Abstract
Toxoplasma gondii, an obligate intracellular protozoan, is the causative agent of toxoplasmosis, which can cause serious public health problems. The current drugs used to treat toxoplasmosis have many limitations. This study evaluated the anti-T. gondii activity and potential mechanism of Licochalcone A (Lico A) in vitro and in vivo. The safe concentration of Lico A in HFF cells was determined by MTT cell viability assays. The presence of T. gondii was assessed by qPCR and Giemsa staining. Azithromycin and sulfadiazine, commonly used effective treatments, served as drug controls. T. gondii ultrastructural alterations were observed by electron microscopy. The anti-T. gondii activity of Lico A was evaluated using an in vivo mouse infection model. In vitro, Lico A had no negative effect on host cell viability at concentrations below 9 μg/mL; however, it did inhibit T. gondii proliferation in a dose-dependent manner, with a 50% inhibitory concentration (IC50) of 0.848 μg/mL. Electron microscopy analyses indicated substantial structural and ultrastructural changes in tachyzoites after Lico A treatment. Nile Red staining assays demonstrated that Lico A caused lipid accumulation. Lico A treatment significantly increased the survival rate of BALB/c mice infected with T. gondii. Lico A achieved the same therapeutic effect as a commonly used clinical drugs (combination of sulfadiazine, pyrimethamine and folinic acid). In conclusion, Lico A has strong anti-T. gondii activity in vitro and in vivo and might be developed into a new anti-T. gondii drug. Moreover, Lico A may exert these effects by interfering with lipid metabolism in the parasite.
Keywords: Licochalcone A, Anti-Toxoplasma gondii, Ultrastructural, Cytotoxicity, Survival curve
Graphical abstract
Highlights
-
•
Lico A inhibit the proliferation of T. gondii in a dose- and time-dependent manner.
-
•
Lico A likely functions by affecting lipid metabolism.
-
•
Lico A can significantly increase the survival rate of infected mice.
1. Introduction
Toxoplasmosis is a zoonotic disease caused by Toxoplasma gondii (Dubey and Jones, 2008). T. gondii is an obligate intracellular and apicomplexan protozoan parasite with a complex life cycle; it can infect a wide range of warm-blooded animals, including humans, and occasionally causes serious diseases (Chemoh et al., 2013; Dupont et al., 2012). It has been estimated that the disease may affect one-third of the world's population (Montoya and Liesenfeld, 2004). Although most infections in healthy humans are asymptomatic and self-limiting, severe complications may occur in immunocompromised patients after congenital T. gondii infection (Ajzenberg et al., 2016; Dupont et al., 2012).
Although T. gondii infections are prevalent in humans and animals, clinical treatments are limited. In 1942, sulfonamides were reported to be effective against murine toxoplasmosis (Sabin and Warren, 1942). The combination of sulfonamides and pyrimethamine was confirmed to effectively treat toxoplasmosis in 1953 (Eyles and Coleman, 1953), and it has since been a standard treatment for toxoplasmosis in humans and animals. Subsequently, spiramycin was shown to have anti-T. gondii activity in mice in 1958 (Garin and Eyles, 1958) and became a recommended treatment for pregnant women to reduce transmission of the parasite from mother to foetus (Desmonts and Couvreur, 1974). For patients allergic to sulfonamides, clindamycin can replace sulfonamides in conjunction with other drugs in the treatment of T. gondii (Mcmaster et al., 1973; Araujo and Remington, 1974). However, combination of sulfadiazine and pyrimethamine or clindamycin and pyrimethamine often results in side effects such as skin rash, fever and bone marrow suppression (Dannemann et al., 1992; Georgiev, 1994; Luft and Remington, 1992). Atovaquone, azithromycin, and clarithromycin are anti-Toxoplasma-targeted drugs; however, only atovaquone exhibits therapeutic activity against cysts. It is also useful for patients who cannot tolerate sulfadiazine or clindamycin (Farthing et al., 1992; Godofsky, 1994; Kovacs, 1992; Raffi et al., 1995; Wynn et al., 1993). Nevertheless, the cure rate and side effects, such as myasthenia, of atovaquone cannot be ignored (Pijpers and Schrey, 1996). Thus, new drugs with fewer or no side effects are necessary for the treatment of toxoplasmosis in the clinic.
Despite efforts to date to investigate many natural products to find effective treatments for toxoplasmosis over the last several decades (Sepulveda-Arias et al., 2014; Si et al., 2016; Zhang et al., 2016), there are limited options to cure toxoplasmosis. Liquorice, the root and rhizome of several Glycyrrhiza species (Fabaceae), is an important natural drug and is widely used as an herbal medicine in China. Licochalcone A (Lico A) is a novel flavonoid isolated from liquorice root that has activity against malaria and Leishmania (Chen et al., 1993, 1994). In addition, studies have shown that Lico A has antioxidant, antibacterial, anti-angiogenesis and antitumour effects (Kim et al., 2010; Liu et al., 2008; Xiao et al., 2011). Here, we report that Lico A inhibits the growth of the T. gondii RH strain in vitro and in vivo.
2. Materials and methods
2.1. Parasites
The tachyzoites used in this study were from the virulent RH strain of T. gondii, which was kindly provided by Dr. Xingquan Zhu (Lanzhou Veterinary Research Institute, Chinese Academy of Agricultural Sciences). The tachyzoites were maintained in human foreskin fibroblast (HFF) cells in Dulbecco's Modified Eagle's Medium (DMEM) containing 1% heat-inactivated foetal bovine serum (FBS), 1% GlutaMAX and 1% MEM Non-Essential Amino Acids (MEM NEAA). Cultures were kept in humidified incubators at 37 °C with 5% CO2. When the host cell monolayer was destroyed, the tachyzoites were collected and centrifuged at 1500 × g for 20 min. The tachyzoites in the pellet were released by forceful passage through a 27-gauge needle and filtered through a 5 μm polycarbonate membrane filter to remove any host cells. The tachyzoites were then centrifuged at 1500 × g for 20 min and the supernatant was discarded. The pellet was resuspended in infection medium, and a haemocytometer was used to calculate the number of tachyzoites.
