Abstract
RAS effector signaling instead of being simple, unidirectional and linear cascade, is actually recognized as highly complex and dynamic signaling network. RAF-MEK-ERK cascade, being at the center of complex signaling network, links to multiple scaffold proteins through feed forward and feedback mechanisms and dynamically regulate tumor initiation and progression. Three isoforms of Ras harbor mutations in a cell and tissue specific manner. Besides mutations, their epigenetic silencing also attributes them to exhibit oncogenic activities. Recent evidences support the functions of RAS oncoproteins in the acquisition of tumor cells with Epithelial-to-mesenchymal transition (EMT) features/ epithelial plasticity, enhanced metastatic potential and poor patient survival. Google Scholar electronic databases and PubMed were searched for original papers and reviews available till date to collect information on stimulation of EMT core inducers in a Ras driven cancer and their regulation in metastatic spread. Improved understanding of the mechanistic basis of regulatory interactions of microRNAs (miRs) and EMT by reprogramming the expression of targets in Ras activated cancer, may help in designing effective anticancer therapies. Apparent lack of adverse events associated with the delivery of miRs and tissue response make ‘drug target miRNA’ an ideal therapeutic tool to achieve progression free clinical response.
Keywords: Epithelial-to-mesenchymal transition (EMT), EMT-activating transcription factors, MicroRNAs, RAS activation and effector proteins, RAS signaling
Introduction
RAS proteins are monomeric membrane localized GDP/GTP binding (G) proteins and serve as molecular switch that link receptor and non-receptor tyrosine kinase activation to downstream targets through RAS dependent signaling. External stimuli including growth factors, hormones, cytokines bind with receptor tyrosine kinases (RTKs) and command the cell through RAS signaling and its crosstalk with intracellular signaling cascades (McKay and Morrison 2007; Rozengurt 2007). Some of its essential functions include progression of cell through cell cycle, promotion of angiogenesis and regulation of cell morphology, differentiation, cellular adhesion, cell invasion and migration (Oktay et al. 1999).
About 30% human cancers are known to harbor mutations in Ras protooncogene. Mutationally activated Ras genes were first detected in human cancers in 1982 (Cox and Der 2010; Atreya et al. 2015). Besides R-Ras (Related rat sarcoma viral oncogene homolog) and M-Ras (Muscle rat sarcoma viral oncogene homolog) genes, three potential oncogenes namely H-Ras (Harvey rat sarcoma viral oncogene homolog), N-Ras (Neuroblastoma rat sarcoma viral oncogene homolog) and K-Ras (Kirsten rat sarcoma viral oncogene homolog) code for highly related protein products namely H-Ras, N-Ras and K-Ras (two protein isoforms: K-RasA and K-RasB) respectively (Varras et al. 1996). Oncogenic functions of mutated Ras genes are implicated in their constitutive activity, stimulation of cell proliferation, inhibition of apoptosis and tumorigeneisis (Capon et al. 1983). Predominant occurrence of specific point mutations has been identified in K-Ras in colon and pancreatic cancer, H-Ras in cancer of urinary tract and bladder, and N-Ras in leukemia (Cox et al. 2014). Presence of mutations in all the three genes in thyroid carcinomas further suggests that their tumorigenic functions are highly tissue and tumor dependent. Analysis of 1168 primary K-Ras-mutant tumors across 30 different cancer types identifies the significance of relative expression of mutant and wild type (WT) K-Ras in tumorigenic and therapeutic responses (Tothova and Ebert 2017). Amplification of mutant Ras confers cells a clonal advantage compared to cells with WT RAS allele which makes it a frequent event in cancer development (Burgess et al. 2017).
Studies have established a link between Ras and Epithelial-to-mesenchymal transition (EMT) through the activation of extracellular inducers; EMT-activating transcription factors (EMT-ATFs); and its downstream effectors. EMT confers tumor cells with enhanced cellular plasticity and it is characterized by the bidirectional conversions of tumor cells with epithelial (E), mesenchymal (M) and hybrid E/M phenotype (Garg 2017). The conserved process of EMT is defined by the loss of cell-cell adhesions, increased migratory abilities and invasiveness (Qi et al. 2014). Aberrant activation of EMT renders tumor cells with enhanced metastatic potential. It leads to the acquisition of therapeutic resistance and imposes significant clinical challenges in the treatment of cancer.
