Graphical abstract
Keywords: Thevetia peruviana, Plant cell culture, Phenolic compounds, Methyl jasmonate, Salicylic acid
Highlights
-
•
Cell suspension cultures from T. peruviana were used to produce secondary metabolites.
-
•
Production of phenolic compounds in plant cell cultures was time-dependent.
-
•
Elicitation with salicylic acid and MeJA increased biosynthesis of phenolics compounds.
-
•
Plant cells exposed to MeJA produced the higher levels of phenolics and antioxidants.
Abstract
The objective was to enhance the production of the phenolic compounds in plant cell suspension cultures of T. peruviana at shake flask scale. The effects of salicylic acid (SA), methyl-jasmonate (MeJA) and the combination of both (SA/MeJA) were studied. Elicitor concentration, elicitation time and harvest time of cells were optimized. Phenolic compound content (PCC), flavonoid content (FC) and antioxidant activity (AA) were determined by the folin-ciocalteu method, flavonoid-aluminum complexation method and the ABTS assay, respectively. Differences between intracellular metabolite profiles due to the mentioned treatments were analyzed by Thin-layer chromatography and High-performance liquid chromatography. Highest PCC, FC and AA were obtained under the following treatments: 3 μM MeJA > 3 μM MeJA/300 μM SA > 300 μM SA > control, when elicited on the 4th day and harvested 96-h post-elicitation. It was demonstrated that exposure to 3 μM MeJA increase 1.49-fold of PCC, 1.66-fold of AA and 2.55-fold of FC compared to the control culture.
1. Introduction
Thevetia peruviana (Pers.) K. Schum is an ornamental shrub belonging to the Gentianales order, Apocynaceae family. It is widely distributed in the tropical and sub-tropical regions of Central and South America, Asia, and Africa [1]. This plant is pharmacologically recognized for containing cardiotonic glycosides such as peruvoside and thevetin [2,3], especially concentrated in its seeds. These metabolites have a positive inotropic effect, like in digoxin [1,4]. Therefore, the un-regulated consumption of the fruits has been reported as toxic [5]. T. peruviana also produces phenolic compounds with potential usage as antimicrobial [6,7] and antineoplastic drugs [8,9]. Additionally, flavonoids have been identified in the fruits and leaves of this plant, and they are capable of inhibit the integrase enzyme and reverse transcriptase associated to DNA polymerase of the human immunodeficiency virus HIV-1 [10].
Phenolic compounds (PC) are one of the most important secondary metabolites in plants. These compounds are related to the mechanisms of environmental adaptation and stress under in vivo growth conditions [11]. However, the quality and quantity of the PC and other secondary metabolites produced in field crops is extremely variable and depends on the biotic and abiotic conditions [12]. In vitro cultivation of plant cells is a viable alternative to increase the growth rate of biomass and the stability during the continuous production of PC and other metabolites. Additionally, in vitro cultivation allows for the manipulation of growth variables, as well as the use of precursors and/or elicitors. These variables might change the biosynthetic pathways of the compounds, optimizing its production [13].
An elicitor can be defined as a compound (natural or synthetic) that initiates or improves the biosynthesis of specific metabolites when introduced in small concentrations to a living cells system [14,15]. Jasmonates (JA) and salicylic acid (SA) are signaling molecules that respond to the biotic and abiotic stress of the plants. These molecules can be used to induce catalytic reactions by specific enzymes involved in the biosynthesis of PC [16]. JA and SA have been used in the stimulation of flavonoids and polyphenols production in cell suspension, calluses and tissue cultures of diverse plant families [[17], [18], [19], [20], [21]]. A recent study published by Rincón et al. [22] reported an increase in the production of PC from callus culture of T. peruviana elicited with a combination of 100 μM of JA and 10 μM of abscisic acid. Similarly, MeJA, a derivative of jasmonic acid, has also been used to elicitate cell suspension culture of T. peruviana, resulting in an increased production of peruvoside, a cardiotonic glycoside [23].
In this study, the effect of SA and MeJA was evaluated on the production of PC in a plant cell suspension culture of T. peruviana at shake flask scale. The concentration and time of addition of the elicitors that improve the production of PC was established, as well as the time of cultivation of the cells post-elicitation.
2. Materials and methods
2.1. Reagents and materials
Folin-Ciocalteu reagent (2.0 N), salicylic acid, methyl jasmonate (95%) and quercetin were purchased from Sigma-Aldrich Chemicals (St. Louis, MO). Gallic acid, acid 2,2′-azino-bis (3-ethylbenzothiazoline-6-sulfonic, ABTS) and glass HPTLC (High Performance TLC) Silica Gel 60 F254 plates were purchased from Merck (Darmstadt, Germany).
2.2. Callus culture
Calluses were obtained from fruit pulp of T. peruviana plants cultivated at the Universidad Nacional de Colombia, Medellin (6°15′44″N 75°34′37″O). Fruits were disinfected following the protocol outlined previously by Arias et al. [23]. Briefly, fruits were submerged in ethanol (70%) for 5 min. Then transfer to a solution of NaClO at 10% (v/v) for 5 min. In-between and after the disinfectants, the fruits were rinsed three times with sterile distilled water. Afterwards, the explants were transferred aseptically onto Schenk and Hildebrandt solid medium (SH) supplemented with 2 mg/L 2,4-D, 0.5 mg/L kinetin, 7 g/L agar, 30 g/L sucrose and 1 g/L myoinositol (pH 5.8), sterilized at 20 psi for 15 min. Cultures were maintained on photoperiod (12 h light/12 h darkness) at 25 °C. Sub-cultures were performed every 3 weeks until friable calluses were obtained.
2.3. Cell suspension culture
Ten grams of fresh friable calluses (g FW) were transferred to 100 mL of SH liquid sterile medium supplemented with 2 mg/L 2,4-D, 0.5 mg/L kinetin, 30 g/L sucrose and 1 g/L myoinositol (pH 5.8), in 250 mL flasks. Cell suspension cultures were maintained on an orbital shaker at 110 rpm (New Brunswick™ Innova® 2300), natural photoperiod and 25 °C. Sub-cultures were made every 2 weeks.
2.4. Growth kinetics
Growth kinetic was done in 250 mL flasks, using an inoculum (cell suspension culture) of 6 days from the last subculture, and an initial concentration of 2–3 g dry weight per liter (DW/L) in 100 mL of supplemented SH liquid sterile medium. The other conditions of the cultures were maintained as previously described. Cellular growth was determined by measuring the DW of biomass every 2 days for 18 days, in duplicate. The content of each flask was filtered using a vacuum system and quantitative filter paper. Biomass was rinsed three times with distilled water and was dried in a convection oven at 60 °C for 48 h [24]. Results were expressed as g DW/L.