2.2. Animals
BALB/c mice raised in specific pathogen-free (SPF) conditions and weighing 18–20 g were purchased from the animal breeding facility of Lanzhou Veterinary Research Institute, CAAS (Lanzhou, China). The animals were housed in stainless steel cages in a ventilated room. A light/dark cycle of 12 h/12 h was maintained, and the living temperature was (22 ± 2) °C with a relative humidity of (55 ± 10) %. Standard compressed mouse feed from Beijing Keao Xieli Co., Ltd. (Beijing, China) and drinking water were supplied ad libitum. The study was performed in compliance with the US National Institutes of Health Guidelines for the Care and Use of Laboratory Animals and approved by Institutional Animal Care and Use Committee of Lanzhou Institute of Husbandry and Pharmaceutical Science of CAAS. The animals were allowed a 1-week quarantine and acclimation period prior to start of the study.
2.3. HFF cells
Primary HFF cells were cultured in DMEM supplemented with 10% FBS, 1% GlutaMAX and 1% MEM NEAA and were maintained at 37 °C and 5% CO2. The cells were kindly provided by the Stem Cell Bank, Chinese Academy of Sciences, Shanghai, China. Parasite infections were performed in subconfluent cultures in 6-well cell culture plates.
2.4. Licochalcone A
Lico A was obtained as a powder from PUSH BIO-TECHNOLOGY Co. Ltd. The purity of Lico A was more than 99%, which was ensured by ultra-performance liquid chromatography (UPLC).
2.5. Cell viability assay
The possible toxic effects of Lico A on the host cells were estimated based on the reduction of MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide]. For these assays, 1 × 104 cells per well were seeded in 96-well plates and cultured in DMEM supplemented with 10% FBS. After 24 h, the cells were washed and directly subjected to Lico A treatment at concentrations derived from serial dilutions in DMEM supplemented with 1% FBS; the dilutions included concentrations from 10 to 1 μg/mL. As a negative control, the cells were cultured in DMEM supplemented with 1% FBS without the addition of the compound.
After 48 h of treatment, the culture supernatant was removed, and 15 μL of MTT solution (5 mg/mL) in DMEM was added to each well for 4 h. The formazan crystals were subsequently solubilized by the addition of 100 μL of pure DMSO. The plate was centrifuged at 400 × g for 7 min, and 100 μL of the supernatant was collected, transferred to a new 96-well plate, and read at 570 nm in a Multiskan GO instrument (Thermo Fisher Scientific, MA, USA).
2.6. Antiproliferative assays
Approximately 1 × 105 HFF cells per well were seeded in 6-well cell culture plates 1 day before the assay. On the day of infection, the cells were infected with parasites in DMEM (5 × 103 parasites per well) for 6 h; the cell monolayer was then washed twice with phosphate-buffered saline (PBS) to remove non-adhered parasites. Subsequently, Lico A was added at different concentrations (8, 7, 6, 5, 4, 3, 0.8 and 0.6 μg/mL). The positive control group was incubated in medium lacking Lico A. As positive drug controls, azithromycin (8.6 μg/mL) and sulfadiazine (0.4 μg/mL) were added separately under the same conditions (Neville et al., 2015). In the negative control group, uninfected cells were cultured in drug-free medium. After 24 h of treatment, the cells were fixed with fresh 4% paraformaldehyde in PBS, stained with Giemsa stain, and observed by light microscopy. For measurements of parasite burden, the cells were infected with tachyzoites (1 × 105 per well) for 6 h, treated with different concentrations of Lico A (6, 5, 4, 3, 2.5, 2, 1.5, 1, 0.8, 0.6, 0.4 and 0 μg/mL), azithromycin (8.6 μg/mL) or sulfadiazine (0.4 μg/mL) for 24 h, and washed twice with PBS. Subsequently, 3.6 × 106 Pze21-MCS-1 plasmids were added to each sample as a reference gene for normalization, and total genomic DNA was isolated from the samples using DNAiso Reagent (Takara). The 529-bp repeat element (RE) of T. gondii was measured by quantitative PCR (qPCR) with the following primers: Tox-F (5′- AGG AGA GAT ATC AGG ACT GTA G-3′), Tox-R (5′-GCG TCG TCT CGT CTA GAT CG-3′) and the Taqman probe Tox-TP (6-Fam CCG GCT TGG CTG CTT TTC CT BHQ1) (Homan et al., 2000). The reference gene was evaluated using the following primers: pZE-F (5′- GCAGCCACTGGTAACAGGATT-3′), pZE-R (5′-CCGTAGTTAGGCCACCACTT-3′) and pZE-TP (6-Fam CAGAGCGAGGTATGTAGG BHQ1). The amplification reactions were performed under the following conditions: 50 °C for 2 min, 95 °C for 10 min, 40 cycles of 95 °C for 15 s, 58 °C for 1 min, 60 °C for 1 min. The fluorescent-labelled reaction products were analysed with a QuantStudio 6 Flex Real-Time PCR System (Life Technologies). Relative quantification of target genes was performed. The number of parasites was determined from a standard curve (Y = 2.0589x-7.3947; R2 = 0.9882) (Fig. S1) obtained with DNA samples from a range of serial dilutions (1 × 106 to 3.9 × 103/mL) of RH strain tachyzoites (3.6 × 106 Pze21-MCS-1 plasmids added per sample). The data were plotted using GraphPad Prism 5.0 software (GraphPad Software Inc., San Diego, USA). The results represent the means ± standard deviations of the results from at least three independent experiments, and differences were considered statistically significant at a P value of ≤0.05. To calculate the 50% inhibitory concentration (IC50), the percentage of tachyzoite growth inhibition was plotted as a function of the drug concentration by fitting the values for nonlinear curve analysis. Regression analyses were performed using SPSS 19.0 software (SPSS Inc., Chicago, USA).
