Abstract
Fungisome® (F), a liposomal amphotericin B (AmB) product, is marketed in India as a safe and effective therapeutic for the parasitic infection visceral leishmaniasis. Its potential in the treatment of cutaneous leishmaniasis (CL), a disfiguring form of the disease affecting the skin, is currently unknown. Here, we report the evaluation of the efficacy of F in the Leishmania major BALB/c murine model of CL, including a head-to-head comparison with the standard liposomal AmB formulation AmBisome® (A). Upon intravenous administration at dose levels of 5, 10 and 15 mg/kg of body weight (on days 0, 2, 4, 6 and 8), F showed clear signs of toxicity (at 15 mg/kg), while A did not. After complete treatment (day 10), the tolerated doses of 5 and 10 mg/kg F had significant antileishmanial activity (ED50 = 4.0 and 12.8 mg/kg for qPCR-based parasite load and lesion size, respectively), although less than that of A at identical doses (ED50 = 3.0 and 8.8 mg/kg). The efficacy of F was inferior compared to A because lower levels of the active agent AmB accumulated within the infected lesion. In conclusion, despite possibly being less safe and efficacious than A at equivalent doses, the moderate in vivo activity of F could indicate a role in the systemic pharmacotherapy of CL.
Keywords: Cutaneous leishmaniasis, Amphotericin B, Liposome, Efficacy, Pharmacokinetics
Graphical abstract
1. Introduction
With more than 2 million new cases per year and 350 million people at risk in over 100 countries, leishmaniasis is a major public health problem affecting the poorest of the poor in many parts of the world (Alvar et al., 2012). Leishmaniasis is a disease complex caused by over 20 species of the protozoan parasite Leishmania that are transmitted via female sand flies. Different types include visceral leishmaniasis (VL, also known as kala-azar, an often fatal-if-not-treated condition of the reticuloendothelial system predominantly caused by L. donovani), and the most common form, cutaneous leishmaniasis (CL). In simple CL, skin lesions caused by species such as L. major or L. mexicana are localized and self-healing, but leave disfiguring wounds and scars. Infections by other species can lead to rare but severe chronic, diffuse or mucosal CL symptoms (complex CL) (Reithinger et al., 2007). Another form in the clinical spectrum of skin syndromes is post-kala-azar-dermal leishmaniasis (PKDL), a complication of VL where recovered, otherwise-healthy patients develop pigmentation disorders and a diffuse macular or nodular rash (Zijlstra et al., 2003).
For CL, the pentavalent antimonials sodium stibogluconate (Pentostam®) and meglumine antimoniate (Glucantime®) remain the standard treatment, despite being associated with a painful and lengthy series of injections, severe side effects and variable treatment outcomes (Aronson et al., 2016). One of the available second-line drugs is the intravenous (IV) polyene antibiotic amphotericin B (AmB), which kills Leishmania through pore formation after complexation with ergosterol in its cell membrane (Cohen, 2016). The clinical use of the conventional deoxycholate salt form is limited by infusion-related side effects such as fever and rigor, as well as chronic toxicity (Tonin et al., 2017). AmBisome® (A), a liposomal formulation of AmB, is better tolerated and effective against VL in single high dose (7.5–10 mg/kg, Mondal et al., 2014) and in multiple doses against CL (3 mg/kg daily for a total of 21 mg/kg, Wortmann et al., 2010) and PKDL (two cycles of 4 × 5 mg/kg, Basher et al., 2017). Despite A being listed on the World Health Organisation Essential Medicines List, the requirement for cold chain and the high price (at least 18 $ per vial via donation programmes) often make availability and access in primary health care settings problematic (Bhattacharya and Ali, 2016). Fungisome® (F) is the brand name of an alternative liposomal AmB formulation developed and commercialized since 2003 in India (Sanath et al., 2005). F contains different lipids and has a different formulation, vesicle size, preparation and pharmacokinetic profile compared to A (Kshirsagar, 2014). Assuring pharmaceutical and/or biological equivalence of F to A is complicated because of a lack of clear regulatory guidelines on liposomal generics (Gaspani, 2013). Clinical studies reported effectiveness and safety of F in VL (Bodhe et al., 1999; Mondal et al., 2010) and recent phase II trials have demonstrated > 90% cure rates following short (2 days) or single high dosed (10 mg/kg) therapy (Sundar et al., 2015; Goswami et al., 2016).
A has been used to treat patients with simple (Guery et al., 2017) and mucosal (Rocio et al., 2014) CL. To investigate the pharmacology of A in CL, we have recently applied an approach based on pharmacokinetic pharmacodynamic (PK PD) relationships in mice (Wijnant et al., 2018). For F, in contrast, there is no such information available to describe the link between drug levels in the infected skin and therapeutic outcomes. This is not only a crucial consideration for potential F treatment of CL, but also of PKDL (for which no animal models of disease are available, Desjeux et al., 2013). Our aim was therefore to (i) evaluate the intralesional drug accumulation and efficacy of intravenously administered F at three dose levels in the L. major BALB/c murine model of CL and (ii) provide a head-to-head comparison with the standard liposomal AmB formulation A.
