Abstract
WNT1 inducible signaling pathway protein 1 (WISP-1/CCN4) is a novel adipokine, which is upregulated in obesity, and induces a pro-inflammatory response in macrophages in-vitro. Preclinical observations suggested WISP-1/CCN4 as a potential candidate for novel obesity therapy targeting adipose tissue inflammation. Whether circulating levels of WISP-1/CCN4 in humans are altered in obesity and/or type 2 diabetes (T2DM) and in the postprandial state, however, is unknown. This study assessed circulating WISP-1/CCN4 levels in a) paired liquid meal tests and hyperinsulinemic- euglycemic clamps (cohort I, n = 26), b) healthy individuals (cohort II, n = 207) and c) individuals with different stages of obesity and glucose tolerance (cohort III, n = 253). Circulating plasma and serum WISP-1/CCN4 concentrations were measured using a commercially available ELISA. WISP-1/CCN4 levels were not influenced by changes in insulin and/or glucose during the tests. In healthy individuals, WISP-1/CCN4 was detectable in 13% of plasma samples with the intraclass correlation coefficient of 0.93 (95% CI: 0.84–0.96) and in 58.1% of the serum samples in cohort III. Circulating WISP-1/CCN4 positively correlated with body mass index, body fat percentage, leptin and triglyceride levels, hip circumference and fatty liver index. No differences in WISP-1/CCN4 levels between individuals with normal glucose tolerance, impaired glucose tolerance and T2DM were found. The circulating concentrations of WISP-1/CCN4 showed no acute regulation in postprandial state and correlated with anthropometrical obesity markers and lipid profiles. In healthy individuals, WISP-1/CCN4 levels are more often below the detection limit. Thus, serum WISP-1/CCN4 levels may be used as a suitable biomarker of obesity.
Keywords: CCN proteins, Insulin resistance, Obesity, WISP-1/CCN4
Introduction
WNT-inducible signaling pathway protein-1 (WISP-1, also known as CCN4) belongs to the CCN family of extracellular matrix proteins and is a downstream target gene of the canonical WNT signaling pathway (Brigstock, 2003). CCN proteins stimulate mitosis, adhesion, apoptosis, extracellular matrix production, growth arrest and migration of multiple cell types. As a result, they play essential roles in cell proliferation, angiogenesis, cardiovascular and skeletal development, tumorigenesis and wound healing (Jun & Lau, 2011). Moreover, experimental evidence suggests that other CCN family members such as WISP-2/CCN5 and NOV/CCN3 participate in the pathogenesis of obesity and associated diseases (Gustafson et al., 2013; Pakradouni et al., 2013).
WISP-1/CCN4 shares structural features with the other CCN proteins in that it contains an N-terminal secretory signal peptide and four functional domains: (i) an insulin-like growth factor binding protein-like module (IGFBP); (ii) a von Willebrand factor type C repeat module (VWC); (iii) a trombospondin type-1 repeat module (TSP-1); and (iv) a cysteine-knot-containing module (CT) (Holbourn et al., 2008). The VWC module is important for protein-to-protein interactions, the TSP domain binds different targets, including collagen, fibronectin, CD36, TGF-beta and heparin, and the cysteine knot (CT) mediates dimerization and binding on Wnt signaling pathway receptors and also heparin (Holbourn et al., 2008; Leask & Abraham, 2006). In addition to the full-length 80-kDa WISP-1/CCN4 protein (Stephens et al., 2015), multiple alternative splice forms have been reported. In detail, WISP-1v lacks the VWC domain and is overexpressed in scirrhous gastric carcinomas (Tanaka et al., 2001). A second isoform (Perbal, 2009) was found in the human chondrosarcoma-derived chondrocytic cells and encodes a single IGFBP module in which eight authentic amino acids at the C-terminus were replaced by 14 other residues (Yanagita et al., 2007). Furthermore, in human hepatoma cells two shorter transcript of 943 bp and 750 bp (Perbal, 2009), respectively, and a variant containing only the signal sequence and cysteine knot motifs have been described (Stephens et al., 2015).
Under physiological conditions, WISP-1/CCN4 plays an important role in embryonic development, wound healing and tissue repair (Brigstock, 2003). Aberrant WISP-1/CCN4 expression is associated with various pathologies including osteoarthritis, fibrosis and cancer (Berschneider & Konigshoff, 2011; Gurbuz & Chiquet-Ehrismann, 2015; Blom et al., 2009; Zhong et al., 2017). Functionally, WISP-1/CCN4 has been shown to induce proliferation and drive epithelial to mesenchymal transition in alveolar epithelial cells whilst increasing the synthesis of extracellular matrix components (ECM) in fibroblasts (Blom et al., 2009). Nevertheless, despite the emerging evidence for a role for WISP-1/CCN4 in fibrosis, the biology of the protein remains poorly understood.
Since WISP-1/CCN4 is a matricellular protein, it may be sequestered in the extracellular matrix. Yet, WISP-1/CCN4 is found in the circulation (Stephens et al., 2015), but the mechanism via which WISP-1/CCN4 enters the circulation is still unclear. Because the shorter forms are mainly expressed in cell lines derived from carcinomas, it seems likely that 80-kDa homodimer is the main form found in the circulation (Stephens et al., 2015).
Importantly, we recently described WISP-1/CCN4 as an adipokine that induces a pro-inflammatory response in macrophages (Murahovschi et al., 2015a). Furthermore, WISP-1/CCN4 gene expression and protein production are up-regulated during human adipocyte differentiation in humans (Tanaka et al., 2001). Interestingly, in humans, WISP-1/CCN4 mRNA expression in adipose tissue and circulating WISP-1/CCN4 levels are downregulated by weight loss (Murahovschi et al., 2015a), and are increased in patients with gestational diabetes (Sahin Ersoy et al., 2016) and obese subjects with insulin resistance and radiological signs of visceral adipose tissue fibrosis (Barchetta et al., 2017).
The aims of this study were to assess the detectability of reliable blood measures of WISP-1/CCN4 in a population-based sample of healthy individuals and to evaluate its suitability as a circulating marker of obesity in individuals with different stages of glucose tolerance.
Materials and methods
Study cohorts
Circulating WISP-1/CCN4 was measured in samples collected from three independent cohorts of the Berlin-Brandenburg area in Germany. Studies protocols were approved by the Ethical Committee of Potsdam University, Charité Medical University of Berlin and the Ethical Committee of the Medical Association of the State of Brandenburg, Germany. Studies were carried out in accordance with the principles of the Declaration of Helsinki. All participants received written and oral information regarding the nature and potential risks and gave their informed consent before the start of studies.