2.7. Electron microscopy analysis
For scanning electron microscopy, cells grown on coverslips were infected with 4 × 103 tachyzoites per well. After 8 h of infection, Lico A was added at a concentration of 3 μg/mL or 4 μg/mL. The cells were cultured for an additional 8 h and then fixed for 1 h in a solution containing 2.5% glutaraldehyde in 0.01 mol/L PBS (pH 7.4), washed with PBS until the glutaraldehyde was removed, and dehydrated in a graded alcohol series. Isoamyl acetate was then replaced with alcohol. The cells were subjected to critical point drying and mounted on stubs, and the upper portion of the cells was removed with adhesive tape, revealing the internal organization of the parasitophorous vacuole (PV). Subsequently, the samples were coated with gold (20–30 nm) and observed using a Hitachi SU-8010 scanning electron microscope (HITACHI, Japan).
To observe the ultrastructure of intracellular parasites, transmission electron microscopy was used. Cells in culture plates were infected with 4 × 103 tachyzoites per well. After 8 h of infection, 4 μg/mL Lico A was added, and the cells were cultured for 2, 4, 8 and 16 h. The cells were digested with TrypLE Express for 3 min, washed twice with PBS, fixed for 1 h in a solution containing 2.5% glutaraldehyde in 0.01 mol/L PBS (pH 7.4), washed with PBS until the glutaraldehyde was removed and post fixed for 1 h in the dark with a solution containing 1% osmium tetroxide in 0.01 M PBS. The cells were subsequently washed in the same buffer, dehydrated in acetone, and embedded in Epon. Ultrathin sections were stained with uranyl acetate and lead citrate and observed under a Hitachi H-7500 transmission electron microscope (HITACHI, Japan).
2.8. Lipid staining and fluorescence analysis
HFF cells were seeded in 150-cm2 culture flasks and infected with tachyzoites. After 24 h of infection, the samples were treated with 4 μg/mL Lico A (2 h, 4 h, 8 h, 16 h), 0.848 μg/mL Lico A (16 h) or left untreated for 40 h (control). T. gondii cells were mechanically isolated and purified by the method described above and incubated at 37 °C for 10 min in 5 μg/mL Nile Red in PBS (stock solution 0.5 mg/mL in DMSO). The tachyzoites were washed in PBS and examined using a Zeiss LSM 800 confocal laser scanning microscope and flow cytometry at a laser excitation wavelength of 488 nm (recording 10,000 events per sample) to evaluate lipid accumulation.
2.9. Anti-Toxoplasma activity of Lico A in vivo
Lico A was dissolved in solution 1 (physiological saline containing 13% Tween 80) to final concentrations of 5, 10 and 15 mg/mL. The positive control group contained 10 mg/mL sulfadiazine, 5 mg/mL pyrimethamine and 1.5 mg/mL folinic acid diluted in solution 2 (physiological saline containing 1% CMC-Na).
Female BALB/c mice were injected with 100 tachyzoites each in the abdominal cavity and were divided into 11 groups consisting of 10 animals each. After 4 h, mice in 4 of the groups were treated with different concentrations of Lico A as follows: 50 mg/kg (IL1 group), 100 mg/kg (IL2 group), 150 mg/kg (IL3 group), and solvent 1 as a control (IS group). These treatments were given as 0.2 mL volumes administered intraperitoneally twice a day. Four other groups of mice were treated by oral administration as follows: 50 mg/kg Lico A (OL1 group), 100 mg/kg Lico A (OL2 group), 150 mg/kg Lico A (OL3 group) and solvent 1 as a control (OS group). The positive control group (PS group) and solvent 2 group (S2 group) were orally treated with 0.2 mL volumes once a day. The negative group (NG) was treated with no drug as a control.
3. Results
3.1. Cell viability assays
Prior to investigating the pharmacological potential of Lico A to prevent the proliferation of T. gondii, we first assayed the cytotoxicity of Lico A by treating HFF cells with Lico A at various concentrations (1, 2, 3, 4, 5, 6, 7, 8, 9 and 10 μg/mL) for 48 h and then performing an MTT assay. Lico A improved the proliferation of HFF cells significantly at concentrations below 9 μg/mL, while the opposite effect was observed when the dose was higher than 9 μg/mL (Fig. S2). We concluded that HFF cell viability was not inhibited by Lico A at concentrations below 9 μg/mL.