2.5. Elicitor treatment method
SA and MeJA were prepared in an aqueous solution of ethanol 50% (v/v) and were filter-sterilized through a 0.45 μm Millipore filter (Minisart®, Sartorius, Germany). Experiments were done with 6 days cell suspensions at a concentration of 2–3 g DW/L in 250 mL flasks with 100 mL of SH liquid sterile medium, under the same conditions described previously. A factorial experiment based on completely randomized design was used to study the effect of the elicitation conditions on the PC production. The evaluated factors were: elicitor concentration, elicitation time and harvest time of the cells post-elicitation (see supplementary file). All the experiments were carried out in triplicate using a destructive sampling method, which consisted of processing the complete sample at each harvest time. Ethanol solution 50% (v/v) was used as control in these experiments. Non-significant elicitor effect was found when ethanol 50% was used when compared to a culture without ethanol (data not shown).
2.5.1. Effect of elicitor concentration on PC production
Concentration of each elicitor was initially evaluated. MeJA was used in concentrations of 0, 1, 3 and 5 μM; while SA was used in concentrations of 0, 100, 300 and 600 μM. Elicitor concentration ranges used in these experiments were chosen by reviewing published studies [17,18] and by a previous screening performed in our laboratory. Elicitors were added during the exponential growth phase (day 8). Cells were harvested 24 h and 96 h after the addition of the elicitor.
2.5.2. Effect of the elicitation time on PC production
Elicitors were added at three different times: at the beginning of the culture (day 0) and during the exponential phase (days 4 and 8), using the best concentration of each elicitor, as it was previously established. Cells were harvested 24 h and 96 h after the addition of the elicitor.
2.5.3. Effect of the harvest time on PC production
The best cultivation time (24–48 – 72–96 and 120 h) after addition of the elicitor was determined, using the concentration and elicitation time that were previously established. In addition, flavonoid content (FC) and antioxidant activity (AA) were measured for each cultivation time.
2.6. Analytical methods
2.6.1. Intracellular metabolites
The biomass was dried in a convection oven at 60 °C for 48 h and was pulverized using mortar and a pestle. The powder (0.3 g) was extracted with 15 mL of an aqueous solution of ethanol 50% (v/v), in an ultrasonic bath (40 kHz) at 30 °C for 30 min, to obtain the intracellular metabolites. The extracts were centrifuged at 3000 rpm for 10 min; the supernatant was collected and stored in polypropylene tubes at - 4 °C, protected from light. The extracts were used for the quantitative determination of phenols and flavonoids. An extract of the dried and powder fruit pulp of T. peruviana was also prepared to determine the metabolites present in the initial explant, following the methodology prescribed for the intracellular extraction of the T. peruviana biomass.
2.6.2. Extracellular metabolites
Extracellular metabolites were determined directly from the cell culture medium without any previous extraction step.
2.6.3. Phenolic compounds content (PCC)
These compounds were determined with the folin-ciocalteu method, using gallic acid as standard [25]. A volume of 2 mL of samples (culture medium or intracellular extracts) were mixed with 2.5 mL of the folin-ciocalteu reagent at 10% (v/v), in different test tubes. After 2 min, 2 mL of 7.5% (p/v) Na2CO3 were added; followed by incubation during 10 min at 50 °C. The absorbance of each reaction was measured at a wavelength of 765 nm in a Genesys 20 Spectronic spectrophotometer (Thermo Fisher Scientific®). The equipment was adjusted to zero absorbance using the following blank: 2 mL of water plus 2.5 mL of 10% folin-ciocalteu reagent and 2 mL of 7.5% Na2CO3. A standard calibration was prepared using gallic acid (80 – 40 – 20 - 10 and 5 μg/mL) and the PCC in each sample was calculated and expressed as milligrams equivalent to gallic acid per gram of dry biomass weight, mg GAE/g DW.
2.6.4. Flavonoid content (FC)
Flavonoid compounds were determined by flavonoid-aluminum (AlCl3) complexation method [26], using quercetin as a standard. Briefly, a volume of 1 mL of samples were added in different test tubes and 0.3 mL of 5% (p/v) NaNO2 was added to each sample, followed by 5 min of incubation. Then, 0.5 mL of 2% (p/v) AlCl3 was added and the sample was softly shaken and neutralized 6 min later with 0.5 mL of 1 N NaOH. After 10 min, the absorbance was read at a wavelength of 425 nm. Samples without AlCl3 were used as blank. FC was calculated using a standard calibration of quercetin alcoholic solution (200 – 100 – 25 – 12.5 and 6.25 μg/mL) and expressed as milligrams of quercentin equivalent per gram of dry biomass weight, mg QE/g DW.
2.6.5. Antioxidant activity (AA)
Antioxidant activity was determined using the ABTS radical cation decolorization assay [27]. Previously, an aqueous solution of 7 mM of ABTS was prepared and the ABTS radical cation was obtained mixing equal volumes of 7 mM ABTS solution and 2.45 mM potassium persulfate. The mix was incubated in the dark for 16 h at 24 °C. Concentrated solution of ABTS•+ was diluted in distilled water until an absorbance of 0.70 ± 0.1 was achieved at a 734 nm wavelength.
A volume of 100 μL of each sample was mixed with 3 mL of the ABTS•+ solution. The mixture was incubated for 10 min in the dark and then the absorbance was measured at 734 nm, using methanol as blank. AA was calculated using a standard calibration of Trolox methanolic solution (150 – 75 – 37.5 – 18.8 and 9.4 μg/mL) and expressed as milligrams equivalent to Trolox per gram of dry biomass weight (mg ET/g DW).
2.7. Thin-layer chromatography (TLC)
A preliminary analysis of the intracellular PC in the cell suspensions before and after the treatments with the elicitors was performed by TLC. A 10 mL volume of each intracellular extract was concentrated in a rotary evaporator (IKA® HB10) under reduce pressure (145 mbar), at 40 °C and 110 rpm. Concentrated extracts were brought to 2 mL with ethanol. A volume of 5 μL of each concentrated extract was applied on HPTLC silica gel 60 F254 plates. The solvents used as mobile phases were: butanol:acetic acid:water (4:1:5 v/v/v) and hexane: ethyl acetate: water (20:19:1 v/v/v). Detection of phenolic compounds was performed by spraying 10% (v/v) folin-Ciocalteau reagent in an aqueous solution of methanol 50% (v/v). For flavonoids detection the plates were sprayed with an ethanolic solution of AlCl3 10% (w/v) and visualized under UV light at 366 nm. After staining, the plates were photograph with the ChemiDoc™ MP System equipment and analyzed with ImageLab™(Bio-Rad®) software, and the free access software JustQuantify (http://justquantify.eu/DefaultHD.aspx).
