formulations tested in vitro and in vivo.
| Formulation ID |
Encapsulated Drug |
Maximum Drug Content in Microsphere Tested (ug/mg) |
Mean Sizes Made (µm) |
Utilized In | Modality |
|---|---|---|---|---|---|
| MS-1 | IR 820 Fluorescent Dye | 10.0 | 30 | IVIS Imaging | In Vivo |
| MS-2 | Betamethasone | 10.0 | 30 | Mus musculus localization and histology, dissolution testing | In Vivo |
| MS-3 | Betamethasone | 10.0 | 40, 50, 60 | Dissolution testing | In Vitro |
| MS-4 | Betamethasone valerate | 498 | 20, 30 | Drug loading optimization | In Vitro |
| MS-5 | Betamethasone acetate | 412 | 20, 30 | Drug loading optimization | In Vitro |
| MS-6 | Dexamethasone | 0.0225 | 40 | FFA Dissolution testing | In Vitro |
| MS-7 | Penicillin G Sodium Salt | 2.62 | 40 | Particle and FFA Dissolution testing | In Vitro |
| MS-8 | Bovine Albumin | 174 | 40 | Particle Dissolution testing | In Vitro |
| MS-9 | DNA | 0.0528 | 40 | Particle and FFA Dissolution testing | In Vitro |
| MS-10 | SiRNA | 0.0763 | 40 | Particle and FFA Dissolution testing | In Vitro |
Figure 2.
Microscope images of microspheres with mean diameter of (A) 40 µm, (B) 50 µm, and (C) 60 µm.
3.2 Film Forming Agent (FFA) Evaluation
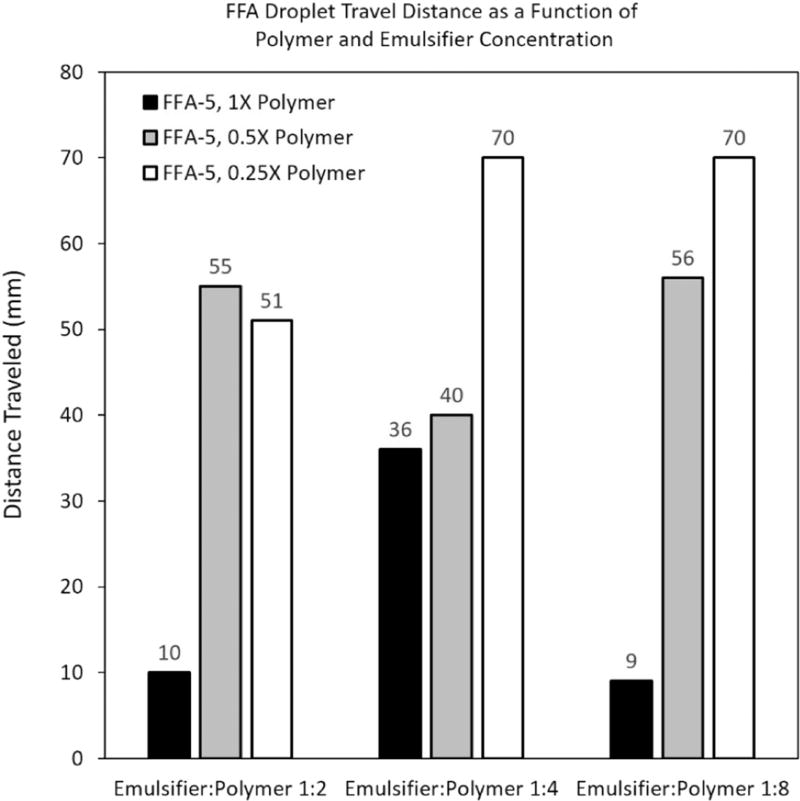
Evaluation of film forming agents revealed that one chemical species, when combined with surfactant, exhibited the shortest overall drying times, while maintaining acceptable film radius and flexible consistency, as opposed to a brittle or rigid makeup (Table 2). Film radius was compared to estimated round window membrane and inner ear anatomy. Localization tests indicated that a 2:1 ratio of film former-to-emulsifier was optimal for reduction of lateral droplet travel (Fig. 3). The main variable that affected drying time looked to be polymer concentration with higher polymer concentration decreasing overall drying time. The optimized formulation was shown to dry on exposed skin within 15 minutes in ambient temperature and humidity (Fig. 4).