3.2. Antiproliferative assay
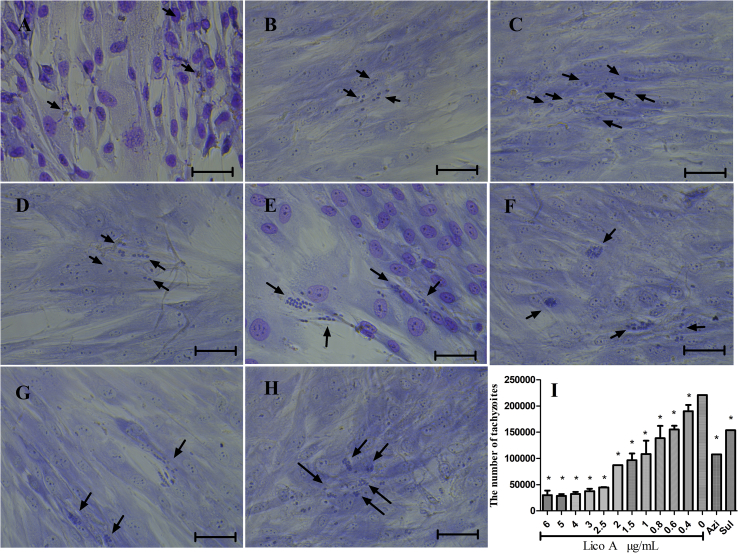
The activity of Lico A against the growth of T. gondii tachyzoites within HFF cells was evaluated after 24 h of treatment. Fig. 1 shows that Lico A induced a dose-dependent inhibition of parasite growth between 0 and 5 μg/mL (Fig. 1A–F). Using light microscopy, we found that the amount of tachyzoites and the average number of tachyzoites within the PV decreased with increasing concentrations of Lico A. At concentrations above 5 μg/mL, typical tachyzoites were not observed, but vestigial tachyzoites were observed; no tachyzoites was observed after removing the drug medium and maintaining the cultures for 10 days (Fig. S3). According to our qPCR results (Fig. 1I), the IC50 of Lico A was 0.848 μg/mL (correlation coefficient = 0.986) following 24 h of exposure. An antiproliferative assay indicated that Lico A inhibited the proliferation of T. gondii in vitro in a dose-dependent manner.
Fig. 1.
The inhibition of T. gondii tachyzoites in HFF cells by Lico A. HFF cells were infected with T. gondii and treated with either 5 μg/mL Lico A (Fig. 1A), 4 μg/mL Lico A (Fig. 1B), 3 μg/mL Lico A (Fig. 1C), 0.8 μg/mL Lico A (Fig. 1D), or 0.6 μg/mL Lico A (Fig. 1E). T. gondii-infected cells without treatment were used as a control (Fig. 1F). Cells infected with T. gondii and treated with Azi (Fig. 1G) and Sul (Fig. 1H) were used as drug controls. The number of tachyzoites per well was detected by qPCR after treatment with different concentrations of Lico A for 24 h (Fig. 1I). The presented results represent the means ± standard deviations of the results from at least three independent experiments. *P ≤ 0.05 compared with the positive group. Scale bars: 50 μm.
3.3. Electron microscopy analysis
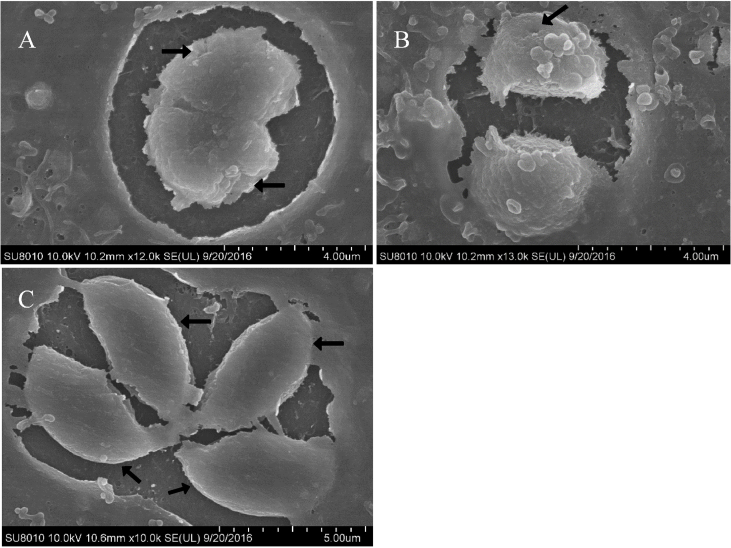
Scanning electron microscopy (SEM) confirmed the organization of the parasites into PVs after 8 h of treatment. Untreated cells contained many tachyzoites with uniform size and smooth surfaces (Fig. 2C). After treatment, the tachyzoites decreased in size and became rounded, with surface depressions (Fig. 2A and B).
Fig. 2.
Scanning electron microscopy micrograph of treated or untreated HFF cells infected with T. gondii. The cells were treated with 3 μg/mL Lico A (Fig. 2A) or 4 μg/mL Lico A (Fig. 2B) or left untreated (Fig. 2C). After 8 h of treatment, the tachyzoites in the treated cells became atrophied and rounded and showed surface depressions compared with those in untreated cells, as indicated by the arrows. Scale bars: 4 μm (Fig. 2A and B); 5 μm (Fig. 2C).
Transmission electron microscopy (TEM) of the control cells revealed preserved tachyzoite structures (Fig. 3A). Longitudinal sections of tachyzoites were banana- or half-moon-shaped, with an enlarged centre and pointed ends. The tachyzoites were arranged in a fence-like manner, the cell membrane and nuclear membrane consisted of two layers, and the structure was clear and complete. The nucleus (Nu) was round or oval, and the chromatin structure was normal. The conoid (Co) and microneme (Mn) were visible in the front of the tachyzoite, and the rhoptry (Rh) extended backward from the conoid to the front of the nucleus and expanded. Some clear dense granules (Dg) could also be seen. After 2 h of treatment with 4 μg/mL Lico A (Fig. 3B), the tachyzoites were organized in rosettes, many lipid bodies (Lb) were observed in the cytoplasm, the dense granules disappeared, and the surface of the cell membrane was not smooth. After 4 h of treatment (Fig. 3C), the lipid bodies became larger, the membrane exhibited protrusions (P), and the rhoptry disappeared. After 8 h of treatment (Fig. 3D), the conoid had almost disappeared, and the membrane system structure became indistinct; the nuclear membrane was loose, and there was a gap between the membrane and cytoplasm in tachyzoites. There were alterations in the cytoplasm of tachyzoites, such as vacuoles with unclear boundaries and irregular shapes, which are hallmarks of a dissolved cytoplasm. The condition of the rhoptry was the same as at 4 h of treatment. After 16 h of treatment (Fig. 3E), the cytoplasmic structure of tachyzoites completely disappeared, and extensive clefts were observed.
