Abstract
Poly(dimethylsiloxane) (PDMS) is a material applicable for tissue and biomedical engineering, especially based on microfluidic devices. PDMS is a material used in studies aimed at understanding cell behavior and analyzing the cell adhesion mechanism. In this work, biological characterization of the modified PDMS surfaces based on cell attachment and toxicity assays was performed. We studied Balb 3T3/c, HMEC-1, and HT-29 cell adhesion on poly(dimethylsiloxane) surfaces modified by different proteins, with and without pre-activation with plasma oxygen and UV irradiation. Additionally, we studied how changing of a base and a curing agent ratios influence cell proliferation. We observed that cell type has a high impact on cell adhesion, proliferation, as well as viability after drug exposure. It was tested that the carcinoma cells do not require a highly specific microenvironment for their proliferation. Cytotoxicity assays with celecoxib and oxaliplatin on the modified PDMS surfaces showed that normal cells, cultured on the modified PDMS, are more sensitive to drugs than cancer cells. Cell adhesion was also tested in the microfluidic systems made of the modified PDMS layers. Thanks to that, we studied how the surface area to volume ratio influences cell behavior. The results presented in this manuscript could be helpful for creation of proper culture conditions during in vitro tests as well as to understand cell response in different states of disease depending on drug exposure.
I. INTRODUCTION
Cell adhesion plays an important role in the communication and regulation of cell functions. The cells have the ability to adhere to other cells or to the extracellular matrix (ECM).1,2 Cell-cell and cell-ECM communications are responded through adhesion molecules, integrin, and cytoskeleton. Additionally, cellular behavior is regulated by the physical and mechanical properties of the culture microenvironment.3 A mechanism analysis of cell adhesion could be helpful to understand the cell interaction and behavior in cell health and pathology.4 Alterations in ECM-cell and cell-cell interactions are cell-type specific. It should be emphasized that properties and features of the cells are dependent on their tissue origin, morphology, and disease state.5,6 Culturing various cell types on the same culture surface could result in a different growth rate, proliferation, differentiation, migration, and viability.7,8 A change in cell adhesion can also define the pathogenesis of diseases. Therefore, it is important to investigate the adhesion and growth of various cells on biomaterials, which could have medical applications.
Poly(dimethylsiloxane)—PDMS has recently been used as a material applicable for tissue and biomedical engineering, especially based on the microfluidic devices.9–11 Because of the biocompatibility of PDMS, it can be successfully utilized in cell and tissue cultures. It should be noted that the surface area to media volume (SA/V) ratio is much smaller in macroscale than in microfluidic devices. It could have an important influence on the behavior of the cells which are cultured in the microsystem. Although, there are some reports which could indicate that PDMS with high SA/V may not be inert for the cells,12–14 PDMS is known to be a good material for cell cultures in both macro- and microscales.4,15 Additionally, PDMS is a low cost, elastic, gas permeable, and optical transparent material.16,17 Although PDMS has numerous beneficial characteristics, it is a hydrophobic material which adsorbs proteins or small hydrophobic molecules.15 This is a significant drawback to PDMS application in adherent cell cultures. The PDMS surface can be modified to change its physicochemical properties and culture microenvironment. Thanks to this, PDMS is also a good material for understanding cell behavior and analyzing the cell adhesion mechanism. Various techniques used for modifying PDMS surfaces are described in the literature.11,18,19 Gas phase processing is a commonly used method for modification of PDMS surfaces. This method involves four techniques: plasma treatment, ultraviolet (UV) irradiation, chemical vapor deposition (CVD), and metal and metal oxide coating.18–21 Wet chemical methods are the other main type of techniques used for PDMS surface modification. They include: dynamic surface modification, deliberate protein adsorption, layer-by-layer (LBL) deposition, silanization, and sol-gel coating.22–25 These methods cause an increase in the number of hydroxyl groups (–OH) and a decrease in the water contact angle (WCA), which finally increases the hydrophilicity of the PDMS surface. Such a modified PDMS can be used to investigate cell behavior with and without external stimulation as well as for fabrication of microfluidic devices applied to biomedical applications.
Some studies based on the PDMS surface modification using proteins such as: fibronectin, laminin, collagen, and proliferation were performed on various cells (HeLa cells, stem cells, and HaCaT cells).26–29 PDMS surfaces are most often coated with proteins and only cell attachment is studied. The variety of the obtained results suggested that the physicochemical properties of PDMS strongly affect cell attachment depending on the cell type. Therefore, studying the adhesion of other cells is important to understand the mechanical properties of the cells and the interaction between the cell-substrate. In this work, we studied Balb 3T3/c, human microvascular endothelial (HMEC-1), and HT-29 cell adhesion on poly(dimethylsiloxane) surfaces modified by different proteins, with and without pre-activation with plasma oxygen and UV irradiation. We have chosen these cell types because of their different origin and morphology. The research has shown how physicochemical properties and cell type influences cell adhesion and proliferation. PDMS is composed of two components, a base and a curing agent. The literature states that a 10:1 ratio of these components is the best for microsystem fabrication and biological application.30,31 In addition to other studies, here we also studied how changing these component ratios influence cell proliferation. Additionally, cytotoxicity assays with celecoxib and oxaliplatin were performed on the modified PDMS surfaces. Cell adhesion was also tested in the microfluidic systems. We observed that cell type has a high impact on cell adhesion, proliferation, as well as viability after drug exposure. Such research is important to create the proper culture conditions during in vitro tests as well as to understand cell response in different states of the disease depending on drug exposure.
II. MATERIALS AND METHODS
A. Preparation of the modified PDMS samples
The PDMS mixture was prepared by mixing the PDMS prepolymer (base) and a curing agent (PDMS Sylgard®184, Dow Corning) in 5:1, 10:1, and 20:1 ratios by weight. Next, the degassed PDMS mixture was poured into a 24-well plate (300 μl per well) and cured at 100 °C for 5 h. After that, the PDMS surfaces were modified using surface activation and by adsorbing proteins.32 The samples were activated using oxygen plasma for 30 s (power of 40 W) (Diener) or irradiated with UV light for 60 min (Black Ray). Next, poly-L-lysine, fibronectin, laminin, gelatin or collagen were poured onto the modified surfaces and incubated for 24 h at 37 °C. Poly-L-lysine solution (0.01%) was prepared by the dilution of 0.1% poly-L-lysine (Sigma-Aldrich) in distilled water. 1 mg of fibronectin from human plasma (Sigma Aldrich) was dissolved in 1 ml of distilled water and next diluted in the final concentration—2.5 μg/ml. 0.5 mg/ml of laminin from human placenta (Sigma Aldrich) was diluted in distilled water to the final concentration, which equaled 2.5 μg/ml. 0.2% gelatin solution was made by dissolving gelatin from porcine skin (Sigma Aldrich) in distilled water at 80 °C for 30 min. 0.1% of collagen type I from calf skin (Sigma Aldrich) in acetic acid was diluted ten times in distilled water. Samples modified only by the protein coating (without a surface pre-activation) were also prepared. The unmodified PDMS samples (5:1, 10:1, and 20:1) were used as negative control samples, whereas the polystyrene (PS) surface was used as a positive control.