2.8. HPLC-DAD
A volume of 2 mL of intracellular extracts were diluted to 10 mL in volumetric flask with a 50% ethanol aqueous solution and then were filtered on 0.45 μm membranes. Diluted extracts were analyzed on a Shimadzu Prominence HPLC equipment coupled to a diode array detector (SPD-M20 A). A reversed phase column LiChrospher® 250-4 RP-18, 5-μm (Merck S.A) was used for the chromatographic separation at 28 °C, using a flow rate 1.5 mL min−1 and the injection volume 20 μL. The mobile phase consisted of: A (formic acid 1% in water) and B (formic acid 1% in acetonitrile). The elution gradient was as follows: 0 min (80% A + 20% B); 7 min (75% A + 25% B); 13 min (70% A + 30% B); 7 min (65% A + 35% B); 3 min (80% A + 20% B). For the data analysis, the wavelength of 280 nm was selected. The retention times (tR) and the UV–vis absorbance data of the peaks present in the samples and those obtained from analytical standards of flavonoid and phenolic compounds previously reported in T. peruviana [28], were compared. The analytical standards were HPLC grade and included: chlorogenic acid (≥95%), trans-sinapic acid (≥99.0%), quercetin (≥95%), (±)-hesperetin (≥98.0%) and kaempferol (≥97.0%).
2.9. Statistical analysis
All data obtained was analyzed with the free statistic software RStudio Version 1.1.383. The results of PCC, FC and AA were present as mean ± standard deviation (SD). Differences between treatments were evaluated with a one-way variance analysis (ANOVA) with a significance level of 0.05. Multiple (pair-wise) comparisons was done by Tukey’s honest significantly test (Tukey’s HSD) and results were presented as confidence intervals at 95% (95% CI).
3. Results and discussion
3.1. Growth kinetics
The exponential growth phase of cell suspension culture of T. peruviana lasted 12 days, followed by a stationary phase until day 16, at which point the cells enter the cellular death phase. The maximum observed concentration of biomass was 14.81 ± 0.32 g DW/L. This kinetic behavior is comparable to that reported in previous reports [23,24], indicating the in vivo stability of these plant cell specie.
3.2. Production of PC in cell suspensions
PC were measured in the culture medium (extracellular compounds) and in the biomass (intracellular compounds) every two days for 18 days. Extracellular PC increased as a function of time, reaching a maximum concentration on day 4 (8.54 ± 0.1 mg GAE/g DW), and then decreased progressively to a minimum of 2.41 ± 0.04 mg GAE/g DW on day 18 of culture (Fig. 1). It is possible that after day 4, the levels of phenolic compounds began to decline due to extracellular enzymatic activity. Berlin et al. [29]. showed that cinnamic acid and benzoic acid derivatives decreased in the first 40 h from the extracellular medium in cell suspension cultures of bean and soy, because of elimination reactions and oxidative decarboxylation.
Fig. 1.
Growth curve and production of extracellular phenolic compounds in cell suspension cultures of T. peruviana. Results are presented as value of mean ± SD of two independent experiments.
By contrast, intracellular PC were relatively constant during cell growth. The higher production of these compounds was at 4th day during the exponential phase (3.71 ± 0.11 mg GAE/ g DW). The lowest content was found in the fruit pulp extracts (2.47 ± 0.07 mg GAE/ g DW) (Table 1). These results prove that establishing cell suspension culture of T. peruviana increases intracellular production of PC compared with the original source of the cultures.
Table 1.
Intracellular production of phenolic compounds (PC), in cell suspension culture and fruit pulp of T. peruviana.
| Time (day) |
PC (mg GAE/g DW)* |
|---|---|
| 0 | 2.561 ± 0.11c |
| 2 | 3.226 ± 0.48abc |
| 4 | 3.705 ± 0.11a |
| 6 | 3.382 ± 0.09abc |
| 8 | 2.651 ± 0.31bc |
| 10 | 3.656 ± 0.24ab |
| 12 | 3.299 ± 0.34abc |
| Fruit (pulp) | 2.472 ± 0.07c |
3.3. Effect of elicitor concentration on PC production
Table 2 shows the effects of SA and MeJA concentration on the production of extracellular and intracellular PC. At the extracellular level, both SA and MeJA did not significantly affect the accumulation of PC (p > 0.05). On the other hand, at the intracellular level, MeJA affected the accumulation of PC in a dose dependent manner. Treatments with 3 μM MeJA (mean difference: 1.226; 95% CI: 0.337–2.115) and 300 μM SA (mean difference: 0.748; 95% CI: 0.188–1.310) significantly increased the content of intracellular PC, 24 h post-elicitation. After 96 h only 3 μM of MeJA caused a significant difference in the PC (mean difference: 1.026; 95% CI: 0.137–1.914).
Table 2.
Intracellular and extracellular phenolic content and biomass accumulation in cell suspension culture of T. peruviana growth with different concentration of salicylic acid (SA) and methyl jasmonate (MeJA).
