Abstract
The Kallikrein Kinin System (KKS) is a vasoactive peptide system with known functions in the maintenance of tissue homeostasis, renal function and blood pressure. The main effector peptide of KKS is Bradykinin (BK). This ligand has two receptors: a constitutive B2 receptor (B2R), which has been suggested to have anti-fibrotic effects in renal and cardiac models of fibrosis; and the inducible B1 receptor (B1R), whose expression is induced by damage and inflammation. Inflammation and fibrosis are hallmarks of Duchenne muscular dystrophy (DMD), therefore we hypothesized that the KKS may play a role in this disease. To evaluate this hypothesis we used the mdx mouse a model for DMD. We blocked the endogenous activity of the KKS by treating mdx mice with B2R antagonist (HOE-140) or B1R antagonist (DesArgLeu8BK (DALBK)) for four weeks. Both antagonists increased damage, fibrosis, TGF-β and Smad-dependent signaling, CTGF/CCN-2 levels as well as the number of CD68 positive inflammatory cells. B2R blockade also reduced isolated muscle contraction force. These results indicate that the endogenous KKS has a protective role in the dystrophic muscle. The KKS may be a new target for future therapies to reduce inflammation and fibrosis in dystrophic muscle.
Electronic supplementary material
The online version of this article (10.1007/s12079-017-0439-x) contains supplementary material, which is available to authorized users.
Keywords: Kallikrein kinin system, Bradykinin receptors B1 and B2, Muscular dystrophy, Fibrosis, Inflammation
Introduction
Duchenne muscular dystrophy (DMD) is a genetic disease with no cure. It is caused by the lack of the dystrophin protein that leads to the weakening and damage of muscle fibers during the contraction-relaxation cycle. These events result in chronic inflammation and the replacement of functional fibers by connective tissue, a process known as fibrosis (Davies and Nowak 2006). Among the major pro-fibrotic factors involved in the progression of fibrosis are transforming growth factor type beta (TGF-β), Connective tissue growth factor (CTGF/CCN-2) and Angiotensin-II (Ang-II) the main effector of the pro-fibrotic arm of the renin angiotensin system (RAS) (Kharraz et al. 2014; Kisseleva and Brenner 2008a, b; Wynn 2007, 2008).
In dystrophic muscle there is an increased level of TGF-β (Bernasconi et al. 1999; Gosselin et al. 2004) and Smad dependent signaling (Acuña et al. 2014; Cohn et al. 2007). The inhibition or reduction of TGF-β or TGF-β signaling results in decreased muscle fibrosis and increased muscle strength (Acuña et al. 2014; Cohn et al. 2007). CTGF/CCN-2 also plays a major role in skeletal muscle damage and fibrosis progression, its levels are increased in skeletal muscle from DMD patients (Sun et al. 2008), and its overexpression can induce a fibrotic phenotype of wild type muscle (Morales et al. 2011), on the other hand, the inhibition or reduction of CTGF/CCN-2 activity results in a reduced dystrophic phenotype (Morales et al. 2017, b). Regarding the RAS, there are increased levels of Angiotensin converting enzyme (ACE) and Angiotensin type 1 (AT1) receptor in DMD muscle, suggesting increased Ang-II activity in dystrophic muscle (Sun et al. 2009). On the other hand, the blockade of ACE with inhibitors (ACEi) such as enalapril, or treatment with antagonists of AT1 like losartan promotes improvement of the fibrotic phenotype and increases strength of isolated muscles (Cabello-Verrugio et al. 2012; Cohn et al. 2007; Cozzoli et al. 2011; Morales et al. 2013a).
The function of the RAS is modulated by another vasoactive peptide system, the kallikrein kinin system (KKS) that has opposite effects of Ang-II. The main effector of KKS is Bradykinin (BK) a nonapeptide produced from kininogen, which is processed by kallikrein to form BK. ACE can degrade BK with high efficiency, which is why ACE is also known as kininase-II (Bhoola et al. 1992; Madeddu et al. 2007). BK acts through two G protein coupled receptors, the B2 receptor (B2R) which is constitutively expressed in most tissues, including skeletal muscle (Bhoola et al. 1992; Figueroa et al. 1996; Madeddu et al. 2007), and the B1 receptor (B1R) whose expression is induced in response to damage and inflammatory processes (Leeb-Lundberg et al. 2005).
Through the activation of B2R KKS antagonizes Ang-II actions, resulting in antihypertensive, natriuretic, antiproliferative and antifibrotic effects (Chao et al. 2010). Also, it has been suggested that some of the beneficial effects of ACEis are mediated by BK, which is increased by ACE inhibition (Madeddu et al. 2007; Tom et al. 2003).
Different studies suggest that BK has a role as an antifibrotic agent. In vivo studies in hepatic, renal and cardiac fibrosis models show that the infusion of BK improves the fibrotic phenotype and reduces TGF-β levels (Chao et al. 2007; Sancho-Bru et al. 2007; Yao et al. 2007). On the other hand, the blockade of kallikrein increases damage and fibrosis in kidney (Liu et al. 2010). B2R knock out mice show increased cardiac and renal fibrosis (Chao et al. 2010; Emanueli et al. 1999; Schanstra et al. 2002). On the other hand, overexpression of kallikrein reduces the fibrotic phenotype and TGF-β levels in renal and cardiac fibrosis models (Tu et al. 2008; Yao et al. 2007; Zhu et al. 2016).
Since KKS has anti-fibrotic effects in other tissues and its components are present in skeletal muscle (Figueroa et al. 1996) the aim of the present study was to evaluate the effect of blocking endogenous KKS using B2R and B1R antagonists in the mdx mice. We found that blocking the BK receptors causes increased damage and fibrosis and reduction of dystrophic skeletal muscle strength, unraveling a beneficial role of endogenous KKS in skeletal muscle.
Materials and methods
Mice and tissue harvest
C57BL/10ScSn-Dmdmdx(mdx mice) and C57BL/10 (wild type mice) were purchased from the Jackson Laboratory (Bar Harbor, ME). Male mice were used in all studies. All mouse protocols were conducted in strict accordance and with formal approval of the Animal Ethics Committee of the Pontificia Universidad Católica de Chile. For tissue harvesting, animals were anesthetized and sacrificed by cervical dislocation. Muscles were quickly dissected for cryosectioning, frozen in isopentane cooled in liquid nitrogen and stored at −80 °C until processing.
Treatment with B1R and B2R antagonists
Twelve week old mice were treated with HOE-140 a B2 receptor antagonist or DesArg9Leu8Bradykinin (DALBK) a B1 receptor antagonist, administered by microosmotic pump (Alzet model 1004, USA), for 4 weeks. The dosage used was 500 ng/Kg*min for each antagonist (Marcon et al. 2013). To accelerate damage and fibrosis in the limbs mice were exercised three times a week for 30 min at 12 m/min (Pessina et al. 2014).
Skeletal muscle histology and Sirius red staining
Gastrocnemius (GM) cryosections were placed onto glass slides. Haematoxylin and eosin staining was performed to assess muscle architecture and histology. Total collagen content was detected by staining with 1% Sirius red in picric acid (Cabello-Verrugio et al. 2012; Morales et al. 2011). For Sirius red quantification we used the ImageJ software, we calculated percentage of Sirius red stained area (% of fibrosis) and intensity (integrated density) by using Otsu threshold parameter (Otsu 1979), the data is expressed relative to wild type.
Immunofluorescence microscopy
For muscle immunofluorescence, GM cryosections (7 μm) were fixed in paraformaldehyde 4%, blocked for 1 h in 4% fish gelatin +4% BSA in PBS, and incubated overnight at 4 °C with anti-fibronectin 1:500 dilution (Sigma, USA), anti-phosphoSmad3 1:100 dilution (Invitrogen, USA) (Cabello-Verrugio et al. 2012; Morales et al. 2011), anti-CD68 1:100 dilution (Abd Serotec -BioRad, USA), anti-iNOS 1:100 dilution (Abcam, USA). The corresponding Alexa Fluor 488 or 568-conjugated anti IgG were used as secondary antibodies 1:1000 dilution. For nuclear staining, sections were incubated with 1 μg/mL Hoechst 33,258 in PBS for 10 min.
