Abstract
BACKGROUND: Additional prognostic markers are needed for better treatment stratification of stage II colon cancer (CC). We investigated the prognostic value of tumor-infiltrating lymphocytes (TILs) in a true population-based cohort of patients with stage II CC. MATERIAL AND METHODS: A total of 573 patients were included. Tumor blocks representing the deepest invasive part of the primary tumor were used for analysis. CD3+ and CD8+ TILs at the invasive front were evaluated by immunohistochemistry on whole tumor sections. The invasive area was manually outlined, and Visiopharm Integrator System software was used for quantification. Data were dichotomized for comparison with clinical data. The prognostic value was investigated in Cox proportional-hazard models for recurrence-free survival (RFS) and overall survival (OS). RESULTS: Low CD3+ or CD8+ TILs were significantly associated with poor RFS and OS (P = .0021 and P ≤ .0009, respectively, log-rank test). In multiple Cox regression analysis, low CD3+ and CD8+ TILs were associated with reduced RFS with hazard ratio (HR) = 1.386 (95% CI 1.039-1.850), P = .026, and HR = 1.394 (95% CI 1.029-1.890), P = .032, respectively, independent of age, T-stage, localization, perforation, and microsatellite instability (MSI). In the subgroups of patients with low CD3+ or CD8+ TILs, there was no difference in survival between patients with MSI and microsatellite-stable tumors, (P = .821 and P = .907, respectively). CONCLUSION: Low CD3+ and CD8+ TILs in the invasive area are both related to inferior prognosis of stage II CC, and we recommend either of these parameters to be considered as additional high-risk factor.
Introduction
Patients with stage II colon cancer (CC) generally have a good prognosis, with a 5-year overall survival (OS) of 70%-80% after surgery alone [1], and the efficiency of adjuvant chemotherapy is often debated. Current international guidelines (ASCO and ESMO) do not recommend routine adjuvant treatment. Such therapy should be limited to patients at high risk of recurrence [2], [3]. However, the current risk factors are insufficient for ideal selection of adjuvant therapy [4], and there is a need for additional prognostic markers for better clinicoprognostic stratification of stage II CC. In this context, tumor-infiltrating lymphocytes (TILs) may represent a promising marker. So far, the TNM classification is considered the most valuable system for categorization, and TILs have been proposed as a component for classification of primary colorectal carcinomas [5]. TILs have been investigated in various settings in colorectal cancer (CRC), but most studies include heterogeneous study populations with both colon and rectal cancer. Preoperative treatment of rectal cancer with radiotherapy is known to decrease the number of TILs [6] and thus might complicate the interpretation of the prognostic value. Few studies have exclusively investigated CC [7], [8], [9], and they include different stages of the disease. Hence, conclusions regarding stage II CC are difficult to extract.
To facilitate TILs as a clinical biomarker in CRC, an Immunoscore has been proposed, and a multinational investigation of the practical implementation and clinical impact is in progress [10]. The Immunoscore is based on estimates of two different populations of T-lymphocytes counted in two different areas of the tumor and defined as the central and the invasive front. TILs mainly consist of T-cells (CD3+), including a subgroup of cytotoxic T-cells (CD8+), which have the ability to kill target cells. For prognostic purposes, CD3+ and CD8+ TILs have appeared to be informative. Galon et al. investigated CD3+ cells in the central part of the tumor and at the invasive margin with an improved prognostic value obtained by the latter [11].
Several studies of CRC have demonstrated high occurrence of CD3+ [12], [13] and CD8+ TILs [11], [14], [15] to be associated with improved survival, but separate data for stage II CC are limited. Only one study by Lee et al. [16] has exclusively investigated TILs in stage II CC. They demonstrated a prognostic value of CD3+ TILs, but their cohort was small (N = 87), and their results were not of independent significance in a multiple Cox analysis. Furthermore, they did not investigate CD8+ TILs. Thus, the prognostic value of CD3+ and CD8+ TILs in stage II CC remains largely unknown.
With this motivation, the objective of the present study was to evaluate the prognostic impact of CD3+ and CD8+ TILs in the invasive tumor front in a nationwide population-based cohort of stage II CC.
Material and Methods
This study is reported in accordance with the REMARK criteria [17].
Patient Population
All patients treated surgically for stage II CC in 2002 in Denmark (N = 746) were identified by a search in the nationwide registry administered by the Danish Colorectal Cancer Group (DCCG). The database contains prospectively collected surgical and pathological data. All pathology departments in Denmark agreed to participate in the study, and thus the cohort represents the entire Danish population of stage II CC in 2002. The following exclusion criteria applied: tumor blocks missing in archives (N = 11), insufficient tissue for analysis (N = 1), stage II not recognized in new sections cut (N = 21), suspicion of stage IV (N = 4), patients treated with adjuvant chemotherapy (N = 26)/radiotherapy (N = 1), and death within 90 days of surgery (N = 75). Patients with loco-advanced disease (N = 8) were excluded, and the same applied to patients diagnosed with another malignancy before primary CC (N = 26). The final study population comprised 573 patients.
Tumors located from the cecum to the transversum were defined as right-sided, and those located from the left colonic flexure to the sigmoideum were defined as left-sided.