| Elicitor (μM) |
Harvest Time (hour) |
Phenolic Content (mg GAE/g DW)* |
Biomass (g DW/L) |
|
|---|---|---|---|---|
| Intracellular | Extracellular | |||
| MeJA | ||||
| 0 | 24 | 3.250 ± 0.381b | 6.118 ± 1.586ab | 10.850 ± 1.282ab |
| 1 | 3.638 ± 0.129ab | 7.750 ± 1.142a | 8.386 ± 0.146c | |
| 3 | 4.476 ± 0.097a | 7.697 ± 0.500a | 7.934 ± 0.150c | |
| 5 | 3.257 ± 0.129b | 6.128 ± 0.975ab | 8.270 ± 1.012c | |
| 0 | 96 | 3.258 ± 0.537b | 5.256 ± 0.752ab | 11.263 ± 0.499a |
| 1 | 3.920 ± 0.201ab | 3.295 ± 0.268b | 9.674 ± 1.40abc | |
| 3 | 4.282 ± 0.051a | 4.785 ± 1.422ab | 8.742 ± 0.358bc | |
| 5 | 3.812 ± 0.519ab | 4.755 ± 0.564ab | 8.994 ± 0.152bc | |
| SA | ||||
| 0 | 24 | 5.235 ± 0.408bc | 2.739 ± 0.346bc | 8.311 ± 0.790b |
| 100 | 4.890 ± 0.267c | 2.908 ± 0.232bc | 8.049 ± 0.331b | |
| 300 | 5.983 ± 0.074a | 3.008 ± 0.532bc | 8.247 ± 0.282b | |
| 600 | 5.613 ± 0.021ab | 2.161 ± 0.329c | 8.470 ± 0.076b | |
| 0 | 96 | 5.131 ± 0.766bc | 4.691 ± 0.483a | 11.328 ± 0.086a |
| 100 | 4.830 ± 0.048c | 4.652 ± 1.093a | 12.073 ± 1.551a | |
| 300 | 4.928 ± 0.239c | 3.780 ± 0.439ab | 10.925 ± 0.533a | |
| 600 | 5.192 ± 0.059bc | 3.378 ± 0.065abc | 10.818 ± 0.257a | |
Additionally, MeJA significantly decreased the accumulation of biomass of T. peruviana in both times, 24 h (p value = 0.0116) and 96 h post-elicitation (p value = 0.0153). The inhibiting effect of MeJA on the biomass accumulation was also reported in cell suspension cultures of Taxus [30] and strawberries [31]. Two independent studies showed that MeJA inhibits the mitotic cycle in plant cells, halting them in G1-phase before the transition to S-phase. This reduces the progression of the cellular cycle and consequently, the number of cells that are actively divided [30,32]. These findings would explain the reduction in the cellular growth that was observed in cell suspensions of T. peruviana treated with MeJA. As for the treatment with SA, under our experimental conditions, this elicitor did not affect the biomass accumulation in the cultures of T. peruviana (Table 2). For this reason, it is implied that the treatment with MeJA and/or SA is not recommended when the objective is only the biomass production.
3.4. Effect of the elicitation time on intracellular PC production
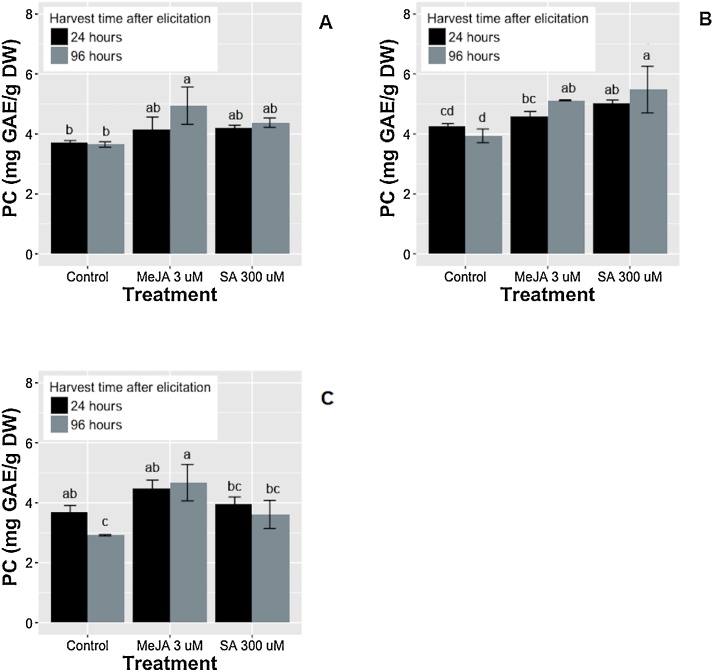
To evaluate this parameter, the elicitors (3 μM MeJA and 300 μM SA) were added to the cell suspensions at the beginning of the culture growth (day 0) and during the exponential phase (day 4 and day 8). Fig. 2 shows the intracellular phenolic content in the cultures 24 and 96 h after elicitor addition. PC increased significantly compared to the control cultures when SA was added on day 4 of growth; when SA was added at the beginning (day 0) or at the end (day 8) of the exponential phase, a non-significant increase in PC was produced compared to control. On the other hand, MeJA significantly increased the content of PC compared to the control, in the three times of elicitor addition and 96 h post-elicitation. Increasing the accumulation of PC, after the addition of MeJA in the exponential phase had been previously described by Wang et al. [33]. They specifically observed the rise in the content and production of flavonoids in cell cultures of Hypericum perforatum. Remarkably, day 4 of growth proved to have the greatest accumulation of PC after the treatment with MeJA and SA. Therefore, to produce these metabolites, day 4 is taken as the optimum time for addition of these elicitors to cell suspension cultures of T. peruviana.
Fig. 2.
Effect of elicitor addition times on the intracellular phenolic compounds (PC) production in cell suspension cultures of T. peruviana. Elicitors were added at the beginning of the culture growth (day 0) (Fig. 2A) and during the exponential phase (days 4 and 8) (Fig. 2B and C); controls were cell cultures without elicitor. Bars represent the means ± SD of three independent experimental replicates. For each parameter, values with different letters are significantly different (p < 0.05).