Immunohistochemistry for CTGF/CCN2
GM cryosections (7 μm) were fixed in ethanol rinsed in 0.05 M Tris-phosphate-saline (TPS) buffer, pH 7.6, and incubated overnight with a primary antibody against CTGF/CCN2 (sc-14939, Santa Cruz Biotechnology, Santa Cruz, CA). The secondary antibody and peroxidase-anti-peroxidase (PAP) complex (MP Biomedicals, Aurora, OH) were applied for 30 min each at 22 °C. The immunoperoxidase reaction was visualized after incubation of sections in 0.1% (wt/vol) diaminobenzidine and 0.03% hydrogen peroxide for 2 min. The sections were washed with tap water and counterstained with hematoxylin, dehydrated in an ethanol gradient and cleared with xylene.
Immunoblot analysis
For immunoblot analyses GM muscles were homogenized in 10 volumes of buffer Tris-EDTA pH 7.4 with 1 mM PMSF. Then, one volume of buffer containing 2% glycerol, 4% SDS, and 0.125 M Tris pH 6.8 was added to the homogenates. Aliquots were subjected to SDS gel electrophoresis in 8 or 10% polyacrylamide gels, electrophoretically transferred onto PVDF membranes (Millipore, USA) and probed with specific antibodies against fibronectin 1:5000 dilution, (Sigma-Aldrich, USA), TGFβ-1,2,3 1:1000 dilution (R&D systems, USA), CTGF/CCN-2 1:500 dilution (sc-14939, Santa Cruz Biotechnology, USA) and GAPDH 1:5000 dilution (Chemicon, USA). All immunoreactions were visualized by enhanced chemiluminescence (Thermo Pierce, USA).
RNA isolation, reverse transcription and quantitative real-time PCR
Total RNA was isolated from tibial anterior (TA) using Trizol (Invitrogen, USA) according to the manufacturer’s instructions. Total RNA (2 μg) was reverse-transcribed to cDNA using random hexamers and MMLV reverse transcriptase (Invitrogen). Quantitative real-time PCR reactions were performed in duplicate on a Step one Plus Real Time PCR System (Applied Biosystems, USA) using TaqMan predesigned primer sets for mouse TGF-β1 (Mm01178820-m1), TNF-α (Mm99999068-m1) and the housekeeping gene 18 s (Mm03928990-g1) (TaqMan, Thermo Fisher, USA). Sybr green designed primers were used for CTGF/CCN2 (Fwd: 5’-CAG-GCT-GGA-GAA-GCA-GAG-TCGT-3′; Rev.: 5’-CTG-GTG-CAG-CCA-GAA-AGC-TCAA-3′) (Acuña and Brandan, 2017) and GAPDH (Fwd: 5’AGG-TCG- GTG-TGA-ACG–GAT-TTG-3′; Rev.: 5′-TGT-AGA-CCA-TGT-AGT-TGA-GGT-CA-3′) as reference gene. For CCN1 (Fwd: 5′-GAA-AGA-GAC-CCG-GAT-CTG-TG-3′; Rev.: 5’-ACT-GGA-GCA-TCC-TGC-ATA-AG-3′), for CCN3 (Fwd: 5’-GTC-ACC-AAC-AGG-AAT-CGC-CAG-T-3′; Rev.: 5’GTA-GGT-GGA-TGG-CTT-TCA-GGG-A-3′); and 18 s (Fwd: 5′-TGA-CGG-AAG-GGC-ACC-ACCAG-3′; Rev.: 5′-CAC-CAC-CAC-CCA-CGG-AAT-CG-3′) that was used as a reference gene for cnn1 and ccn3. mRNA expression was quantified using the comparative dCt method (2-ΔΔCT), using the corresponding reference gene. mRNA levels were expressed relative to the mean expression in the mdx mice without treatment.
Muscle strength assessment
After treatment, the mice were sacrificed and the Diaphragm (DIA) and TA rapidly excised and placed into a dish containing oxygenated Krebs-Ringer solution. Muscle strength was determined as described previously (Cabello-Verrugio et al. 2012; Morales et al. 2011). The optimum muscle length (Lo) and stimulation voltage were determined from micromanipulation of muscle length to produce the maximum isometric twitch force. Maximum isometric specific tetanic force was determined from the curve’s plateau of the relationship between specific isometric force (mN/mm2) with a stimulation frequency (Hz) ranging from 1 to 200 Hz for 450 ms, with 2 min of rest between stimuli. Muscle mass and Lo were used to calculate the specific net force (force normalized per total muscle fiber cross-sectional area, mN/mm2).
Quantification of phospho-Smad3 and CD68 positive cells
The number of nuclei positive for phospho-Smad3 (pSmad3) or CD68 cell staining was quantified from 5 different frames for each analyzed muscle at 20× magnification using the ImageJ v1.51n “cell counter” plugin. The data obtained were corrected for total area.
Statistics
The statistical significance of the differences between the means of the experimental groups was evaluated using one-way ANOVA with a post hoc Bonferroni multiple comparison test (Graph Pad Prism 6.01). A difference was considered statistically significant at p < 0.05. A two-way ANOVA was performed for the in vitro strength test in which two parameters (frequency and strength) were analyzed for each data group.
Results
BK receptors blockade increases damage and fibrosis of dystrophic mice
Since it has been described that BK reduces fibrosis in cardiac and renal damage models trough B2R (Chao et al. 2007; Sancho-Bru et al. 2007; Yao et al. 2007) and that B1 receptor expression is induced by damage and inflammation (Bhoola et al. 1992), we hypothesized that KKS could be playing a role in DMD. To study if the endogenous KKS plays a role in the progression of muscle dystrophy we treated mdx mice with HOE-140 to block B2R or Des-Arg9-Leu8-Bradykinin (DALBK) to block B1R by systemic infusion with micro-osmotic pumps for 4 weeks. We observed that treatment with the antagonists increased tissue damage in the mdx skeletal muscle as assessed with H&E staining. There was an increase of mononucleated cells that infiltrated the muscle, and also an evident increase of necrotic-degenerating foci (Morales et al. 2017) in comparison with the control mdx mice (Fig. 1a upper panels). The pictures on the left show the normal muscle structure of wild type muscle. We performed Sirius red staining to asses total collagen content, which was increased in the muscles from mice treated with the BK receptors antagonists (Fig. 1a bottom panels). We also observed Sirius red under polarized light (Supplementary Fig. 1) and noticed that HOE-140 treated muscles had thicker collagen fibers (red color) compared with mdx control (yellow color). We also observed areas with increased collagen accumulation in the muscles treated with DALBK in comparison with the control mdx. We quantified Sirius red staining by intensity of the red signal (Fig. 1b) and by the percentage of fibrosis (as percentage of red stained area) (Fig. 1c), and we found that total collagen is increased in HOE-140 and DALBK treated animals when compared to mdx control mice. In order to evaluate another fibrotic component we performed immunofluorescence staining for fibronectin (FN) and found increased levels of FN in the muscles from mdx mice treated with the antagonists when compared with control mdx mice (Supplementary Fig. 2A). We next performed western blots analyses for FN in muscle extracts and found there was a significant induction of FN in HOE-140 treated muscle compared to control mdx mice (Supplementary Fig. 2B and C) and there was no significant increase in the DALBK treated muscles (Supplementary Fig. 2D and E). When we treated the mice with a mixture of both antagonists we observed an increase of collagen and damage, but not for FN (Data not shown). These results suggest that the endogenous KKS has a protective role in the dystrophic muscle mainly via B2R, since its blockade increases damage and fibrosis.