Patients with histologically verified recurrence were identified through PatoBanken, i.e., the national registry containing all pathology reports in Denmark. Patients diagnosed with other histologically verified malignancies were also identified using this registry and subsequently censored from the recurrence-free survival (RFS) analysis on the date of their new cancer diagnosis. Information on treatment with adjuvant chemotherapy, nonhistologically verified recurrence, or diagnosis of other malignancies was obtained from the National Patient Registry containing information on all Danish citizens' contacts to the healthcare system linked by the unique personal identification number.
The follow-up period was 7 years and began at the date of surgery. This interval was selected since most patients experienced recurrence within 5 years from being diagnosed with their primary disease and 7 years thus encompasses the majority of recurrences. Data on T-category, malignancy grade, localization, number of lymph nodes, and neural and vascular invasion were obtained from the pathology reports. Whenever inconclusive on T-stage or differentiation, the decision was made by microscopy of new sections. Information on tumor perforation, ileus, and type of surgery was obtained partly from the pathology reports and partly from the DCCG Registry based on the surgeon's report.
The study was approved by The Regional Committees on Health Research Ethics for Southern Denmark (S-20140119) and the Danish Data Protection Agency (14/26345). All patients were screened in the Danish Registry of Tissue Utilization before enrolment in the study.
Samples
Archived formalin-fixed, paraffin-embedded tissue blocks were stored and transported at room temperature. One tumor block representing the deepest invasive margin was selected from each tumor by microscopy performed by first a trainee and afterwards an experienced pathologist.
Immunohistochemistry
Serial 4-μm sections were cut from the selected tumor blocks and mounted on FLEX IHC Microscope Slides (K8020, DAKO, Glostrup, Denmark). The pretreatment processes were performed using PT Link (DAKO). Heat-induced epitope retrieval was achieved with Envision Target Retrieval Solution (DAKO) at pH 9 and 97°C for 20 minutes.
Staining was performed using a DAKO Autostainer Link 48 (DAKO). Endogenous peroxidase activity was blocked by Envision FLEX Peroxidase-Blocking Reagent (DAKO). The primary antibodies were mouse monoclonal anti-CD3 (code M7254, DAKO) diluted 1:600 and anti-CD8 (code M7103, DAKO) diluted 1:300. For mismatch repair, we used monoclonal mouse antibody against MLH1 (Novocastra, Leica, Germany, clone ES05, dilution 1:100, product code NCL-L-MLH1), MSH2 (Novocastra, Leica, clone 25D12, dilution 1:100, product code NCL-L-MSH2), MSH6 (BD Transduction Laboratories, clone 44/MSH6, dilution 1:200, material number 610919), and PMS2 (BD Pharmingen, clone A16-4, dilution 1:500, material number 556415).
The primary antibodies were diluted with Envision Flex antibody diluent (code S2022 DAKO) and incubated for 30 minutes at room temperature. For amplification, Envision Flex+ Mouse(Linker) (DAKO) was used for 20 minutes. Bound antibodies were detected using Envision FLEX/HRP (DAKO) and visualized by Envision FLEX DAB (DAKO) and chromogene diluted in Envision Flex Substrate Buffer (DAKO). To enhance the immunohistochemical stains, the sections were incubated in 0.5% CuSO4 in TBS buffer pH 7.6 for 10 minutes. Meyer's hematoxylin (Merck, Damstadt, Germany) was used as counterstain, and finally, the histological slides were cover-slipped with Tissue-Tek PERTEX (Histolab Products AB, Göteborg, Sweden).
Quantification of TILs
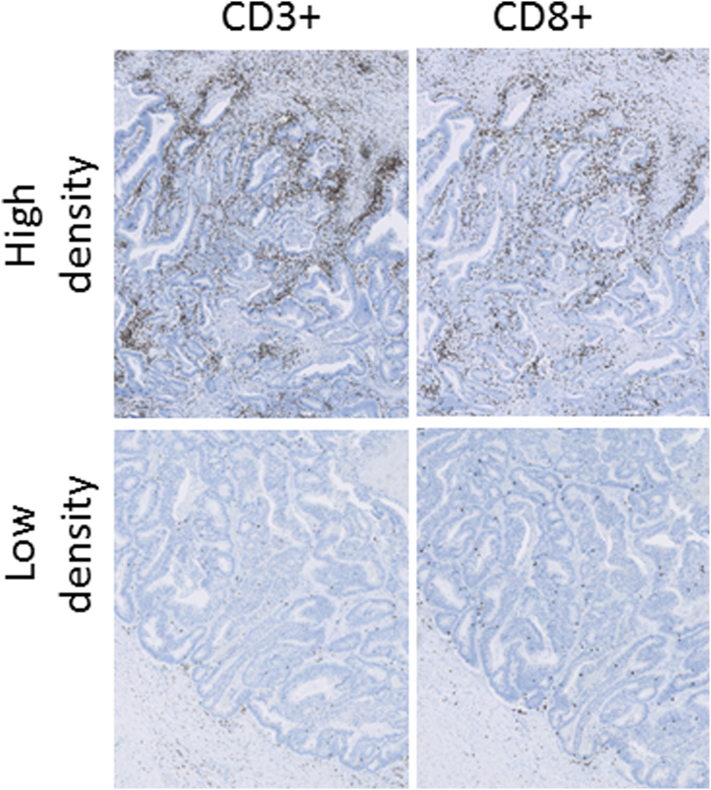
Sections were scanned at 40× magnification using a NanoZoomer XR scanner (Hamamatsu, Japan). The image format was NanoZoomer Digital Pathology Image (*.ndpi) with a resolution of 226 nm/pixel (112,389 dots per inch, i.e., 4.4 × 4.4 pixels/μm corresponding to a final magnification of ×1.558). The quantification of TILs was performed by digital image analysis using Visiopharm Integrator System software (VIS; Visiopharm A/S, Hoersholm, Denmark) (Figure 1). Regions of interest were manually delineated by the observer in the software as an invasive area outlined as the outermost front of the adenocarcinoma, including the deepest invasive front of the tumor, areas with tumor budding and/or irregular tumor islands. TILs were quantified as immune densities (cells/mm2) within both cancer epithelium (intratumoral) and tumor-associated stroma (peritumoral) as previously described [18]. In brief, we used an app-based image analysis algorithm developed specifically for counting of CD3+ and CD8+ lymphocytes. Artifacts, including tissue folds and clefts, mucin, fatty tissue, and necrosis, were automatically excluded to avoid contributions from regions of no interest.