B. Cell culture
The unmodified and modified PDMS samples were used for in vitro studies. Balb 3T3/c (mouse fibroblast), HMEC-1 (human microvascular endothelial), and HT-29 (human colon carcinoma) cell lines were used in the experiments. All cell lines were manipulated under sterile standard culture flasks and maintained in a 5% CO2 humidified incubator at 37 °C (HeraCell 150, Thermo Scientific). The Balb 3T3/c and HT-29 cells were purchased from American Type Culture Collection (ATCC, Virginia, USA), whereas HMEC-1 cells were obtained from Emory University (Georgia, USA). The Balb 3T3/c, HMEC-1, and HT-29 cells were cultured in DMEM with high glucose (Biowest, Cytogen, Poland), Endopan 3 (PAN Biotech, Germany) and RPMI 1640 (Biowest, Cytogen, Poland), respectively, according to the supplier's recommendations. The culture media were supplemented with 10% of fetal bovine serum (Gibco), 1% of stable glutamine (Sigma Aldrich), and 1% of antibiotic-antimycotic solutions (Thermo Fisher). HMEC-1 cells were additionally supplemented with endothelial growth factors (Sigma Aldrich).
C. Multiwell plate tests
Adhesion/proliferation of Balb 3T3/c, HMEC-1, and HT-29 cells on the modified PDMS samples was evaluated. To prepare the cell culture, the cells were seeded into 24-well plates filled with the modified PDMS samples. For this purpose, 500 μl of cell suspension (0.2–0.3 × 106 cells/ml) was added to each well. Next, the well plates were incubated at 37 °C in a humidified 5% CO2 atmosphere overnight. 48 h after cell seeding, cell adhesion/proliferation was analyzed. For this purpose, the colorimetric 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT, Sigma-Aldrich) assay was used. The MTT assay assesses the cell metabolic activity on the basis of the ability of the mitochondrial succinate tetrazolium reductase system to convert the yellow dye (MTT) to purple-colored formazan in living cells. The metabolic activity of the cell is proportional to the formed color intensity.
To analyze cell adhesion/proliferation, 50 μl of 5 mg/ml MTT solution was added to each well of the 24-well plate. The plates were incubated for 4 h at 37 °C. After that time, the cells were lysed with 10% acid sodium dodecyl sulfate (SDS, Sigma) solution overnight. Finally, absorbance of the supernatant was measured at 570 nm using a microplate reader (Bio-Tek, Winooski, VT, USA).
D. Cytotoxicity assays
Cytotoxicity assays were performed using two different drugs: oxaliplatin (Sigma, St. Louis, MI, USA) and celecoxib (Sigma, St. Louis, MI, USA). The PDMS surface modified using a surface plasma activation and protein coating (gelatin, fibronectin and collagen) were selected for these experiments. The cell culture on the polystyrene well served as a positive control. To prepare the drug solutions (with a concentration range of 40–120 μM), the drugs were dissolved in 100% dimethylsulfoxide (DMSO; Sigma) and then diluted with the culture medium for further experiments. The final concentration of DMSO was maintained at 0.2% which had no effect on cellular growth and survival. The cells, after being cultured for 24 h on the PDMS samples, were incubated (37 °C, 5% CO2) with 250 μl of oxaliplatin or celecoxib for the next 24 h. Finally, 48 h after cell seeding, the cell viability was analyzed using the MTT assay as described above.
E. Immunostaining
For immunostaining, the cells were fixed in 4% paraformaldehyde (in PBS) for 15 min. Next, the fixed cells were permeabilized with 0.5% Triton X-100 (in PBS) for 15 min. Finally, 3% BSA in PBS was used for blocking unspecific binding for 60 min. Each step was followed by washing the cell with PBS three times and was conducted at room temperature (RT). Polyclonal rabbit antibody anti-vinculin conjugated with fluorescein isothiocyanate (FITC, Bioss antibodies, MA, USA) was employed in 3% BSA of PBS at RT for 60 min for vinculin staining. Additionally, the cells were stained with NucRed® Dead 647 ReadyProbes® Reagent (Invitrogen, ThermoFisher Scientific, USA) according to the manufacturer's instructions. The cells were examined using an Olympus FV500 confocal microscope equipped with a 40× objective. The dyes were excited with Ar-blue 488 nm and He-Ne 633 nm lasers. The green and red fluorescence of the dyes were detected in the sequential mode to avoid crosstalk using 505–525 nm and 660 nm bandpass filters, respectively.
F. Microsystem tests
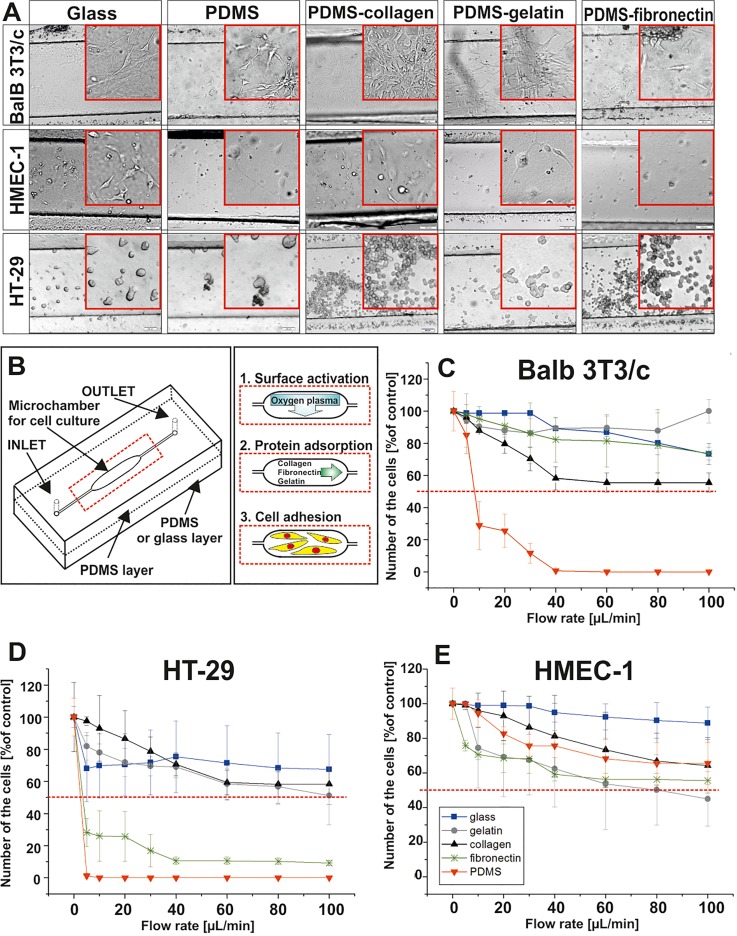
Attachment/detachment of the cells was also tested in a microfluidic system (Fig. 6). The microsystem was composed of two layers: PDMS with a microstructure and modified PDMS. To prepare negative and positive controls, a non-modified PDMS layer or a glass layer were used instead of a modified PDMS layer. The PDMS layer with a microstructure consisted of one microchamber (a width of 500 μm, a depth of 50 μm, and a length of 1 cm) connected with an inlet and an outlet. The microchamber was fabricated in PDMS using photolithography and replica molding techniques. In short, to obtain a replication stamp, a capillary film (Pro/Cap 50, Chromaline) was deposited on a sodium glass plate and next irradiated by a UV lamp for 30 s. The stamp with a microchannel was obtained by washing the uncured capillary film with water. Next, the PDMS prepolymer was mixed with a curing agent with a weight ratio of 10:1. The liquid mixture was poured over the stamp and cured for 5 h at 75 °C to form a 4–5 