Cohort I
26 subjects with different stages of glucose tolerance (five subjects with normal glucose tolerance (NGT), 11 subjects with impaired fasting glucose/impaired glucose tolerance (IFG/IGT), and 10 subjects with type 2 diabetes (T2DM)) were selected from our previously published study (ISRCTN40281673) (Rudovich et al., 2011). All study subjects underwent both 2-h hyperinsulinemic-euglycemic glucose clamps on two separated days after 10-h fasting (EC; capillary blood glucose at 5.5 mmol/l by variable infusion of 20% glucose (Serag Wiessner, Naila, Germany) and constant infusion of 100 mU ∙m2 body surface ∙ min−1 human insulin (Actrapid; Novo Nordisk, Bagsværd, Denmark)) and 4-h liquid meal challenges tests (LMCT; using a commercial liquid meal preparation (Biosorb Energie®; Pfrimmer Nutricia, Germany; 77.6 g carbohydrate, 22.3 g fat, 24 g protein, 600 kcal per 400 ml)). For this study, only serum samples (at 3rd freeze-thaw cycle) collected at baseline were used.
Cohort II
207 apparently healthy participants (124 women and 83 men) below the age of 64 years were randomly selected from the European Prospective Investigation of Cancer and Nutrition (EPIC) Potsdam cohort, which was designed to investigate the association between nutrition, cancer and other chronic diseases (Riboli et al., 2002). Participants were excluded, if they had a history of heart disease (myocardial infarction, heart failure, cardiomyopathy, stroke and angina pectoris), suffered from impaired mobility, used β-blockers and had systolic or diastolic blood pressure above 180 mmHg or 110 mmHg, respectively. Blood samples were collected on two occasions four months apart with the first blood collection in the period of October 2007 to March 2008 and the second between February and July 2008. Plasma samples (at 3rd freeze-thaw cycle) were collected on EDTA and stored at −80 °C.
Cohort III
253 subjects with different stages of glucose tolerance (51 subjects with NGT, 184 with IFG/IGT and 18 subjects with T2DM) were selected from two intervention studies (NCT: 00579657 and NCT: 01681173) and circulating WISP-1/CCN4 levels were assessed in serum samples (at 2nd or 3rd freeze-thaw cycle) obtained at baseline of each study. Body composition was determined by air displacement plethysmography (BOD POD®, COSMED, Italy). MRI and 1H–MRS were performed on a 1.5 T whole body scanner (Magnetom Avanto, Siemens Healthcare, Germany) for quantification of visceral fat depots (VAT) and intrahepatic lipids (IHL), respectively.
Analytical procedures
All venous blood samples were immediately centrifuged and frozen at −80 °C until analysis. Routine markers were measured in serum using ABX Pentra 400 (Horiba, Japan). Capillary blood glucose concentrations were measured using a glucose oxidase method on a Dr. Müller Super GL (Dr. Müller Glucose Analyzer, Germany). HbA1c was measured using a Hi-Auto A1C HA-8140 system (Menarini Diagnostics, Germany). Commercially available ELISA kits were used for the measurements of insulin (Insulin ELISA, Mercodia AB, Sweden) and leptin (Quantikine® Human Leptin Immunoassay, R&D Systems, Minneapolis, USA).
WISP-1/CCN4 levels were measured by human direct sandwich WISP-1/CCN4 DuoSet ELISA kit (DY1627; R&D Systems, Germany) in combination with bovine serum albumin (A7030, Sigma, Germany) or human serum albumin (A1887, Sigma, Germany) and performed on 96-well high-binding assay plates (82.1581, Sarstedt, Germany). The ELISA utilizes a monoclonal capture antibody and polyclonal detection antibody. According to the supplier’s information, the assay exhibited no cross-reactivity or interference with NOV/CCN3, the WISP-3/CCN6/Fc chimera, as well as biglycan and decorin. In each plate, the standard dilution series were used to generate a four parameter logistic (4-PL) curve fit and to calculate the sample concentration. The lower detection limit of this assay was defined as 15 pg/ml. The inter-assay coefficient of variation (CV) was 4.3% - 23.6% for different pooled samples, whereas the intra-assay CV was 9.5% - 17.6% for serum, and 6.9% - 17.4% for EDTA plasma, respectively.
Statistical analyses
All data are expressed as mean ± S.D. Statistical significance was defined as p < 0.05. Index of whole-body insulin resistance (HOMA-IR) was calculated as: fasting insulin [μU/ml] x fasting glucose in [mM] / 22.5. The Fatty Liver Index (FLI) was calculated from serum triglyceride, body mass index, waist circumference, and gamma-glutamyltransferase [FLI = (e 0.953*loge (triglycerides) + 0.139*BMI + 0.718*loge (ggt) + 0.053*waist circumference - 15.745) / (1 + e 0.953*loge (triglycerides) + 0.139*BMI + 0.718*loge (ggt) + 0.053*waist circumference - 15.745) * 100] (Bedogni et al., 2006) and was used as a noninvasive predictive index for liver fat content.
Gaussian distribution of the data was examined by the Kolmogorov–Smirnov test. Comparisons between two groups were tested by Student’s t test in the case of Gaussian-distributed data or Mann-Whitney U test in case of skewed data sets. Pearson’s coefficient or Spearman’s rank correlation coefficients were used for correlation analysis for Gaussian and skewed datasets, respectively. The repeated measures ANOVA was performed to determine changes in mean blood glucose, insulin and WISP-1/CCN4 level. The intraclass correlation coefficients (ICC-s) were used in the EPIC sub-cohort for assessment of the WISP-1/CCN4 reliability over a 4-month period. ICC-s were calculated as ratios of between-person variance and total variance (between person variance + within person variance). Following established cut points estimated reproducibility is rated as excellent (ICCs ≥0.75), good (ICC: 0.74–0.60), fair (ICC: 0.59–0.40) or poor (ICC < 0.40). Stepwise linear regression was performed to determine the dependence of WISP-1/CCN4 concentrations on markers of obesity. Statistical analyses were performed with SAS (Version 9.4, Enterprise Guide 6.1, SAS Institute Inc., Cary, NC, USA) and SPSS Statistics version 20.0 (IBM Corporation, USA).