Figure 1.
Photomicrographs of CD3 and CD8 immunohistochemistry. The panels on the top show high cell densities, and the panels on the bottom show low densities.
Statistics
OS was defined as the time between date of primary surgery and date of death from any cause or the date of last follow-up. RFS was defined as the time from date of primary surgery until date of death from any cause or the date of first locoregional or distant recurrence. Patients diagnosed with another cancer were censored at the date of diagnosis. The Kaplan-Meier method was used to present survival curves, and the log-rank test was used to test for significant differences in survival time among the groups. We used the multiple Cox regression model with a hazard ratio (HR) of 1.0 as reference and a 95% confidence interval (CI). A cutoff significance level of 0.10 was prespecified for a parameter to be included in the multiple Cox regression model.
The continuous TILs data were categorized into high and low using cutoffs at 350 cells /mm2 for CD3+ and at 200 cells/mm2 for CD8+ corresponding to the lower tertiles in both cases. Analyses of associations between clinicopathological variables were carried out using the chi-square test. All statistical analyses were performed using the STATA software version 14.0 (StataCorp, College Station, TX), and all statistical tests were two-sided with P values less than .05 considered significant.
Results
Patient Characteristics
Patient characteristics are summarized in Table 1. In the follow-up period of 7 years, 266 (46.4%) patients died; 110 (19.2%) recurred, and 78 (13.6%) patients were diagnosed with another cancer. The median age at time of surgery was 73 years (range 29-95), and the mean follow-up time was 6.9 years (range 3-84 months).
Table 1.
Patient Characteristics and Association to CD3+ and CD8+ TILs⁎
| Number (n = 573) (%) | CD3+ TILs |
CD8+ TILs |
|||||
|---|---|---|---|---|---|---|---|
| High (%) | Low (%) | P Value | High (%) | Low (%) | P Value | ||
| Age (years) at diagnosis | |||||||
| Median | 73 | ||||||
| Range | 29-95 | ||||||
| ≥73 | 268 (47) | 185 (48) | 83 (45) | .475 | 211 (48) | 57 (42) | .165 |
| <73 | 305 (53) | 202 (52) | 103 (55) | 225 (52) | 80 (58) | ||
| Gender | |||||||
| Male | 284 (50) | 185 (48) | 99 (53) | .224 | 217 (50) | 67 (49) | .860 |
| Female | 289 (50) | 202 (52) | 87 (47) | 219 (50) | 70 (51) | ||
| T-stage | |||||||
| pT3 | 501 (87) | 342 (88) | 159 (85) | .329 | 383 (88) | 118 (86) | .598 |
| pT4 | 72 (13) | 45 (12) | 27 (15) | 53 (12) | 19 (14) | ||
| Histology (WHO) | |||||||
| Adenocarcinoma NOS | 516 (89) | 353 (91) | 163 (88) | .076 | 393 (90) | 123 (90) | .687 |
| Mucinous adenocarcinoma | 55 (10) | 34 (9) | 21 (11) | 42 (10) | 13 (9) | ||
| Signet-ring cell carcinoma | 2 (1) | 0 | 2 (1) | 1 (0.2) | 1 (1) | ||
| Malignancy grade | |||||||
| Medium + low | 451 (79) | 298 (77) | 153 (82) | .150 | 334 (77) | 117 (85) | .028 |
| High⁎ | 122 (21) | 89 (23) | 33 (18) | 102 (23) | 20 (15) | ||
| Localization | |||||||
| Right | 273 (48) | 195 (50) | 78 (42) | .058 | 219 (50) | 54 (39) | .027 |
| Left | 300 (52) | 192 (50) | 108 (58) | 217 (50) | 83 (60) | ||
| Tumor perforation | |||||||
| Yes | 17 (3) | 9 (2) | 8 (4) | .192 | 12 (3) | 5 (4) | .629 |
| No | 531 (93) | 361 (98) | 170 (96) | 402 (97) | 129 (96) | ||
| Unknown | 25 (4) | ||||||
| Lymph nodes | |||||||
| Median | 10 | ||||||
| Range | 0-41 | ||||||
| <12 nodes | 352 | 218 (56) | 134 (72) | <.001 | 256 (59) | 96 (70) | .017 |
| ≥12 nodes | 221 | 169 (44) | 52 (28) | 180 (41) | 41 (30) | ||
| Perineural invasion | |||||||
| Yes | 26 (5) | 13 (5) | 13 (10) | .068 | 13 (5) | 13 (13) | .003 |
| No | 360 (63) | 243 (95) | 117 (90) | 275 (95) | 85 (87) | ||
| Not assessed | 187 (32) | ||||||
| Vascular invasion | |||||||
| Yes | 43 (7) | 27 (9) | 16 (11) | .538 | 33 (10) | 10 (9) | .823 |
| No | 387 (68) | 261 (91) | 126 (89) | 291 (90) | 96 (91) | ||
| Not assessed | 143 (25) | ||||||
| Mismatch repair status | |||||||
| MSS | 400 (70) | 243 (63) | 157 (84) | <.001 | 279 (64) | 121 (88) | <.001 |
| MSI | 173 (30) | 144 (37) | 29 (16) | 157 (36) | 16 (12) | ||
Abbreviations: MSI, microsatellite instability; MSS, microsatellite stable; NOS, not otherwise specified.