mm thick PDMS layer. The PDMS replica was peeled off from the stamp and the PDMS layer with microstructure was obtained. Finally, inlet and outlet holes were drilled in this layer. The PDMS layer without the microstructure was prepared by pouring the liquid mixture over a glass plate and curing for 5 h at 75 °C. Both prepared layers were sterilized (using UV light and 70% of ethanol) and bonded using an oxygen plasma treatment (Diener) (see Fig. 1S in the supplementary material). Next, the tubings were placed in the drilled holes. To modify PDMS surfaces, solutions of the proteins were introduced into the microsystems using syringe pumps (a flow rate of 2.0 μl/min) immediately after a plasma oxygen exposure. Fibronectin from human plasma (2.5 μg/ml), gelatin from porcine skin or collagen type I from calf skin (0.1%) were used in the experiments. The microsystems with the proteins were incubated at 37 °C for 24 h and they were washed with a fresh culture medium. Balb 3T3/c, HMEC-1, and HT-29 cell suspensions (a density of 1 × 106 cells/ml) were prepared according to a standard protocol and introduced into the microsystems with a flow rate of 5 μl/min. Next, the microsystems were sealed and placed in an incubator. 48 h after cell seeding, cell attachment/detachment tests were performed. For this purpose, the microsystems were rinsed with a fresh culture medium at different flow rates in a range of 5–100 μl/min for 5 min. After each step, photos of the remaining cells were taken using an inverted microscope coupled with a CCD camera (Olympus IX70). The same experiments were performed for the microsystems composed of PDMS-glass layers (a positive control) and PDMS-non-modified PDMS layers (a negative control). Additionally, cytotoxicity tests using 80 μM of oxaliplatin were performed in the microsystems. Three types of the microsystems were utilized to test the influence of the toxic agent on cell adhesion. The microsystems were made of PDMS with the microstructure and (1) PDMS modified with collagen, (2) PDMS modified with gelatin or (3) glass layer instead of the modified PDMS layer. The microsystems with the cultured Balb 3T3/c cells were washed with oxaliplatin solution (a flow rate of 1 μl/min for 10 min) and after that they were placed in an incubator for the next 24 h. Finally, cell attachment/detachment tests were performed according to the procedure described above. All fluids were introduced into the microsystems using peristaltic pumps (Ismatec Reglo-Digital MS-4/12).
FIG. 6.
(a) Balb 3T3/c, HMEC-1, and HT-29 cell adhesion on the non-modified PDMS surface, glass, and PDMS surfaces modified with gelatin, collagen, and fibronectin. (b) Scheme of the experiments performed in the microsystems. (c) Balb 3T3/c, (d) HMEC-1, and (e) HT-29 cell detachment test performed in the microsystems 48 h after cell seeding. Flow rates in the range of 5–100 μl/min were tested. (n ≥ 3).
G. Gas chromatography
The absorption of oxaliplatin and celecoxib in PDMS samples was measured according to the previously elaborated procedure.33,34 In short, PDMS samples were cut into small pieces and placed in 3 ml of DMSO overnight. Next, the DMSO extract was analyzed using gas chromatography (Agilent Technology).
H. Statistical analysis
All data were expressed as means with the standard deviation (SD) based on at least three independent experiments. Statistical analysis was performed using the t-Student test. A value of p 0.05 or lower was considered to indicate a significant difference (asterisk indicate p < 0.05).
III. RESULTS AND DISCUSSION
A. Cell adhesion/proliferation on the modified PDMS surface
PDMS is a biocompatible material widely used for fabrication of Lab-on-a-chip systems, especially for biological applications.30,35 PDMS is a hydrophobic and porous material. Therefore, to maintain stable cell culture conditions and to reduce the effects of absorption into PDMS, the modification of its surface from hydrophobic to hydrophilic seems to be a reasonable solution. We tested how different modification methods of PDMS influences cell adhesion/proliferation. In our preliminary studies, we discussed the physicochemical properties of the modified PDMS samples.32 The main conclusion was that the usage of oxygen plasma treatment as a pre-activation step of the PDMS samples was better than the usage of UV irradiation. It enabled to generate a high number of hydrophilic groups, which reacted with the tested proteins. Usually, optimal physical properties of PDMS were achieved when a 10:1 ratio of base to curing agent was used. However, it was confirmed that the change of the base to curing agent ratio could have a significant influence on both the physicochemical and biological properties of PDMS.4,32 In the literature, there are some reports which indicate that the change of ratio of PDMS components could be toxic for the cells.17,36 Therefore, we decided to test how it influences cell proliferation and adhesion of three different cell types: Blab 3T3/c, HMEC-1, and HT-29. Based on that and our previous studies, here we propose a biological analysis of PDMS surfaces made of three different PDMS prepolymer and curing agent ratios: 5:1, 10:1, and 20:1. The PDMS samples were modified by surface activation, oxygen plasma or UV light. Next, the chemically modified PDMS samples were additionally coated with proteins such as fibronectin, laminin, collagen, Poly-L-Lysine, and gelatin.