Fig. 1. Bradykinin receptor blockade increases damage and fibrosis in mdx muscle.
a GAST cryosections were stained with H&E to asses for tissue damage (upper panel). Sirius red staining was performed to asses for tissue fibrosis (bottom panel). Bar 100 μm. b Quantification of Sirius red staining intensity, the integrated density of the red stain was measured. One way ANOVA *p < 0,05 vs wild type; ϕ p < 0,05 vs mdx control, n = 9 mdx control; n = 5 mdx HOE-140, n = 6 mdx DALBK and WT n = 3 (8 to10 images from different fields were quantified per mice). c Quantification of Sirius red staining, % of fibrosis is the total red stained area. One way ANOVA *p < 0,05 vs wild type; ϕ p < 0,05 vs mdx control, n = 9 mdx control; n = 5 mdx HOE-140, n = 6 mdx DALBK and WT n = 3 (10 images from different fields were quantified per mice)
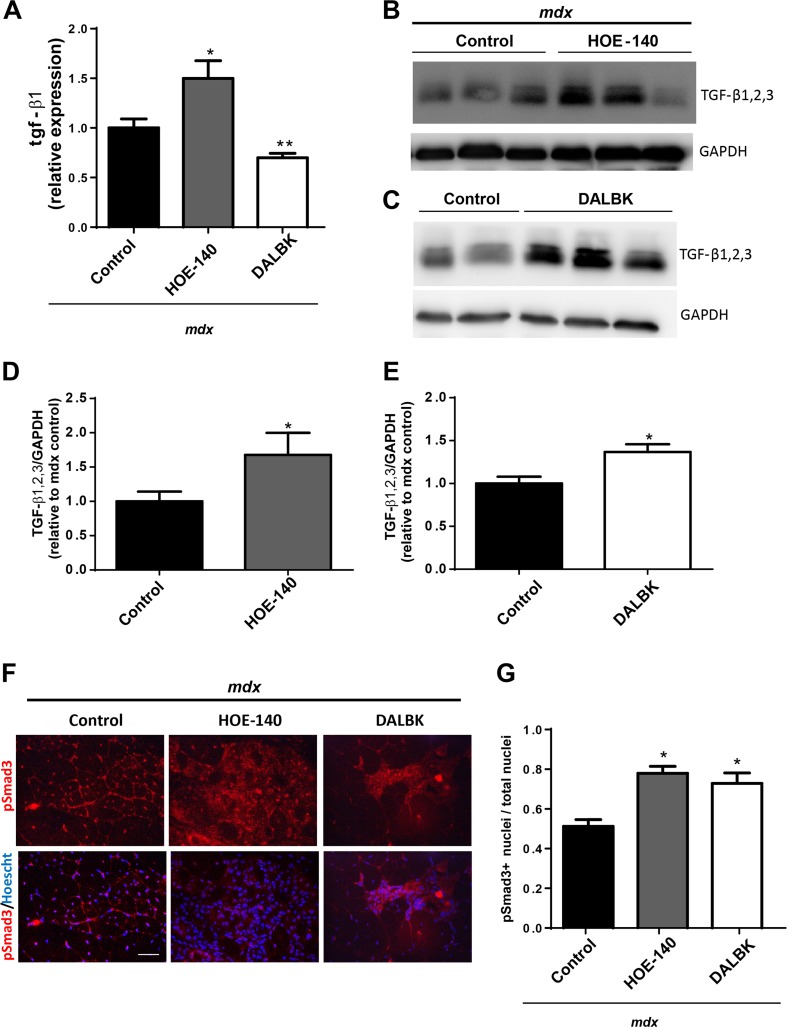
BK receptor blockade increases TGF-β levels, phospho-Smad3 dependent signaling and CTGF/CCN-2 in dystrophic muscle
Since we found increased fibrosis in our previous experiments, we quantifed mRNA levels of the profibrotic factor TGF-β1 by qPCR. We found increased tgf-β1 mRNA levels in the HOE-140 treated muscles. Interestingly, we observed a reduction of tgf-β1transcripts in the DALBK (Fig. 2a). Next we perform western blot analyses for TGF-β1, 2, 3 and found that its levels were increased with both treatments (Fig. 2b, c, d and e), this suggests that in the DALBK treated muscles there are post-transcriptional effects that modulate TGF-β levels.
Fig. 2. Bradykinin receptor blockade increases TGF-β and phospho-Smad3 positive cells.
a qPCR analysis of tgf-β1 and 18 s (as reference gene), from total RNA purified from TA. One way ANOVA *p < 0,05 vs mdx control; n = 10 mdx control; n = 8 mdx HOE-140, n = 6 mdx DALBK. b Western blot analysis for TGF-β1,2,3 and GAPDH as loading control of GAST extracts from mdx mice and mdx mice treated with HOE-140. c Western blot analysis for TGF-β1,2,3; and GAPDH as loading control of GAST extracts from mdx mice and mdx mice treated with DALBK. d and e Quantitation of western blots analyses for TGF-β1,2,3. T-test *p < 0,05 vs mdx. n = 4 mdx control; n = 4mdx HOE-140, n = 5 mdx DALBK. F) GAST cryosections from mdx control, mdx HOE-140 and mdx DALBK treated mice were immunostained for pSmad3 (upper panel). Hoechst dye was used to stain nuclei (lower panel). Bar 100 μm. G) Quantification of pSmad3 positive cells. One way ANOVA *p < 0,05 vs mdx control, n = 5 mice for each condition (five photographs were quantified for each mice)
In previous work we had observed that there was a correlation between the activity of Smad dependent signaling, damage and fibrosis in dystrophic muscle (Acuña et al. 2014). Therefore, we established the levels of pSmad3 through immunostaining analyses. We found an increased staining for pSmad3 in mice treated with HOE-140 and DALBK (Fig. 2f). We quantified the number of positive nuclei for pSmad3 and found there was a 1,5 fold increase of positive nuclei in the muscles from mice treated with the BK antagonists (Fig. 2g), we observed the same induction of pSmad3 when we treated mice with a mixture of both antagonists (data not shown). These increase in pSmad3 correlates with tissue damage and fibrosis, again suggesting that Smad signaling correlates with these pathological features in the dystrophic muscle.
Next we evaluated the profibrotic factor CTGF/CCN2 mRNA, and we found that ctgf/ccn-2 levels were increased by treatment with HOE-140 (Fig. 3a) and found a non-significative induction in the DALBK treated muscles. Western blot analyses for CTGF/CCN2 reveal there was a significant induction with both treatments (Fig. 3b, c, d and e). Immunohistochemistry analysis for CTGF/CCN2 also indicate an increase of the matricellular factor with both treatments (Fig. 3f).
Fig. 3. Bradykinin receptor blockade increases CTGF/CCN2 mRNA and protein levels.
a qPCR analysis of ctgf/ccn-2 and gapdh (as reference gene), from total RNA purified from TA. One way ANOVA *p < 0,05 vs mdx control; n = 10 mdx control; n = 8 mdx HOE-140, n = 6 mdx DALBK. b Western blot analysis for CTGF/CCN2 and GAPDH as loading control of GAST extracts from mdx mice and mdx mice treated with HOE-140. c Western blot analysis for CTGF/CCN2 and GAPDH as loading control of GAST extracts from mdx mice and mdx mice treated with DALBK. d and e Quantitation of western blots analyses for CTGF/CCN-2. T-test *p < 0,05 vs mdx. n = 4 mdx control; n = 4 mdx HOE-140, n = 4 mdx DALBK. f Immunohistochemical analysis for CTGF/CCN2 of GAST cryosections from WT control and mdx Control, HOE-140 and DALBK treated muscles. Bar 100 μm
These results indicate that TGF-β protein levels and its downstream signaling are increased in HOE-140 and DALBK treated muscles; the profibrotic factor CTGF/CCN-2 is also increased in these mice. All these markers correlate with the damage of the dystrophic muscle.
Since CTGF/CCN2 was modulated by the treatment with the antagonists,we further evaluated other CCN protein family members, CCN1 and CCN3 by qPCR analysis, and we found that ccn1 and ccn3 mRNA levels were reduced with HOE-140 (Supplementary Fig. 3A, C) and a non-significative change in the DALBK treated muscles (Supplementary Fig. 3B, D).