Non coding conserved RNAs/microRNAs (miRs) are the master regulators of many pathological and physiological processes including EMT. MiRs form mutually inhibitory feedback loop with EMT inducers/ ATFs and a crosstalk between distinct signaling cascades (Garg 2015). RAS mediated RAF/MEK/ERK pathway is emerged as a potential inducer of EMT. It stimulates the nuclear expression of EMT inducers and reprograms the expression of genes that are involved in cell-cell adhesion, cytoskeletal arrangement, invasion and migration. The current paper focuses on the molecular basis of RAS mediated signaling, its crosstalk with intracellular cascades, stimulation of EMT program and its contribution in tumor development. This comprehensive review provides insights towards localizing the miR based potential therapeutic approaches which might help in designing new anti-cancer drugs to RAS mediated acquisition of EMT and to prevent cancer metastasis and its relapse.
Ras oncoproteins in human cancer
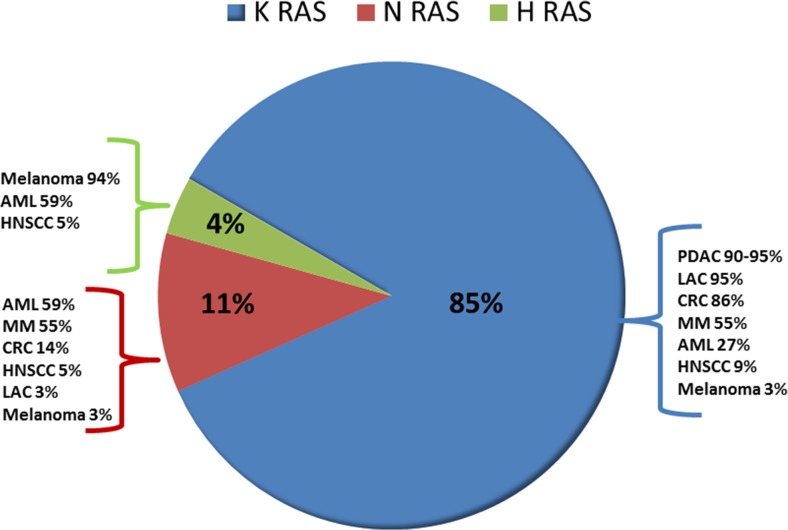
Number of studies confirms the oncogenic functions of mutant Ras gene. Rodent fibroblast transfected with mutant Ras gene and genetically engineered mouse models harboring mutant Ras gene exhibit increased incidences of cancer progression. Withdrawal of Ras expression using RNA interference (RNAi) both in vivo and in vitro leads to regression of tumor (Brummelkamp et al. 2002; Lim and Counter 2005; Singh et al. 2009). This increases the therapeutic usefulness of mutant Ras in advanced cancers. High frequency of mutations in Ras genes in humans is identified in a wide variety of tissue specific cancer types (Fig. 1 and Table 1). Missense mutations at codon 12, 13, 59 and 61 in cancer associated Ras genes abolish GAP-induced GTP hydrolysis of Ras proteins, make it constitutively active and stimulate cellular proliferation. Activated K-Ras oncogene has been examined to modulate tumor angiogenesis/ neovascularization in chronic pancreatitis (CP) and pancreatic adenocarcinoma (PAC) (Banerjee et al. 2000). More than 99% of the early stage and grade pancreatic intraepithelial neoplasms, the most common precursors of pancreatic ductal adenocarcinoma (PDAC) harbor mutations in K-Ras, p16/CDKN2A, GNAS, or BRAF (Kanda et al. 2012; Cancer Genome Atlas Research Network. Electronic address: andrew_aguirre@dfci.harvard.edu; Cancer Genome Atlas Research Network 2017). Since the recent researches associate Ras activation with the cell migration and increased invasion properties, therefore, it is essential to examine molecular functions of Ras oncogenes and its downstream effects (Chen et al. 2016; Lu et al. 2016; Saitoh et al. 2016; Krebs et al. 2017).
Fig. 1.
Frequency distribution of K-Ras, H-Ras and N-Ras mutations in human cancer. Among Ras-driven cancers, 86% of mutations observed in K-Ras isoform followed by 11% in N-Ras and 3% in H-Ras. Ras gene mutations are detected in percent frequencies among different Ras isoforms in different human cancers
Table 1.