Results
Assay validation
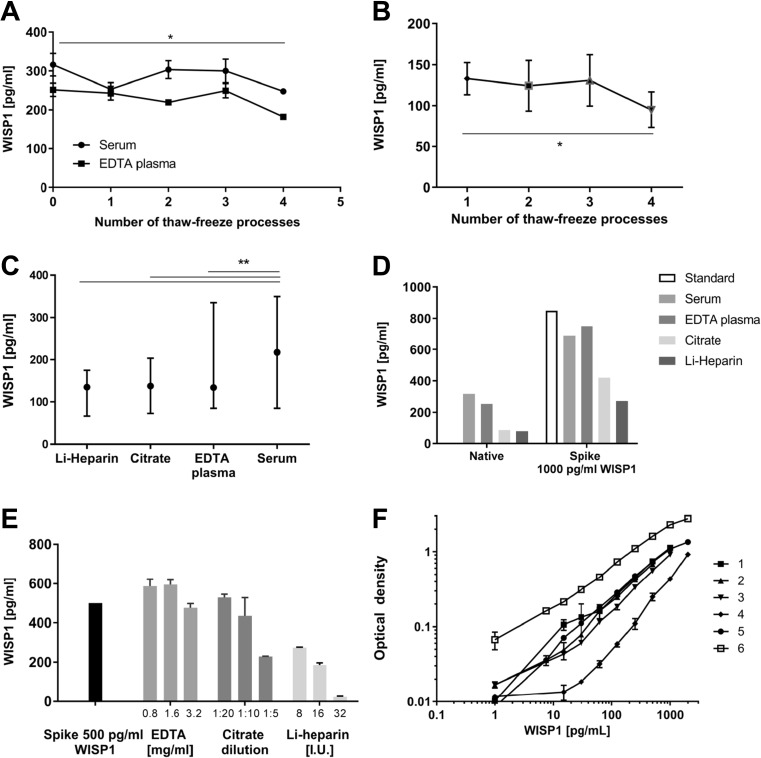
According to the supplier, the human WISP-1/CCN4 DuoSet ELISA (R&D Systems, Germany) is validated for analysis of WISP-1/CCN4 levels in cell culture supernatants, serum, and plasma samples. Nevertheless, we performed additional validation to ensure reliable results in the analysis of circulating WISP-1/CCN4 in human clinical studies. First, we investigated the stability of WISP-1/CCN4 protein during the storage and thawing process. For this, freshly collected and pooled serum and plasma samples from 9 overweight non-critically ill volunteers underwent four consecutive freeze-thaw cycles. In both types of samples, the measured WISP-1/CCN4 levels were not changed after three freeze-thaw cycles. After the 4th freeze-thaw cycle, we observed a reduction in the amount of WISP-1/CCN4 that was detected (p < 0.05, Figure 1a). Similar results were observed in a freeze-thaw experiment with pooled serum samples from 8 subjects from cohort III (Figure 1b).
Fig. 1.
Validation of WISP-1/CCN4 assay. WISP-1/CCN4 concentrations were measured (a) after multiple freeze-thaw cycles in fresh collected and pooled serum and plasma samples (n = 9); (b) after multiple freeze-thaw cycles in pooled serum samples from cohort III (n = 8); (c) in serum, EDTA plasma, citrate plasma and Li-heparin plasma samples at the same freeze-thaw cycle (n = 14); (d) in pooled serum and plasma samples spiked with 1000 pg/mL of recombinant human WISP-1/CCN4 (data shown as median ± interquartile range); (e) in reagent diluent samples with different concentrations of clotting inhibitors and 500 pg/mL of recombinant human WISP-1/CCN4. (f) Example figure of standard curves in the human WISP-1/CCN4 DuoSet ELISA kit (DY1627) assay. *p < 0.05, ** p < 0.01
We then evaluated whether the detection of WISP-1/CCN4 was similar in serum and plasma samples. Therefore, we used serum, EDTA plasma and citrate plasma samples from the same freeze-thaw cycle from 14 subjects from cohort III. As shown in Figure 1c, the highest WISP-1/CCN4 concentrations were found in the serum samples (p = 0.0001 vs. EDTA/citrate plasma and p = 0.01 vs. Li-heparin plasma).
We further performed a spike-and-recovery assessment in pooled serum and plasma samples spiked with 1000 pg/mL of recombinant human WISP-1/CCN4. We observed a recovery of 81.3% for serum, 88.3% for EDTA plasma, 49.4% for citrate plasma and 31.8% for Li-heparin (Figure 1d), respectively. These findings suggest that clothing inhibitors in plasma samples may impact on the detection of WISP-1/CCN4. To substantiate this, an additional analysis of samples spiked with 500 pg/ml of recombinant human WISP-1/CCN4 together with common concentrations of clotting inhibitors – 3.2 mg/mL, 1.6 mg/mL, 0.8 mg/mL EDTA (No: 02.1066, Sarstedt, Germany), 1:5, 1:10, 1:20 dilution citrate from the 32% stock solution (No. 02.1067, Sarstedt, Germany), 32 I.U., 16 I.U., 8 I.U. Li-heparin (No. 02.1065, Sarstedt, Germany) was conducted. We did not find differences between EDTA spiked (0.8–1.6 mg/dl) or citrate spiked samples (1:20–1:10) vs. control without clotting inhibitors. However, we observed decreasing WISP-1/CCN4 concentrations in samples spiked with Li-heparin, namely a dose-dependent reduction of WISP-1/CCN4 concentration of 45%, 63% and 95% in samples with 8 I.U., 16 I.U. and 32 I.U. Li-heparin (Figure 1e). Collectively, these data show that serum samples are preferred over plasma EDTA or citrate samples for optimal detection of circulating WISP-1/CCN4. Furthermore, the presence of heparin in plasma markedly hampered the detection of WISP-1/CCN4.
WISP-1/CCN4 reliability in healthy individuals
We assessed the WISP-1/CCN4 reliability in plasma samples obtained from healthy individuals (n = 207) within the EPIC-Potsdam cohort (cohort II, Table 1). In this sub-cohort, plasma concentrations of WISP-1/CCN4 were detectable in only 27 individuals (13 men and 14 women, 13% of the full sample). No correlation of plasma WISP-1/CCN4 levels with waist circumference was found in this cohort. There was a trend towards a difference in WISP-1/CCN4 concentrations according to sex, with male individuals having higher concentrations compared to females (155.5 ± 190.4 pg/ml vs. 55.0 ± 58.9 pg/ml, respectively, NS). The intraclass correlation coefficient used for assessment of the WISP-1/CCN4 reliability over a 4-month period was 0.93 (95% CI: 0.84–0.96) in all individuals, 0.94 (95% CI: 0.78–0.98) in men and 0.91 (95% CI: 0.70–0.97) in women.