3.5. Effect of the cultivation time on intracellular PC production
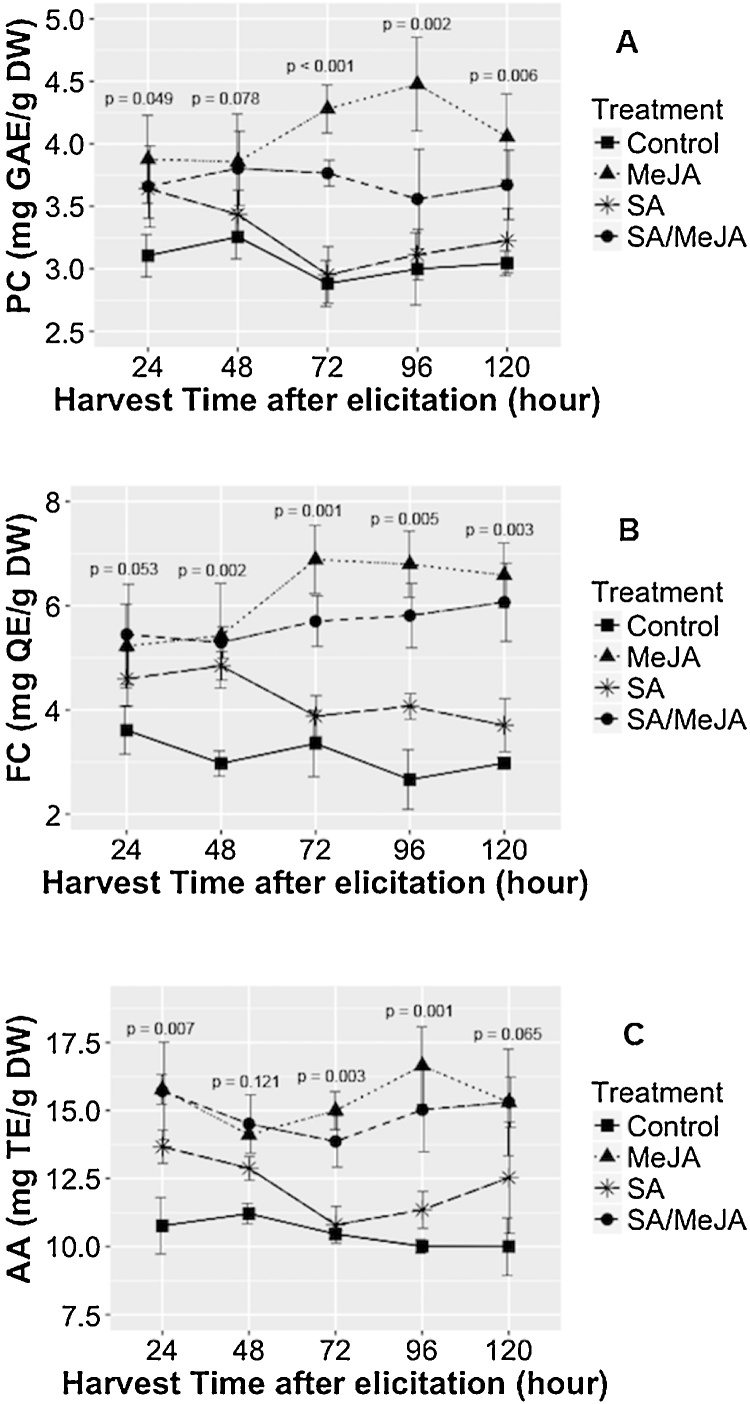
This parameter was evaluated on the treated cultures with 3 μM MeJA, 300 μM SA, and a combination of both, 300μM SA + 3μM MeJA (SA/MeJA). Elicitors were added to the suspension cultures on day 4 of growth, according to previous results. Besides the effect on the intracellular accumulation of PCC, FC and AA were evaluated at 24–48 – 72–96 and 120 h post-elicitation (Fig. 3).
Fig. 3.
Effect of the harvest time on the intracellular production of metabolites in the cell suspension cultures of T. peruviana. Fig. 3A corresponds to the results of the phenolic compounds-PC; Fig. 3B to the flavonoids content-FC; Fig. 3C to the antioxidant activity-AA in cell culture exposed to 3 μM MeJA, 300 μM SA and SA/MeJA mixture. Data are presented as mean ± SD of three independent experimental replicates.
Treatment with SA, MeJA and SA/MeJA increased the biosynthesis of PC, compared to the control (without elicitation) in all evaluated times. Additionally, direct relationship between PCC, FC, and AA was observed. The highest levels of PCC and AA were found in the treatments with 3 μM MeJA at 96 h post-elicitation (PCC = 4.47 ± 0.37 mg GAE/ g DW; AA = 16.65 ± 1.43 mg TE/ g DW), representing a rise of 49.25% and 66.28% compared to the control, respectively. Additionally, the higher content of FC was found 72 h post-elicitation with 3 μM of MeJA (FC = 6.88 ± 0.65 mg QE/ g DW), representing a 105.26% increase compared to cultures without treatment with MeJA.
On the other hand, the maximum elicitor effect of SA was reached during the first hours of cultivation, achieving an increase of 21.41% of PCC and 36.5% of AA at 24 h post-elicitation, compared to the control while FC increased 81.9% at 48 h post-elicitation. The combination of elicitors SA and MeJA also increased the production of these metabolites compared to the culture without elicitor, but these levels were lower than those obtained for MeJA alone and were higher than those with SA alone. These results suggest that SA and MeJA have different mechanism of action on plant cell suspension culture of T. peruviana.
T. peruviana is an important source of phenolic compounds and antioxidants, especially in its fruit [34]. In cell suspension cultures of T. peruviana, the production of PC has also been reported. Rincón et al. [22] reported a PC content of 0.95 ± 0.01 mg GAE/ g dried extract (DE) of T. peruviana calluses, which increased to 2.8 ± 0.02 mg GAE/ g DE after the treatment with jasmonic acid (100 μM) plus abscisic acid (10 μM). Arias et al. [24] reported the production of PC in cellular suspension of T. peruviana cultured in light with different wavelengths and in the darkness and found a total phenolic content between 7.21–7.91 mg GAE/g DW under light conditions and 9.46 mg GEA/g DW in darkness, at 23 days of growth. This study also found a direct relation between the TPC and the antioxidant capacity determined by the FRAP (Ferric Reducing Antioxidant Power) and ABTS assay.
3.6. TLC
Intracellular extracts obtained before elicitation showed 8 signals with folin-ciocalteu reagent and AlCl3/UV 366-nm, confirming the presence of phenolic and flavonoid compounds, respectively. Analysis of the Rf in the plate sprayed with AlCl3 proved that the signal with Rf = 0.527 increased its concentration as the time of cultivation passes. On the contrary, the signal with Rf = 0.083 decreased with time, which suggested that some metabolites are synthesized differently as the cells grow (Fig. 4). Differential biosynthesis of PC has been previously reported in Larrea divaricata Cav. suspension cultures, with metabolites such as p-coumaric, ferulic acid and synaptic alcohol. These differences were attributed to changes in the maximum speed of enzymes involved in the biosynthesis and degradation of phenylpropanoids [35]. Differential expression of genes involved in the phenylpropanoids biosynthesis could be another explanation for the variance in the intracellular concentrations of phenolic and flavonoids compounds [36]. Additionally, there were notorious differences in the TLC profile of the extracts from cell suspensions and fruit pulp of T. peruviana. Cell suspensions showed a complex profile of compounds that were not visualized in the fruit extract. Previous studies have reported that plant cells cultured in vitro can synthesize new compounds that may or may not be related to those previously isolated in the whole plant [37,38].
Fig. 4.