2. Materials and methods
2.1. Drugs
Sealed vials of Fungisome® (F, Lifecare Innovations, India) were sonicated in a Camlab TransSonic T460/H water bath for 45 min at 25 ± 5 °C to transform the large multilamellar vesicles (2700–3500 nm, Serrano et al., 2013) into smaller unilamellar liposomes (≈220 nm, Jadhav et al., 2011), as per the manufacturer's instructions. For AmBisome® (A, Gilead, UK), 12 ml sterile water was added to the lyophilized powder to reconstitute the unilamellar liposomes (≈70 nm, Walsh et al., 1998; Dupont, 2002), also following the manufacturer's protocol. Saline 0.85% and dextrose 5% were used to dilute stocks of F and A respectively to the desired concentrations. Paromomycin sulphate (Sigma, UK) was prepared in phosphate buffered saline (PBS). Dilutions were prepared one day before starting the experiment and stored at 4 °C in the dark.
2.2. Murine model of CL
L. major MHOM/SA85/JISH118 parasites were cultured in Schneider's insect medium (Sigma, UK) supplemented with 10% heat-inactivated fetal calf serum (HiFCS, Sigma UK). These were passaged each week at a 1:10 ratio of existing culture to fresh media in 25 ml culture flasks without filter and incubated at 26 °C. Stationary phase parasites were centrifuged for 10 min at 2100 rpm and 4 °C. The supernatant was removed and the pellet re-suspended in RPMI medium (Sigma, UK). Cell number was estimated by microscopic counting with a Neubauer haemocytometer. Female BALB/c mice around 6–8 weeks old were purchased from Charles River Ltd. (Margate, UK). These mice were kept in humidity- and temperature controlled rooms (55–65% and 25–26 °C, respectively) and fed water and rodent food ad libitum. After acclimatization for 1 week, mice were randomized and subcutaneously (s.c.) injected in the shaven rump above the tail with 200 μl of a parasite suspension containing 4 × 107 low-passage-number (p < 5), stationary-phase L. major promastigotes in RPMI medium. Twelve days later, when a 5- to 6-mm non-ulcerating nodule had formed, mice were allocated to the different experimental arms to ensure comparable lesion sizes in each group. All animal experiments were conducted under licence X20014A54 according to UK Home Office regulations under the Animals (Scientific Procedures) Act 1986 and EC Directive 2010/63/E.
2.3. Treatment and sample collection
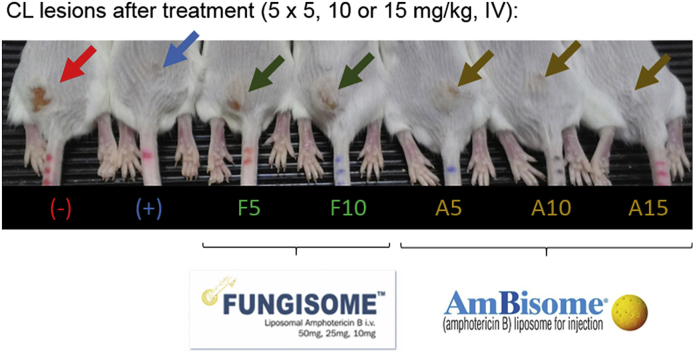
L. major -infected BALB/c mice (n = 4–5 per group) each received an intravenous bolus (200 μl) of A or F at 5, 10, 15 mg/kg or 0.85% saline (untreated negative control, (−)) over a 1–2 min period on days 0, 2, 4, 6 and 8. The dosing regimen for F and A was based on earlier data on the efficacy of A in the L. major-BALB/c model of CL (Wijnant et al., 2018). Due to previous reports of F's toxicity, the dose of 15 mg/kg was not exceeded (Szoka et al., 1987). The positive control group (+) received 10 daily doses of intraperitoneal (IP) 50 mg/kg paromomycin, a regimen with proven efficacy in this CL model (Wijnant et al., 2017; El-On and Hamburger, 1987). On day 10, the experiment was terminated, animals were sacrificed and lesion skin samples were collected.
2.4. Measurement of lesion size and intralesional AmB levels
The methodologies to determine lesion size, homogenize skin samples and measure intralesional AmB levels have been described in full detail in earlier publications (Wijnant et al., 2018). Samples in this study were treated identically. In brief, lesion size (average of width and length) was measured daily during treatment using digital callipers. On day 10, animals were sacrificed and the collected lesions were ground mechanically with zirconium oxide beads in 1 ml of PBS. The drug (AmB) was then extracted from tissue homogenates with 84:16 methanol:DMSO, followed by LC-MS/MS quantification.