Relative frequency of specific missense mutations in RAS genes in organ specific human cancer types
| RAS genes | Missense mutation (s) | Relative frequency of missense mutation (s) | Affected organs with cancer | References |
|---|---|---|---|---|
| K RAS | G12 | 83% | Pancreas, Lung, Colorectal Upper aero, Urinary tract, Skin, Prostate, Thyroid, Lymphoid |
Prior et al. 2012; Ouerhani et al. 2013 |
| G13 | 14% | Lymphoid, Thyroid, Prostate Colorectal, Upper aerodigestive tract, Urinary tract, Lung, Skin, Pancreas |
Cox et al. 2014; Hobbs et al. 2016 | |
| Q61 | 2% | Skin,Thyroid, Urinary tract Upper aerodigestive tract, Lymphoma |
Hobbs et al. 2016 | |
| G15, K16, G18, L19, D30,T20,Q22 E31, K117, A146, R164, D173 | 1% | Colorectal, Adrenal, Blood | Lin et al. 1998; Miyakura et al. 2002; Akagi et al. 2007; Smith et al. 2010; Naguib et al. 2011 | |
| H RAS | G12 | 33% | Colorectal, Upper aerodigestive tract, Urinary Tract, Skin,Thyroid, Lung, Prostate | Prior et al. 2012; Ouerhani et al. 2013 |
| G13 | 27% | Lymphoid, Urinary tract, lung, Prostate, Thyroid, Upper aerodigestive tract, Skin | Cox et al. 2014; Hobbs et al. 2016 | |
| Q61 | 37% | Hemato/Lymphoid, Prostate, Lung, Skin, Thyroid, Urinary tract, Upper aero | Hobbs et al. 2016 | |
| K117, A146 | 3% | Adrenal gland, Head and neck, Skeletal muscles, Urinary tract, Pituitary | Lin et al. 1998; Miyakura et al. 2002; Akagi et al. 2007; Smith et al. 2010; Naguib et al. 2011 | |
| N RAS | G12 | 27% | Urinary tract, Prostate, Haemato/Lymphoid, Colorectal, Upper aerodigestive tract, Skin, Thyroid | Prior et al. 2012; Ouerhani et al. 2013 |
| G13 | 12% | Haemato/Lymphoid, Lung, Pancreas, Colorectal, Skin | Cox et al. 2014; Hobbs et al. 2016 | |
| Q61 | 63% | Thyroid, Upper aero, Skin, Lung, Pancreas, Colorectal, Haemato/lymphoid, Prostate, Urinary tract | Hobbs et al. 2016 |
Prenylation and processing of RAS protein
Each RAS protein is a 190 amino acid long and is highly conserved at N and C termini. However, different functions assigned to RAS proteins could be due to differences in 5 amino acid long hypervariable domain near C-terminus. Prenylation facilitates the interaction of membrane with small hydrophilic protein like RAS which is involved in cell signaling. Transfer of farnesyl group from farnesyl-pyrophosphate (FPP) to terminal cysteine in the motif CAAX [C = cysteine; A = aliphatic amino acid (Lysine, Valine, Isoleucine); X = Glutamine, Leucine, Serine, Methionine] located at carboxyl end of Ras proteins is catalyzed by farnesyl transferase (FT) (Cox et al. 2014). Depending upon ‘X’, geranylgeranyl transferase type 1 (GGT-I) catalyzes the transfer of geranylgeranyl group to the proteins from geranylgeranyl pyrophosphate (GGPP). This is followed by the cleavage of ‘AAX’ by specific endoprotease in endoplasmic reticulum and methylation of exposed carboxyl group by prenyl protein specific methyl transferase (Clarke 1992). Transfer of geranylgeranyl groups from GGPP by geranylgeranyl transferase type 2 (GGT-II) to the proteins containing CXC motif (members of RAB family) has been examined. Farnesylated/ geranylgeranylated proteins undergo palmitoylation on –SH group of penultimate cysteine residue and is the only reversible event for H-RAS, N-RAS and K-RAS proteins containing CAAX motif. This increases the affinity of RAS proteins with the cell membrane (Fig. 2a). Palmitoylation further increases affinity of K-RasB with membrane due to presence of lysine rich sequences which can electrostatically interact with acidic phospholipids on inner membrane surface (Kato et al. 1992). Local lipid composition within the membrane influences the differential activation of RAS effectors (Zhou et al. 2017). Growth of H-Ras driven tumors was shown to be blocked by farnesyl transferase inhibitors (FTIs) in preclinical mouse studies. Two FTIs (lonafarnib and tipifarnib) failed to show any antitumor activity in phase III clinical trials in K-Ras and N-Ras driven pancreatic and colon cancers. The reason for this failure could be alternative prenylation where K-RAS and N-RAS proteins are catalyzed by the related prenyl transferase, geranylgeranyl transferase I (GGTase I) (Whyte et al. 1997).