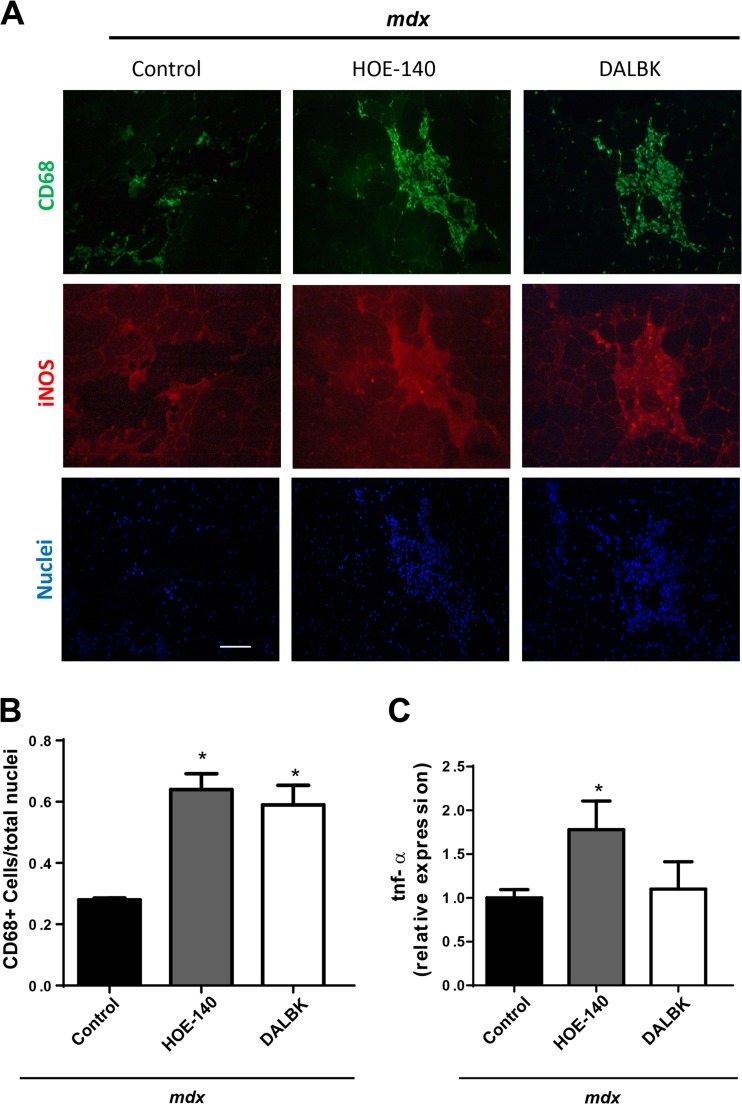
BK receptor blockade increases inflammatory cells in dystrophic muscle
H&E staining showed that there is an increase in the number of mononucleated cells that infiltrate the muscle. Since B1R is induced by inflammation and could play a role in the inflammatory process (Duchene and Ahluwalia 2009), we performed immunofluorescence studies to detect CD68 a marker of monocytes/macrophages and iNOS that is produced by pro-inflammatory and necrotic M1 type macrophages (Wynn and Vannella 2016). We observed increased CD68 staining in the muscles that were treated with the BK receptor antagonists (Fig. 4a upper panels). The staining for CD68 matched iNOS staining indicating that most of the macrophages stained with CD68 are also iNOS positive (Fig. 4a middle panels). We quantified the CD68+ cells and found that there was an increment of these cells in the muscles that had been treated with the BK receptor antagonists compared with control mdx mice (Fig. 4b). We also assessed mRNA levels of the pro-inflammatory cytokine TNF-α, and found an increase in the HOE-140 treated muscles (Fig. 4c), but not with the DALBK treatment. Together, these results indicate that the BK receptor blockade increases inflammation of the dystrophic muscle, suggesting that BK receptors could be reducing the inflammatory response.
Fig. 4. Bradykinin receptor blockade increases inflammatory CD68+ cells and tnf-α mRNA levels inmdx muscle.
a GAST cryosections from mdx control, mdx HOE-140 and mdx DALBK treated mice, were immunostained for CD-68 (upper panel), iNOS (middle panel). Hoechst dye was used to stain nuclei (lower panel). Bar 100 μm. b Quantitation of CD-68 positive cells. One way ANOVA *p < 0,05 vs mdx control, n = 5 mice (five photographs were quantified for each mice). C) qPCR analysis of tnf-α and 18 s as reference gene, from total RNA purified from TA. One way ANOVA *p < 0,05 vs mdx control; n = 10 mdx control; n = 7 mdx HOE-140, n = 5 mdx DALBK
B2R blockade reduces the strength of dystrophic muscle
Because we found increased damage in the limbs of mice treated with the BK antagonists, we determined the strength of isolated dystrophic muscle under these experimental conditions. Specifically, we evaluated muscle strength in vitro on isolated DIA strips and tibialis anterior TA muscle at increasing stimulation frequencies. We observed that HOE-140 treatment reduced the strength of DIA (Fig. 5a): in contrast, the treatment with DALBK did not produce any changes on muscle strength (Fig. 5b). We obtained similar results when we analyzed the TA muscle (Figs. 5c and d).
Fig. 5. B2R blockade reduces strength in diaphragm and tibial anterior muscle of mdx mice.
In vitro strength measurement of diaphragm strips (a and b) or tibial anterior muscles (c and d) stimulated at increasing frequencies. A and C) Muscle strength of mdx control (open circles) and mdx + HOE-140 treated mice (closed squares) Two way ANOVA *p < 0,05 vs mdx control. B and D) Muscle strength of mdx control (open circles) and mdx + DALBK treated mice (closed triangles) n = 6 per treatment
Discussion
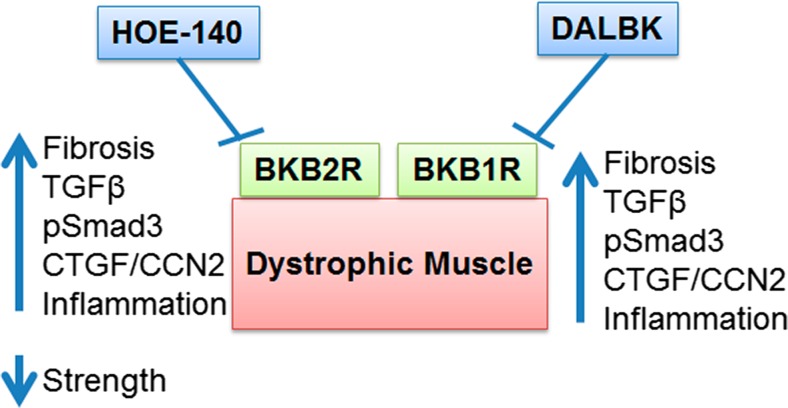
In this study we show that there is a protective role for the endogenous KKS in dystrophic muscle, since B2R and B1R blockade with specific antagonists worsens the dystrophic phenotype, Fig. 6 shows a summary of the effects found by the antagonists treatments These findings provides a new target for anti-fibrotic and anti-inflammatory therapies in muscular dystrophy.
Fig. 6.
Blockade of BK receptors worsens dystrophic phenotype. Summary diagram showing that HOE-140 antagonizes B2R (BKB2R), on the other hand DALBK antagonizes B1R (BKB1R) and in dystrophic muscle this treatment increases fibrosis, TGF-β, pSmad3, CTGF/CCN2 and inflammation, also with HOE-140 we found a reduction of strength
The dystrophic muscle has reduced capillary density (Matsakas et al. 2013), which causes ischemia leading to tissue damage and disease progression. One of the mechanisms by which the KKS may be protecting the dystrophic muscle is by increasing capillary density. There is evidence that tissue kallikrein overexpression leads to increased neovascularization of limbs and in a model of hind limb ischemia (Emanueli et al. 2001; Emanueli et al. 2000). This effect was blocked by treatment with HOE-140 or DALBK and L-NAME, a nitric oxide synthase inhibitor, suggesting that BK induces the vascularization of muscle through B2R and B1R via NO production. Cyclooxygenase-2 was also required for neovascularization induced by kallikrein (Emanueli et al. 2000).
In line with these observations, mdx mice that overexpress VEGF or nNOS show increased muscle strength and reduced damage (Messina et al. 2007; Rebolledo et al. 2016; Wehling et al. 2001). It would be interesting to evaluate capillary density along with VEGF and nNOS levels in the mdx mice treated with the BK antagonists to determine if, as expected, these parameters are reduced. Furthermore, BK is related to skeletal muscle metabolism. It is released from skeletal muscle during physical activity in response to pH changes and lactate (Dietze et al. 1996; Stebbins et al. 1990); the release of BK is associated with insulin dependent glucose transport and with the induction of prostacyclin and NO production via B2R, inducing a vasodilator response to increase muscle blood flow and glucose uptake (Dietze et al. 1996; Kishi et al. 1998; Miyata et al. 1998). Therefore, antagonism of the BK receptor could also be causing damage due to a further reduction in blood flow and increased hypoxia.