P values are obtained using chi2 test.
Including mucinous adenocarcinomas and signet-ring cell carcinomas.
Tumor-Infiltrating Lymphocytes
The mean density of TILs was 607 (SD ± 441) cells/mm2 for CD3+ and 470 (SD ± 394) cells/mm2 for CD8+. After dichotomizing the continuous data, 186 (32.5%) CCs were classified as low-CD3+ and 387 (67.5%) CCs were classified as high-CD3+. Regarding CD8, 185 (32.3%) and 338 (67.7%) CCs were classified as low-CD8+ and high-CD8+, respectively. A total of 124 tumors were classified as low TILs for both CD3+ and CD8+, while 374 CCs were classified as high TILs for both CD3+ and CD8+. The remaining 75 tumors were classified as combined high and low.
Low occurrence of CD3+ and CD8+ TILs was related to left-sided CC (P = .058 and .027), low number of retrieved lymph nodes (P < .001 and .017), and microsatellite stability (MSS) (P < .001). Furthermore, low CD8+ TILs were associated with perineural invasion (P = .003) and medium/low malignancy grade (P = .028).
Survival Analyses
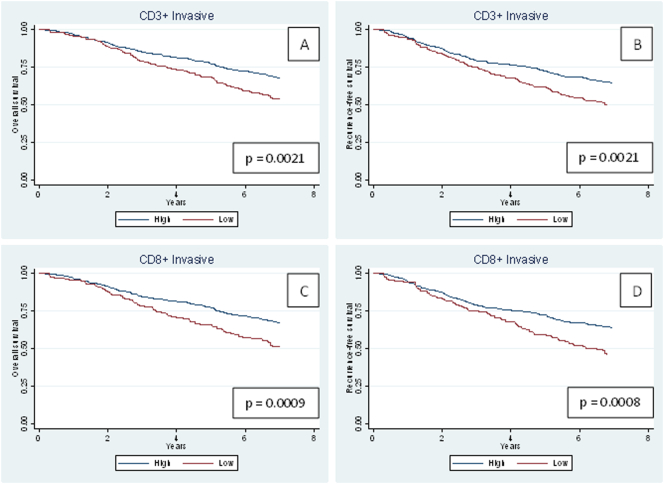
The 5-year RFS rate for the population with low CD3+ TILs was 61.7% versus 72.7% in the group of patients with high CD3+ TILs, and OS was 68.5% versus 77.4%. Similarly, the 5-year RFS rate for the population with low CD8+ TILs was 59.5% versus 72.2% in the group of patients with high CD8+ TILs, and OS was 65.6% versus 77.3%. Significant differences were observed for both RFS and OS (Figure 2).
Figure 2.
Kaplan-Meier survival curves for overall survival and recurrence-free survival.
(A-B) CD3+ TILs and (C-D) CD8+ TILs in the total patient population (n = 573).
Invasive refers to the area of the outermost front of the adenocarcinoma, including the deepest invasive front of the tumor, areas with tumor budding, and/or irregular tumor islands.
Results from the corresponding simple and multiple Cox regression analyses are shown in Table 2, Table 3. Age ≥ 73, T4 tumor, and perforation were significantly related to an adverse outcome regarding OS. In addition, localization and MSI status were nearly significantly related to RFS. In the simple analysis, low CD3+ and CD8+ TILs were related to adverse outcome of both OS and RFS. In the multiple analyses, low CD3+ and CD8+ TILs were associated with reduced RFS, HR = 1.386 (1.039-1.850), P = .026 and HR = 1.394 (1.029-1.890), P = .032, respectively, and reduced OS, HR = 1.532 (1.142-2.054), P = .004 and HR = 1.591 (1.169-2.166), P = .003, respectively, independent of age, T-stage, localization, perforation, and MSI status.
Table 2.