2.5. Measurement of parasite load
A 2-step RT qPCR was used to determine parasite load in murine CL lesions. RNA from a 200 μl volume of skin homogenate was extracted using the GRS FullSample Purification kit (Grisp Research Solutions, Portugal). Briefly, samples were lysed, DNA was removed via a genomic DNA mini spin column and the collected flow-through was transferred to an RNA mini spin column and processed following the manufacturer's protocol. After washing, RNA was eluted from the column with 30 μl RNase-free water. Quality and purity of the RNA extract was estimated with a Nanodrop ND1000 (Thermo Fisher Scientific, USA). To avoid contamination or degradation of RNA, all workbench surfaces and materials were cleaned with RNase AWAY® (Thermo Fisher, USA) and samples kept on ice and stored at −80 °C until further use. Complementary DNA (cDNA) was then generated from 10 μl of the RNA-extract using the iScript™ cDNA Synthesis Kit (Bio-Rad, USA) on a T100 Thermal Cycler (Bio-Rad, USA) according to the manufacturer's instructions. TaqMan qPCR reactions of 10 μl were performed in duplicate including 2 μl of cDNA, 5 μl GRISP Xpert Fast PROBE master mix (Grisp Research Solutions, Portugal), 0.25 μM probe (StabVida, Portugal) and 0.4 μM of each primer in 96-well PCR plates (VWR, Portugal). Assays were performed in a CFX Connect™ Real-Time PCR Detection System (Bio-Rad, USA). Cycling conditions were as follows: 40 cycles at a denaturation setting of 95 °C for 5 min followed by a two-step amplification cycle of 95 °C for 10 s and 60 °C for 30 s. Each run included triplicates of a negative control, a no template control and the calibration standards (L. major DNA from 108 to 102 parasites). Bio-Rad CFX Manager 3.1 software was used for analysis. The lower limit of detection was 100 parasites. The qPCR methodology (based on amplification of the 18S ribosomal Leishmania region), probe and primer sequences (Van Der Meide et al., 2008) and preparation of calibration standards were as described before (Wijnant et al., 2017).
2.6. Dose-response curves
Non-linear fit models (log(agonist) versus normalized response with variable slope) in GraphPad Prism version 7.02 were used to calculate ED50 and LD50 data. Response in treated groups was expressed as relative reduction compared to untreated controls ((signal untreated – signal treated)/signal untreated × 100%).
2.7. Statistical analysis
Analysis of variance (ANOVA) assuming Gaussian distribution (one-way for parasite load and intralesional AmB level, repeated measures for lesion size) followed by Tukey's multiple comparison tests was used to analyse differences between groups. All data is presented as means and standard deviation (SD). A p - value < 0.05 was considered statistically significant. All analyses were performed using GraphPad Prism version 7.02.
3. Results
3.1. Toxic effects of F and A
After L. major-infected mice (n = 5) received slow infusions of 5, 10 or 15 mg/kg of either F or A (200 μl over 2 min), no direct adverse effects and signs of acute toxicity were observed during the first 30 min or 24 h after dosing. However, 48 h after drug administration (day 2), one mouse in the 10 mg/kg F and two mice in the 15 mg/kg F group had died. Among the surviving animals, which had been similarly dosed again on day 2, two more mice in the 15 mg/kg F died 24 h later. By day 3, the one surviving mouse in this highest dose F group showed signs of a hunched posture, pilo-erection and weight loss (data not shown) and it was humanely sacrificed by CO2. No signs of potential CL-related mortality such as severe ulceration, dissemination of the lesion or hepatosplenomegaly (which could confound the cause of death) were observed during autopsies of these F-treated mice. In contrast, in the mice dosed with A (all three dose levels), F (5 and 10 mg/kg) and negative and positive controls, no fatalities were seen during the rest of treatment.
3.2. Efficacy of F and A
3.2.1. Lesion size
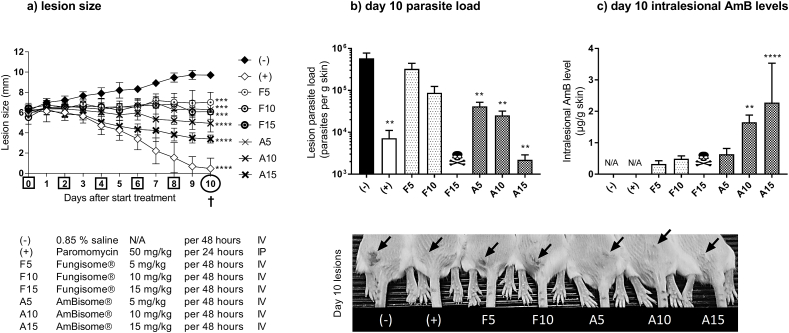
The effect of F and A on lesion size over the course of the 10-day IV treatment in the L. major –BALB/c model of CL is shown in Fig. 1a. Compared to the untreated controls (−), the lesion size for F was significantly lower in 5 mg/kg and 10 mg/kg groups (p = 0.0007 and 0.0002, respectively). Significant reductions in lesion size were also found for A at all dose levels (p < 0.0005). When the activity of A and F were compared at identical dose levels, differences in lesion size were not significant (5 mg/kg: p = 0.99, 10 mg/kg: p = 0.48). No direct comparison between F and A could be made for 15 mg/kg, due to the lethal effects at this dose level for F. Reductions in lesion size in liposomal AmB-treated groups were lower when compared to those in the positive control (+).