Another mechanism of damage caused by blocking the endogenous KKS could be the targeting of the TGF-β Smad signaling. We observed that blocking the endogenous KKS causes an increment in the number of pSmad3 positive nuclei, indicating that TGF-β dependent signaling is enhanced. In models of renal injury caused by diabetes, the overexpression of kallikrein reduces TGF-β levels (Zhu et al. 2016). BK infusion can also decrease TGF-β levels in cardiac, renal and hepatic fibrosis (Chao et al. 2007; Sancho-Bru et al. 2007; Yao et al. 2007). Therefore, the endogenous KKS may be decreasing the TGF-β Smad signaling, and protecting muscle from further damage and fibrosis, similar to what we observed for Angiotensin-(1–7) (Ang-(1–7)), another vasoactive peptide from the RAS (Acuña et al. 2014).
Interestingly, in DALBK treated muscle pSmad3 is increased, even though tgf -β1 mRNA levels are reduced, TGF-β1,2,3 protein level and pSmad3 are increased, suggesting that there is post-transcriptional regulation of TGF-β. There are different mechanisms that can regulate TGF-β activity, such as ligand bioavailability: TGF-β is produced as a latent form that interacts with ECM components trough latent TGF-β-binding protein (LTBP). TGF-β is then released from the ECM by the enzymatic cleavage of the lap peptide associated to LTBP or activated by trombospondin-1 (Sweetwyne and Murphy-Ullrich 2012; ten Dijke and Arthur 2007). Additionally, TGF-β can be sequestered in the ECM by decorin and byglican (Brandan et al. 2008) Furthermore, another regulatory mechanism involves the modulation of its correceptor betaglycan which presents TGF-β to its type II receptor (Lopez-Casillas et al. 2003). Finally, the activation of Smad signaling can be independent of TGF-β, since it can be induced by other factors such as Ang-II (Carvajal et al. 2008; Yang et al. 2009). It would be interesting to evaluate if one of these mechanisms is being modulated by DALBK.
CTGF/CCN-2, a pro-fibrotic factor induced by TGF-β that leads to muscle fibrosis (Morales et al. 2011), is also increased in the HOE-140 and DALBK treated muscles. Interestingly, through gain and loss of function experiments we have shown that the levels of CTGF/CCN2 correlate with the level of skeletal damage and fibrosis in the mdx mice (Morales et al. 2017, 2011, 2013b). Similarly, the HOE-140 and DALBK treated mdx muscles show more damage, correlating with the increased CTGF/CCN-2 levels. It would be interesting to evaluate the phenotype of BK antagonists treatment in the mdx hemizygous for CTGF/CCN-2 (Morales et al. 2013a), one could expect that in these animals there would be less damage with the antagonists. We observed that ccn1 and ccn3 mRNA levels were reduced in HOE-140 treated muscles. It has been established CCN1 as a senescence switch that converts ECM-producing myofibroblasts into ECM-degrading cells, thereby limiting fibrosis in wound healing. Thus, this diminished level of ccn1 mRNA might also be favoring the increase in skeletal muscle fibrosis observed after HOE-140 treatment (Jun and Lau 2010). Regarding the decrease of ccn3 mRNA levels, it has been shown that CCN3 might play an anti-inflammatory effects secondary to inhibition of NF-kappaB nuclear accumulation (Lin et al. 2010). Inflammation is a hallmark of DMD (Acharyya et al. 2007; Cabrera et al. 2014), thus the potential decrease in CCN3 might be enhancing inflammation in the muscle treated with HOE-140. Further studies are required to establish the role of CCN matricellular proteins in DMD.
We also observed that there was an increase in the number of inflammatory cells in the dystrophic muscle treated with de BK receptor antagonists. In addition, we found an increase of tnf-α transcript levels in the HOE-140 treated mice This result suggests that the endogenous KKS may be reducing the inflammatory response in the mdx background. In line with this evidence, infusion of kallikrein reduces inflammation in a model of renal fibrosis induced by gentamicin and mediated by B2R (Bledsoe et al. 2008). Kallikrein also reduces inflammation in renal fibrosis induced by salt and DOCA-salt models of injury (Xia et al. 2005; Zhang et al. 2004), and the inflammatory response in cardiac injury (Chao et al. 2010; Savvatis et al. 2010). On the other hand, there is evidence showing that B1R leads to increased inflammation and fibrosis and that its blockade has beneficial effects (Huart et al. 2015; Klein et al. 2010; Savvatis et al. 2010). The B1R is inducible by inflammatory cues, such as IL-1β, TNF-α, etc., via the activation of NF-κB transcription factor, and acts promoting neutrophil infiltration (Duchene and Ahluwalia 2009). Therefore, B1R could have a dual effect on inflammatory responses since its chronic induction may lead to persistent inflammation worsening the fibrotic phenotype. In this study, the blockade of B1R alone induces damage, fibrosis and inflammation, thus the role played by B1R should be elucidated, since it appears that in the mdx model B1R has a protective role.
The blockade of the B2R decreased the strength of mdx mice muscles. However, the blockade of B1R did not compromise mdx muscle strength. It could be speculated that B2R has a more potent protective role than B1R. Therefore, even though DALBK treatment increase damage, fibrosis and inflammation it does not an effect on strength. On the other hand, blockade of B2R has an impact on dystrophic muscle strength suggesting that this receptor plays a major role in protecting muscle affected by dystrophy pathology. Alternatively, the blockade of B2R may cause the endogenous KKS to exclusively activate B1R, which could lead to increased damage due to the inflammatory function of B1R. Further experiments, using B2R and B1R specific agonists or in dystrophic mice that have deletions for B1R o B2R should clarify this question.
This study provides evidence that the KKS plays a protective role in the dystrophic muscle. It is interesting that BK and Ang-(1–7) both vasoactive peptides with opposite functions to Ang-II, have protective effects in different pathologies including the dystrophic muscle. BK and Ang-(1–7) may be protecting the mdx muscle by reducing ACE/Ang-II/AT1 and it crosstalk with TGF-β (Acuña et al. 2014; Cardenas et al. 2015). It is interesting to note that the mdx phenotype is milder than the phenotype of DMD patients. Since the blockade of Ang-(1–7) Mas receptor (Acuña et al. 2014) and BK receptors leads to a more deleterious phenotype resembling the human phenotype, it is tempting to speculate that in humans Ang-(1–7) and BK could be less active than in the mdx mice model. Therefore, it would be interesting to evaluate these peptides in DMD patients.
In summary, we found that blockade of BK receptors leads to an aggravated dystrophic phenotype, indicating that the endogenous KKS has a protective role in muscular dystrophy. Our results also suggest that the KKS may be a new target for therapy intervention in DMD. Future experiments need to be conducted in order to clarify the protective role of both receptors and the potential therapeutic use of BK or BK receptors specific agonists.
Electronic supplementary material
BK receptors blockade increases total collagen staining. Sirius red staining reconstruction of gastrocnemius (GAST) from mdx control, mdx HOE-140 and mdx DALBK treated mice. The photographs were taken under polarized light. The red-orange color corresponds to thicker collagen fibers, the yellow to intermediate and the greenish color to thinner. (PDF 1576 kb)
Fibronectin is increased in HOE-140 treated mice. A) GAST cryosections were immunostained for fibronectin (FN) to asses for tissue fibrosis. Bar 100 μm. B) and D) are representative western blot analysis for FN from total GAST muscle extracts GAPDH was used as the loading control: B) wild type, mdx control and mdx HOE-140, D) wild type, mdx control and mdx DALBK. C) and E) Quantitation of western blots analyses for FN. One way ANOVA *p < 0,05 vs wild type; ϕ p < 0,05 vs mdx control; n = 4 mdx control; n = 6 mdx HOE-140, n = 6 mdx DALBK and WT n = 5. (PDF 6081 kb)
CCN1 and CCN3 mRNA levels are decreased in dystrophic muscle treated with HOE-140. Total RNA purified from TA was analyzed by qPCR for ccn1 A) and B) and ccn3 C) and D). 18 s was used as reference gene. T-test *p < 0,05 vs mdx control; n = 6 mdx control; n = 7 mdx HOE-140, n = 6 mdx DALBK (PDF 223 kb)
Acknowledgements
The authors are grateful to Victor Troncoso, Tamara Ramirez, Darling Vera, Lina Correa and Eduardo Ramirez for technical assistance.