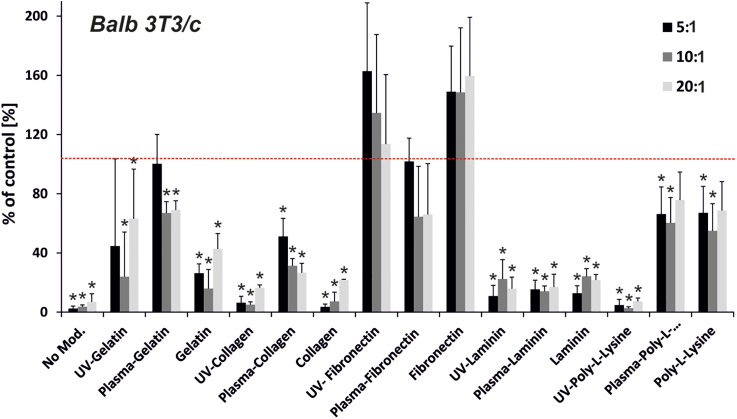
Each cell line has specific properties which determine their adhesion and proliferation, therefore it is important to optimize the surface on which the cells are cultured and tested. We selected Blab 3T3/c, HMEC-1, and HT-29 cells, because they differ in tissue origin and represent non-malignant, immortalized, and cancer cell types, respectively. The tested cells exhibit different sensitivities and have different requirements for the growth microenvironment. Blab 3T3/c, HMEC-1, and HT-29 are fibroblast, endothelial-like, and epithelial cells, respectively. We have studied how cell origin and disease state influences cell adhesion to the modified surfaces. We supposed that different cell proliferation will be observed on the modified PDMS surfaces. Figure 1 show MTT results obtained for Blab 3T3/c cells, which were maintained on different PDMS surfaces for 48 h. Cell adhesion/proliferation was dependent on the PDMS curing agent weight as well as the protein used for coating the growth surface. Adverse result was observed for three un-modified PDMS substrates having different base to curing agent ratios (w/w) 5:1, 10:1, and 20:1. The MTT results show an over 90% decrease in cell attachment properties for the unmodified surfaces of PDMS (Fig. 1). The same dependence was also observed for HMEC-1 and HT-29 cell lines (see Figs. 2 and 3).
FIG. 1.
Proliferation of Balb 3T3/c cells on the modified PDMS samples (prepolymer to curing agent ratio—5:1, 10:1, and 20:1). PDMS samples were modified using UV irradiation or oxygen plasma activation and protein adsorption: gelatin, collagen, fibronectin, laminin, and poly-L-Lysine. The red line indicates proliferation for the positive control—polystyrene surface. (n ≥ 3, asterisk indicates p < 0.05).
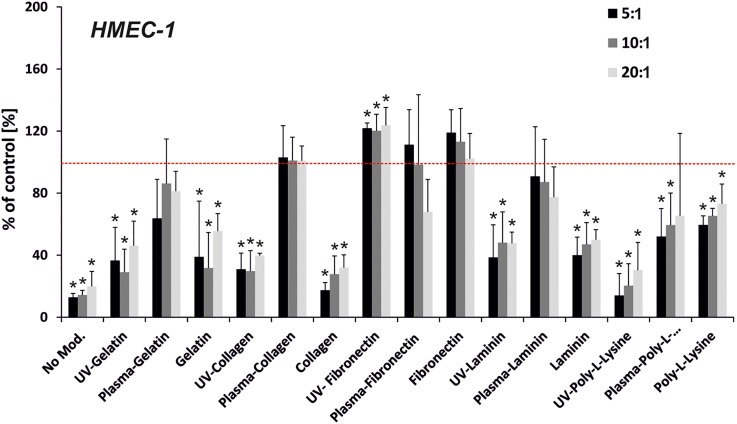
FIG. 2.
Proliferation of HMEC-1cells on the modified PDMS samples (prepolymer to curing agent ratio—5:1, 10:1, and 20:1). PDMS samples were modified using UV irradiation or oxygen plasma activation and protein adsorption: gelatin, collagen, fibronectin, laminin, and poly-L-Lysine. The red line indicates proliferation for the positive control—polystyrene surface. (n ≥ 3, asterisk indicates p < 0.05).
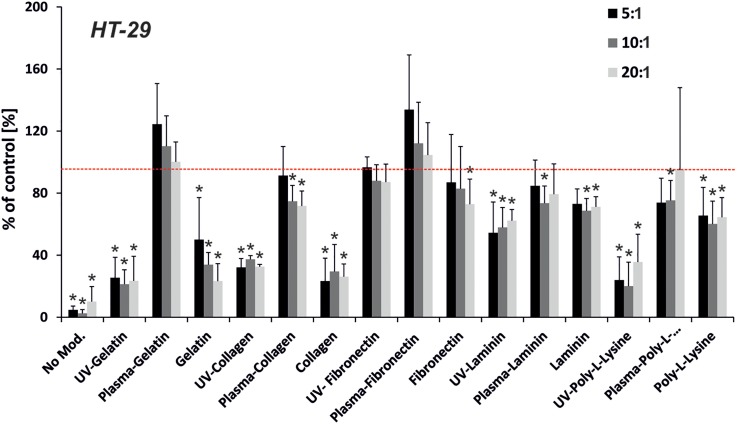
FIG. 3.
Proliferation of HT-29 cells on the modified PDMS samples (pre-polymer to curing agent ratio—5:1, 10:1, and 20:1). PDMS samples were modified using UV irradiation or oxygen plasma activation and protein adsorption: gelatin, collagen, fibronectin, laminin, and poly-L-Lysine. The red line indicates proliferation for the positive control—polystyrene surface. (n ≥ 3, asterisk indicates p < 0.05).
Each of the tested proteins excluding laminin improved Blab 3T3/c cell proliferation to compare with the un-modified PDMS surfaces. The usage of Poly-L-Lysine on non-activated and those pre-activated with plasma oxygen, enhanced Blab 3T3/c cell proliferation more than the unmodified PDMS samples. Cell proliferation/attachment equaled about 80% of the control (polystyrene surface) for each type of modification. It was noticed that, the usage of gelatin (with plasma exposure) and fibronectin (with plasma and UV exposure) improved Blab 3T3/c cell proliferation even better than the usage of a standard polystyrene plate. Surprisingly, higher proliferation was observed for the PDMS surfaces coated only with fibronectin or pre-activated with UV irradiation than for the surfaces pre-activated with plasma.
The analysis of HMEC-1 cell proliferation/adhesion on different PDMS surfaces is shown in Fig. 2. Each of the used proteins enhanced the proliferation of these cells compared to the unmodified PDMS samples. However, the rate of proliferation was dependent on the used modification. The lowest cell proliferation was noticed for the samples with Poly-L-Lysine. For almost each tested protein (gelatin, collagen, laminin), pre-activation with oxygen plasma induced an increase in cell proliferation/adhesion compared to the negative control. HMEC-1 cell attachment increased for PDMS surfaces modified with collagen (plus oxygen plasma) and fibronectin (plus oxygen plasma, plus UV irradiation, and without modification).
The analysis of HT-29 cell proliferation/adhesion on the modified PDMS surfaces is shown in Fig. 3. It is quite difficult to draw clear conclusions based on the obtained results. Each tested modification of PDMS surfaces enhanced cell proliferation/adhesion. However, this dependence varied for different methods. Pre-activation of the PDMS surface with oxygen plasma enhanced HT-29 cell proliferation for all tested proteins. The highest cell proliferation was obtained for gelatin and fibronectin. For these modifications, HT-29 cell proliferation/adhesion was higher than 100% compared to the positive control (PS plate). The obtained results showed that HT-29 cells do not have specific requirements for the protein used for PDMS surface coating to improve cell growth. This could result from the fact that HT-29 cells are carcinoma cells and they do not require a highly specific microenvironment.