Table 1.
Baseline characteristics of study participants
| Cohort I | Cohort II | Cohort III | |
|---|---|---|---|
| Total N | 26 | 207 | 253 |
| NGT/IFG + IGT/T2DM (N) | 5/11/10 | 207/0/0 | 51/184/18 |
| Sex (% male) | 55.6 | 40 | 36.8 |
| Age (years) | 59.1 ± 9.1 | 56 ± 4.2 | 58.5 ± 9.6 |
| Weight (kg) | 90.1 ± 14.7 | n.a. | 89.0 ± 16.6 |
| BMI (kg/m2) | 31.5 ± 4.9 | 26.5 ± 4.0 | 31.6 ± 5.0 |
| Waist circumference (cm) | 103.7 ± 10.9 | 93.0 ± 12.8 | 102.3 ± 12.4 |
| Hip circumference (cm) | 108.9 ± 11.7 | n.a. | 111.5 ± 10.9 |
| Waist-to-hip ratio | 0.90 ± 0.09 | n.a. | 0.92 ± 0.08 |
| HOMA-IR | 3.75 ± 6.28 | n.a. | 2.22 ± 1.22 |
| HbA1c (%) | 5.92 ± 0.69 | n.a. | 5.41 ± 0.43 |
| HDL (mmol/L) | 1.21 ± 0.28 | n.a. | 1.28 ± 0.30 |
| LDL (mmol/L) | 3.35 ± 1.04 | n.a. | 3.51 ± 0.85 |
| Triglycerides (mmol/L) | 1.88 ± 1.02 | n.a. | 1.42 ± 0.81 |
| Total cholesterol (mmol/L) | 5.46 ± 1.24 | n.a. | 5.43 ± 1.02 |
| ALT (U/L) | 38.9 ± 110.5 | n.a. | 27.3 ± 18.4 |
| Body fat (%) | 37.3 ± 8.3 | n.a. | 37.7 ± 8.2 |
| VAT (%) | n.a. | n.a. | 4.61 ± 2.05 |
| IHL (%) | 14.22 ± 10.37 | n.a. | 8.20 ± 8.36 |
| Fatty Liver Index (FLI) | n.a. | n.a. | 66.7 ± 26.8 |
Data are mean ± S.D.; n.a. = not available. Cohort I - 26 subjects with different stages of glucose tolerance, who underwent liquid meal test and euglycemic-hyperinsulinemic clamp; cohort II - European Prospective Investigation of Cancer and Nutrition (EPIC) Potsdam study; cohort III - subjects with different stages of obesity and glucose tolerance
Effect of acute insulin and glucose changes on circulating WISP-1/CCN4
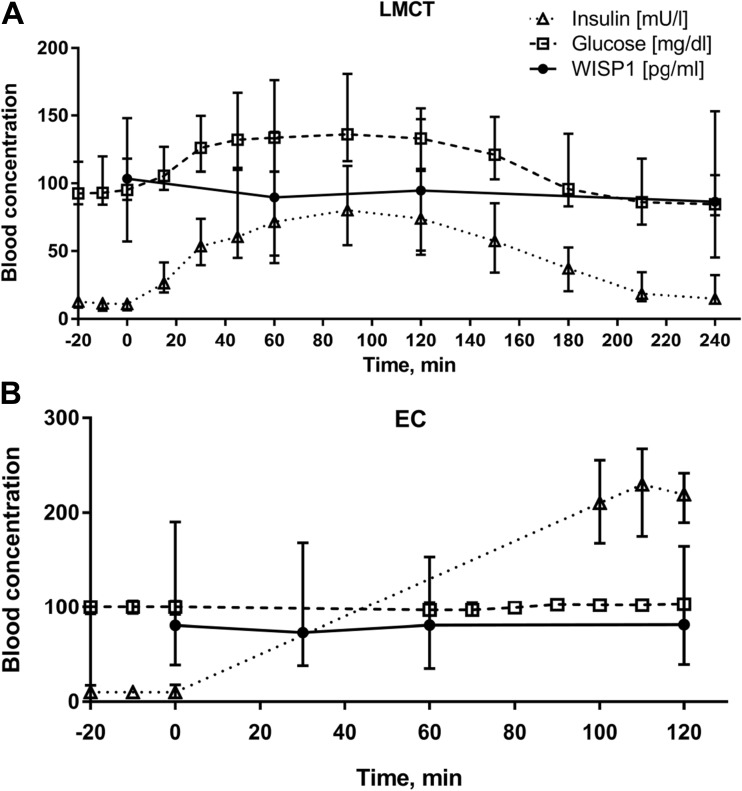
Because insulin treatment was found to increase WISP-1/CCN4 expression in adipocytes in-vitro (Murahovschi et al., 2015a), we next investigated whether acute changes in insulin and glucose levels affect circulating WISP-1/CCN4 in humans in vivo. For this, we assessed circulating serum WISP-1/CCN4 levels in 26 subjects (cohort I, Table 1) at the start and at several time points (60, 120, and 240 min) after ingestion of a standardized liquid meal. While blood glucose and insulin acutely increased after the test meal ingestion (p < 0.0001 for both), WISP-1/CCN4 levels were not affected (p = 0.16) (Fig. 2a). To investigate the effect of acute hyperinsulinemia on circulating WISP-1/CCN4, we also measured serum WISP-1/CCN4 levels at baseline and 30, 60, and 120 min after the start of the insulin infusion during a euglycemic-hyperinsulinemic clamp. Although WISP-1/CCN4 levels changed during the test (p < 0.01), regression analysis revealed no association (p = 0.216) with rising insulin levels (p = 0.01) (Fig. 2b).
Fig. 2.