Figure 3.
Incline testing to optimize FFA formulation 5 (FFA-5). Optimization investigated varying polymer and surfactant content and ratios thereof. Testing indicated that drying time was mainly a function of polymer concentration, where high polymer concentration was responsible for fast drying times. Surfactant content also improved drying times, when approaching a ratio of 1:2 to the polymer.
Figure 4.
Pilot testing on skin (with 50 µL droplet) indicated rapid drying of the optimized FFA-5 within 15 minutes. Drying in vivo was also observed to happen within 15 minutes, though in vitro testing on artificial surfaces reported a 4X increase in drying time. An increase in drying time in vitro is most likely attributed to reduced surface temperature and surface porosity.
3.3 Syringeability Studies
Injection studies with microsphere variants indicated that as microsphere diameter increased, a lower suspension concentration was required for administration at any given needle gauge (data not shown). Depending on microsphere size, concentrations of up to 100 mg/mL through an 88.9 mm 26 GA needle.
3.4 In Vitro Dissolution
Dissolution kinetics demonstrated the ability of microspheres to control release of multiple classes of chemical species including a steroids, protein, peptide, and nucleic acid (Fig. 5a). These results showed longevity in all four chemical species out to day 35 when the microspheres were used in conjuncture with the FFA. Release of all species, when suspended freely in the FFA component, was rapid (Fig. 5b), with water-soluble species, such as nucleic acids, exhibited the fastest release. Release was also affected by microsphere size, where larger microspheres released drug more slowly (Fig. 5c).
Figure 5.
(A) Various drug release rates from microsphere and FFA combinations, (B) Various drug release rates from a single FFA formulation, and (C) Drug release rates of betamethasone from microspheres of different sizes, when suspended in an FFA component. The microsphere component retains drug and releases it over long periods of time, whereas the FFA component makes the drug readily available for therapeutic effect.
3.5 Dosing of mice and in vivo imaging
Observed drying times for the FFA in vivo appeared to be approximately 10–15 minutes. All mice administered the optimized microsphere-in-FFA formulations were recovered from anesthesia without complications.
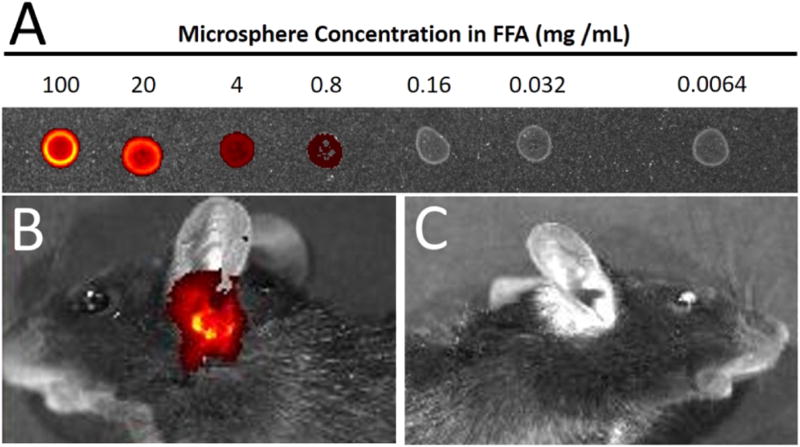
IVIS system imaging indicated that signal of the IR820 dye could be detected when the microsphere concentration was as low as 0.8 mg/mL, but preferable signal was at > 20 mg/mL (Fig. 6a). Imaging of mus musculus indicated that signal was visible through the ear space following dosing (Fig. 6b). Signal of IR820 dye in FFA was undetectable after day 10, after which localization ability was determined by necroscopy at 21 and 35 days. The control mice had no identification of signal (Fig. 6c).
Figure 6.
(A) Droplets of IR820-encapsulated microspheres in the optimized FFA were tested on the IVIS system for quantification of signal strength. (B) Pilot RWM localization in mus musculus (with 2 µL droplet) indicated rapid drying of the optimized FFA-5 within 15 minutes. (C) Mice with no ORB-202 injection exhibited no IR signal.