The obtained results indicated that PDMS did not support attachment of the tested cell lines as conventional cell culture treated polystyrene surfaces. However, chemical modification of PDMS and additional coating of the growth surface with proteins improve cell adhesion. Some reports suggested that, the change of the ratio of PDMS components (ratio larger or smaller than 10:1) could be toxic for the cells. Based on our results, it is too difficult to define an unambiguous dependency between the ratio of PDMS components and cell attachment. However, we can conclude that the tested ratios did not have a toxic effect on Blab 3T3/c, HMEC-1, and HT-29. Furthermore, for some modifications, the proliferation of the cells was higher for 5:1 or 20:1 than for the 10:1 ratio. This means that the surface chemical composition plays a pivotal role in affecting the evaluated cell line adhesion on PDMS. The obtained results indicated that plasma treatment is an important factor which increases cell proliferation. The MTT tests show that in most cases, the best surface modification for cell attachment was an oxygen plasma treatment combined with solutions of gelatin or fibrous ECM proteins, i.e., fibronectin, type I collagen coating the surface. We observed at least 100% cell viability/attachment properties in comparison with the control PS surface. Cell behavior is highly dependent on surface factors such as: surface wettability, topography, and surface bond-proteins.37 The same results were obtained in our study, in which we observed that all of the cells tested above prefer hydrophilic surfaces. Moreover, our observations are similar to those obtained by Wang et al. for the Caco-2 cell line. The authors showed that plasma treatment alone resulted in an insignificant increase in cell adhesion on 10:1 PDMS and, when pre-coated with fibronectin or collagen, the oxygen plasma treated PDMS surface greatly promoted Caco-2 adhesion and proliferation.25 We also noticed that each cell line prefers specific properties of the culture surface. Balb 3T3/c cells, contrary to HT-29 cells, which grew on different kinds of surfaces, are the most demanding for the surface properties' hydrophilicity.
B. Vinculin staining
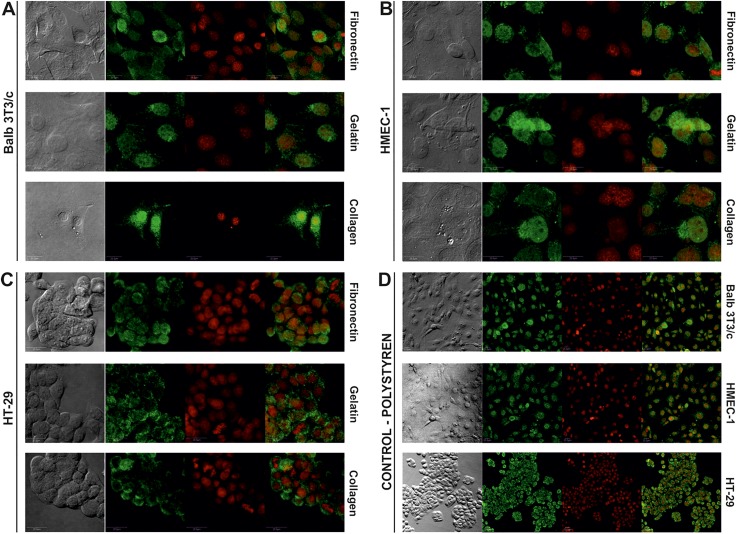
The obtained results showed that the proposed modification of PDMS surfaces influences cell proliferation. Cell spreading and movement occur through the process of binding the cell surface integrin receptors to extracellular matrix adhesion molecules. Vinculin is a membrane-cytoskeletal protein associated with cell-cell and cell-matrix junctions.38 It is also based on focal adhesion plaques, which in turn take part in communication between integrin adhesion molecules and the actin cytoskeleton. To show how the proposed modifications enhanced cell adhesion, vinculin was immunostained. The experiments were performed for the samples, which were modified using the plasma oxygen treatment and coated with gelatin, fibronectin or collagen. These samples were chosen based on the results obtained for Balb 3T3/c, HMEC-1, and HT-29 cell adhesion/proliferation. The highest cell proliferation/adhesion was noticed in the cases described above. The results indicate that the plasma modification of 5:1 PDMS pre-coated with gelatin, fibronectin or collagen support proliferation and adhesion of the tested cells to a similar extent as traditional cell culture PS. Figure 4 shows the detection of vinculin, a soluble cytoplasmic protein that localizes to focal adhesions.
FIG. 4.
Immunostaining with vinculin of (a) Balb/3T3c, (b) HMEC-1, and (c) HT-29 cells cultured on different PDMS surfaces modified with plasma oxygen and proteins. (d) Immunostaining with vinculin of Balb/3T3c HMEC-1 and HT-29 cultured on polystyrene (green colour—anti-vinculin conjugated with FITC, red colour—NucRed® Dead 647 ReadyProbes® Reagent).
The formation of focal adhesion plaques is a prerequisite process for the development of signaling transduction in cell attachment.39 Generally, the expression of vinculin increases the adhesion of cells to the extracellular matrix and slows cellular migration speed. The photographs show that the formations of vinculin-positive focal adhesion are visible on all evaluated types of the modified growth surfaces. The presence of vinculin was noticed for each of the samples mentioned above. However, the most intense staining was observed for the HT-29 cells on the PDMS-gelatin surface and it can be connected with the cell morphology. These cells are able to form a multilayer of nonpolarized cells that display an undifferentiated phenotype with tight junctions between cells.40 PS surface with Balb 3T3/c, HMEC-1, and HT-29 cells were used as a positive control [Fig. 4(d)]. The negative control (PDMS) was not tested because of the low number of cells adhered to the unmodified PDMS during proliferation/adhesion assays.