Fig. 1.
Comparative efficacy of the liposomal amphotericin B (AmB) formulations AmBisome® (A) and Fungisome® (F) in the L. major-BALB/c model of CL. Mice received 10 doses of 50 mg/kg paromomycin (IP, (+)) or five doses of either 0.85% saline (untreated negative control (−)) or 5, 10 and 15 mg/kg F and A (IV). During treatment, lesion size was measured daily (a). On day 10, lesion skin samples were collected and parasite load (b) and AmB levels (c) in the tissue were evaluated. The photo on the bottom shows the CL lesions (arrow) on the rump of the mice on day 10. Each point represents the mean ± SD (n = 4–5 per group). ANOVA (1-way for parasite load and intralesional AmB level, repeated measures for lesion size) followed by Tukey's multiple comparison tests was used to analyse differences between untreated controls and experimental groups. A p-value < 0.05 was considered statistically significant (*: p < 0.05, **: p < 0.001, ***: p < 0.0005, ****: p<0.0001), p>0.05 not significant (no marking above bar). †: day of sacrifice. Skull: no data available due to lethal toxicity at this dose level. N/A: not applicable.
3.2.2. Parasite load
The efficacy of F and A, as evaluated by RT qPCR parasite load on day 10, is shown in Fig. 1b. Compared to the untreated controls, parasite load was lower in the 5 mg/kg and 10 mg/kg F groups, but the differences were not significant (p = 0.51 and 0.06 respectively). In contrast, increasing doses of A resulted in significant reductions in parasite load (all p-values <0.05). At identical dose levels, the differences between A and F in parasite load were not significant (5 mg/kg: p = 0.39, 10 mg/kg: p = 0.99) and again, no comparison could be made at 15 mg/kg due to toxicity of F at this dose level. All liposomal AmB-treated groups had lower parasite load reductions compared to the positive control (+), except for the highest dose of A where there was no significant difference (p > 0.999).
3.2.3. Intralesional amphotericin B levels
We determined the drug levels of the active compound AmB within the infected lesion at the end of the experiment (Fig. 1c). At identical dose levels of 5 and 10 mg/kg, intralesional AmB levels were lower for F than for A, but the differences between the groups were not significant (p = 0.96 and 0.18, respectively). Due to fatal toxicity of F at 15 mg/kg, no direct comparison with A could be made at this dose level. As expected, no AmB was detectable in samples from untreated (−) and positive controls (+).
3.3. Therapeutic window of F and A
After the logarithm of the dose level was plotted against percentage response for A and F (data from toxicity section and Fig. 1), 50% and 90% effective (ED) and lethal (LD) doses were calculated (Table 1). Based on the number of lethal events per group over the course of drug administration (A: 0/5 for 5, 10 and 15 mg/kg; F: 0/5 for 5 mg/kg, 1/5 for 10 mg/kg, 4/5 for 15 mg/kg F), LD50 and LD90 could not be calculated for A, but were found to be 12.3 and 16.9 mg/kg for F. Both 50% and 90% effective doses for parasite load and lesion size were higher for F than for A, indicating inferior efficacy. Therapeutic indices (LD/ED) were over 10-fold lower for F compared to A.
Table 1.
50% and 90% effective (ED) and lethal (LD) doses (mg/kg) for the liposomal amphotericin B (AmB) formulations AmBisome® (A) and Fungisome® (F) in the L. major-BALB/c model of CL, based on toxicity and Fig. 1 data. The number of lethal events per group over the full course of treatment was monitored during treatment for calculation of LD values. At day 10, parasite load (PL) and lesion size (LS) were determined and effect expressed as relative percentage of reduction compared to the untreated control group, which was used to calculate ED values. Therapeutic indices (TI) were calculated as the LD over ED ratio. As no lethal events occurred for A at the tested dose levels, reference LD values from mice (*) were used from the AmBisome® FDA pharmacological review document, application number 050740 (Drug Approval Package).
| AMBISOME® |
FUNGISOME® |
|||
|---|---|---|---|---|
| 50% | 90% | 50% | 90% | |
| LD | 133.0* | 150.0* | 12.3 | 16.9 |
| EDPL | 3.0 | 4.5 | 4.0 | 11.0 |
| EDLS | 8.8 | 51.3 | 12.8 | 102.8 |
| TIPL | 44.9 | 33.0 | 3.1 | 1.5 |
| TILS | 15.1 | 2.9 | 1.0 | 0.2 |
4. Discussion
The liposomal multilamellar amphotericin B (AmB) formulation Fungisome® (F) is intravenously administered after sonication for the treatment of VL (Goswami et al., 2016). Little is known about its potential role in CL pharmacotherapy. Here, we evaluated efficacy, toxicity and intralesional drug accumulation of F in direct comparison to the standard liposomal AmB product AmBisome® (A) in the L. major-BALB/c model of CL.