Fig. 3.
Transmission electron microscopy images of HFF cells infected with T. gondii and treated with 4 μg/mL Lico A or untreated.T. gondii within untreated HFF cells after 16 h (Fig. 3A). The cell membrane, nucleus (Nu), dense granules (Dg), rhoptries (Rh), and conoids (Co) in tachyzoites were clear and integral. The tachyzoite shape was typical. After 2 h of treatment (Fig. 3B), the parasites were organized in rosettes, lipid bodies (Lb) were observed in the cytoplasm, dense granules disappeared, and the surface of the cytoplasm was not smooth. After 4 h of treatment (Fig. 3C), the tachyzoite lipid bodies became larger, protrusions (P) emerged on the membrane, and the rhoptries disappeared. After 8 h of treatment (Fig. 3D), the conoids and rhoptries of tachyzoites disappeared, the membrane system structure became indistinct, the nuclear membrane loosened, and a gap between the membrane and cytoplasm emerged. Vacuoles (V) appeared in the cytoplasm and exhibited unclear boundaries and irregular shapes, which are hallmarks of a dissolved cytoplasm. After 16 h of treatment (Fig. 3E), the tachyzoite cytoplasm completely disappeared, and extensive clefts arose. Scale bars: 0.5 μm (Fig. 3A, D and E); 1 μm (Fig. 3B and C).
3.4. Lipid staining and fluorescence analysis
Nile Red staining was used to evaluate the possible interference with lipid metabolism in tachyzoites after Lico A treatment. When infected HFF cell were treated with 4 μg/mL Lico A for 16 h, increased lipid accumulation in the cytoplasm of tachyzoites was observed by confocal laser scanning microscopy (Fig. 4A and C). In addition, the tachyzoites became smaller in size and rounded in shape, with surface depressions (Fig. 4B and D), which was also observed at 0.848 μg/mL Lico A (Figs. S4C and D). The fluorescence intensity of tachyzoites treated with 4 μg/mL Lico A at different times was assessed by flow cytometry. Interestingly, the fluorescence intensity increased gradually with time, which indicated that Lico A induced an increase in intracellular lipid levels in a time-dependent manner (Fig. 4E and F).
Fig. 4.
Confocal microscopy images and flow cytometry analysis. Tachyzoites without treatment were used as control (Fig. 4A and B) or treated with 4 μg/mL Lico A after 16 h (Fig. 4C and D) and then stained with Nile Red. After 16 h of treatment, lipid bodies were obvious in the cytoplasm of tachyzoites (arrows) compared with the control group. Differential interference contrast (DIC) microscopy images (Fig. 4B and D). Fluorescence intensity was detected by flow cytometry after treatment with 4 μg/mL Lico A for different durations (Fig. 4E and F). The presented results represent the means ± standard deviations of the results from at least three independent experiments. *P ≤ 0.05 compared with the control group. Scale bars: 20 μm.
3.5. The effect of Lico A on the survival rate of T. gondii-infected mice
To determine the effect of Lico A on T. gondii in mice, we examined the survival rate of T. gondii-infected mice. Intraperitoneal injection of Lico A in mice infected intraperitoneally with RH tachyzoites resulted in a significant dose-dependent protection against death (Fig. 5A). Lico A protected 80% (P = 0.0003), 90% (P < 0.0001) and 100% (P < 0.0001) of infected mice in the IL1, IL2 and IL3 groups, respectively. All mice in the PS group survived. All NG, IS and S2 mice died by day 13 of infection. Oral treatment of mice intraperitoneally infected with RH tachyzoites also significantly prolonged the survival time (OL1, P = 0.0048; OL2, P = 0.0045; OL3, P = 0.0027) (Fig. 5B). NG, OS and S2 mice began to die on the 10th or 11th day of infection. Mice orally treated with Lico A began to die on the 12th day, and the longest survival time was 14 days. Oral treatment (Fig. 5C) with Lico A significantly increased the average survival rate compared with NG mice; however, there was no significant difference in survival time with higher doses.
Fig. 5.
Effect of intraperitoneal (Fig. 5A) or oral (Fig. 5B) administration of Lico A on the survival rate of mice with acute toxoplasmosis and the average survival days of BALB/c mice with toxoplasmosis orally treated with Lico A (Fig. 5C). *, significantly different from NG mice (P ≤ 0.05) using analysis of variance (ANOVA). The presented results represent the means ± standard deviations of the results from at least three independent experiments.
4. Discussion
Lico A is a novel flavonoid isolated from the root of Glycyrrhiza species belonging to the family Fabaceae; it can reduce inflammation (Cui et al., 2008), act as an antibacterial (Liu et al., 2008), inhibit tumourigenesis and induce cell cycle arrest and apoptosis in various cancer cell lines both in vitro and in vivo (Cho et al., 2014; Kim et al., 2010; Xiao et al., 2011). Recently, many studies have shown that Lico A has not only a broad antibacterial effect but also anti-protozoal activity, including against Plasmodium falciparum, Leishmania major and Leishmania donovani promastigotes and amastigotes. Lico A can inhibit the growth of P. falciparum at all stages (Chen et al., 1994), markedly reduce the infection rate of human peripheral blood monocyte-derived macrophages and U937 cells with L. major promastigotes and exhibit a strong intracellular killing effect on the parasite (Chen et al., 1993). Both P. falciparum and L. major are intracellular parasites. Thus, we can infer that Lico A may act against intracellular parasites. It is necessary to further explore whether Lico A has anti-T. gondii activity in vitro and in vivo. In this study, the anti-T. gondii effects of Lico A were evaluated in vitro and in vivo. Lico A exhibited strong anti-T. gondii activity with low cytotoxic effects in vitro; therefore, it should be considered as a promising lead for the development of much-needed anti-toxoplasma drugs.