C. Cytotoxicity assays on the modified PDMS
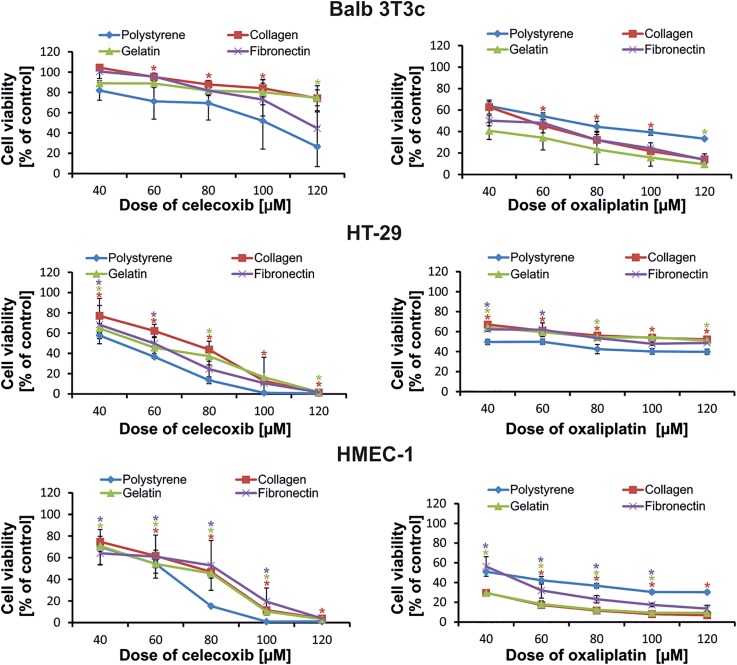
The second type of the experiments was planned to estimate how the response of the cell growing on plasma modified 5:1 PDMS coated with gelatin fibronectin or collagen surfaces (and pre-activated with plasma oxygen) will change after exposure to two cytotoxic drugs such as celecoxib and oxaliplatin. We chose such types of modification based on the results obtained for cell proliferation/adhesion assays. The best results of cell growth were noted for these modification methods. Cell proliferation was not only statistically higher than for the un-modified PDMS surface but also statistically higher than for standard PS surfaces for some cases. Celecoxib and oxaliplatin were chosen because of their toxicity mechanism differences. The first one is a selective nonsteroidal anti-inflammatory drug usually used in rheumatoid arthritis treatment, but also has cytotoxic activity in cancer. Celecoxib inhibits the transformation of arachidonic acid to prostaglandin precursors which results in its antipyretic and anti-inflammatory properties as well as influences its genes and pathways involved in inflammation and malignant transformation in colon tumors. Oxaliplatin is a known chemotherapeutic drug commonly used in the treatment of colorectal cancer. Oxaliplatin inhibits DNA synthesis in the cells by forming inter- and intra-strand cross links in DNA, which prevent DNA replication and transcription, causing cell death.41
The obtained results show that normal cells such as Balb 3T3/c and HMEC-1 are more sensitive to the oxaliplatin than the HT-29 colorectal cancer cell line. The viability of Balb 3T3/c and HMEC-1 cells on collagen and gelatin pre-coated surfaces decreased by 20%–30%, depending on the dose of the drug in comparison to the viability of the cells growing on the traditional PS surface [Fig. 5(a)]. The surfaces pre-coated with proteins imitate the environment of ECM and, as a consequence, create better conditions for the proliferation and adhesion of cells. This fact may influence the response of the cells to stress factors such as oxaliplatin. The results of the MTT assay show that cancer cells, in the same cultivation conditions, are less sensitive to the oxaliplatin, especially at the lowest dose. The same effect was observed for the second drug, celecoxib [Fig. 5(b)]. The HT-29 cell trend in sensitivity to both evaluated drugs was similar, whereas the response of Balb 3T3/c and HMEC-1 cells was different after treatment with celecoxib. Both normal cell lines cultured on the modified PDMS surfaces were less sensitive to the celecoxib in comparison to the PS surface. The effect was especially pronounced in the case of HMEC-1 cells which was a surprise and concern. Nevertheless, we think that the major factor in such a response results from the cell type and their environmental requirements for growth and, in less degree, from both drugs' different mechanisms of action. The coated surfaces have cytoprotective properties which slow down the cytotoxic effect of the celecoxib action. Celecoxib, along with having anti-inflammatory properties, also induces intracellular reactive oxygen species (ROS) generation.42 For instance, increased ROS formation and oxidative stress cause unfavorable changes in the extracellular matrix (ECM). This results in ECM remodeling and higher susceptibility to degradation. Consequently, the ECM becomes thinner. Moreover, after activation of vascular cells by cytokines, growth factors or mitogens, COX-2 is rapidly expressed (i.e., 1–2 h) resulting in an enhanced capacity of the cells to generate prostaglandins (PG) which influence cells' adhesive properties and the progression of cancer.43 Inhibition of COX-2 by celecoxib results in a decrease in the PG level, an increase in apoptosis, and a decrease in the proliferation of endothelial cells. Because the evaluated PDMS surfaces are coated (and in this way imitating the natural environment of ECM), the effect of celecoxib on the cells may be extended (Fig. 5), therefore it is not as high as on a PS surface.44 Because of the PDMS porosity, it is possible that the tested compounds could absorb in the modified PDMS surfaces. Therefore, it was important to determine oxaliplatin and celecoxib absorption into the tested PDMS samples. For this purpose, gas chromatography was utilized according to the procedure elaborated previously.33 The obtained results indicated that neither of the tested drugs absorb in the PDMS.
FIG. 5.
Cytotoxicity of celecoxib and oxaliplatin on Balb 3T3/c, HT-29, and HMEC-1 cells. The viability of the cells was determined 24 h after drug exposure. (n ≥ 3, asterisk indicates p < 0.05).
D. Cell attachment/detachment tests in the microsystems
PDMS is widely used for cell culture and cytotoxicity assays in the microsystems.9–11 Because the SA/V ratio is higher in the microsystems than in the macroscale, it could have an influence on cell behavior/viability.12–14 Therefore, we tested how SA/V value influences Balb 3T3/c, HMEC-1, and HT-29 cell adhesion. For this purpose, we used the microsystems in which the SA/V ratio was 200 times higher than in the well of 24-well plate. Based on the results obtained in macroscale, we decided to test cell adhesion on PDMS modified with oxygen plasma and proteins such as: fibronectin, gelatin or collagen. Similarly as in the macroscale, the lowest adhesion of each tested cell line was noticed for non-modified PDMS (Fig. 6). The usage of proteins improved cell attachment; furthermore, collagen enhanced attachment of the cells even more than the positive control (glass surface). The highest adhesion was obtained for Balb 3T3/c cells whereas the lowest for HMEC-1 cells.