Compared to A, F was more toxic and less efficacious; thus, it had a narrower therapeutic index. As the main aim of this work was to evaluate efficacy and not toxicity, we did not perform dose-escalating studies. However, LD50 values were still calculable as multiple unexpected fatal events occurred at the highest dose of F. The observed LD50 for F (12.3 mg/kg) likely underestimates its safety window, as other researchers found a higher murine LD50 (17 mg/kg, Szoka et al., 1987) and doses up to 10–15 mg/kg are tolerated by patients (Sundar et al., 2015). Chronic side effects of the active compound AmB (such as nephrotoxicity, hepatotoxicity and anaemia) were plausible causes of death in the 15 mg/kg F group, because the lipids of the F liposome are non-toxic (Kshirsagar et al., 2005) and we saw no typical signs of acute AmB overdose in mice (convulsions followed by coma). Although F might be more toxic than A (possibly due to higher uptake in the kidney and/or liver, or increased leakage of AmB from the liposome to the blood), its LD50 is still 5-fold higher than that of non-liposomal AmB deoxycholate (LD50 = 2.3 mg/kg, Szoka et al., 1987).
F was mildly efficacious at 5 × 5 mg/kg and 5 × 10 mg/kg, as it caused a significant suppressive effect on lesion development, despite a non-significant reduction in parasite loads. The inferior efficacy of F compared to A could be related to lower intralesional drug concentrations. Levels of AmB in the infected skin have been shown to correlate well to therapeutic outcomes (Wijnant et al., 2018), which can be expected because of the concentration-dependency of its antimicrobial activity (Lestner et al., 2010). The higher accumulation of A over F at the infected skin site could be explained by differences in a number of physicochemical and pharmacokinetic parameters between the two formulations. After intravenous administration of A, 97% of AmB in the bloodstream remains liposome-associated during the first 4 h (Bekersky et al., 2002) and systemic exposure levels are high (1 mg/kg: Cmax ≈ 10 μg/ml, AUC ≈70 μg.h/ml in mice, Wijnant et al., 2018). The small liposome size (≈70 nm) could facilitate extravasation through the leaky capillaries in the inflamed lesion skin (Romero and Morilla, 2008), followed by uptake by the parasitized dermal macrophages. Alternatively, phagocytic monocytes in the blood could carry ingested A while migrating to the infection site (Voak et al., 2017). In contrast to A, the liposomal stability of F in plasma is unknown (possibly releasing ‘free’ AmB after interaction with (lipo)proteins, Romero and Morilla, 2008) and its systemic exposure is 7- fold lower (1 mg/kg: Cmax≈1 μg/ml, AUC≈11 μg.h/ml in humans, Gokhale et al., 1993). The 3-fold larger size of F (≈220 nm) compared to A enhances its clearance by the reticuloendothelial system following opsonin-coating (80% of the total F dose accumulates in the liver within 30 min, Jadhav et al., 2011; Ownes and Peppas, 2006), and could possibly also affect the size-dependent processes of extravasation (Poh et al., 2015), macrophage uptake (Champion et al., 2008) and drug retention in tissues (Tang et al., 2014). The dependency of the final diameter of the small unilamellar F liposomes on the water bath equipment (type, condition, settings) used for sonication of the multilamellar vesicles could be a concern for safety and quality-assurance. The importance of a strict manufacturing process to control particle size is illustrated by the increased toxicity of other liposomal AmB products Anfrogen and Lambin compared to A, even though their lipid composition is identical. Of the several generic liposomal AmB formulations currently available, F is the only one that has been tested in clinical trials for the treatment of (visceral) leishmaniasis (Adler-Moore et al., 2016).
Our data has implications for both the clinical use of F and drug development for CL. The relatively small scale (n = 4–5), murine model of L. major CL (non-cure BALB/c rather than self-curing C57BL/6) and single tested treatment regimen (5 alternate day administrations over 10 days) are limitations of this work. However, the lower tolerated doses, inferior efficacy and much smaller therapeutic window of F compared to A we observed in our murine study might indicate an increased risk of CL treatment failure and/or duration. The proof of low but significant drug accumulation of F in L. major skin lesions and already known activity against L. donovani in VL raises the possibility of use in PKDL treatment, be it with similar caveats as for CL. However, the immunological and histopathological nature of the inflammatory skin response in PKDL is different compared to that of localized CL (Mondal et al., 2010; Scott and Novais, 2016; Nylén and Eidsmo, 2012), which could affect the pharmacokinetics of liposomal formulations. Finally, many researchers over the last decades have proposed the encapsulation of antileishmanial drugs into liposomes as a strategy for passive targeting of the CL infection site (Gutiérrez et al., 2016). The data in the present study suggests an advantage for smaller liposomes (70 > 220 nm) with higher stability and exposure in the bloodstream after IV administration.
In conclusion, F could play a minor role in the systemic treatment of (mainly complex) CL, as we observed moderate efficacy in a murine disease model. However, compared to A, the therapeutic index was narrower and the in vivo activity was inferior due to lower levels of the active compound AmB delivered to the infected lesion site. Future research should also investigate the effects of the alternative topical formulation Fungisome® Gel (Lifecare Innovations), as it has potential for local treatment for simple, small CL lesions with little risk of complication.