In a cell viability assay, Lico A had no cytotoxic effects on HFF cells in the concentration range of 1–9 μg/mL. In addition, it promoted HFF cell proliferation at suitable concentrations. Interestingly, this study is the first to report the impact of Lico A on normal primary cells, though previous studies have shown that Lico A can inhibit cancer cell growth (Bortolotto et al., 2016; Cho et al., 2014). An in vitro anti-proliferative assay indicated that Lico A can significantly affect the proliferation of tachyzoites in a dose-dependent manner compared with the positive control group. Notably, no typical tachyzoites were observed when cells were treated with 4 μg/mL Lico A, but qPCR results indicated that tachyzoites were still present. This finding could indicate the presence of residual DNA from dead tachyzoites or that there were too few tachyzoites to be observed microscopically. In this experiment, we used the IC50 of Azi and Sul as the treatment dose (Neville et al., 2015). qPCR results showed that the amount of tachyzoites in the Azi and Sul groups was approximately 50% of that in the nontreatment group, demonstrating that the method was accurate and that the results are reliable. In addition, the IC50 of Lico A was 0.848 μg/mL, which was lower than that of Azi, indicating that at the same concentration, the anti-T. gondii activity of Lico A was stronger than that of Azi. Compared with other recently described natural products, the activity of Lico A was stronger than matrine (ME) (Zhang et al., 2016), ginkgolic acids (Chen et al., 2008) and other plant extracts (Choi et al., 2008; Sepulveda-Arias et al., 2014).
To further study the impact of Lico A on T. gondii, electron microscopy (SEM and TEM) analyses were conducted. SEM analysis showed that the tachyzoites were deformed, sunken and atrophied after Lico A treatment. Furthermore, TEM analysis showed that over time, lipid bodies developed, the nuclear membrane thickened, the cell surface became rough, a gap between the cell membrane and the cytoplasm appeared, and the cytoplasmic structure completely disappeared in tachyzoites after Lico A. After Nile Red staining, the emergence of neutral lipid aggregation in the cytoplasm of tachyzoites treated with Lico A was markedly increased, as assessed by confocal laser scanning microscopy. In addition, DIC microscopy images showed that tachyzoites were smaller in size and rounded in shape, with surface depressions; all of these observations are in agreement with the results observed by SEM. The correlation between fluorescence intensity and treatment time of Lico A was explored by flow cytometry, and the results showed that the increase in intracellular lipid levels occurred in a time-dependent manner. Therefore, Lico A may exert its effects against toxoplasmosis by interfering with lipid metabolism. Our results were inconsistent with a previous study showing that Lico A altered the ultrastructure and function of the mitochondria of Leishmania parasites (Zhai et al., 1995). This may be due to the differences between T. gondii and Leishmania in structural characteristics and physiological functions, though both are protozoans. Although the possible function of Lico A was deduced from the electron microscopy analysis, the specific mechanism of Lico A against T. gondii should be further studied in the future.
We also determined whether Lico A could exert anti-T. gondii effects in acute infections in vivo using a mouse model. Mice were infected with the virulent T. gondii RH strain, and Lico A extended the life of mice infected with a lethal dose of tachyzoites. Control mice succumbed to infection by day 11, while Lico A effectively improved the survival rate of mice injected with the treatment. The survival rate of the IL3 group was 100%, equal to that of the PS group, indicating that 150 mg/kg Lico A could achieve the same therapeutic effect as a commonly used clinical drug (combination of sulfadiazine, pyrimethamine and folinic acid); thus, Lico A could be further studied as a promising drug. However, under these same conditions, the effect of oral administration was far less than that of injection. In mice that received oral Lico A administration, Lico A prolonged the survival time but did not protect the mice from death, and the inhibitory effect was not significantly different among treatment groups. This limited efficacy may be a result of low absorption when the treatment is given orally. Together, our data obviously indicated that Lico A has potential as a novel anti-T. gondii agent and that injection administration was effective.
In summary, Lico A can effectively inhibit the proliferation of T. gondii in a dose-dependent and time-dependent manner with low cytotoxicity against HFF host cells. Electron microscopy analysis indicated that Lico A likely functions by affecting lipid metabolism. Importantly, the injection administration of a suitable concentration of Lico A can significantly increase the survival rate of mice infected with T. gondii, and Lico A achieved the same therapeutic effect as a commonly used clinical drug (combination of sulfadiazine, pyrimethamine and folinic acid). Thus, our results clearly demonstrate that Lico A exhibits potency in vitro and in vivo against T. gondii, warranting its possible evaluation as a treatment for toxoplasmosis in the future.
Funding
This work was supported by Key Projects in the National Science & Technological Pillar Program during the Twelfth Five-year Plan Period (2015BAD1101) and the earmarked fund for the China Agriculture Research System (CAR-38).
Conflicts of interest
None to declare.
Acknowledgements
We thank Dr. Xingquan Zhu for kindly providing the virulent RH stain of T. gondii and Donghui Zhou for technical assistance. We also thank Xukun Zhang (Shenyang Pharmaceutical University) for valuable suggestions.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.ijpddr.2018.02.006.