Thin layer chromatography (TLC) of the intracellular extracts of T. peruviana before elicitation. Fig. 4A - TLC was development with butanol:acetic acid:water (4:1:5 v/v/v) and was revealed with an ethanolic solution of AlCl3 10% (w/v) and UV light at 366 nm. Lane 1 correspond to fruit pulp extract; lanes 2–11 correspond to cell suspensions at 0, 2, 4, 6, 8, 10, 12, 14, 16 and 18 days of culture. The most intense signals are shown. Fig. 4B - TLC chromatograms, signals with Rf = 0.928, 0.527 and 0.083 showed changes on intensity as time went by in the cell culture.
In addition, TLC of the intracellular extracts of biomass harvested at 96 h post-elicitation, showed 19 signals with the folin-ciocalteu reagent (Fig. 5). TLC signal comparison showed some differences in the metabolites profile between elicited cultures and control. TLC analysis allowed for a quick view of the effect of the elicitors on the metabolite profile produced by cell suspensions. However, TLC has the disadvantage of low resolution, mainly in the separation of complex samples containing many components that differ widely in adsortivities.
Fig. 5.
Thin layer chromatography (TLC) of the intracellular extracts of plant cell culture of T. peruviana at 96 h post-elicitation. TLC was development with hexane:ethyl acetate:water (20:19:1 v/v/v) and revealed with 10% (v/v) folin-ciocalteu reagent. Lanes 1–3 correspond to cell suspensions culture treated with 300 μM SA, 3μM MeJA and 3μM MeJA/300μM SA; lane 4 correspond to cell suspension culture control.
3.7. HPLC-DAD
Cell suspensions treated with the elicitors showed differences in the HPLC profile compared with the control, independently of the harvest time. A total of 17 peaks were detected, however, tR and UV–vis absorbances of these peaks did not correlate with the available analytical standards (Table 3). Therefore, it was not possible to identify these peaks. The results showed an early response of the cells to SA treatment, at 24 h post-elicitation there was an increase in the production of two compounds (peaks 3 and 4 with tR = 7.45 and 7.96 min, respectively) that were not present in the control (Fig. 6). After 48 h, there was an increase in the production of a compound with tR = 11.62 min (peak 10) in the cultures elicited with SA/MeJA. This compound was detected in the control and all treatments, but only increased in the suspensions treated with the combination SA/MeJA. This result suggests a synergistic effect of both elicitors for the biosynthesis of the compound (peak 10). Synergistic elicitor effect between SA and MeJA was also observed for the compounds with tR = 12.5 and 12.85 min (peaks 11 and 12). On the other hand, compounds with tR = 8.89 and 13.29 min (peaks 5 and 13) only increase in the MeJA treatments after 72 h. Interestingly, the same compounds are not increase after elicitation with SA/MeJA, suggesting an antagonistic effect of SA over MeJA for these compounds. Therefore, our results indicated that simultaneous treatment of cell suspension with SA and MeJA produce an antagonistic effect in some, but not all PC in cell suspension cultures of T. peruviana.
Table 3.
Retention time (Rt) and UV-VIS absorbance of standards and peaks in the samples analyzed by HPLC.
| Compound | Molecular formula |
tR (min) | Maximum Absorbance (UV/VIS) |
|---|---|---|---|
| Chlorogenic acid | C16H18O9 | 9.096 | 214/324 |
| (±)-Hesperetin | C16H14O6 | 18.884 | 220/286 |
| trans-Sinapic acid | C11H12O5 | 12.015 | 222/321 |
| Quercetin | C15H10O7 | 16.573 | 218/369 |
| Kaempferol | C15H10O6 | 19.210 | 224/365 |
| Peak 1 | Unidentified | 1.749 | 222/258 |
| Peak 2 | Unidentified | 5.605 | 222/282 |
| Peak 3 | Unidentified | 7.456 | 222/236 |
| Peak 4 | Unidentified | 7.968 | 222/236 |
| Peak 5 | Unidentified | 8.896 | 222/236/323 |
| Peak 6 | Unidentified | 9.824 | 222/237 |
| Peak 7 | Unidentified | 10.058 | 222/237 |
| Peak 8 | Unidentified | 10.442 | 222/237 |
| Peak 9 | Unidentified | 11.051 | 222/237 |
| Peak 10 | Unidentified | 11.626 | 222/237 |
| Peak 11 | Unidentified | 12.501 | 222/237 |
| Peak 12 | Unidentified | 12.853 | 222/237 |
| Peak 13 | Unidentified | 13.291 | 222/237 |
| Peak 14 | Unidentified | 14.101 | 222/237 |
| Peak 15 | Unidentified | 14.698 | 222/237 |
| Peak 16 | Unidentified | 16.309 | 222/237 |
| Peak 17 | Unidentified | 17.824 | 222/237 |
Fig. 6.
HPLC profiles obtained for intracellular extracts of plant cell suspensions of T. peruviana. Chromatograms were recorded at 280 nm. Fig. 6A–E corresponds to the harvested cells at 24, 48, 72, 96 and 120 h post-elicitation.
Several studies have been shown that antagonism between endogenous SA and jasmonates (JA) plays a central role in the modulation of the plant immune signaling network [39]. Similarly, it has been reported that the exogenous treatment with both SA and JA can result in an antagonistic effect on the production of PC and antioxidants in plants, because the salicylates can override aspects of JA signaling cascade [40,41]. However, antagonistic effect is not generalized and synergistic effect between SA and MeJA on plant secondary metabolism can also be produced [42].
Our study did not investigate the biochemical mechanisms of the elicitation of cell suspension cultures of T. peruviana with SA and MeJA. Future experiments should be carried out to understand these mechanisms. For example, analysis of enzymes of phenylpropanoid metabolism, such as phenylalanine ammonia lyase (PAL), peroxides (POD) and polyphenoloxidase (PPO) should be considered.
4. Conclusion
The metabolic pathways responsible for the synthesis of PC are active in cell suspension cultures of T. peruviana. These pathways produced a complex signal profile that was detected by TLC and HPLC analysis. SA (300 μM) and MeJA (3 μM) increased the content of phenolics and flavonoids compounds, suggesting an inducer effect of these elicitors in the phenylpropanoids metabolic pathway. The addition of 3 μM MeJA on day 4 of growth and the harvest of the cells at 96 h post-elicitation produced the highest content of these secondary metabolites. On the other hand, elicitation with the SA and MeJA mixture can differentially affect the production of some PC. The results obtained in the present study allow us to conclude that this elicitation strategy can be applied jointly with scale up processes to increase the global productivity of these cultures.