Conflicts of interest
None.
Competing interests
No competing interstates to report.
Acknowledgements
Gert-Jan Wijnant's doctoral project is part of the EuroLeish.Net Training Network (www.euroleish.net) and has received funding from the European Horizon's 2020 Research and Innovation Programme under the Marie Sklodowska-Curie grant agreement number 642609. The authors thank Drs. L. and J. Verma, Lifecare Innovations (Lucknow, India) for the generous donation of Fungisome® and Dr. Ana Domingos for laboratory facilities at IHMT.
References
- Adler-Moore J.P., Gangneux J.-P., Pappas P.G. Comparison between liposomal formulations of amphotericin B. Med. Mycol. 2016;54:223–231. doi: 10.1093/mmy/myv111. [DOI] [PubMed] [Google Scholar]
- Alvar J., Vélez I.D., Bern C., Herrero M., Desjeux P., Cano J., Jannin J., Boer M. den, Team, the W.L.C Leishmaniasis worldwide and global estimates of its incidence. PLoS One. 2012;7 doi: 10.1371/journal.pone.0035671. e35671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aronson N., Herwaldt B.L., Libman M., Pearson R., Lopez-Velez R., Weina P., Carvalho E.M., Ephros M., Jeronimo S., Magill A. Diagnosis and treatment of leishmaniasis: clinical practice guidelines by the infectious diseases society of America (IDSA) and the American society of tropical medicine and hygiene (ASTMH) Clin. Infect. Dis. 2016;63:e202–e264. doi: 10.1093/cid/ciw670. [DOI] [PubMed] [Google Scholar]
- Basher A., Maruf S., Nath P., Hasnain M.G., Mukit M.A., Anuwarul A., Aktar F., Nath R., Hossain A.A., Milton A.H., Mondal D., Mohammad Sumsuzzaman A.K., Rahman R., Faiz M.A. Case report: treatment of widespread nodular post kala-azar dermal leishmaniasis with extended-dose liposomal amphotericin B in Bangladesh: a series of four cases. Am. J. Trop. Med. Hyg. 2017;97:1111–1115. doi: 10.4269/ajtmh.16-0631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bekersky I., Fielding R.M., Dressler D.E., Lee J.W., Buell D.N., Walsh T.J. Plasma protein binding of amphotericin B and pharmacokinetics of bound versus unbound amphotericin B after administration of intravenous liposomal amphotericin B (AmBisome) and amphotericin B deoxycholate. Antimicrob. Agents Chemother. 2002;46:834–840. doi: 10.1128/AAC.46.3.834-840.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharya P., Ali N. Treatment of visceral leishmaniasis: anomalous pricing and distribution of AmBisome and emergence of an indigenous liposomal amphotericin B, FUNGISOME. J. Parasit. Dis. 2016;40:1094–1095. doi: 10.1007/s12639-014-0607-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodhe P.V., Kotwani R.N., Kirodian B.G., Pathare A.V., Pandey A.K., Thakur C.P., Kshirsagar N.A. Dose-ranging studies on liposomal amphotericin B (L-AMP-LRC-1) in the treatment of visceral leishmaniasis. Trans. R. Soc. Trop. Med. Hyg. 1999;93:314–318. doi: 10.1016/s0035-9203(99)90036-6. [DOI] [PubMed] [Google Scholar]
- Champion J.A., Walker A., Mitragotri S. Role of particle size in phagocytosis of polymeric microspheres. Pharm Res. 2008;25:1815–1821. doi: 10.1007/s11095-008-9562-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen B.E. The role of signaling via aqueous pore formation in resistance responses to amphotericin B. Antimicrob. Agents Chemother. 2016;60:5122–5129. doi: 10.1128/AAC.00878-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desjeux P., Ghosh R.S., Dhalaria P., Strub-Wourgaft N., Zijlstra E.E. 2013. Report of the Post Kala-azar Dermal Leishmaniasis (PKDL) Consortium Meeting, New Delhi, India; pp. 27–29. June 2012. Parasit Vectors 6, 196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drug Approval Package: Ambisome (Amphotericin B) NDA# 050740 [WWW Document], n.d. URL https://www.accessdata.fda.gov/drugsatfda_docs/nda/97/050740_ambisome_toc.cfm (accessed 11.16.2017).