Circulating serum WISP-1/CCN4 levels during (a) liquid meal challenge test (LMCT, n = 26) and (b) hyperinsulinemic euglycemic clamps (EC, n = 26). Median values with interquartile range (I50) for serum insulin, glucose and WISP-1/CCN4 are shown
Circulating WISP-1/CCN4 and markers of insulin resistance and obesity
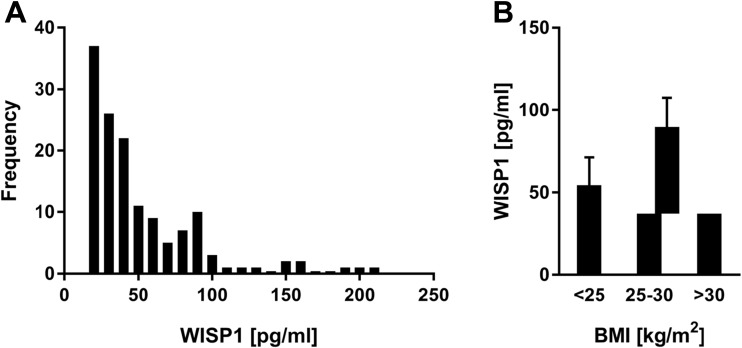
We further analysed circulating serum WISP-1/CCN4 levels in subjects with different stages of glucose tolerance including 51 subjects with NGT, 184 subjects with IFG/IGT and 18 subjects with T2DM according to WHO criteria for diabetes (WHO, 2006) (n = 253, cohort III, Table 1). In this cohort, circulating WISP-1/CCN4 levels were above the detection limit in 58.1% of subjects. In these subjects, we found that WISP-1/CCN4 was not normally distributed. We observed a left skewed distribution of WISP-1/CCN4 values, and 95% of all values were up to 249.42 pg/mL or lower (median = 38.5 pg/ml, I50 = 47.5 pg/ml) (Fig. 3a). In contrast to the EPIC sub-cohort, no gender differences in WISP-1/CCN4 levels were observed (79.0 ± 161.8 pg/ml vs. 79.5 ± 136.4 pg/ml for men and women, respectively, NS).
Fig. 3.
Circulating serum WISP-1/CCN4 levels in subjects of the cohort III. (a) Distribution diagram of WISP-1/CCN4 values in cohort III. Divided into classes of 10 pg/ml. (b) WISP-1/CCN4 levels in normal weight, overweight and obese subjects. Data are shown as mean ± SEM
Furthermore, we found weak correlations of serum WISP-1/CCN4 levels with BMI (n = 147; r = 0.19, p = 0.02), hip circumference (n = 147; r = 0.20, p = 0.017), body fat percentage (n = 140; r = 0.20, p = 0.025), body lean mass (n = 140; r = −0.20, p = 0.03), triglycerides (n = 147; r = 0.22, p = 0.007), FLI (n = 91; r = 0.28, p = 0.008), and circulating leptin (n = 96; r = 0.26, p = 0.01), but not with HbA1c, hepatic triglyeride content, visceral fat mass (both measured in MRI), HOMAIR, cholesterol, alanine transaminase and aspartate transaminase. In stepwise linear regression analysis, only BMI remained an independent parameter associated with WISP-1/CCN4 concentration, whereas other variables were excluded from the predictive model (Table 2). However, the model could explain only 5% (adjusted R2 of the model) of the WISP-1/CCN4 variability. Differences in the WISP-1/CCN4 levels between normal weight, overweight and obese subjects did not reach statistical significance (p = 0.123) (Fig. 3b). In addition, we analyzed differences in subjects with WISP-1/CCN4 concentrations under the detection limit to subjects with measurable WISP-1/CCN4 (Table 3). Total, LDL and HDL cholesterol are lower in subjects with undetectable WISP-1/CCN4 levels, but no differences in BMI and other anthropological markers of obesity were observed.
Table 2.
Stepwise linear regression analysis of association between WISP-1/CCN4 levels and metabolic parameters
| Beta-coefficient | p-value | Adjusted R2 for model | |
|---|---|---|---|
| BMI | 0.230 | 0.036 | 0.053 |
| Excluded variables | |||
| Hip circumference | −0.320 | 0.145 | |
| Body fat (%) | 0.064 | 0.633 | |
| Triglycerides | −0.038 | 0.726 | |
| FLI | −0.160 | 0.366 | |
Bold values relate to variable that remained independent predictors of WISP1/CCN4 levels
Abbreviations: FLI, Fatty Liver Index
Table 3.
Characteristics of subjects with WISP-1/CCN4 over and under detection limit
| WISP-1/CCN4 detectable | WISP-1/CCN4 not detectable | p-value | |
|---|---|---|---|
| Total N | 147 | 106 | |
| NGT/IFG + IGT/T2DM (N) | 29/108/10 | 22/76/8 | |
| Sex (% male) | 36.1 | 37.7 | |
| Age (years) | 58.8 ± 9.3 | 58.0 ± 10.0 | 0.527 |
| Weight (kg) | 88.4 ± 16.4 | 89.8 ± 16.8 | 0.513 |
| BMI (kg/m2) | 31.5 ± 5.0 | 31.8 ± 4.9 | 0.479 |
| Waist circumference (cm) | 102.3 ± 12.6 | 102.2 ± 12.1 | 0.937 |
| Hip circumference (cm) | 111.3 ± 11.3 | 111.8 ± 10.5 | 0.653 |
| Waist-to-hip ratio | 0.92 ± 0.08 | 0.92 ± 0.08 | 0.605 |
| HOMA-IR | 2.23 ± 1.21 | 2.21 ± 1.24 | 0.920 |
| HbA1c (%) | 5.44 ± 0.43 | 5.35 ± 0.44 | 0.098 |
| HDL (mmol/L) | 1.32 ± 0.29 | 1.23 ± 0.30 | 0.025 |
| LDL (mmol/L) | 3.60 ± 0.89 | 3.38 ± 0.78 | 0.041 |
| Triglycerides (mmol/L) | 1.44 ± 0.84 | 1.38 ± 0.75 | 0.606 |
| Total cholesterol (mmol/L) | 5.58 ± 1.09 | 5.23 ± 0.88 | 0.007 |
| ALT (U/L) | 26.4 ± 14.8 | 28.4 ± 22.4 | 0.513 |
| Body fat (%) | 37.8 ± 8.3 | 37.5 ± 8.1 | 0.765 |
| VAT (%) | 4.57 ± 1.96 | 4.68 ± 2.20 | 0.760 |
| IHL (%) | 8.81 ± 8.68 | 7.26 ± 7.84 | 0.310 |
| Fatty Liver Index (FLI) | 66.3 ± 26.5 | 67.4 ± 27.5 | 0.805 |
Subjects of cohort III were divided in two groups dependent on the serum WISP-1/CCN4 level – over and under detection limit (15 pg/ml). Data are mean ± S.D.; n.a. = not available
Finally, WISP-1/CCN4 concentrations were statistically not significantly different among individuals with NGT (257.3 ± 793.4 pg/ml) and IGT (104.8 ± 247.0 pg/ml) or T2DM (56.7 ± 30.2 pg/ml).