3.6 Necroscopy
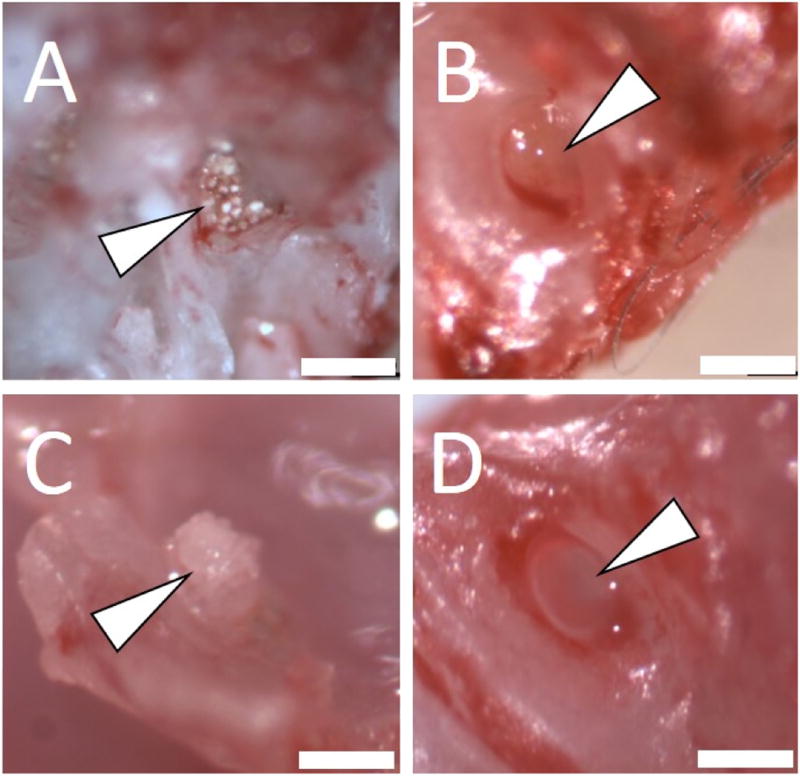
At both three (Day 21) and five (Day 35) weeks, necroscopy revealed that animals with the FFA component retained drug-containing microspheres on the RWM (Fig. 7a—Day 21 and 7c—Day 35), whereas animals administered microspheres in saline (no FFA localization ability) did not have microsphere localization (Fig. 7b—Day 21 and 7d—Day 35).
Figure 7.
Necroscopy of mus musculus at 21 days (A,B) and 35 days (C,D). Surgical resection revealed that mice administered formulations with an FFA component (A,C) had microspheres still localized to the RWM, and those without an FFA component (B,D) had no microsphere localization. Microspheres in the non-FFA group could not be located during necroscopy. Arrow indicates round window niche. Scale bar = 400 µm.
3.7 Immunohistochemistry Evaluation
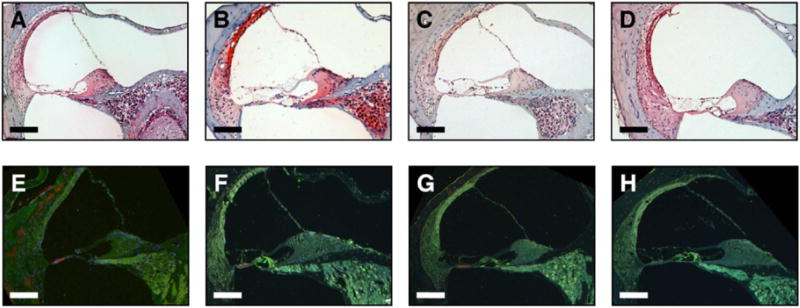
Compared to the negative controls (Fig. 8a–b, e–f), there was no pro-inflammatory cytokines staining in the cochlea for TNF-α or IL-6 in ORB-202 treated groups (Fig. 8c–d, g–h).
Figure 8.
Immunohistochemistry and histology of the cochlea in mice following 28 days with localized ORB-202 formulation. No signs of inflammation were seen with H&E staining in negative controls (A,B) compared to treatment groups (C,D). Double immunolabeling of TNF-α (red) and IL-6 (green) revealed minimal inflammation in animals receiving the ORB-202 formulation (G,H) compared to the negative controls (E,F). Scalebar = 100µm.