- Dupont B. Overview of the lipid formulations of amphotericin B. J. Antimicrob. Chemother. 2002;49(Suppl 1):31–36. doi: 10.1093/jac/49.suppl_1.31. [DOI] [PubMed] [Google Scholar]
- El-On J., Hamburger A.D. Topical treatment of New and Old World cutaneous leishmaniasis in experimental animals. Trans. R. Soc. Trop. Med. Hyg. 1987;81:734–737. doi: 10.1016/0035-9203(87)90011-3. [DOI] [PubMed] [Google Scholar]
- Gaspani S. Access to liposomal generic formulations: beyond AmBisome and Doxil/Caelyx. Generics and Biosimilars Initiative Journal. 2013;2:60–62. doi: 10.5639/gabij.2013.0202.022. [DOI] [Google Scholar]
- Gokhale P.C., Barapatre R.J., Advani S.H., Kshirsagar N.A., Pandya S.K. Pharmacokinetics and tolerance of liposomal amphotericin B in patients. J. Antimicrob. Chemother. 1993;32:133–139. doi: 10.1093/jac/32.1.133. [DOI] [PubMed] [Google Scholar]
- Goswami Rama P., Goswami Rudra P., Das S., Satpati A., Rahman M. Short-course treatment regimen of indian visceral leishmaniasis with an indian liposomal amphotericin B preparation (Fungisome™) Am. J. Trop. Med. Hyg. 2016;94:93–98. doi: 10.4269/ajtmh.14-0657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guery R., Henry B., Martin-Blondel G., Rouzaud C., Cordoliani F., Harms G., Gangneux J.-P., Foulet F., Bourrat E., Baccard M., Morizot G., Consigny P.-H., Berry A., Blum J., Lortholary O., Buffet P., Network, the F.C.L.S. group & the L Liposomal amphotericin B in travelers with cutaneous and muco-cutaneous leishmaniasis: not a panacea. PLoS Neglected Trop. Dis. 2017;11 doi: 10.1371/journal.pntd.0006094. e0006094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutiérrez V., Seabra A.B., Reguera R.M., Khandare J., Calderón M. New approaches from nanomedicine for treating leishmaniasis. Chem. Soc. Rev. 2016;45:152–168. doi: 10.1039/c5cs00674k. [DOI] [PubMed] [Google Scholar]
- Jadhav M.P., Nagarsenker M.S., Gaikwad R.V., Samad A., Kshirsagar N.A. Formulation and evaluation of long circulating liposomal amphotericin B: a scinti-kinetic study using 99mTc in BALB/C mice. Indian J. Pharmaceut. Sci. 2011;73:57–64. doi: 10.4103/0250-474X.89757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kshirsagar N. Different liposomal amphotericin B formulations for visceral leishmaniasis–Author’s reply. The Lancet Global Health. 2014;2 doi: 10.1016/S2214-109X(14. e450. [DOI] [PubMed] [Google Scholar]
- Kshirsagar N.A., Pandya S.K., Kirodian G.B., Sanath S. Liposomal drug delivery system from laboratory to clinic. J. Postgrad. Med. 2005;51(Suppl 1):S5–S15. [PubMed] [Google Scholar]
- Lestner J.M., Howard S.J., Goodwin J., Gregson L., Majithiya J., Walsh T.J., Jensen G.M., Hope W.W. Pharmacokinetics and pharmacodynamics of amphotericin B deoxycholate, liposomal amphotericin B, and amphotericin B lipid complex in an in vitro model of invasive pulmonary aspergillosis. Antimicrob. Agents Chemother. 2010;54:3432–3441. doi: 10.1128/AAC.01586-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mondal D., Alvar J., Hasnain M.G., Hossain M.S., Ghosh D., Huda M.M., Nabi S.G., Sundar S., Matlashewski G., Arana B. Efficacy and safety of single-dose liposomal amphotericin B for visceral leishmaniasis in a rural public hospital in Bangladesh: a feasibility study. The Lancet Global Health. 2014;2:e51–e57. doi: 10.1016/S2214-109X(13. [DOI] [PubMed] [Google Scholar]
- Mondal S., Bhattacharya P., Rahaman M., Ali N., Goswami R.P. A curative immune profile one week after treatment of Indian kala-azar patients predicts success with a short-course liposomal amphotericin B therapy. PLoS Neglected Trop. Dis. 2010;4:e764. doi: 10.1371/journal.pntd.0000764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nylén S., Eidsmo L. Tissue damage and immunity in cutaneous leishmaniasis. Parasite Immunol. 2012;34:551–561. doi: 10.1111/pim.12007. [DOI] [PubMed] [Google Scholar]
- Owens D.E., Peppas N.A. Opsonization, biodistribution, and pharmacokinetics of polymeric nanoparticles. Int. J. Pharm. 2006;307:93–102. doi: 10.1016/j.ijpharm.2005.10.010. [DOI] [PubMed] [Google Scholar]
- Poh S., Chelvam V., Low P.S. Comparison of nanoparticle penetration into solid tumors and sites of inflammation: studies using targeted and nontargeted liposomes. Nanomedicine (Lond) 2015;10:1439–1449. doi: 10.2217/nnm.14.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reithinger R., Dujardin J.-C., Louzir H., Pirmez C., Alexander B., Brooker S. Cutaneous leishmaniasis. Lancet Infect. Dis. 2007;7:581–596. doi: 10.1016/S1473-3099(07. [DOI] [PubMed] [Google Scholar]