Declarations of interest
None.
Funding
This study was supported by the Universidad Nacional de Colombia, Sede Medellín (Grant No. 35515-2017) and by the Departamento Administrativo de Ciencia, Tecnología e Innovación de Colombia – COLCIENCIAS (Grant No. FP44842-006-2018).
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.btre.2018.e00273.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- 1.Bandara V., Weinstein S.A., White J., Eddleston M.V. A review of the natural history, toxinology, diagnosis and clinical management of Nerium oleander (common oleander) and Thevetia peruviana (yellow oleander) poisoning. Toxicon. 2010;56:273–281. doi: 10.1016/j.toxicon.2010.03.026. [DOI] [PubMed] [Google Scholar]
- 2.Kohls S., Scholz B., Teske J., Zark P., Rullkötter J. Cardiac glycosides from yellow oleander (Thevetia peruviana) seed. Phytochemistry. 2012;75:114–127. doi: 10.1016/j.phytochem.2011.11.019. [DOI] [PubMed] [Google Scholar]
- 3.Kohls S., Scholz-Böttcher B.M., Teste J., Rullkötter J. Isolation and quantification of six cardiac glycosides from the seeds of Thevetia peruviana provide a basis for toxicological survey. Indian J. Biochem. Biophys. 2015;54:1502–1510. [Google Scholar]
- 4.Kumar P., Atreya A., Tanuj T. Thevetia peruviana. Wilderness Environ. Med. 2015;26:590–591. doi: 10.1016/j.wem.2015.07.007. [DOI] [PubMed] [Google Scholar]
- 5.Bose T.K., Basu R.K., Biswas B., De J.N., Majumdar B.C., Datta S. Cardiovascular effects of yellow oleander ingestion. J. Indian Med. Assoc. 1999;97:407–410. [PubMed] [Google Scholar]
- 6.Hassan M.M., Saha A.K., Khan S.A., Islam A., Mahabub-Uz-Zaman M., Ahmed S.S.U. Studies on the antidiarrhoeal, antimicrobial and cytotoxic activities of ethanol-extracted leaves of yellow oleander (Thevetia peruviana) Open Vet. J. 2011;1:28–31. [PMC free article] [PubMed] [Google Scholar]
- 7.Dabur R., Gupta A., Mandal T.K., Singh D.D., Bajpai V., Gurav A.M., Lavekar G.S. Antimicrobial activity of some Indian medicinal plants. Afr. J. Tradit. Complement. Altern. Med. 2007;4:313–318. doi: 10.4314/ajtcam.v4i3.31225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Haldar S., Karmakar I., Chakraborty M., Ahmad D., Haldar P.K. Antitumor potential of Thevetia peruviana on Ehrlich’s ascites carcinoma-bearing mice. J. Environ. Pathol. Toxicol. Oncol. 2015;34:105–113. doi: 10.1615/jenvironpatholtoxicoloncol.2015012017. [DOI] [PubMed] [Google Scholar]
- 9.Ramos-Silva A., Tavares-Carreón F., Figueroa M., De la Torre-Zavala S., Gastelum-Arellanez A., Rodríguez-García A., Galán-Wong L.J., Avilés-Arnaut H. Anticancer potential of Thevetia peruviana fruit methanolic extract. BMC Complement. Altern. Med. 2017;2:241. doi: 10.1186/s12906-017-1727-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tewtrakul S., Nakamura N., Hattori M., Fujiwara T., Supavita T. Flavanone and flavonol glycosides from the leaves of Thevetia peruviana and their HIV-1 reverse transcriptase and HIV-1 integrase inhibitory activities. Chem. Pharm. Bull. (Tokyo) 2002;50:630–635. doi: 10.1248/cpb.50.630. [DOI] [PubMed] [Google Scholar]
- 11.Buchanan B.B., Gruissem W., Jones R.L. 2nd edition. John Wiley & Sons, Inc.; 2015. Biochemistry and Molecular Biology of Plants; pp. 984–1195. [Google Scholar]
- 12.Ncube B., Finnie J.F., Van Staden J. Quality from the field: the impact of environmental factors as quality determinants in medicinal plants. S. Afr. J. Bot. 2012;82:11–20. [Google Scholar]
- 13.Sajca L., Grubisic D., Vunjak-Novakovic G. Bioreactors for plant engineering: an outlook for further research. Biochem. Eng. J. 2000;4:89–99. [Google Scholar]
- 14.Namdeo A. Plant cell elicitation for production of secondary metabolites: a review. Review. 2007;1:69–79. [Google Scholar]
- 15.Zhao J., Davis L., Verpoorte R. Elicitor signal traduction leading to production of plant secondary metabolites. Biotechnol. Adv. 2005;23:283–333. doi: 10.1016/j.biotechadv.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 16.Beckers G.J., Spoel S.H. Fine-tuning plant defence signalling: salicylate versus jasmonate. Plant Biol. (Stuttg) 2006;8:1–10. doi: 10.1055/s-2005-872705. [DOI] [PubMed] [Google Scholar]
- 17.Manivannan A., Soundararajan P., Park Y.G., Jeong B.R., Manivannan A., Soundararajan P., Park Y., Jeong B. Chemical elicitor-induced modulation and antioxidant metabolism and enhancement of secondary metabolite accumulation in cell suspension cultures of Scrophularia kakudensis Franch. Int. J. Mol. Sci. 2016;17:399. doi: 10.3390/ijms17030399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vuong T.V., Franco C., Zhang W. Treatment strategies for high resveratrol induction in Vitis vinifera L. cell suspension culture. Biotecnol. Rep. 2014;1–2:15–21. doi: 10.1016/j.btre.2014.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Saeed S., Ali H., Khan T., Kayani W., Khan M.A. Impacts of methyl jasmonate and phenyl acetic acid on biomass accumulation and antioxidant potential in adventitious roots of Ajuga bracteosa Wall ex Benth., a high valued endangered medicinal plant. Physiol. Mol. Biol. Plants. 2017;23:229–237. doi: 10.1007/s12298-016-0406-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gadzovska S., Maury S., Delaunay A., Spasenoski M., Hagège D., Courtois D., Joseph C. The influence of salicylic acid elicitation of shoots, callus, and cell suspension cultures on production of naphtodianthrones and phenylpropanoids in Hypericum perforatum L. Plant Cell Tissue Organ Cult. (PCTOC) 2013;113:25–39. [Google Scholar]