Cox Regression Analysis, OS (n = 573)⁎
| Parameter | Simple Analysis |
Multiple Analysis (CD3+ TILs)) |
Multiple Analysis (CD8+ TILs) |
|||
|---|---|---|---|---|---|---|
| HR (95% CI) | P Value | HR (95% CI) | P Value | HR (95% CI) | P Value | |
| Age | ||||||
| <73 | 1 | <.001 | 1 | <.001 | 1 | <.001 |
| ≥73 | 2.405 (1.774-3.260) | 2.650 (1.930-3.638) | 2.639 (1.922-3.622) | |||
| Sex | ||||||
| Male | 1 | .448 | - | - | - | - |
| Female | 0.897 (0.678-1.187) | |||||
| T-category | ||||||
| T3 | 1 | .007 | 1 | .004 | 1 | .003 |
| T4 | 1.662 (1.148-2.407) | 1.748 (1.193-2.561) | 1.777 (1.213-2.602) | |||
| Malignancy grade | ||||||
| Medium/Low | 1 | 0.292 | - | - | - | - |
| High⁎ | 1.193 (0.859-1.656) | |||||
| Localization | ||||||
| Right | 1 | 0.201 | - | - | - | - |
| Left | 1.202 (0.907-1.593) | |||||
| Tumor perforation | ||||||
| No | 1 | .033 | 1 | .017 | 1 | .021 |
| Yes | 2.072 (1.059-4.052) | 2.311 (1.161-4.600) | 2.254 (1.132-4.489) | |||
| Lymph nodes | ||||||
| <12 nodes | 1 | .295 | - | - | - | - |
| ≥12 nodes | 0.856 (0.640-1.145) | |||||
| Perineural invasion | ||||||
| No | 1 | .153 | - | - | - | - |
| Yes | 1.540 (0.851-2.786) | |||||
| Vascular invasion | ||||||
| No | 1 | .409 | - | - | - | - |
| Yes | 1.237 (0.747-2.047) | |||||
| Mismatch repair status | ||||||
| MSS | 1 | .272 | - | - | - | - |
| MSI | 0.839 (0.613-1.148) | |||||
| CD3-invasive | ||||||
| High | 1 | .002 | 1 | .004 | - | - |
| Low | 1.560 (1.173-2.076) | 1.532 (1.142-2.054) | ||||
| CD8-invasive | ||||||
| High | 1 | .001 | - | - | 1 | .003 |
| Low | 1.660 (1.229-2.245) | 1.591 (1.169-2.166) | ||||
Invasive refers to the area of the outermost front of the adenocarcinoma, including the deepest invasive front of the tumor, areas with tumor budding, and/or irregular tumor islands.
Including mucinous adenocarcinomas and signet-ring cell carcinomas.
Table 3.
Cox Regression Analysis, RFS (n = 573)⁎
| Parameter | Simple Analysis |
Multiple Analysis (CD3+ TILs) |
Multiple Analysis (CD8+ TILs) |
|||
|---|---|---|---|---|---|---|
| HR (95% CI) | P Value | HR (95% CI) | P Value | HR (95% CI) | P Value | |
| Age | ||||||
| <73 | 1 | <.001 | 1 | <.001 | 1 | <.001 |
| ≥73 | 1.854 (1.405-2.446) | 1.999 (1.499-2.668) | 2.006 (1.504-2.675) | |||
| Sex | ||||||
| Male | 1 | 0.440 | - | - | - | - |
| Female | 0.901 (0.692-1.174) | |||||
| T-category | ||||||
| T3 | 1 | .009 | 1 | .012 | 1 | .011 |
| T4 | 1.612 (1.128-2.305) | 1.602 (1.108-2.315) | 1.611 (1.115-2.328) | |||
| Malignancy grade | ||||||
| Medium/Low | 1 | .820 | - | - | - | - |
| High⁎ | 1.038 (0.754-1.429) | |||||
| Localization | ||||||
| Right | 1 | .094 | 1 | .159 | 1 | .187 |
| Left | 1.256 (0.962-1.639) | 1.251 (0.916-1.709) | 1.233 (0.903-1.685) | |||
| Tumor perforation | ||||||
| No | 1 | .010 | 1 | .014 | 1 | .016 |
| Yes | 2.301 (1.218-4.349) | 2.257 (1.178-4.325) | 2.230 (1.161-4.280) | |||
| Lymph nodes | ||||||
| <12 nodes | 1 | .453 | - | - | - | - |
| ≥12 nodes | 0.901 (0.685-1.184) | |||||
| Perineural invasion | ||||||
| No | 1 | .112 | - | - | - | - |
| Yes | 1.585 (0.898-2.799) | |||||
| Vascular invasion | ||||||
| No | 1 | .708 | - | - | - | - |
| Yes | 1.100 (0.667-1.816) | |||||
| Mismatch repair status | ||||||
| MSS | 1 | .057 | 1 | .455 | 1 | .400 |
| MSI | 0.746 (0.552-1.009) | 0.870 (0.603-1.255) | 0.855 (0.594-1.231) | |||
| CD3-invasive | ||||||
| High | 1 | .001 | 1 | .026 | - | - |
| Low | 1.549 (1.183-2.030) | 1.386 (1.039-1.850) | ||||
| CD8-invasive | ||||||
| High | 1 | .001 | - | - | 1 | .032 |
| Low | 1.621 (1.219-2.156) | 1.394 (1.029-1.890) | ||||
Invasive refers to the area of the outermost front of the adenocarcinoma, including the deepest invasive front of the tumor, areas with tumor budding, and/or irregular tumor islands.
Including mucinous adenocarcinomas and signet-ring cell carcinomas.