Discussion
In the present study, we characterized WISP-1/CCN4 as a stable circulating marker that is not affected by multiple freeze-thaw cycles and by acute insulin and glucose changes. WISP-1/CCN4 levels demonstrated a high reliability over a 4-month period and are associated with obesity markers such as BMI, body fat, triglycerides, hip circumference, fatty liver index and pro-inflammatory adipokines, such as circulating leptin.
In validation experiments, we found that serum samples are preferable to plasma EDTA or citrate samples for optimal detection of circulating WISP-1/CCN4, and that the presence of heparin in plasma markedly hampered the detection of WISP-1/CCN4. This may be ascribed to the binding of heparin to WISP-1/CCN4. Within the WISP-1/CCN4 protein, three domains, namely the VWC-, the TSP-1 and the CT-domain can theoretically bind to heparin (Holbourn et al., 2008; Leask & Abraham, 2006). Moreover, we observed lower WISP-1/CCN4 concentration in plasma samples compared to serum samples, suggesting that plasma factors as well as extracellular proteins and glycosaminoglycanes, may interfere with WISP-1/CCN4 detection.
To the best of our knowledge, this is the first study to evaluate the methodological utility of measuring WISP-1/CCN4 concentrations in apparently healthy populations. Using data from a large validation sub-study within the EPIC cohort, we found that assessing WISP-1/CCN4 levels in healthy individuals is methodologically challenging due to the high number of samples with WISP-1/CCN4 concentrations below the detection limit. Compared to the study sample of pre-diabetic individuals where approximately half of the WISP-1/CCN4 concentrations were detectable, in the healthy EPIC participants we were able to detect only 13% of the measurements. This finding implicates an important aspect of WISP-1/CCN4 biology and indicates that caution should be paid when planning future research studies taking into account the methodological challenges in measuring WISP-1/CCN4 concentrations in metabolically non-compromised individuals. A higher percentage of samples with WISP-1/CCN4 levels over the assay detection limit was observed in cohort III which included metabolically afflicted subjects compared with the EPIC sub-cohort of metabolically healthy individuals. However, it should be noted that WISP-1/CCN4 concentration measured in serum samples is higher than in plasma samples of the same subjects. Plasma samples were analyzed in cohort II, and serum samples in cohort III; therefore, we cannot exclude an additional effect of this phenomenon on the outcome of the WISP-1/CCN4 measurements.
Although WISP-1/CCN4 expression was upregulated by the insulin treatment in vitro (Murahovschi et al., 2015a), we did not observe this effect in the euglycemic-hyperinsulinemic clamp test in humans. This could be explained by the relatively short period of hyperinsulinemia in vivo together with relatively low insulin concentration compared with in vitro experiments.
We further assume that long-term elevated glucose levels accompanying the insulin resistance state and contributing to the low-grade inflammation in adipose tissue could have an influence on the WISP-1/CCN4 production in the body. In accordance with this hypothesis, the data of the very recently published study of Barchetta et al. (Barchetta et al., 2017) showed a marked association between WISP-1/CCN4 levels and increased IL-8 levels, reduced adiponectin levels, and radiological signs of visceral adipose tissue fibrosis. In line with our data, the authors found no association with type 2 diabetes. We also found no differences in WISP-1/CCN4 concentrations between individuals with NGT and T2DM. This observation may be explained by good therapeutic glycemic control of subjects with diabetes in our cohort. The previously reported increased WISP-1/CCN4 levels in gestational diabetes (Sahin Ersoy et al., 2016) may reflect increased insulin resistance in these patients and is contrary to our observation. Interestingly, women with gestational diabetes have mild hyperglycemia and pathophysiologically placental hormone dependent increase in insulin resistance (Catalano et al., 2003) and altered balance between pro- and anti-inflammatory cytokines with triggering adipose tissue inflammation (Abell et al., 2015) are two major contributor to the disease progress. Taken together, the perturbation in the tissue inflammation seems to be superior to the hyperglycemic state when it comes to an increase in circulating WISP-1/CCN4 levels. Thus, it remains to be investigated whether pro-inflammatory cytokines could be held responsible for the increase in WISP-1/CCN4 levels in gestational diabetes.
In our study, WISP-1/CCN4 levels correlated positively with adiposity-associated metabolic factors such as BMI, body fat, triglycerides, hip circumference, fatty liver index and leptin. Similar associations between WISP-1/CCN4 and BMI were observed in other studies (Sahin Ersoy et al., 2016; Barchetta et al., 2017). In the stepwise multivariate regression, only BMI remained an independent parameter associated with WISP-1/CCN4 level, whereas other variables were excluded from the predictive model. Nevertheless, BMI explained just a small proportion (5%) of WISP-1/CCN4 variability and only a borderline statistical difference was observed in WISP-1/CCN4 concentrations between obese and non-obese subjects. Interestingly, subjects with WISP-1/CCN4 concentrations above the detection limit of the assay showed higher cholesterol levels with similar BMI and other obesity markers in our study. Moreover, gene expression of low-density lipoprotein receptor-related protein 5 (LRP5) and LRP6 was increased after six weeks of high fat diet increased expression in our pilot study (Murahovschi et al., 2015b). Other members of the CCN family, such, as NOV3/CCN3, showed association with circulating cholesterol or LDL cholesterol (Pakradouni et al., 2013), and the overexpression of NOV3/CCN3 inhibits inflammation and progression of atherosclerosis in animal models (Liu et al., 2014). In accordance with our data, Barchetta et al. reports circulating IL-8 as the main determinant of increased WISP-1/CCN4 and no association between WISP-1/CCN4 and classical parameters of metabolic syndrome such as blood pressure, fasting blood glucose, age and blood lipids (Barchetta et al., 2017). Interestingly, we observed the strong positive correlation between circulating leptin and WISP-1/CCN4. Leptin, an adipose tissue derived cytokine, hormone and satiety factor, regulates body weight by suppressing appetite and stimulating energy expenditure and modulates a wide range of immune and inflammatory processes including adipose tissue inflammation and reorganization (Zhou et al., 2015). Therefore, one may speculate that an increase in WISP-1/CCN4, under conditions of adipose tissue inflammation, may, together with leptin, induce adipose tissue remodelling and strengthen local fibrosis. Thus, other obesity-associated factors beyond BMI, such as adipose tissue fibrosis markers or circulating cytokines, should be examined in further studies as potential causal factors of circulating WISP-1/CCN4.