4. DISCUSSION
Results demonstrate the feasibility of an extended-release drug delivery to the inner ear by characterizing the ability to formulate the microspheres and FFA along with data supporting the capability of adhering microspheres to the RWM. In vitro, the microsphere component can release drug for more than 35 days, depending on the molecular weight of the polymer used and water solubility of the species encapsulated. Combined with extensive existing knowledge surrounding in vitro-in vivo correlations for PLGA microspheres,18–21 such a kinetic profile might translate to nearly one month of potential efficacy, without accounting for the relatively gradual elimination of the API itself once released into the cochlear perilymph. The betamethasone would not be expected to reside in the RWM indefinitely, rather, the steroid would diffuse across the RWM of the cochlea due to concentration gradients between the perilymph and FFA. It is unknown, in this particular circumstance, what the residence time in the perilymph would be, but one would expect release from the microsphere to be the rate limiting factor, and not the clearance, due to the fact that the literature shows steroid only delivery (in the absence of controlled release formulations) results in rapid elimination of drug from the perilymph.22
Evaluated FFAs also exhibited drying times that are heavily dependent upon the type of aqueous polymer utilized, and resistance to flow after FFA droplet deposition is proportional to polymer concentration. Future iterations of the FFA component could be used to modulate or slow drug release in the same manner as microspheres, enabling other combinations of otic delivery systems. As demonstrated, the physical properties of the FFA can also be adjusted for use in other otolaryngological sites where hydration levels differ from the inner ear space.
With IVIS imaging indicating that the FFA was dry and secure on RWM immediately following administration, and for several days following, clinical impact could come in the form of reduced procedure time, in addition to an overall reduction in number of treatment visits for steroid-responsive hearing disorders. Lack of signal following day 10 is expected to be a result of degradation of the infrared fluorescing dye due to the humidity of the ear, as IR-820 degrades in aqueous solutions.23 Fine needle gauges and microsphere diameters below 20 µm might also decrease patient discomfort. The lack of presentation of TNF-α and IL-6 in the stria vascularis and the spiral ganglion cells at the base of the organ of Corti in treated groups (Fig. 8g–h), compared to the negative controls (Fig. 8e–f), signified acceptable tolerability by the lack of inflammatory cytokines.24,25 Though the pilot toxicology evaluation described here (Fig. 8) indicates no significant inflammation or discomfort in mice subject to treatment, future studies in guinea pigs will evaluate toxicology as a function of dose over several weeks using cytocochleograms and auditory brainstem response (ABR) testing. In addition, the water solubility of the FFA components would enable reversal of the ORB-202 delivery system with a saline gavage to the dosing site should a patient experience an adverse event.
Due to the exceedingly small volumes of the mouse scala typani (~0.32 µL) and scala vestibule (~0.3 µL),26 concentrations of betamethasone from the perilymph were not quantifiable in the current study, but physical localization of ORB-202 is encouraging. Moreover, this study served as a pilot investigation to demonstrate proof of concept. Microsphere morphology indicated that although several weeks had elapsed, the physical integrity of the formulation was still maintained, suggesting localization and release could potentially extend beyond one month (Fig. 7). Future perilymph and plasma pharmacokinetic analyses in guinea pigs and sheep will assist with characterizing potential efficacy through dose range finding studies and associated allometric scaling, as the sheep cochlear length and structure is closer to that of a human.27
5. CONCLUSION
Described here is a successful demonstration of the feasibility of formulating microspheres for use in the inner ear along with FFA for localization of drug to the RWM. As part of these studies, betamethasone was studied in order to create an extended-release steroid formulation, denoted ORB-202, which utilizes an FFA to adhere betamethasone-loaded microspheres to the RWM. The authors do note, however, that the data presented herein is of small scale with low replicates, which presents limitations on definitive conclusions. With the successful formulation of a betamethasone product for the inner ear and evidence of long-term adhesion of infrared dye-loaded microspheres to the RWM, ORB-202 is now ready for pharmacokinetic and toxicological evaluation in small and large animal models. The delivery platform utilized by ORB-202 may reduce or eliminate the need for physicians to prescribe off-label use of high concentrations of oral steroids or ad hoc formulations of suspended steroids that they deliver through intratympanic injection. Regardless of the route of administration, off-label use of steroids is problematic for a number of reasons including: (i) lack of both clinical and scientific-based understanding of the appropriate steroid concentrations to achieve a therapeutic effect in the inner ear, (ii) insufficient pharmacokinetic data to determine the appropriate dose, leaving patients at risk for both under and over dose, (iii) lack of safety data, leaving patients exposed to unnecessary risks that could potentially worsen their hearing, and (iv) minimal clarity on the appropriate treatment duration, which further contributes to the potential risks to patients suffering from these diseases. The present study establishes proof of concept for an extended release otic delivery platform, which is adjustable to fit each patient’s needs, and to address a handful of otic diseases for which transtypmanic injection is a viable treatment option.