Abbreviations
- Ang-II
Angiotensin-II
- Ang-(1–7)
Angiotensin-(1–7)
- ACE
Angiotensin converting enzyme
- AT1
Angiotensin receptor 1
- BK
Bradykinin
- B1R
Bradykinin Receptor 1
- B2R
Bradykinin Receptor 2
- CTGF/CCN-2
Connective tissue growth factor
- DALBK
DesArg9Leu8-Bradykinin
- DMD
Duchenne muscular dystrophy
- ECM
Extracellular matrix
- KKS
Kallikrein kinin system
- LTBP
Latent TGF-β-binding protein
- RAS
Renin Angiotensin system
- TGF-β
Transforming growth factor type-β
- TNF-α
Tumor necrosis factor alpha
Author contributions
M.J.A., C.V. and E.B. conceived the concepts, designed the study and wrote the manuscript. M.J.A. performed the experiments, drafted the manuscript and analyzed the data. A.C-C. helped with western blot and Sirius red quantitation analyses. C.C. did the immunohistochemical stain for CTGF/CCN2, M.C-S and D.S. did the qPCR experiments.
Funding
FONDECYT Grant 3,140,323 to M.J.A and 1,150,106 to EB; CARE-PFB-12/2007 grant to E.B. and C.P.V and SQM grant to C.P.V. The funding agencies had no role in the design of the study, data collection and analysis, decision to publish, or preparation of the manuscript.
Compliance with ethical standards
Conflict of interest
The authors declare no competing financial interests.
Footnotes
Electronic supplementary material
The online version of this article (10.1007/s12079-017-0439-x) contains supplementary material, which is available to authorized users.
Contributor Information
María José Acuña, Email: mjacuna1@uc.cl.
Daniela Salas, Email: danielasalas@gmail.com.
Adriana Córdova-Casanova, Email: acordovacasanova@gmail.com.
Meilyn Cruz-Soca, Email: mcruz6@uc.cl.
Carlos Céspedes, Email: labcvio@bio.puc.cl.
Carlos P. Vio, Email: cvio@uc.cl
Enrique Brandan, Email: ebrandan@bio.puc.cl.
References
- Acharyya S, Villalta SA, Bakkar N, Bupha-Intr T, Janssen PM, Carathers M, Li ZW, Beg AA, Ghosh S, Sahenk Z, Weinstein M, Gardner KL, Rafael-Fortney JA, Karin M, Tidball JG, Baldwin AS, Guttridge DC. Interplay of IKK/NF-kappaB signaling in macrophages and myofibers promotes muscle degeneration in Duchenne muscular dystrophy. J Clin Invest. 2007;117:889–901. doi: 10.1172/JCI30556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acuña MJ, Brandan E. Analysis of pathological activities of CCN2/CTGF in muscle dystrophy. Methods Mol Biol. 2017;1489:513–521. doi: 10.1007/978-1-4939-6430-7_43. [DOI] [PubMed] [Google Scholar]
- Acuña MJ, Pessina P, Olguin H, Cabrera D, Vio CP, Bader M, Munoz-Canoves P, Santos RA, Cabello-Verrugio C, Brandan E. Restoration of muscle strength in dystrophic muscle by angiotensin-1-7 through inhibition of TGF-beta signalling. Hum Mol Genet. 2014;23:1237–1249. doi: 10.1093/hmg/ddt514. [DOI] [PubMed] [Google Scholar]
- Bernasconi P, Di Blasi C, Mora M, Morandi L, Galbiati S, Confalonieri P, Cornelio F, Mantegazza R. Transforming growth factor-beta1 and fibrosis in congenital muscular dystrophies. Neuromuscul Disord. 1999;9:28–33. doi: 10.1016/S0960-8966(98)00093-5. [DOI] [PubMed] [Google Scholar]
- Bhoola KD, Figueroa CD, Worthy K. Bioregulation of kinins: kallikreins, kininogens, and kininases. Pharmacol Rev. 1992;44:1–80. [PubMed] [Google Scholar]
- Bledsoe G, Shen B, Yao YY, Hagiwara M, Mizell B, Teuton M, Grass D, Chao L, Chao J. Role of tissue kallikrein in prevention and recovery of gentamicin-induced renal injury. Toxicol Sci. 2008;102:433–443. doi: 10.1093/toxsci/kfn008. [DOI] [PubMed] [Google Scholar]
- Brandan E, Cabello-Verrugio C, Vial C. Novel regulatory mechanisms for the proteoglycans decorin and biglycan during muscle formation and muscular dystrophy. Matrix Biol. 2008;27:700–708. doi: 10.1016/j.matbio.2008.07.004. [DOI] [PubMed] [Google Scholar]
- Cabello-Verrugio C, Morales MG, Cabrera D, Vio CP, Brandan E. Angiotensin II receptor type 1 blockade decreases CTGF/CCN2-mediated damage and fibrosis in normal and dystrophic skeletal muscles. J Cell Mol Med. 2012;16:752–764. doi: 10.1111/j.1582-4934.2011.01354.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabrera D, Gutierrez J, Cabello-Verrugio C, Morales MG, Mezzano S, Fadic R, Casar JC, Hancke JL, Brandan E. Andrographolide attenuates skeletal muscle dystrophy in mdx mice and increases efficiency of cell therapy by reducing fibrosis. Skelet Muscle. 2014;4:6. doi: 10.1186/2044-5040-4-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardenas A, Campos J, Ehrenfeld P, Mezzano S, Ruiz-Ortega M, Figueroa CD, Ardiles L. Up-regulation of the kinin B2 receptor pathway modulates the TGF-beta/Smad signaling cascade to reduce renal fibrosis induced by albumin. Peptides. 2015;73:7–19. doi: 10.1016/j.peptides.2015.08.003. [DOI] [PubMed] [Google Scholar]
- Carvajal G, Rodriguez-Vita J, Rodrigues-Diez R, Sanchez-Lopez E, Ruperez M, Cartier C, Esteban V, Ortiz A, Egido J, Mezzano SA, Ruiz-Ortega M. Angiotensin II activates the Smad pathway during epithelial mesenchymal transdifferentiation. Kidney Int. 2008;74:585–595. doi: 10.1038/ki.2008.213. [DOI] [PubMed] [Google Scholar]
- Chao J, Li HJ, Yao YY, Shen B, Gao L, Bledsoe G, Chao L. Kinin infusion prevents renal inflammation, apoptosis, and fibrosis via inhibition of oxidative stress and mitogen-activated protein kinase activity. Hypertension. 2007;49:490–497. doi: 10.1161/01.HYP.0000255925.01707.eb. [DOI] [PubMed] [Google Scholar]
- Chao J, Shen B, Gao L, Xia CF, Bledsoe G, Chao L. Tissue kallikrein in cardiovascular, cerebrovascular and renal diseases and skin wound healing. Biol Chem. 2010;391:345–355. doi: 10.1515/bc.2010.042. [DOI] [PubMed] [Google Scholar]
- Cohn RD, van Erp C, Habashi JP, Soleimani AA, Klein EC, Lisi MT, Gamradt M, ap Rhys CM, Holm TM, Loeys BL, Ramirez F, Judge DP, Ward CW, Dietz HC. Angiotensin II type 1 receptor blockade attenuates TGF-beta-induced failure of muscle regeneration in multiple myopathic states. Nat Med. 2007;13:204–210. doi: 10.1038/nm1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cozzoli A, Nico B, Sblendorio VT, Capogrosso RF, Dinardo MM, Longo V, Gagliardi S, Montagnani M, De Luca A. Enalapril treatment discloses an early role of angiotensin II in inflammation- and oxidative stress-related muscle damage in dystrophic mdx mice. Pharmacol Res. 2011;64:482–492. doi: 10.1016/j.phrs.2011.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies KE, Nowak KJ. Molecular mechanisms of muscular dystrophies: old and new players. Nat Rev Mol Cell Biol. 2006;7:762–773. doi: 10.1038/nrm2024. [DOI] [PubMed] [Google Scholar]
- Dietze GJ, Wicklmayr M, Rett K, Jacob S, Henriksen EJ. Potential role of bradykinin in forearm muscle metabolism in humans. Diabetes. 1996;45(Suppl 1):S110–S114. doi: 10.2337/diab.45.1.S110. [DOI] [PubMed] [Google Scholar]
- Duchene J, Ahluwalia A. The kinin B(1) receptor and inflammation: new therapeutic target for cardiovascular disease. Curr Opin Pharmacol. 2009;9:125–131. doi: 10.1016/j.coph.2008.11.011. [DOI] [PubMed] [Google Scholar]