WISP-1/CCN4 was overexpressed in visceral adipose tissue in our previously published study (Murahovschi et al., 2015a). In this study we observed no correlation between visceral fat content, measured with MRI technology, and circulating WISP-1/CCN4 levels. Similarly, data from Barchetta et al. showed no correlation with visceral fat content but a significant association with the homogeneity of visceral fat in MRI, as a radiological marker of visceral adipose tissue inflammation (Barchetta et al., 2017), suggesting that the quality of visceral adipose tissue influences WISP-1/CCN4 production in the body.
Nonalcoholic fatty liver disease (NAFLD) is strongly associated with visceral obesity and diabetes. Circulating WISP-1/CCN4 was positively correlated with a surrogate index of liver fat content but not with liver fat content itself. This is in part contrary to previously published studies showing no association of hepatic WISP-1/CCN4 expression with liver fat content (Murahovschi et al., 2015a).
Our study has several strengths: we evaluated WISP-1/CCN4 in various populations covering healthy and pre-diseased population groups. The study populations were reasonably large and included both sexes in acceptable proportions. However, the study population we used does not allow for generalization of our findings in other ethnic groups. Future studies should take into account potential differences in biomarker assays, laboratories, plasma sample types, storage time and repeated samplings.
Nevertheless, several technical limitations should be also mentioned. Although the assay exhibited no cross-reactivity or interference with NOV/CCN3 and WISP3/CCN6, no other CCN family members were tested according to the manufacturer’s information. Furthermore, multiple variants for WISP-1/CCN4 have been reported (Tanaka et al., 2001; Perbal, 2009; Yanagita et al., 2007). Since the expression of these variants seems to be confined to carcinoma, it seems likely the main circulating form of WISP-1/CCN4 is the full-length protein. Nevertheless, interactions with circulating forms of these splice variants cannot be completely excluded.
Taken together, this study has identified WISP-1/CCN4 as a protein secreted in significant amounts in overweight or adipose subjects, independent of glucose intolerance. In healthy individuals, a high number of subjects with WISP-1/CCN4 concentrations below the detection limit were observed. This finding may possibly implicate WISP-1/CCN4 as an early diagnostic rather than pre-diagnostic marker. BMI explained a small part of WISP-1/CCN4 variability and major determinants of WISP-1/CCN4 concentrations remain obscure. However, the diagnostic and therapeutic potential of WISP-1/CCN4 has to be evaluated in future studies.
Acknowledgements
We thank all study participants for their cooperation. We thank the Human Study Centre at the DIfE for data collection and biological sample logistics. We express thanks to Dr. Manuela Bergmann for her contribution by leading the underlying processes of data generation, as well as to Silke Navia Fruth and Herbert Piechot for their valuable assistance with biosamples management. Particular thanks are given to the EPIC Potsdam data manager - Ellen Kohlsdorf. We gratefully acknowledge the excellent technical assistance of Katrin Sprengel, and Tanja Ahrens. We thank Stephanie Sucher for her extensive advice regarding nutritional counselling and June Inderthal for reading and correcting the manuscript.
Abbreviations
- ALT
Alanine transaminase
- CCN
CTGF/Cyr61/Nov) family proteins
- EPIC
European Prospective Investigation into Cancer and Nutrition
- FLI
Fatty Liver Index
- HOMA-IR
Homeostatic model assessment insulin resistance
- HEC
Hyperinsulinemic euglycemic clamp
- IFG
Impaired fasting glucose
- IGT
Impaired glucose tolerance
- IHL
Intrahepatic liver fat
- LMCT
Liquid meal challenges tests
- NGT
Normal glucose tolerance
- T2DM
Type 2 diabetes mellitus
- VAT
Visceral adipose tissue
- WHR
Waist-to-hip ratio
- WISP-1/CCN4
WNT-inducible signaling pathway protein-1
Author contribution
Study concept and design: AFHP, OP, and NR. Acquisition of data: CT, KA, TH, MK, SH, CG and SK. Analysis and interpretation of data: CT, KA, DMO, VM, MM, CG, MR, DMO, TH, MOW, HB, AFHP, OP, and NR. Drafting of the manuscript: CT, AFHP, OP, and NR. Critical revision of the manuscript: CT, KA, MR, VM, MM, MK, SH, UK, CH, SK, TH, DMO, MOW, HB, AFHP, OP and NR. Obtained funding: DMO and NR. All authors contributed to and approved the final version of the manuscript. AFHP, OP, and NR are the guarantors of this work and as such had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Funding
This work was financially supported by grant from German Diabetes Center (DZD) (NR, DMO) and grant of European Foundation for Study of Diabetes (EFSD) (NR, DMO). The funding source had no role in study design, data collection, analysis or interpretation, report writing, or the decision to submit this paper for publication.
Compliance with ethical standards
Duality of interest
The authors declare that there is no duality of interest associated with this manuscript.
Conflict of interest
The authors declare no conflict of interest.
Footnotes
Olga Pivovarova and Natalia Rudovich contributed equally to this work.
Some of the data were presented as abstracts at the D·A·CH meeting 2015 and at the European Association for the Study of Diabetes annual meeting in 2016.