- Rocio C., Amato V.S., Camargo R.A., Tuon F.F., Nicodemo A.C. Liposomal formulation of amphotericin B for the treatment of mucosal leishmaniasis in HIV-negative patients. Trans. R. Soc. Trop. Med. Hyg. 2014;108:176–178. doi: 10.1093/trstmh/tru011. [DOI] [PubMed] [Google Scholar]
- Romero E.L., Morilla M.J. Drug delivery systems against leishmaniasis? Still an open question. Expet Opin. Drug Deliv. 2008;5:805–823. doi: 10.1517/17425247.5.7.805. [DOI] [PubMed] [Google Scholar]
- Sanath S.S., Gogtay N.J., Kshirsagar N.A. Post-marketing study to assess the safety, tolerability and effectiveness of Fungisome: an Indian liposomal amphotericin B preparation. J. Postgrad. Med. 2005;51(Suppl 1):S58–S63. [PubMed] [Google Scholar]
- Scott P., Novais F.O. Cutaneous leishmaniasis: immune responses in protection and pathogenesis. Nat. Rev. Immunol. 2016;16:581–592. doi: 10.1038/nri.2016.72. [DOI] [PubMed] [Google Scholar]
- Serrano D., Ballesteros M., Schätzlein A., Torrado J., Uchegbu I. Amphotericin B formulations – the possibility of generic competition. Pharm. Nanotechnol. 2013;1:250–258. doi: 10.2174/2211738501999131118125018. [DOI] [Google Scholar]
- Sundar S., Singh A., Rai M., Chakravarty J. Single-dose indigenous liposomal amphotericin B in the treatment of Indian visceral leishmaniasis: a phase 2 study. Am. J. Trop. Med. Hyg. 2015;92:513–517. doi: 10.4269/ajtmh.14-0259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szoka F.C., Milholland D., Barza M. Effect of lipid composition and liposome size on toxicity and in vitro fungicidal activity of liposome-intercalated amphotericin B. Antimicrob. Agents Chemother. 1987;31:421–429. doi: 10.1128/aac.31.3.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang L., Yang X., Yin Q., Cai K., Wang H., Chaudhury I., Yao C., Zhou Q., Kwon M., Hartman J.A., Dobrucki I.T., Dobrucki L.W., Borst L.B., Lezmi S., Helferich W.G., Ferguson A.L., Fan T.M., Cheng J. Investigating the optimal size of anticancer nanomedicine. Proc. Natl. Acad. Sci. U. S. A. 2014;111:15344–15349. doi: 10.1073/pnas.1411499111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonin F.S., Steimbach L.M., Borba H.H., Sanches A.C., Wiens A., Pontarolo R., Fernandez-Llimos F. Efficacy and safety of amphotericin B formulations: a network meta-analysis and a multicriteria decision analysis. J. Pharm. Pharmacol. 2017;69:1672–1683. doi: 10.1111/jphp.12802. [DOI] [PubMed] [Google Scholar]
- van der Meide W., Guerra J., Schoone G., Farenhorst M., Coelho L., Faber W., Peekel I., Schallig H. Comparison between quantitative nucleic acid sequence-based amplification, real-time reverse transcriptase PCR, and real-time PCR for quantification of Leishmania parasites. J. Clin. Microbiol. 2008;46:73–78. doi: 10.1128/JCM.01416-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voak A.A., Harris A., Qaiser Z., Croft S.L., Seifert K. Pharmacodynamics and biodistribution of single-dose liposomal amphotericin B at different stages of experimental visceral leishmaniasis. Antimicrob. Agents Chemother. 2017;61 doi: 10.1128/AAC.00497-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh T.J., Yeldandi V., McEvoy M., Gonzalez C., Chanock S., Freifeld A., Seibel N.I., Whitcomb P.O., Jarosinski P., Boswell G., Bekersky I., Alak A., Buell D., Barret J., Wilson W. Safety, tolerance, and pharmacokinetics of a small unilamellar liposomal formulation of amphotericin B (AmBisome) in neutropenic patients. Antimicrob. Agents Chemother. 1998;42:2391–2398. doi: 10.1128/aac.42.9.2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wijnant G.-J., Bocxlaer K.V., Yardley V., Murdan S., Croft S.L. Efficacy of paromomycin-chloroquine combination therapy in experimental cutaneous leishmaniasis. Antimicrob. Agents Chemother. 2017;61 doi: 10.1128/AAC.00358-17. e00358-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wijnant G.-J., Bocxlaer K.V., Yardley V., Harris A., Murdan S., Croft S.L. Relation between skin pharmacokinetics and efficacy in AmBisome treatment of murine cutaneous leishmaniasis. Antimicrob. Agents Chemother. 2018;62 doi: 10.1128/AAC.02009-17. e02009-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wortmann G., Zapor M., Ressner R., Fraser S., Hartzell J., Pierson J., Weintrob A., Magill A. Lipsosomal amphotericin B for treatment of cutaneous leishmaniasis. Am. J. Trop. Med. Hyg. 2010;83:1028–1033. doi: 10.4269/ajtmh.2010.10-0171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zijlstra E.E., Musa A.M., Khalil E.a.G., el-Hassan I.M., el-Hassan A.M. Post-kala-azar dermal leishmaniasis. Lancet Infect. Dis. 2003;3:87–98. doi: 10.1016/s1473-3099(03)00517-6. [DOI] [PubMed] [Google Scholar]