- Emanueli C, Maestri R, Corradi D, Marchione R, Minasi A, Tozzi MG, Salis MB, Straino S, Capogrossi MC, Olivetti G, Madeddu P. Dilated and failing cardiomyopathy in bradykinin B(2) receptor knockout mice. Circulation. 1999;100:2359–2365. doi: 10.1161/01.CIR.100.23.2359. [DOI] [PubMed] [Google Scholar]
- Emanueli C, Zacheo A, Minasi A, Chao J, Chao L, Salis MB, Stacca T, Straino S, Capogrossi MC, Madeddu P. Adenovirus-mediated human tissue kallikrein gene delivery induces angiogenesis in normoperfused skeletal muscle. Arterioscler Thromb Vasc Biol. 2000;20:2379–2385. doi: 10.1161/01.ATV.20.11.2379. [DOI] [PubMed] [Google Scholar]
- Emanueli C, Minasi A, Zacheo A, Chao J, Chao L, Salis MB, Straino S, Tozzi MG, Smith R, Gaspa L, Bianchini G, Stillo F, Capogrossi MC, Madeddu P. Local delivery of human tissue kallikrein gene accelerates spontaneous angiogenesis in mouse model of hindlimb ischemia. Circulation. 2001;103:125–132. doi: 10.1161/01.CIR.103.1.125. [DOI] [PubMed] [Google Scholar]
- Figueroa CD, Dietze G, Muller-Esterl W. Immunolocalization of bradykinin B2 receptors on skeletal muscle cells. Diabetes. 1996;45(Suppl 1):S24–S28. doi: 10.2337/diab.45.1.S24. [DOI] [PubMed] [Google Scholar]
- Gosselin LE, Williams JE, Deering M, Brazeau D, Koury S, Martinez DA. Localization and early time course of TGF-beta 1 mRNA expression in dystrophic muscle. Muscle Nerve. 2004;30:645–653. doi: 10.1002/mus.20150. [DOI] [PubMed] [Google Scholar]
- Huart A, Klein J, Gonzalez J, Buffin-Meyer B, Neau E, Delage C, Calise D, Ribes D, Schanstra JP, Bascands JL. Kinin B1 receptor antagonism is equally efficient as angiotensin receptor 1 antagonism in reducing renal fibrosis in experimental obstructive nephropathy, but is not additive. Front Pharmacol. 2015;6:8. doi: 10.3389/fphar.2015.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jun JI, Lau LF. Cellular senescence controls fibrosis in wound healing. Aging (Albany NY) 2010;2:627–631. doi: 10.18632/aging.100201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kharraz Y, Guerra J, Pessina P, Serrano AL, Munoz-Canoves P. Understanding the process of fibrosis in Duchenne muscular dystrophy. Biomed Res Int. 2014;2014:965631. doi: 10.1155/2014/965631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishi K, Muromoto N, Nakaya Y, Miyata I, Hagi A, Hayashi H, Ebina Y. Bradykinin directly triggers GLUT4 translocation via an insulin-independent pathway. Diabetes. 1998;47:550–558. doi: 10.2337/diabetes.47.4.550. [DOI] [PubMed] [Google Scholar]
- Kisseleva T, Brenner DA. Fibrogenesis of parenchymal organs. Proc Am Thorac Soc. 2008;5:338–342. doi: 10.1513/pats.200711-168DR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kisseleva T, Brenner DA. Mechanisms of fibrogenesis. Exp Biol Med (Maywood) 2008;233:109–122. doi: 10.3181/0707-MR-190. [DOI] [PubMed] [Google Scholar]
- Klein J, Gonzalez J, Decramer S, Bandin F, Neau E, Salant DJ, Heeringa P, Pesquero JB, Schanstra JP, Bascands JL. Blockade of the kinin B1 receptor ameloriates glomerulonephritis. J Am Soc Nephrol. 2010;21:1157–1164. doi: 10.1681/ASN.2009090887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leeb-Lundberg LM, Marceau F, Muller-Esterl W, Pettibone DJ, Zuraw BL. International union of pharmacology. XLV. Classification of the kinin receptor family: from molecular mechanisms to pathophysiological consequences. Pharmacol Rev. 2005;57:27–77. doi: 10.1124/pr.57.1.2. [DOI] [PubMed] [Google Scholar]
- Lin Z, Natesan V, Shi H, Hamik A, Kawanami D, Hao C, Mahabaleshwar GH, Wang W, Jin ZG, Atkins GB, Firth SM, Rittie L, Perbal B, Jain MK. A novel role of CCN3 in regulating endothelial inflammation. J Cell Commun Signal. 2010;4:141–153. doi: 10.1007/s12079-010-0095-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Bledsoe G, Hagiwara M, Yang ZR, Shen B, Chao L, Chao J. Blockade of endogenous tissue kallikrein aggravates renal injury by enhancing oxidative stress and inhibiting matrix degradation. Am J Physiol Renal Physiol. 2010;298:F1033–F1040. doi: 10.1152/ajprenal.00518.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Casillas F, Riquelme C, Perez-Kato Y, Ponce-Castaneda MV, Osses N, Esparza-Lopez J, Gonzalez-Nunez G, Cabello-Verrugio C, Mendoza V, Troncoso V, Brandan E. Betaglycan expression is transcriptionally up-regulated during skeletal muscle differentiation. Cloning of murine betaglycan gene promoter and its modulation by MyoD, retinoic acid, and transforming growth factor-beta. J Biol Chem. 2003;278:382–390. doi: 10.1074/jbc.M208520200. [DOI] [PubMed] [Google Scholar]
- Madeddu P, Emanueli C, El-Dahr S. Mechanisms of disease: the tissue kallikrein-kinin system in hypertension and vascular remodeling. Nat Clin Pract Nephrol. 2007;3:208–221. doi: 10.1038/ncpneph0444. [DOI] [PubMed] [Google Scholar]
- Marcon R, Claudino RF, Dutra RC, Bento AF, Schmidt EC, Bouzon ZL, Sordi R, Morais RL, Pesquero JB, Calixto JB. Exacerbation of DSS-induced colitis in mice lacking kinin B(1) receptors through compensatory up-regulation of kinin B(2) receptors: the role of tight junctions and intestinal homeostasis. Br J Pharmacol. 2013;168:389–402. doi: 10.1111/j.1476-5381.2012.02136.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsakas A, Yadav V, Lorca S, Narkar V. Muscle ERRgamma mitigates Duchenne muscular dystrophy via metabolic and angiogenic reprogramming. FASEB J. 2013;27:4004–4016. doi: 10.1096/fj.13-228296. [DOI] [PubMed] [Google Scholar]
- Messina S, Mazzeo A, Bitto A, Aguennouz M, Migliorato A, De Pasquale MG, Minutoli L, Altavilla D, Zentilin L, Giacca M, Squadrito F, Vita G. VEGF overexpression via adeno-associated virus gene transfer promotes skeletal muscle regeneration and enhances muscle function in mdx mice. FASEB J. 2007;21:3737–3746. doi: 10.1096/fj.07-8459com. [DOI] [PubMed] [Google Scholar]
- Miyata T, Taguchi T, Uehara M, Isami S, Kishikawa H, Kaneko K, Araki E, Shichiri M. Bradykinin potentiates insulin-stimulated glucose uptake and enhances insulin signal through the bradykinin B2 receptor in dog skeletal muscle and rat L6 myoblasts. Eur J Endocrinol. 1998;138:344–352. doi: 10.1530/eje.0.1380344. [DOI] [PubMed] [Google Scholar]
- Morales MG, Cabello-Verrugio C, Santander C, Cabrera D, Goldschmeding R, Brandan E. CTGF/CCN-2 over-expression can directly induce features of skeletal muscle dystrophy. J Pathol. 2011;225:490–501. doi: 10.1002/path.2952. [DOI] [PubMed] [Google Scholar]
- Morales MG, Cabrera D, Cespedes C, Vio CP, Vazquez Y, Brandan E, Cabello-Verrugio C. Inhibition of the angiotensin-converting enzyme decreases skeletal muscle fibrosis in dystrophic mice by a diminution in the expression and activity of connective tissue growth factor (CTGF/CCN-2) Cell Tissue Res. 2013;353:173–187. doi: 10.1007/s00441-013-1642-6. [DOI] [PubMed] [Google Scholar]
- Morales MG, Gutierrez J, Cabello-Verrugio C, Cabrera D, Lipson KE, Goldschmeding R, Brandan E. Reducing CTGF/CCN2 slows down mdx muscle dystrophy and improves cell therapy. Hum Mol Genet. 2013;22:4938–4951. doi: 10.1093/hmg/ddt352. [DOI] [PubMed] [Google Scholar]