Acknowledgments
This work was made possible through the National Institutes of Health (NIH) National Institute on Deafness and Other Communication Disorders (NIDCD) under grant numbers R43DC012749 and R44DC012749. Special thanks to the lab of George Vielhauer, Pharm.D., Ph.D. for access and navigation of the IVIS Spectrum unit.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Matsuoka AJ, Harris JP. Autoimmune Inner Ear Disease: A Retrospective Review of Forty-Seven Patients. Audiology & Neuro-otology. 2013;(18):228–239. doi: 10.1159/000351289. [DOI] [PubMed] [Google Scholar]
- 2.Harris DA, Mikulec AA, Carls SL. Autoimmune inner ear disease preliminary case report: audiometric findings following steroid treatments. American journal of Audiology. 2013;(22):120–124. doi: 10.1044/1059-0889(2012/12-0040). [DOI] [PubMed] [Google Scholar]
- 3.Trune DR, Canlon B. Corticosteroid Therapy for Hearing and Balance Disorders. The Anatomical Record: Advances in Integrative Anatomy and Evolutionary Biology. 2012;(295):1928–1943. doi: 10.1002/ar.22576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Haynes DS, O'Malley M, Cohen S, Watford K, Labadie RF. Intratympanic dexamethasone for sudden sensorineural hearing loss after failure of systemic therapy. The Laryngoscope. 2007;(117):3–15. doi: 10.1097/01.mlg.0000245058.11866.15. [DOI] [PubMed] [Google Scholar]
- 5.Rauch SD, Halpin CF, Antonelli PJ, et al. Oral vs intratympanic corticosteroid therapy for idiopathic sudden sensorineural hearing loss: a randomized trial. JAMA: the Journal of the American Medical Association. 2011;(305):2071–2079. doi: 10.1001/jama.2011.679. [DOI] [PubMed] [Google Scholar]
- 6.Swan EE, Mescher MJ, Sewell WF, Tao SL, Borenstein JT. Inner ear drug delivery for auditory applications. Advanced Drug Delivery Reviews. 2008;(60):1583–1599. doi: 10.1016/j.addr.2008.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berkland C, Pollauf E, Varde N, Pack DW, Kim KK. Monodisperse liquid-filled biodegradable microcapsules. Pharmaceutical Research. 2007;(24):1007–1013. doi: 10.1007/s11095-006-9197-9. [DOI] [PubMed] [Google Scholar]
- 8.Berkland C, Pollauf E, Raman C, Silverman R, Kim K, Pack DW. Macromolecule release from monodisperse PLG microspheres: control of release rates and investigation of release mechanism. Journal of Pharmaceutical Sciences. 2007;(96):1176–1191. doi: 10.1002/jps.20948. [DOI] [PubMed] [Google Scholar]
- 9.Berkland C, Pollauf E, Pack DW, Kim K. Uniform double-walled polymer microspheres of controllable shell thickness. Journal of Control Release. 2004;(96):101–111. doi: 10.1016/j.jconrel.2004.01.018. [DOI] [PubMed] [Google Scholar]
- 10.Berkland C, Kipper MJ, Narasimhan B, Kim KK, Pack DW. Microsphere size, precipitation kinetics and drug distribution control drug release from biodegradable polyanhydride microspheres. Journal of Controlled Release. 2004;(94):129–141. doi: 10.1016/j.jconrel.2003.09.011. [DOI] [PubMed] [Google Scholar]
- 11.Berkland C, Cox A, Kim K, Pack DW. Three-month, zero-order piroxicam release from monodispersed double-walled microspheres of controlled shell thickness. Journal of Biomedical Material Research – Part A. 2004;(70):576–584. doi: 10.1002/jbm.a.30114. [DOI] [PubMed] [Google Scholar]
- 12.Berkland C, Kim K, Pack DW. PLG microsphere size controls drug release rate through several competing factors. Pharmaceutical Research. 2003;(20):1055–1062. doi: 10.1023/a:1024466407849. [DOI] [PubMed] [Google Scholar]