Combining MSI status and TILs revealed a better prognosis for MSS tumors with high TILs than MSI tumors with low TILs (Figure 3). There was no difference in survival between patients with MSI and MSS tumors in the subgroups of patients with low CD3+ (N = 186) or CD8+ (N = 137) TILs, P = .821 and P = .907, respectively. The subgroup of patients with MSI tumors (N = 173) may experience decreased survival in case of low CD3+ or CD8+ TILs compared to MSI tumors with high CD3+ or CD8+ TILs. However, the difference did not reach statistical significance (P = .0763 and P = .1060, respectively).
Figure 3.
Kaplan-Meier survival curves for recurrence-free survival for different combinations of TILs and MSI status (N = 573).
(A) High CD3+ TILs versus low CD3+ TILs and (B) high CD8+ TILs versus low CD8+ TILs.
Invasive refers to the area of the outermost front of the adenocarcinoma, including the deepest invasive front of the tumor, areas with tumor budding, and/or irregular tumor islands.
Discussion
We investigated the prognostic value of CD3+ and CD8+ TILs in the invasive front of adenocarcinomas in an unbiased, nationwide, population-based cohort of stage II CC patients treated exclusively with surgery. We demonstrated an independent prognostic impact on both OS and RFS with similar prognostic power afforded by CD3+ and CD8+ TILs.
Previous studies reporting data on stage II CRC and CD3+ TILs have documented a prognostic value [12], [13]; however, these studies only included a subgroup of patients with stage II CC. Furthermore, CD3+ TILs were evaluated as area fractions [12] or semiquantitatively [13], while we evaluated TILs as density estimates using automatic digital image analysis. Other studies using image analysis report conflicting results. Nosho et al. [19] did not find densities of either CD3+ or CD8+ TILs to be associated with survival in their study of CRC. They included all stages, and stage II disease only represented a subgroup (N = 236). TILs were evaluated by image analysis but only quantified in the neoplastic epithelial cells, not in the tumor-associated stroma. Consequently, the TILs count by Nosho et al. might not reflect the real immune reaction against the tumor cells occurring in the tumor microenvironment. Turksma et al. [8] evaluated TILs in stage II and III CC using image analysis and demonstrated a prognostic value of both CD3+ and CD8+ TILs. In this study, the prognostic impact was related to stromal CD3+ and tumor epithelial CD8+ T-cells. We did not distinguish between stromal and epithelial localization of T-cells but quantified the two groups of TILs together. In accordance with previous studies [16], our impression is that the highest density of TILs is in the stromal compartment. We found mean densities of CD3+ to be 607 cells/mm2 in the invasive area, which are comparable with results from the stromal compartment in the study by Lee et al. [16]. They evaluated TILs separately in the intraepithelial and stromal compartments in stage II CC and found a significant association between CD3+ TILs and OS as well as disease-free survival when combining measurements from both compartments. However, their study only included 87 patients, and their results were not significant in the multivariate analysis. We investigated a large population-based cohort, and our result remained significant in the multivariate analysis independent of age, T-stage, localization, perforation, and MSI status. Also, we investigated TILs in the entire invasive area of whole tumor slides, while Lee et al. used hotspot sampling [16].
The Immunoscore project [5] includes combinations of TILs. We investigated the prognostic value of the combination of CD3+ and CD8+ TILs, but this did not add any additional prognostic value (data not shown).
We found MSI tumors to have a significantly higher occurrence of TILs compared to MSS tumors, which is in agreement with previous studies [8], [20]. The marked intratumoral infiltration of inflammatory cells in MSI tumors might be related to the genetic instability in this group of tumors. The unreliable DNA repair in MSI tumors results in the production of a greater number of abnormal peptides (i.e., tumor specific antigens) that may trigger the immune system, including recruitment and activation of cytotoxic T-cells [21]. MSI status is known to have a strong prognostic value, and stage II CRC patients with MSI tumors are often found to have a more favorable prognosis [22]. This may partly be explained by the benefit from T-cell infiltration.
The activated cytotoxic T-cells are capable of killing the cancer cells, either by production and release of cytotoxic proteins such as perforin and granzymes or destruction via Fas/FasL interactions [23].
Consistent with previous studies [8], [13], [21], we may hypothesize the subgroup of patients with MSI tumors and low presence of CD3+ or CD8+ TILs to have decreased survival compared to MSI tumors with high presence of CD3+ or CD8+ TILs. Interestingly, we found no difference in RFS between patients with MSI and MSS tumors in the subgroup of patients with low TILs. Dahlin et al. found no difference in cancer-specific survival between MSI and MSS patients with a similar degree of CD3+ T-cell infiltration [13], but another study of all stages of CRC [14] reported MSS tumors with high infiltration of CD8+ TILs to have a better OS. In our study, the subgroups of patients with MSI and low TILs were small. This was expected, but particular attention should be paid to this group of patients since TILs may be an important prognostic marker in combination with MSI status.
Some MSS tumors display a marked inflammatory reaction with high presence of TILs.
Giannakis et al. [24] performed whole exome sequencing of 619 CRC and showed significant association between high neoantigen load and high degree of TILs in analysis restricted to MSS tumors. Thus, also within MSS tumors, the neoantigen load is a determinant for the immune cell infiltration. On the other hand, some MSI tumors display low TILs. This might be related to low neoantigen load; however, the mutational burden in this group of patients remains to be investigated.
The present study is limited by the retrospective design, as we had no influence on the preanalytical phase of the IHC. Moreover, data on recurrence and death were obtained from registers, and the individual patient records have not been evaluated. Especially the cause of death may be questionable, and therefore, we did not use a cancer-specific endpoint.