- 21.Sudha G., Ravishankar G.A. Elicitation of anthocyanin production in callus cultures of Daucus carota and the involvement of methyl jasmonate and salicylic acid. Acta Physiol. Plant. 2003;25:249–256. [Google Scholar]
- 22.Rincón-Pérez J., Rodríguez-Hernández L., Ruíz-Valdiviezo V.M., Abud-Archila M., Luján-Hidalgo M.C., Ruiz-Lau N., González-Mendoza D., Gutiérrez-Miceli F.A. Fatty acids profile, phenolic compounds and antioxidant capacity in elicited callus of Thevetia peruviana (Pers.) K. Schum. J. Oleo Sci. 2016;65:311–318. doi: 10.5650/jos.ess15254. [DOI] [PubMed] [Google Scholar]
- 23.Arias M., Angarita M., Restrepo J.M., Caicedo L.A., Perea M. Elicitation with methyl-jasmonate stimulates peruvoside production in cell suspention culture of Thevetia peruviana. In vitro Cell. Dev. Biol. Plant. 2010;46:233–238. [Google Scholar]
- 24.Arias J.P., Zapata K., Rojano B., Arias M. Effect of light wavelength on cell growth, content of phenolic compounds and antioxidant activity in cell suspension cultures of Thevetia peruviana. J. Photochem. Photobiol. B. 2016;163:87–91. doi: 10.1016/j.jphotobiol.2016.08.014. [DOI] [PubMed] [Google Scholar]
- 25.Slinkard K., Singleton V.L. Total phenol analysis: automation and comparison with manual methods. Am. J. Enol. Vitic. 1977;28:49–55. [Google Scholar]
- 26.Pękal A., Pyrzynska K. Evaluation of aluminium complexation reaction for flavonoid content assay. Food Anal. Methods. 2014;7:1776–1782. [Google Scholar]
- 27.Re R., Pellegrini N., Proteggente A., Pannala A., Yang M., Rice-Evans C. Antioxidant activity applyingan improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999;26:1231–1237. doi: 10.1016/s0891-5849(98)00315-3. [DOI] [PubMed] [Google Scholar]
- 28.Dr. Duke’s Phytochemical and Ethnobotanical databases. https://phytochem.nal.usda.gov/phytochem/search (Accessed 12 June 2018).
- 29.Berlin J., Barz W., Harms H., Haider K. Degradation of phenolic compounds in plant cell cultures. FEBS Lett. 1971;16:141–146. doi: 10.1016/0014-5793(71)80353-8. [DOI] [PubMed] [Google Scholar]
- 30.Patil R.A., Lenka S.K., Normanly J., Walker E.L., Roberts S.C. Methyl jasmonate represses growth and affects cell cycle progression in cultured Taxus cells. Plant Cell Rep. 2014;33:1479–1492. doi: 10.1007/s00299-014-1632-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Miyanaga K., Seki M., Furusaki S. Quantitative determination of cultured strawberry-cell heterogeneity by image analysis: effects of medium modification on anthocyanin accumulation. Biochem. Eng. J. 2000;5:201–207. doi: 10.1016/s1369-703x(00)00059-0. [DOI] [PubMed] [Google Scholar]
- 32.Noir S., Bömer M., Takahashi N., Ishida T., Tsui T.L., Balbi V., Shanahan H., Sugimoto K., Devoto A. Jasmonate controls leaf growth by repressing cell proliferation and the onset of endoreduplication while maintaining a potential stand-by mode. Plant Physiol. 2013;161:1930–1951. doi: 10.1104/pp.113.214908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang J., Qian J., Yao L., Lu Y. Enhanced production of flavonoids by methyl jasmonate elicitation in cell suspension culture of Hypericum perforatum. Bioresour. Bioprocess. 2015;2:5. [Google Scholar]
- 34.Dixit A., Singh H., Sharma R.A., Sharma A. Estimation of antioxidant and antibacterial activity of crude extracts of Thevetia peruviana (Pers.) K. Schum. Int. J. Pharm. 2015;7:55–59. [Google Scholar]
- 35.Palacio L., Cantero J.J., Cusidó R., Goleniowski M. Phenolic compound production by Larrea divaricate Cav. plant cell cultures and effect of precursor feeding. Process Biochem. 2011;46:418–422. [Google Scholar]
- 36.Md-Mustafa N.D., Khalid N., Gao H., Peng Z., Alimin M.F., Bujang N., Ming W.S., Mohd-Yusuf Y., Harikrishna J.A., Othman R.Y. Transcriptome profiling shows gene regulation patterns in a flavonoid pathway in response to exogenous phenylalanine in Boesenbergia rotunda cell culture. BMC Genomics. 2014;15:984. doi: 10.1186/1471-2164-15-984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Verpoorte R., van der Heijden R., Hoge J.H.C., ten Hoopen H.J.G. Plant cell biotechnology for the production of secondary metabolites. Pure Appl. Chem. 1994;66:2307–2310. [Google Scholar]
- 38.Schmeda-Hirschmann G., Jordan M., Gerth A., Wilken D. Secondary metabolite content in rhizomes, callus cultures and in vitro regenerated plantlets of Solidago chilensis. Z. Naturforsch. C. 2005;60:5–10. doi: 10.1515/znc-2005-1-202. [DOI] [PubMed] [Google Scholar]
- 39.Niki T., Mitsuhara I., Seo S., Ohtsubo N., Ohashi Y. Antagonistic effect of salicylic acid and jasmonic acid on the expression of pathogenesis-related (PR) protein genes in wounded mature tobacco leave. Plant Cell Physiol. 1998;39:500–507. [Google Scholar]
- 40.Ncho X.E., Doumbia M.L., Traore S., Konan Y.K.F., Kone M., Kouakou T.H. Estimation of total phenolic compounds in treated leaves with methyl jasmonate and salicylic acid of banana (Musa acuminata L. AAA group cv. Grand Naine) susceptible to the black leaf streak disease. Agric. Sci. Res. J. 2016;6:175–181. [Google Scholar]
- 41.Considine M., Gordon C., Croft K., Ching S. Salicylic acid overrides the effect of methyl jasmonate on the total antioxidant capacity of table grapes. Acta Hortic. 2009;841:495–498. [Google Scholar]
- 42.Sukito A., Tachibana S. Effect of methyl jasmonate and salycilic acid synergism on enhancement of bilobalide and ginkgolide production by immobilized cell cultures of Ginkgo biloba. Bioresour. Bioprocess. 2016;3(24) [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.