Fig. 2.
a Prenylation and processing of RAS proteins. The three Ras genes, K-Ras, H-Ras and N-Ras encode 188–190 amino acids and share 82% - 90% overall sequence identity. These proteins undergo farnesylation at the thiol group of cysteine moiety of CAAX motif. This is followed by cleavage of terminal tripeptide by endopeptidase, methylation and palmitoylation of terminal exposed cysteine residue before membrane localization. b Activation cycle of RAS GTP-GDP. Conformation of RAS proteins at 30–38 amino acid region [Switch I (SI)] and at 60–76 amino acid region [Switch II (SII)] changes during GDP-GTP cycling. GTP hydrolysis is facilitated by RASGAPs while guanine nucleotide exchange activities are stimulated by GNEFs. Binding of ligand to the receptor activates GNEFs, destabilizes nucleotide binding and releases nucleotide. At or above ten-fold higher concentration of GTP in cell cytoplasm, GNEFs facilitate transient formation of RAS-GTP. This is followed by the increased intrinsic GTPase activity of RAS (stimulated by GAPs). As a result RAS-GTP is converted into inactive GDP bound form. Mutations in Ras gene impair GAPs stimulated GTP hydrolysis and allow RAS to persist in GTP bound form. GTP: Guanosine triphosphate; GDP: Guanosine diphosphate; GAPs: GTPase-activating proteins; GNEFs: Guanine nucleotide exchange factors
Ras protein activation
Binding of growth factors, polypeptide hormones, neurotransmitters, chemokines, and phorbol esters to the receptor causes its dimerization and activation of intrinsic receptor tyrosine kinase. Specific tyrosine residue on intracellular portion of receptor undergoes autophosphorylation (Medarde and Santos 2011). Further sequence of events include binding of phosphorylated tyrosine residues with SH2 (sequence homology 2) domain of adaptor protein such as Grb2 and binding of proline rich motifs of protein like son of sevenless (SOS) [a guanine dissociation stimulator (GDS) of RAS/ guanine nucleotide exchange factors (GNEFs)] with SH3 (sequence homology 3) domains of Grb2. Grb2 thus links ligand bound/ activated receptor to SOS, recruits SOS (cytosolic protein) into the vicinity to RAS protein on plasma membrane (Cox et al. 2014). Binding of RAS to SOS activates it by allowing the change in Ras conformations, dissociation of GDP and binding of GTP to it (Cox and Der 2010). Recruitment of downstream effectors including phosphoinositide 3′-kinase (PI3-K), serine-threonine kinase RAF-1 and RalGDS to the active form of RAS in plasma membrane leads to the activation of distinct signaling cascades (Ferro and Trabalzini 2010; Castellano and Downward 2011).
Like other G proteins, RAS behaves as small GTPase, acts as binary molecular switches that cycles between GTP- bound active form and GDP- bound inactive form. RAS in inactive GDP-bound state is stable and undergoes conversion to the active GTP-bound form which is stimulated by GEFs (Simanshu et al. 2017). This is followed by reconversion to inactive form via stimulation of GTP hydrolysis by GTPase-activating proteins (GAPs) (Cherfils and Zeghouf 2013). Normally RAS protein exists in GDP bound inactive state with intrinsic GTPase activity. GTPase activating domain (often referred to as the GAP-related domain, GRD) of RAS GAPs is highly conserved; stimulates GTP hydrolysis; and plays distinct roles in signal transduction and RAS regulation (Bos et al. 2007). RASA1/p120 RASGAP contains SH2 and SH3 domains that allow it to bind to activated receptors, such as PDGFR and thus enable RASA1/p120 RASGAP to downregulate RAS appropriately during signaling (Kaplan et al. 1990).