The tumor microenvironment is complex, and the presence of specific T-cell subsets should be interpreted with caution, as other factors in the tumor microenvironment may influence the composition of TILs and thus tumor progression. However, CD3+ and CD8+ TILs represent variables investigated in various settings, and the study group behind the Immunoscore recommends analysis of these two particular T-cell groups [11].
Conclusion
In conclusion, a low level of CD3+ or CD8+ TILs in the invasive tumor front is related to an inferior prognosis in patients with stage II CC. We recommend either low CD3+ or CD8+ TILs at the invasive front to be considered as an additional high-risk parameter. Particular attention should be paid to future studies of patients with MSI tumors and low CD8+ TILs, as the prognosis of this subgroup may be unexpectedly poor.
Availability of Data and Material
Data generated and analyzed in this study are included in this article. Raw data can be obtained from the corresponding author.
Competing Interests
The authors declare that there are no conflicts of interest.
Authors' Contributions
Conceived and designed the experiments: A. C. E., F. B. S., S. K. F., J. L., and T. F. H.; performed the experiments: A. C. E.; analyzed the data: A. C. E., R. D., and T. F. H.; wrote the paper: A. C. E., F. B. S., H. H., J. L., and T. F. H. All authors read and approved the final manuscript.
Acknowledgements
We thank Birgit Roed Sørensen for excellent technical assistance and Karin Larsen for linguistic editing of the manuscript. We also thank all pathology departments in Denmark for their support and participation in the study, which have made the population-based design possible. Also, we appreciate the help and advice by OPEN, Odense Patient data Explorative Network, Odense University Hospital, Odense, Denmark.
Footnotes
Funding: The study was conducted and affiliated under Danish Colorectal Cancer Group and Danish Colorectal Cancer Center South, Vejle Hospital (Part of Lillebaelt Hospital), Denmark. Financial support was granted by The Research Council of Lillebaelt Hospital, the Danish Cancer Research Foundation, and A.P. Møller and hustru Chastine Mc-Kinney Møller Foundation. The funding organizations did not play any role in the study design, data collection, analysis or interpretation of the data, decision to publish, or preparation of the manuscript.
References
- 1.Morris M, Platell C, McCaul K, Millward M, van Hazel G, Bayliss E, Trotter J, Ransom D, Iacopetta B. Survival rates for stage II colon cancer patients treated with or without chemotherapy in a population-based setting. Int J Colorectal Dis. 2007;22:887–895. doi: 10.1007/s00384-006-0262-y. [DOI] [PubMed] [Google Scholar]
- 2.Labianca R, Nordlinger B, Beretta GD, Mosconi S, Mandala M, Cervantes A, Arnold D. Early colon cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2013;24:64–72. doi: 10.1093/annonc/mdt354. [DOI] [PubMed] [Google Scholar]
- 3.Benson AB, III, Schrag D, Somerfield MR, Cohen AM, Figueredo AT, Flynn PJ, Krzyzanowska MK, Maroun J, McAllister P, Van Cutsem E. American Society of Clinical Oncology recommendations on adjuvant chemotherapy for stage II colon cancer. J Clin Oncol. 2004;22:3408–3419. doi: 10.1200/JCO.2004.05.063. [DOI] [PubMed] [Google Scholar]
- 4.O'Connor ES, Greenblatt DY, LoConte NK, Gangnon RE, Liou JI, Heise CP, Smith MA. Adjuvant chemotherapy for stage II colon cancer with poor prognostic features. J Clin Oncol. 2011;29:3381–3388. doi: 10.1200/JCO.2010.34.3426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Galon J, Pages F, Marincola FM, Thurin M, Trinchieri G, Fox BA, Gajewski TF, Ascierto PA. The immune score as a new possible approach for the classification of cancer. J Transl Med. 2012;10(1) doi: 10.1186/1479-5876-10-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nagtegaal ID, Marijnen CA, Kranenbarg EK, Mulder-Stapel A, Hermans J, van de Velde CJ, van Krieken JH. Short-term preoperative radiotherapy interferes with the determination of pathological parameters in rectal cancer. J Pathol. 2002;197:20–27. doi: 10.1002/path.1098. [DOI] [PubMed] [Google Scholar]
- 7.Lavotshkin S, Jalas JR, Torisu-Itakura H, Ozao-Choy J, Lee JH, Sim MS, Stojadinovic A, Wainberg Z, Bifulco CB, Fox BA. Immunoprofiling for prognostic assessment of colon cancer: a novel complement to ultrastaging. J Gastrointest Surg. 2015;19:999–1006. doi: 10.1007/s11605-015-2759-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Turksma AW, Coupe VM, Shamier MC, Lam KL, de Weger VA, Belien JA, van den Eertwegh AJ, Meijer GA, Meijer CJ, Hooijberg E. Extent and Location of Tumor-Infiltrating Lymphocytes in Microsatellite-Stable Colon Cancer Predict Outcome to Adjuvant Active Specific Immunotherapy. Clin Cancer Res. 2016;22:346–356. doi: 10.1158/1078-0432.CCR-13-2462. [DOI] [PubMed] [Google Scholar]