References
- Abell SK, De Courten B, Boyle JA, Teede HJ. Inflammatory and Other Biomarkers: Role in Pathophysiology and Prediction of Gestational Diabetes Mellitus. Int J Mol Sci. 2015;16:13442–13473. doi: 10.3390/ijms160613442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barchetta I, Cimini FA, Capoccia D, De Gioannis R, Porzia A, Mainiero F, Di Martino M, Bertoccini L, De Bernardinis M, Leonetti F, et al. WISP1 Is a Marker of Systemic and Adipose Tissue Inflammation in Dysmetabolic Subjects With or Without Type 2 Diabetes. J Endocrine Society. 2017;1:660–670. doi: 10.1210/js.2017-00108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedogni G, Bellentani S, Miglioli L, Masutti F, Passalacqua M, Castiglione A, Tiribelli C. The Fatty Liver Index: a simple and accurate predictor of hepatic steatosis in the general population. BMC Gastroenterol. 2006;6:33. doi: 10.1186/1471-230X-6-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berschneider B, Konigshoff M. WNT1 inducible signaling pathway protein 1 (WISP1): a novel mediator linking development and disease. Int J Biochem Cell Biol. 2011;43:306–309. doi: 10.1016/j.biocel.2010.11.013. [DOI] [PubMed] [Google Scholar]
- Blom AB, Brockbank SM, van Lent PL, van Beuningen HM, Geurts J, Takahashi N, van der Kraan PM, van de Loo FA, Schreurs BW, Clements K, et al. Involvement of the Wnt signaling pathway in experimental and human osteoarthritis: prominent role of Wnt-induced signaling protein 1. Arthritis Rheum. 2009;60:501–512. doi: 10.1002/art.24247. [DOI] [PubMed] [Google Scholar]
- Brigstock DR. The CCN family: a new stimulus package. J Endocrinol. 2003;178:169–175. doi: 10.1677/joe.0.1780169. [DOI] [PubMed] [Google Scholar]
- Catalano PM, Kirwan JP, Haugel-de Mouzon S, King J. Gestational diabetes and insulin resistance: role in short- and long-term implications for mother and fetus. J Nutr. 2003;133:1674S–1683S. doi: 10.1093/jn/133.5.1674S. [DOI] [PubMed] [Google Scholar]
- Gurbuz I, Chiquet-Ehrismann R. CCN4/WISP1 (WNT1 inducible signaling pathway protein 1): a focus on its role in cancer. Int J Biochem Cell Biol. 2015;62:142–146. doi: 10.1016/j.biocel.2015.03.007. [DOI] [PubMed] [Google Scholar]
- Gustafson B, Hammarstedt A, Hedjazifar S, Smith U. Restricted adipogenesis in hypertrophic obesity: the role of WISP2, WNT, and BMP4. Diabetes. 2013;62:2997–3004. doi: 10.2337/db13-0473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holbourn KP, Acharya KR, Perbal B. The CCN family of proteins: structure-function relationships. Trends Biochem Sci. 2008;33:461–473. doi: 10.1016/j.tibs.2008.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jun JI, Lau LF. Taking aim at the extracellular matrix: CCN proteins as emerging therapeutic targets. Nat Rev Drug Discov. 2011;10:945–963. doi: 10.1038/nrd3599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leask A, Abraham DJ. All in the CCN family: essential matricellular signaling modulators emerge from the bunker. J Cell Sci. 2006;119:4803–4810. doi: 10.1242/jcs.03270. [DOI] [PubMed] [Google Scholar]
- Liu J, Ren Y, Kang L, Zhang L. Overexpression of CCN3 inhibits inflammation and progression of atherosclerosis in apolipoprotein E-deficient mice. PLoS One. 2014;9:e94912. doi: 10.1371/journal.pone.0094912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murahovschi V, Pivovarova O, Ilkavets I, Dmitrieva RM, Docke S, Keyhani-Nejad F, Gogebakan O, Osterhoff M, Kemper M, Hornemann S, et al. WISP1 Is a Novel Adipokine Linked to Inflammation in Obesity. Diabetes. 2015;64:856–866. doi: 10.2337/db14-0444. [DOI] [PubMed] [Google Scholar]
- Murahovschi VTC, Pivovarova O, Kruse M, Seltmann AC, Kemper M, Hornemann S, Pfeiffer AFH, Rudovich N. Regulation of WNT signaling receptors and co-receptors by high fat diet in humans. Exp Clin Endocrinol Diabetes. 2015;123:P08_02. [Google Scholar]
- Pakradouni J, Le Goff W, Calmel C, Antoine B, Villard E, Frisdal E, Abifadel M, Tordjman J, Poitou C, Bonnefont-Rousselot D, et al. Plasma NOV/CCN3 levels are closely associated with obesity in patients with metabolic disorders. PLoS One. 2013;8:e66788. doi: 10.1371/journal.pone.0066788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perbal B. Alternative splicing of CCN mRNAs .... it has been upon us. J Cell Commun Signal. 2009;3:153–157. doi: 10.1007/s12079-009-0051-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riboli E, Hunt KJ, Slimani N, Ferrari P, Norat T, Fahey M, Charrondiere UR, Hemon B, Casagrande C, Vignat J, et al. European Prospective Investigation into Cancer and Nutrition (EPIC): study populations and data collection. Public Health Nutr. 2002;5:1113–1124. doi: 10.1079/PHN2002394. [DOI] [PubMed] [Google Scholar]
- Rudovich NN, Weickert MO, Pivovarova O, Bernigau W, Pfeiffer AF. Effects of acarbose treatment on markers of insulin sensitivity and systemic inflammation. Diabetes Technol Ther. 2011;13:615–623. doi: 10.1089/dia.2010.0235. [DOI] [PubMed] [Google Scholar]
- Sahin Ersoy G, Altun Ensari T, Subas S, Giray B, Simsek EE, Cevik O. WISP1 is a novel adipokine linked to metabolic parameters in gestational diabetes mellitus. J Matern Fetal Neonatal Med. 2016;30:1–5. doi: 10.1080/14767058.2016.1192118. [DOI] [PubMed] [Google Scholar]
- Stephens S, Palmer J, Konstantinova I, Pearce A, Jarai G, Day E. A functional analysis of Wnt inducible signalling pathway protein −1 (WISP-1/CCN4) J Cell Commun Signal. 2015;9:63–72. doi: 10.1007/s12079-015-0267-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka S, Sugimachi K, Saeki H, Kinoshita J, Ohga T, Shimada M, Maehara Y, Sugimachi K. A novel variant of WISP1 lacking a Von Willebrand type C module overexpressed in scirrhous gastric carcinoma. Oncogene. 2001;20:5525–5532. doi: 10.1038/sj.onc.1204723. [DOI] [PubMed] [Google Scholar]
- WHO . Definition and Diagnosis of Diabetes Mellitus and Intermediate Hyperglycemia. Geneva: World Health Organization; 2006. pp. 1–46. [Google Scholar]
- Yanagita T, Kubota S, Kawaki H, Kawata K, Kondo S, Takano-Yamamoto T, Tanaka S, Takigawa M. Expression and physiological role of CCN4/Wnt-induced secreted protein 1 mRNA splicing variants in chondrocytes. FEBS J. 2007;274:1655–1665. doi: 10.1111/j.1742-4658.2007.05709.x. [DOI] [PubMed] [Google Scholar]
- Zhong X, Tu YJ, Li Y, Zhang P, Wang W, Chen SS, Li L, Chung AC, Lan HY, Chen HY, et al. Serum levels of WNT1-inducible signaling pathway protein-1 (WISP-1): a noninvasive biomarker of renal fibrosis in subjects with chronic kidney disease. Am J Transl Res. 2017;9:2920–2932. [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Yu X, Chen H, Sjoberg S, Roux J, Zhang L, Ivoulsou AH, Bensaid F, Liu CL, Liu J, et al. Leptin Deficiency Shifts Mast Cells toward Anti-Inflammatory Actions and Protects Mice from Obesity and Diabetes by Polarizing M2 Macrophages. Cell Metab. 2015;22:1045–1058. doi: 10.1016/j.cmet.2015.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]