Appendix A. Supplementary data
The following are the supplementary data related to this article:
figs1.
figs2.
figs3.
figs4.
References
- Ajzenberg D., Lamaury I., Demar M., Vautrin C., Cabie A., Simon S., Nicolas M., Desbois-Nogard N., Boukhari R., Riahi H., Darde M.L., Massip P., Dupon M., Preux P.M., Labrunie A., Boncoeur M.P. Performance testing of PCR assay in blood samples for the diagnosis of toxoplasmic encephalitis in AIDS patients from the French departments of America and genetic diversity of Toxoplasma gondii: a prospective and multicentric study. PLoS Neglected Trop. Dis. 2016;10 doi: 10.1371/journal.pntd.0004790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araujo F.G., Remington J.S. Effect of clindamycin on acute and chronic toxoplasmosis in mice. Antimicrob. Agents Chemother. 1974;5:647–651. doi: 10.1128/aac.5.6.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bortolotto L.F., Barbosa F.R., Silva G., Bitencourt T.A., Beleboni R.O., Baek S.J., Marins M., Fachin A.L. Cytotoxicity of trans-chalcone and licochalcone A against breast cancer cells is due to apoptosis induction and cell cycle arrest. Biomed. Pharmacother. 2016;85:425–433. doi: 10.1016/j.biopha.2016.11.047. [DOI] [PubMed] [Google Scholar]
- Chemoh W., Sawangjaroen N., Nissapatorn V., Suwanrath C., Chandeying V., Hortiwakul T., Andiappan H., Sermwittayawong N., Charoenmak B., Siripaitoon P., Lekkla A., Sukthana Y. Toxoplasma gondii infection: what is the real situation? Exp. Parasitol. 2013;135:685–689. doi: 10.1016/j.exppara.2013.10.001. [DOI] [PubMed] [Google Scholar]
- Chen M., Christensen S.B., Blom J., Lemmich E., Nadelmann L., Fich K., Theander T.G., Kharazmi A. Licochalcone A, a novel antiparasitic agent with potent activity against human pathogenic protozoan species of Leishmania. Antimicrob. Agents Chemother. 1993;37:2550–2556. doi: 10.1128/aac.37.12.2550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M., Theander T.G., Christensen S., Hviid L., Zhai L., Kharazmi A. Licochalcone A, a new antimalarial agent, inhibits in vitro growth of the human malaria parasite Plasmodium falciparum and protects mice from P. yoelii infection. Antimicrob. Agents Chemother. 1994;38:1470–1475. doi: 10.1128/aac.38.7.1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S.-X., Wu L., Jiang X.-G., Feng Y.-Y., Cao J.-P. Anti-Toxoplasma gondii activity of GAS in vitro. J. Ethnopharmacol. 2008;118:503–507. doi: 10.1016/j.jep.2008.05.023. [DOI] [PubMed] [Google Scholar]
- Cho J.J., Chae J.I., Yoon G., Kim K.H., Cho J.H., Cho S.S., Cho Y.S., Shim J.H. Licochalcone A, a natural chalconoid isolated from Glycyrrhiza inflata root, induces apoptosis via Sp1 and Sp1 regulatory proteins in oral squamous cell carcinoma. Int. J. Oncol. 2014;45:667–674. doi: 10.3892/ijo.2014.2461. [DOI] [PubMed] [Google Scholar]
- Choi K.-M., Gang J., Yun J. Anti-Toxoplasma gondii RH strain activity of herbal extracts used in traditional medicine. Int. J. Antimicrob. Agents. 2008;32:360–362. doi: 10.1016/j.ijantimicag.2008.04.012. [DOI] [PubMed] [Google Scholar]
- Cui Y., Ao M., Li W., Hu J., Yu L. Anti-inflammatory activity of licochalcone A isolated from Glycyrrhiza inflata. J. Biosci. 2008;63:361–365. doi: 10.1515/znc-2008-5-609. Zeitschrift fur Naturforschung. C. [DOI] [PubMed] [Google Scholar]
- Dannemann B., Mccutchan J.A., Israelski D., Antoniskis D., Leport C., Luft B., Nussbaum J., Clumeck N., Morlat P., Chiu J. Treatment of toxoplasmic encephalitis in patients with AIDS. A randomized trial comparing pyrimethamine plus clindamycin to pyrimethamine plus sulfadiazine. The California Collaborative Treatment Group. Ann. Intern. Med. 1992;116:33–43. doi: 10.7326/0003-4819-116-1-33. [DOI] [PubMed] [Google Scholar]
- Desmonts G., Couvreur J. Toxoplasmosis in pregnancy and its transmission to the fetus. Bull. N. Y. Acad. Med. 1974;50:146–159. [PMC free article] [PubMed] [Google Scholar]
- Dubey J.P., Jones J.L. Toxoplasma gondii infection in humans and animals in the United States. Int. J. Parasitol. 2008;38:1257–1278. doi: 10.1016/j.ijpara.2008.03.007. [DOI] [PubMed] [Google Scholar]
- Dupont C.D., Christian D.A., Hunter C.A. Immune response and immunopathology during toxoplasmosis. Semin. Immunopathol. 2012;34:793–813. doi: 10.1007/s00281-012-0339-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eyles D.E., Coleman N. Synergistic effect of sulfadiazine and daraprim against experimental toxoplasmosis in the mouse. Antibiot. Chemother. 1953;3:483–490. [PubMed] [Google Scholar]