- 13.Berkland C, King M, Cox A, Kim K, Pack DW. Precise control of PLG microsphere size provides enhanced control of drug release rate. Journal of Controlled Release. 2002;(82):137–147. doi: 10.1016/s0168-3659(02)00136-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Berkland C, Kim K, Pack DW. Fabrication of PLG microspheres with precisely controlled and monodisperse size distributions. Journal of Controlled Release. 2001;(73):59–74. doi: 10.1016/s0168-3659(01)00289-9. [DOI] [PubMed] [Google Scholar]
- 15.Chaw CS, Yang YY, Lim IJ, Phan TT. Water-soluble betamethasone-loaded poly(lactide-co-glycolide) hollow microparticles as a sustained release dosage form. Journal of Microencapsulation. 2003;20(3):349–359. doi: 10.1080/0265204021000058447. [DOI] [PubMed] [Google Scholar]
- 16.Xiong Y, Xiao KP, Rustum AM. Development and validation of a stability-indicating RP-HPLC method to separate low levels of dexamethasone and other related compounds from betamethasone. Journal of Pharmaceutical and Biomedical Analysis. 2009;(49):646–654. doi: 10.1016/j.jpba.2008.12.023. [DOI] [PubMed] [Google Scholar]
- 17.Huber U, Onigbinde AO. HPLC Analysis of Antibacterial Drugs with Penicillin-Like Structure. Agilient Technologies. Published September 10, 2002. [Google Scholar]
- 18.Zhou X, He J, Du H, Fan Y, et al. Pharmacokinetic and pharmacodynamic profiles of recombinant human erythropoietin-loaded poly(lactic-co-glycolic acid) microspheres in rats. Acta Pharmacologica Sinica. 2012;(33):137–134. doi: 10.1038/aps.2011.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.D’Souza S, Faraj JA, Giovagnoli S, et al. In vitro–in vivo correlation from lactide-co-glycolide polymeric dosage forms. Progress in Biomaterials. 2014;3(2):131–142. doi: 10.1007/s40204-014-0029-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cardot J-M, Tomic I. In vitro in vivo correlation basis and application to slow release injectable formulation, a review. Farmacia. 2015;63(6):781–791. [Google Scholar]
- 21.Larsen C, Larsen SW, Jensen H, Yaghmur A, Ostergaard J. Role of in vitro release models in formulation development and quality control of parenteral depots. Expert Opinion on Drug Delivery. 2009;6(12):1283–1295. doi: 10.1517/17425240903307431. [DOI] [PubMed] [Google Scholar]
- 22.Wang X, Fernandez R, Dellamary L, et al. Pharmacokinetics of Dexamethasone Solution following Intratympanic Injection in Guinea Pig and Sheep. Audiology and Neurotology. 2011;16:233–241. doi: 10.1159/000320611. [DOI] [PubMed] [Google Scholar]
- 23.Lei T, Fernandez-Fernandez A, Tang Y, Carvajal D, Manchanda R, Kazmi SZ, McGoron A. A comparative study of IR-820 and indocyanine green (ICG) Journal of Nuclear Medicine. 2010;51(supplement 2):225–225. [Google Scholar]
- 24.Jo M, Kim C, Koh S, Nam G, Jeong H, Lee J, et al. Expression and Distribution of Tumor Necrosis Factor-Alpha in Mice Cochlea Exposed to Noise. Otology. 2010;53(9):527–533. [Google Scholar]
- 25.So H, Kim H, Lee J-H, Park C, Kim Y, Kim E, et al. Cisplatin Cytotoxicity of Auditory Cells Requires Secretions of Proinflammatory Cytokines via Activation of ERK and NF-κB. Journal of the Association for Research in Otolaryngology. 2007;8(3):338–355. doi: 10.1007/s10162-007-0084-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thorne M, Salt A, DeMott J, Henson M, Henson O, Gewalt S. Cochlear fluid space dimensions for six species derived from reconstructions of three-dimensional magnetic resonance images. Laryngoscope. 1999;109(10):1661–1668. doi: 10.1097/00005537-199910000-00021. [DOI] [PubMed] [Google Scholar]
- 27.Schnabl J, Glueckert R, Feuchtner G, et al. Sheep as a large animal model for middle and inner ear implantable hearing devices: a feasibility study in cadavers. Otology & neurotology: The American Otological Society, American Neurotology Society and European Academy of Otology and Neurotology. 2012;(33):481–489. doi: 10.1097/MAO.0b013e318248ee3a. [DOI] [PubMed] [Google Scholar]