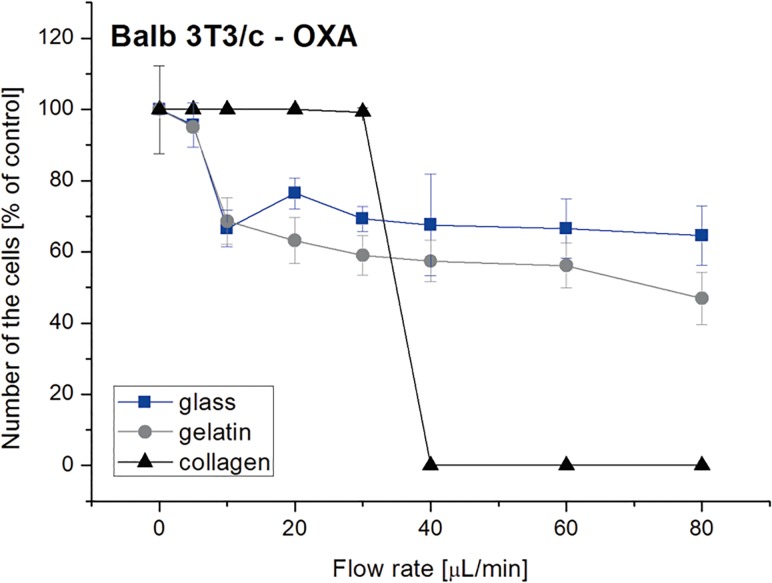
A microfluidic system can also be used as a tool for analysis of cell detachment. The main advantage of the usage of microfluidics, in comparison with other methods, is the possibility to test cell adhesion in a wide range of shear stress. We used the microsystems with the modified PDMS surfaces to test Balb 3T3/c, HMEC-1, and HT-29 cell detachment. For this purpose, the microsystems with the cells were rinsed with a fresh medium at different flow rates in a range of 5–100 μl/min for 5 min. Balb 3T3/c cell adhesion to the PDMS was the same (for fibronectin) or higher (for gelatin) than for glass [Fig. 6(c)]. For the PDMS modified with collagen, the fraction of Balb 3T3/c cells remaining in the microsystem was lower than for the positive control, but still it was under 50%. Non-modified PDMS showed low Balb 3T3/c cell adhesion after the detachment test. For HT-29 cells, it was observed that the modifications with gelatin and collagen caused similar cell adhesion as for the glass surface [Fig. 6(d)]. The number of the remaining cells after the detachment test was a little higher than 50%. In turn, fibronectin enhanced cell attachment a bit higher than non-modified PDMS, but it was at a low level (∼10%). The results obtained for HMEC-1 cells, showed that the usage of PDMS modifications did not significantly enhance adhesion of the cells [Fig. 6(e)]. For the highest value of shear stress, the number of the cells remaining in the microsystem was lower than for glass, and similar (for collagen) or lower (for gelatin and fibronectin) than for non-modified PDMS. However, it should be noted that the number of the cells remaining in the microsystem was still higher than 50%. Based on the obtained results, we conclude that the high value SA/V did not inhibit Balb 3T3/c, HMEC-1, and HT-29 attachment to the modified PDMS surfaces. It was noticed that the selected modifications enhanced cell adhesion in both macro- and microscale. However, for the macroscale, the highest adhesion of the cells was noticed for PDMS modified with fibronectin, whereas for the microsystems, for PDMS modified with collagen. To show that PDMS with modified surfaces can be used for cytotoxicity assays, we performed a detachment test in the microsystems. The microsystems with PDMS modified with collagen, PDMS modified with gelatin and glass substrate were utilized in the experiments. Balb 3T3/c cell detachment was analyzed after incubation with 80 μM of oxaliplatin (Fig. 7). We selected such parameters based on the experiments obtained in the macro- and microscale. The obtained results showed that Balb 3T3/c cells are more sensitive to the drug on the modified PDMS surfaces than on the positive control. Additionally, a large decrease in the number of the cells was observed for PDMS modified with collagen. A similar tendency was also noticed in the macroscale.
FIG. 7.
Balb 3T3/c cell detachment test performed in the microsystems after incubation with 80 μM of oxaliplatin. Flow rates in the range of 5–100 μl/min were tested. (n ≥ 3).
IV. CONCLUSIONS
The aim of our work was to investigate the adhesion of Balb 3T3/c, HMEC-1, and HT-29 cells on poly(dimethylsiloxane) surfaces modified with various proteins. Different physicochemical cell characterizations enabled the testing of how culture surfaces influence cell proliferation/attachment and viability after drug exposure. Such research is important to create the proper culture conditions during in vitro tests. Here, we tested the biological aspect of the PDMS surface modification. The enhancement of cell proliferation's importance in modifying PDMS using surface pre-activation and protein coating was investigated. The best cell proliferation/adhesion was obtained for plasma activation. It enabled to generate a high number of hydrophilic groups (-OH, -NH), which reacted with the tested proteins. Moreover, it was observed that Balb 3T3/c, HMEC-1, and HT-29 cells proliferate well on PDMS samples modified with gelatin, fibronectin, and collagen. For HT-29 cells, it was not easy to select one modification, in which these cells proliferate very well. This could indicate that this type of the cells can adhere to various types of surfaces. The result obtained in the microsystem, where a high SA/V ratio is maintained, show that the proposed modifications can be successfully used in both the macro- and microscale. The obtained results could give important information for creating growth conditions for various cell types. It is especially important to determine which could be used during the fabrication of Lab-on-a-chip systems, where the cell culture microenvironment mimics natural cell growth. Additionally, we show that the microsystems are good tools for analysis of cell attachment/detachment on various modified surfaces.
SUPPLEMENTARY MATERIAL
See supplementary material for the complete a scheme of the microfluidic device used in the experiments.
ACKNOWLEDGMENTS
This work was financially supported within the frame of the SONATA 5 Program No. UMO-2013/09/D/ST5/03887. The authors would like to thank Dr. Anna Jerzak from the Faculty of Chemistry WUT for the GC measurements and Dr. Katarzyna Wiktorska from the Microscope Facility at the National Medicines Institute for the excellent assistance with confocal microscopy.