- Farthing C., Rendel M., Currie B., Seidlin M. Azithromycin for cerebral toxoplasmosis. Lancet (London, England) 1992;339:437–438. doi: 10.1016/0140-6736(92)90132-m. [DOI] [PubMed] [Google Scholar]
- Garin J.P., Eyles D.E. Spiramycin therapy of experimental toxoplasmosis in mice. Presse Med. 1958;66:957–958. [PubMed] [Google Scholar]
- Georgiev V.S. Management of toxoplasmosis. Drugs. 1994;48:179–188. doi: 10.2165/00003495-199448020-00005. [DOI] [PubMed] [Google Scholar]
- Godofsky E.W. Treatment of presumed cerebral toxoplasmosis with azithromycin. N. Engl. J. Med. 1994;330:575–576. doi: 10.1056/nejm199402243300817. [DOI] [PubMed] [Google Scholar]
- Homan W.L., Vercammen M., De Braekeleer J., Verschueren H. Identification of a 200- to 300-fold repetitive 529 bp DNA fragment in Toxoplasma gondii, and its use for diagnostic and quantitative PCR1. Int. J. Parasitol. 2000;30:69–75. doi: 10.1016/s0020-7519(99)00170-8. [DOI] [PubMed] [Google Scholar]
- Kim J.K., Shin E.K., Park J.H., Kim Y.H., Park J.H. Antitumor and antimetastatic effects of licochalcone A in mouse models. J. Mol. Med. 2010;88:829–838. doi: 10.1007/s00109-010-0625-2. [DOI] [PubMed] [Google Scholar]
- Kovacs J.A. Efficacy of atovaquone in treatment of toxoplasmosis in patients with AIDS. Lancet (London, England) 1992;340:637–638. doi: 10.1016/0140-6736(92)92172-c. [DOI] [PubMed] [Google Scholar]
- Liu X.L., Xu Y.J., Go M.L. Functionalized chalcones with basic functionalities have antibacterial activity against drug sensitive Staphylococcus aureus. Eur. J. Med. Chem. 2008;43:1681–1687. doi: 10.1016/j.ejmech.2007.10.007. [DOI] [PubMed] [Google Scholar]
- Luft B.J., Remington J.S. Toxoplasmic encephalitis in AIDS. Clin. Infect. Dis. 1992;15:211–222. doi: 10.1093/clinids/15.2.211. [DOI] [PubMed] [Google Scholar]
- Mcmaster P.R.B., Powers K.G., Finerty J.F., Lunde M.N. The effect of two chlorinated lincomycin analogues against acute toxoplasmosis in mice. Am. J. Trop. Med. Hyg. 1973;22:14–17. doi: 10.4269/ajtmh.1973.22.14. [DOI] [PubMed] [Google Scholar]
- Montoya J.G., Liesenfeld O. Toxoplasmosis. Lancet (London, England) 2004;363:1965–1976. doi: 10.1016/S0140-6736(04)16412-X. [DOI] [PubMed] [Google Scholar]
- Neville A.J., Zach S.J., Wang X., Larson J.J., Judge A.K., Davis L.A., Vennerstrom J.L., Davis P.H. Clinically available medicines demonstrating anti-toxoplasma activity. Antimicrob. Agents Chemother. 2015;59:7161–7169. doi: 10.1128/AAC.02009-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pijpers E., Schrey G. A clarithromycin-induced myasthenic syndrome. Clin. Infect. Dis. 1996;22:175–176. doi: 10.1093/clinids/22.1.175. [DOI] [PubMed] [Google Scholar]
- Raffi F., Struillou L., Ninin E., Reliquet V., Billaud E., Milpied B. Breakthrough cerebral toxoplasmosis in patients with AIDS who are being treated with clarithromycin. Clin. Infect. Dis. 1995;20:1076–1077. doi: 10.1093/clinids/20.4.1076. [DOI] [PubMed] [Google Scholar]
- Sabin A.B., Warren J. Therapeutic effectiveness of certain sulfonamides on infection by an intracellular protozoon (toxoplasma) Exp. Biol. Med. 1942;51:19–23. [Google Scholar]
- Sepulveda-Arias J.C., Veloza L.A., Mantilla-Muriel L.E. Anti-Toxoplasma activity of natural products: a review. Recent Pat. Anti-Infect. Drug Discov. 2014;9:186–194. doi: 10.2174/1574891x10666150410120321. [DOI] [PubMed] [Google Scholar]
- Si K., Wei L., Yu X., Wu F., Li X., Li C., Cheng Y. Effects of (+)-Usnic acid and (+)-Usnic acid-liposome on toxoplasma gondii. Exp. Parasitol. 2016;166:68–74. doi: 10.1016/j.exppara.2016.03.021. [DOI] [PubMed] [Google Scholar]
- Wynn R.F., Leen C.L.S., Brettle R.P. Azithromycin for cerebral toxoplasmosis in AIDS. Lancet (London, England) 1993;341:243–244. doi: 10.1016/0140-6736(93)90107-r. [DOI] [PubMed] [Google Scholar]
- Xiao X.Y., Hao M., Yang X.Y., Ba Q., Li M., Ni S.J., Wang L.S., Du X. Licochalcone A inhibits growth of gastric cancer cells by arresting cell cycle progression and inducing apoptosis. Canc. Lett. 2011;302:69–75. doi: 10.1016/j.canlet.2010.12.016. [DOI] [PubMed] [Google Scholar]
- Zhai L., Blom J., Chen M., Christensen S.B., Kharazmi A. The antileishmanial agent licochalcone A interferes with the function of parasite mitochondria. Antimicrob. Agents Chemother. 1995;39:2742–2748. doi: 10.1128/aac.39.12.2742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X., Jin L., Cui Z., Zhang C., Wu X., Park H., Quan H., Jin C. Antiparasitic effects of oxymatrine and matrine against Toxoplasma gondii in vitro and in vivo. Exp. Parasitol. 2016;165:95–102. doi: 10.1016/j.exppara.2016.03.020. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.