- 9.Markl B, Wieberneit J, Kretsinger H, Mayr P, Anthuber M, Arnholdt HM, Schenkirsch G. Number of Intratumoral T Lymphocytes Is Associated With Lymph Node Size, Lymph Node Harvest, and Outcome in Node-Negative Colon Cancer. Am J Clin Pathol. 2016;145:826–836. doi: 10.1093/ajcp/aqw074. [DOI] [PubMed] [Google Scholar]
- 10.Galon J, Pages F, Marincola FM, Angell HK, Thurin M, Lugli A, Zlobec I, Berger A, Bifulco C, Botti G. Cancer classification using the Immunoscore: a worldwide task force. J Transl Med. 2012;10:205. doi: 10.1186/1479-5876-10-205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Galon J, Costes A, Sanchez-Cabo F, Kirilovsky A, Mlecnik B, Lagorce-Pages C, Tosolini M, Camus M, Berger A, Wind P. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006;313:1960–1964. doi: 10.1126/science.1129139. [DOI] [PubMed] [Google Scholar]
- 12.Laghi L, Bianchi P, Miranda E, Balladore E, Pacetti V, Grizzi F, Allavena P, Torri V, Repici A, Santoro A. CD3+ cells at the invasive margin of deeply invading (pT3-T4) colorectal cancer and risk of post-surgical metastasis: a longitudinal study. Lancet Oncol. 2009;10:877–884. doi: 10.1016/S1470-2045(09)70186-X. [DOI] [PubMed] [Google Scholar]
- 13.Dahlin AM, Henriksson ML, Van Guelpen B, Stenling R, Oberg A, Rutegard J, Palmqvist R. Colorectal cancer prognosis depends on T-cell infiltration and molecular characteristics of the tumor. Mod Pathol. 2011;24:671–682. doi: 10.1038/modpathol.2010.234. [DOI] [PubMed] [Google Scholar]
- 14.Deschoolmeester V, Baay M, Van Marck E, Weyler J, Vermeulen P, Lardon F, Vermorken JB. Tumor infiltrating lymphocytes: an intriguing player in the survival of colorectal cancer patients. BMC Immunol. 2010;11:19. doi: 10.1186/1471-2172-11-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Berntsson J, Svensson MC, Leandersson K, Nodin B, Micke P, Larsson AH, Eberhard J, Jirström K. The clinical impact of tumour-infiltrating lymphocytes in colorectal cancer differs by anatomical subsite: A cohort study. Int J Cancer. 2017;141:1654–1666. doi: 10.1002/ijc.30869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee WS, Park S, Lee WY, Yun SH, Chun HK. Clinical impact of tumor-infiltrating lymphocytes for survival in stage II colon cancer. Cancer. 2010;116:5188–5199. doi: 10.1002/cncr.25293. [DOI] [PubMed] [Google Scholar]
- 17.McShane LM, Altman DG, Sauerbrei W, Taube SE, Gion M, Clark GM. REporting recommendations for tumour MARKer prognostic studies (REMARK) Br J Cancer. 2005;93:387–391. doi: 10.1038/sj.bjc.6602678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eriksen AC, Andersen JB, Kristensson M, dePont Christensen R, Hansen TF, Kjaer-Frifeldt S, Sørensen FB. Computer-assisted stereology and automated image analysis for quantification of tumor infiltrating lymphocytes in colon cancer. Diagn Pathol. 2017;12:65. doi: 10.1186/s13000-017-0653-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nosho K, Baba Y, Tanaka N, Shima K, Hayashi M, Meyerhardt JA, Giovannucci E, Dranoff G, Fuchs CS, Ogino S. Tumour-infiltrating T-cell subsets, molecular changes in colorectal cancer, and prognosis: cohort study and literature review. J Pathol. 2010;222:350–366. doi: 10.1002/path.2774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Phillips SM, Banerjea A, Feakins R, Li SR, Bustin SA, Dorudi S. Tumour-infiltrating lymphocytes in colorectal cancer with microsatellite instability are activated and cytotoxic. Br J Surg. 2004;91:469–475. doi: 10.1002/bjs.4472. [DOI] [PubMed] [Google Scholar]
- 21.Guidoboni M, Gafa R, Viel A, Doglioni C, Russo A, Santini A, Del Tin L, Macrì E, Lanza G, Boiocchi M. Microsatellite instability and high content of activated cytotoxic lymphocytes identify colon cancer patients with a favorable prognosis. Am J Pathol. 2001;159:297–304. doi: 10.1016/S0002-9440(10)61695-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Merok MA, Ahlquist T, Royrvik EC, Tufteland KF, Hektoen M, Sjo OH, Mala T, Svindland A, Lothe RA, Nesbakken A. Microsatellite instability has a positive prognostic impact on stage II colorectal cancer after complete resection: results from a large, consecutive Norwegian series. Ann Oncol. 2013;24:1274–1282. doi: 10.1093/annonc/mds614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Weinberg RA. The biology of Cancer. Second Edition. New York: Garland. Science. 2014:641–689. [Google Scholar]
- 24.Giannakis M, Mu XJ, Shukla SA, Qian ZR, Cohen O, Nishihara R, Bahl S, Cao Y, Amin-Mansour A, Yamauchi M. Genomic Correlates of Immune-Cell Infiltrates in Colorectal Carcinoma. Cell Rep. 2016;15:857–865. doi: 10.1016/j.celrep.2016.03.075. [DOI] [PMC free article] [PubMed] [Google Scholar]