References
- 1. Byron A. and Frame M. C., Curr. Opin. Cell Biol. 39, 93 (2016). 10.1016/j.ceb.2016.02.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Christ K. V. and Turner K. T., J. Adhes. Sci. Technol. 24, 2027 (2010). 10.1163/016942410X507911 [DOI] [Google Scholar]
- 3. Lukashev M. E. and Werb Z., Trends Cell Biol. 8, 437 (1998). 10.1016/S0962-8924(98)01362-2 [DOI] [PubMed] [Google Scholar]
- 4. Wala J., Maji D., and Das S., Biomed. Mater. 12, 065002-1 (2017). 10.1088/1748-605X/aa7e81 [DOI] [PubMed] [Google Scholar]
- 5. Huang S. and Ingber D. E., Nat. Cell Biol. 1, E131 (1999). 10.1038/13043 [DOI] [PubMed] [Google Scholar]
- 6. Parsons J. T., Horwitz A. R., and Schwartz M. A., Nat. Rev. Mol. Cell Biol. 11, 633 (2010). 10.1038/nrm2957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Charras G. and Sahai E., Nat. Rev. Mol. Cell Biol. 15, 813 (2014). 10.1038/nrm3897 [DOI] [PubMed] [Google Scholar]
- 8. Khalili A. A. and Ahmad M. R., Int. J. Mol. Sci. 16, 18149 (2015). 10.3390/ijms160818149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Johnston I. D., McCluskey D. K., Tan C. K. L., and Tracey M. C., J. Micromech. Microeng. 24, 035017_1 (2014). 10.1088/0960-1317/24/3/035017 [DOI] [Google Scholar]
- 10. Wu D., Qin J., and Lin B., Lab Chip 7, 1490 (2007). 10.1039/b708877a [DOI] [PubMed] [Google Scholar]
- 11. Zhou J., Ellis A. V., and Voelcker N. H., Electrophoresis 31, 2 (2010). 10.1002/elps.200900475 [DOI] [PubMed] [Google Scholar]
- 12. Regehr K. J., Domenech M., Koepsel J. T., Carver K. C., Ellison-Zelski S. J., Murphy W. L., Schuler L. A., Alarid E. T., and Beebe D. J., Lab Chip 9, 2132 (2009). 10.1039/b903043c [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Paguirigan A. L. and Beebe D. J., Integr. Biol. 1, 182 (2009). 10.1039/b814565b [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Berthier E., Young E. W. K., and Beebe D., Lab Chip 12, 1224 (2012). 10.1039/c2lc20982a [DOI] [PubMed] [Google Scholar]
- 15. Mehling M. and Tay S., Curr. Opin. Biotechnol. 25, 95 (2014). 10.1016/j.copbio.2013.10.005 [DOI] [PubMed] [Google Scholar]
- 16. Halldorsson S., Lucumi E., Gómez-Sjöberg R., and Fleming R. M. T., Biosens. Bioelectron. 63, 218 (2015). 10.1016/j.bios.2014.07.029 [DOI] [PubMed] [Google Scholar]
- 17. Friend J. and Yeo L., Biomicrofluidics 4, 026502 (2010). 10.1063/1.3259624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Berdichevsky Y., Khandurina J., Guttman A., and Lo Y. H., Sens. Actuators, B Chem. 97, 402 (2004). 10.1016/j.snb.2003.09.022 [DOI] [Google Scholar]
- 19. Zhou J., Khodakov D. A., Ellis A. V., and Voelcker N. H., Electrophoresis 33, 89 (2012). 10.1002/elps.201100482 [DOI] [PubMed] [Google Scholar]
- 20. Tan H. M. L., Fukuda H., Akagi T., and Ichiki T., Thin Solid Films 515, 5172 (2007). 10.1016/j.tsf.2006.10.026 [DOI] [Google Scholar]
- 21. Zhang W., Choi D. S., Nguyen Y. H., Chang J., and Qin L., Sci. Rep. 3, 2332 (2013). 10.1038/srep02332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Farrell M. and Beaudoin S., Colloids Surf. B Biointerfaces 81, 468 (2010). 10.1016/j.colsurfb.2010.07.059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Roman G. T. and Culbertson C. T., Langmuir 22, 4445 (2006). 10.1021/la053085w [DOI] [PubMed] [Google Scholar]
- 24. Tu Q., Wang J. C., Zhang Y., Liu R., Liu W., Ren L., Shen S., Xu J., Zhao L., and Wang J., Rev. Anal. Chem. 31, 177 (2012). 10.1515/revac-2012-0016 [DOI] [Google Scholar]
- 25. Wang L., Sun B., Ziemer K. S., Barabino G. A., and Carrier R. L., J. Biomed. Mater. Res.-Part A 93, 1260 (2010) 10.1002/jbm.a.32621. [DOI] [PubMed] [Google Scholar]
- 26. Kuddannaya S., Bao J., and Zhang Y., ACS Appl. Mater. Interfaces 7, 25529 (2015). 10.1021/acsami.5b09032 [DOI] [PubMed] [Google Scholar]
- 27. Siddique A., Meckel T., Stark R. W., and Narayan S., Colloids Surf. B Biointerfaces 150, 456 (2017). 10.1016/j.colsurfb.2016.11.011 [DOI] [PubMed] [Google Scholar]
- 28. Zhang Q., Xu J.-J., Liu Y., and Chen H.-Y., Lab Chip 8, 352 (2008). 10.1039/B716295M [DOI] [PubMed] [Google Scholar]
- 29. Xue P., Li Q., Sun L., Zhang L., Xu Z., Li C. M., and Kang Y., Microfluid. Nanofluid. 22, 1 (2018). 10.1007/s10404-017-2014-4 [DOI] [Google Scholar]
- 30. Yeo L. Y., Chang H. C., Chan P. P. Y., and Friend J. R., Small 7, 12 (2011). 10.1002/smll.201000946 [DOI] [PubMed] [Google Scholar]
- 31. Andersson H. and Van den Berg A., Sens. Actuators, B Chem. 92, 315 (2003). 10.1016/S0925-4005(03)00266-1 [DOI] [Google Scholar]
- 32. Zuchowska A., Kwiatkowski P., Jastrzebska E., Chudy M., Dybko A., and Brzozka Z., Electrophoresis 37, 536 (2016). 10.1002/elps.201500250 [DOI] [PubMed] [Google Scholar]
- 33. Jastrzebska E., Flis S., Rakowska A., Chudy M., Jastrzebski Z., Dybko A., and Brzozka Z., Microchim. Acta 180, 895 (2013). 10.1007/s00604-013-1009-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ziolkowska K., Jedrych E., Kwapiszewski R., Lopacinska J., Skolimowski M., and Chudy M., Sens. Actuators, B Chem. 145, 533 (2010). 10.1016/j.snb.2009.11.010 [DOI] [Google Scholar]
- 35. Jastrzebska E., Tomecka E., and Jesion I., Biosens. Bioelectron. 75, 67 (2016). 10.1016/j.bios.2015.08.012 [DOI] [PubMed] [Google Scholar]
- 36. Mehling M., Frank T., Albayrak C., and Tay S., Lab Chip 15, 1276 (2015). 10.1039/C4LC01038H [DOI] [PubMed] [Google Scholar]
- 37. Lourenço B. N., Marchioli G., Song W., Reis R. L., van Blitterswijk C. A., Karperien M., van Apeldoorn A., and Mano J. F., Biointerphases 7, 46 (2012). 10.1007/s13758-012-0046-6 [DOI] [PubMed] [Google Scholar]
- 38. Carisey A. and Ballestrem C., Eur. J. Cell Biol. 90, 157 (2011). 10.1016/j.ejcb.2010.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Dasgupta S., Tarafder S., Bandyopadhyay A., and Bose S., Mater. Sci. Eng. C 33, 2846 (2013). 10.1016/j.msec.2013.03.004 [DOI] [PubMed] [Google Scholar]
- 40. Cohen E., Ophir I., and Shaul Y. B., J. Cell Sci. 2657, 2657 (1999). [DOI] [PubMed] [Google Scholar]
- 41. Alcindor T. and Beauger N., Curr. Oncol. 18, 18 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kim C. H., Chung C. W., Lee H. M., Kim D. H., Kwak T. W., Il Jeong Y., and Kang D. H., Int. J. Nanomed. 8, 2173 (2013) 10.2147/IJN.S44394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Eligini S., Habib A., Lebret M., Créminon C., Lévy-Toledano S., and Maclouf J., Br. J. Pharmacol. 133, 1163 (2001). 10.1038/sj.bjp.0704163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Rüegg C., Dormond O., and Mariotti A., Biochim. Biophys. Acta-Rev. Cancer 1654, 51 (2004). 10.1016/j.bbcan.2003.09.003 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
See supplementary material for the complete a scheme of the microfluidic device used in the experiments.