- Morales MG, Acuña MJ, Cabrera D, Goldschmeding R, Brandan E (2017) The pro-fibrotic connective tissue growth factor (CTGF/CCN2) correlates with the number of necrotic-regenerative foci in dystrophic muscle. J Cell Commun Signal. 10.1007/s12079-017-0409-3 [DOI] [PMC free article] [PubMed]
- Otsu N. A threshold selection method from gray-level histograms. IEEE Trans Syst Man Cybern. 1979;9:62–66. doi: 10.1109/TSMC.1979.4310076. [DOI] [Google Scholar]
- Pessina P, Cabrera D, Morales MG, Riquelme CA, Gutierrez J, Serrano AL, Brandan E, Munoz-Canoves P. Novel and optimized strategies for inducing fibrosis in vivo: focus on Duchenne muscular dystrophy. Skelet Muscle. 2014;4:7. doi: 10.1186/2044-5040-4-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebolledo DL, Kim MJ, Whitehead NP, Adams ME, Froehner SC. Sarcolemmal targeting of nNOSmu improves contractile function of mdx muscle. Hum Mol Genet. 2016;25:158–166. doi: 10.1093/hmg/ddv466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sancho-Bru P, Bataller R, Fernandez-Varo G, Moreno M, Ramalho LN, Colmenero J, Mari M, Claria J, Jimenez W, Arroyo V, Brenner DA, Gines P. Bradykinin attenuates hepatocellular damage and fibrosis in rats with chronic liver injury. Gastroenterology. 2007;133:2019–2028. doi: 10.1053/j.gastro.2007.09.023. [DOI] [PubMed] [Google Scholar]
- Savvatis K, Westermann D, Schultheiss HP, Tschope C. Kinins in cardiac inflammation and regeneration: insights from ischemic and diabetic cardiomyopathy. Neuropeptides. 2010;44:119–125. doi: 10.1016/j.npep.2009.11.007. [DOI] [PubMed] [Google Scholar]
- Schanstra JP, Neau E, Drogoz P, Arevalo Gomez MA, Lopez Novoa JM, Calise D, Pecher C, Bader M, Girolami JP, Bascands JL. In vivo bradykinin B2 receptor activation reduces renal fibrosis. J Clin Invest. 2002;110:371–379. doi: 10.1172/JCI0215493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stebbins, C.L., O.A. Carretero, T. Mindroiu, and J.C. Longhurst. 1990. Bradykinin release from contracting skeletal muscle of the cat. J Appl Physiol (1985). 69:1225–1230 [DOI] [PubMed]
- Sun G, Haginoya K, Wu Y, Chiba Y, Nakanishi T, Onuma A, Sato Y, Takigawa M, Iinuma K, Tsuchiya S. Connective tissue growth factor is overexpressed in muscles of human muscular dystrophy. J Neurol Sci. 2008;267:48–56. doi: 10.1016/j.jns.2007.09.043. [DOI] [PubMed] [Google Scholar]
- Sun G, Haginoya K, Dai H, Chiba Y, Uematsu M, Hino-Fukuyo N, Onuma A, Iinuma K, Tsuchiya S. Intramuscular renin-angiotensin system is activated in human muscular dystrophy. J Neurol Sci. 2009;280:40–48. doi: 10.1016/j.jns.2009.01.020. [DOI] [PubMed] [Google Scholar]
- Sweetwyne MT, Murphy-Ullrich JE. Thrombospondin1 in tissue repair and fibrosis: TGF-beta-dependent and independent mechanisms. Matrix Biol. 2012;31:178–186. doi: 10.1016/j.matbio.2012.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ten Dijke P, Arthur HM. Extracellular control of TGFbeta signalling in vascular development and disease. Nat Rev Mol Cell Biol. 2007;8:857–869. doi: 10.1038/nrm2262. [DOI] [PubMed] [Google Scholar]
- Tom B, Dendorfer A, Danser AH. Bradykinin, angiotensin-(1-7), and ACE inhibitors: how do they interact? Int J Biochem Cell Biol. 2003;35:792–801. doi: 10.1016/S1357-2725(02)00273-X. [DOI] [PubMed] [Google Scholar]
- Tu L, Xu X, Wan H, Zhou C, Deng J, Xu G, Xiao X, Chen Y, Edin ML, Voltz JW, Zeldin DC, Wang DW. Delivery of recombinant adeno-associated virus-mediated human tissue kallikrein for therapy of chronic renal failure in rats. Hum Gene Ther. 2008;19:318–330. doi: 10.1089/hum.2007.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wehling M, Spencer MJ, Tidball JG. A nitric oxide synthase transgene ameliorates muscular dystrophy in mdx mice. J Cell Biol. 2001;155:123–131. doi: 10.1083/jcb.200105110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wynn TA. Common and unique mechanisms regulate fibrosis in various fibroproliferative diseases. J Clin Invest. 2007;117:524–529. doi: 10.1172/JCI31487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wynn TA. Cellular and molecular mechanisms of fibrosis. J Pathol. 2008;214:199–210. doi: 10.1002/path.2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wynn TA, Vannella KM. Macrophages in tissue repair, regeneration, and fibrosis. Immunity. 2016;44:450–462. doi: 10.1016/j.immuni.2016.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia CF, Bledsoe G, Chao L, Chao J. Kallikrein gene transfer reduces renal fibrosis, hypertrophy, and proliferation in DOCA-salt hypertensive rats. Am J Physiol Renal Physiol. 2005;289:F622–F631. doi: 10.1152/ajprenal.00427.2004. [DOI] [PubMed] [Google Scholar]
- Yang F, Chung AC, Huang XR, Lan HY. Angiotensin II induces connective tissue growth factor and collagen I expression via transforming growth factor-beta-dependent and -independent Smad pathways: the role of Smad3. Hypertension. 2009;54:877–884. doi: 10.1161/HYPERTENSIONAHA.109.136531. [DOI] [PubMed] [Google Scholar]
- Yao YY, Yin H, Shen B, Chao L, Chao J. Tissue kallikrein and kinin infusion rescues failing myocardium after myocardial infarction. J Card Fail. 2007;13:588–596. doi: 10.1016/j.cardfail.2007.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang JJ, Bledsoe G, Kato K, Chao L, Chao J. Tissue kallikrein attenuates salt-induced renal fibrosis by inhibition of oxidative stress. Kidney Int. 2004;66:722–732. doi: 10.1111/j.1523-1755.2004.00794.x. [DOI] [PubMed] [Google Scholar]
- Zhu D, Zhang L, Cheng L, Ren L, Tang J, Sun D. Pancreatic Kininogenase ameliorates renal fibrosis in Streptozotocin induced-diabetic nephropathy rat. Kidney Blood Press Res. 2016;41:9–17. doi: 10.1159/000368542. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
BK receptors blockade increases total collagen staining. Sirius red staining reconstruction of gastrocnemius (GAST) from mdx control, mdx HOE-140 and mdx DALBK treated mice. The photographs were taken under polarized light. The red-orange color corresponds to thicker collagen fibers, the yellow to intermediate and the greenish color to thinner. (PDF 1576 kb)
Fibronectin is increased in HOE-140 treated mice. A) GAST cryosections were immunostained for fibronectin (FN) to asses for tissue fibrosis. Bar 100 μm. B) and D) are representative western blot analysis for FN from total GAST muscle extracts GAPDH was used as the loading control: B) wild type, mdx control and mdx HOE-140, D) wild type, mdx control and mdx DALBK. C) and E) Quantitation of western blots analyses for FN. One way ANOVA *p < 0,05 vs wild type; ϕ p < 0,05 vs mdx control; n = 4 mdx control; n = 6 mdx HOE-140, n = 6 mdx DALBK and WT n = 5. (PDF 6081 kb)
CCN1 and CCN3 mRNA levels are decreased in dystrophic muscle treated with HOE-140. Total RNA purified from TA was analyzed by qPCR for ccn1 A) and B) and ccn3 C) and D). 18 s was used as reference gene. T-test *p < 0,05 vs mdx control; n = 6 mdx control; n = 7 mdx HOE-140, n = 6 mdx DALBK (PDF 223 kb)