Ras mutations at codons 12, 13 and 61 (replacement of glycine at 12 and 13 with any amino acid except proline) either reduce intrinsic GTPase activity or completely abolish GAP induced activation (Trahey and McCormick 1987) (Fig. 2b). As a result RAS becomes the constitutive activator of downstream effectors/ targets. GAPs attenuate normal RAS functions and activate it by mediating RAS- induced disruption of actin stress filaments through its SH2 and SH3 domains at N terminus (Maruta and Burgess 1996). Mutations in Ras such as A146 mutations (4% in colorectal cancer), T158A, R164Q, and K176Q decrease affinity for nucleotide; allow GDP to dissociate rapidly; and lead to abnormal accumulation of RAS in the GTP-form at the plasma membrane without any assistance from upstream signals and GEFs (Edkins et al. 2006). Abnormal RAS activity may also play a significant role in various developmental conditions, autism and other neurological disorders (Simanshu et al. 2017).
GTP-competitive inhibitors function like ATP-competitive inhibitors, have the ability to impair stimulatory activity of GAP and thus target mutant Ras. However, the efforts were not successful due to difference in binding affinity of ATP (micromolar) with protein kinases and of GTP (picomolar) with RAS oncoproteins (Stephen et al. 2002). Understanding the functions of RAS proteins in context with plasma membrane and characterization of RAS interactions with downstream effectors are required for interventions aimed at RAS diseases.
RAS and its cytosolic targets
Eleven distinct classes of RAS effectors are identified and many of them have functionally related isoforms. They are known to possess RAS-association (RA) domains or RAS-binding domains (RBD). Binding of effectors to the activated RAS-GTP promotes their activation, recruits to plasma membrane in high concentration and enhances their intrinsic catalytic activity.
RAS and RAF 1
Activated RAS (RAS GTP) recruits serine-threonine kinase RAF-1 (rapidly accelerated fibrosarcoma1) to the plasma membrane. Fusion of RAF to the C-terminal membrane localization signal of K-RAS has been examined in murine cells (Kolch et al. 1991). Nevertheless, dominant negative mutants of RAF are studied to impair Ras transforming activity (Schaap et al. 1993). MEK or MAPKK [mitogen activated protein kinase (MAPK)/ extracellular signal regulated kinase (ERK)] can either be phosphorylated by MEK kinase or by all the three forms of activated RAF (ARAF, BRAF and CRAF) and in turn phosphorylates ERK. High MAPK activity brings about the phosphorylation and activation of ribosomal S6 kinase and transcription factors including c-MYC, c-JUN, c-FOS. This stimulates the expression of proliferator genes (Lusk et al. 2017). Analysis of RASopathies (group of clinical syndromes like neurofibromatosis type 1; autism spectrum disorders; cancer) in humans examines similar phenotypes exerted by either germline-activated alleles of H-Ras, N-Ras, and K-Ras; or by activated BRAF, CRAF, MEK1/ MEK2. This explains the central importance of RAF/MAPK cascade in RAS biology (Simanshu et al. 2017).
Non-steroidal anti-inflammatory drug, sulindac sulphide although potentially inhibits RAS-RAF interaction by binding at RAF binding site, decreases the phosphorylation of ERK and inhibits the proliferation of RAS transformed cells but due to its off target activities, it is no more considered as useful drug (Karaguni et al. 2002).
RAS and serine – Threonine kinase (MEKK1)
MEKK1 and its downstream targets, MAP kinases including c-JUN N-terminal kinases/stress activated kinases (JNK/SAPK) are activated by RAS-GTP through Ral/CDC42 pathway and regulate stress-response. Despite JNK being the primary targets of MEKK1, experimental studies examine the role of MEKK1 in MEK/ERK pathway activation independent of RAF-1(Lange-Carter and Johnson 1994). Hyperactivation of the RAS/MAPK pathway causes RASopathies in humans. Targeting MEK relieves feedback suppression of upstream signaling; increased RTK signaling and ERK activity (that can off-set the effects of MEK inhibition). Minimal therapeutic window between normal cells and K-Ras cancer cells is due to the fact that only small fraction of normal cells exhibit high MEK activity while large fraction of K-Ras cancer cells show high MEK activity due to chronic Ras activation (Zhang et al. 2015; Lake et al. 2016).
RAS and RAC/ RHO
The G protein RAC and RHO are regulated by factors analogous to GAPs and GNEFs and are activated by Ras-GTP (Buday and Downward 1993). Critical functions of RAC and RHO include membrane ruffling, formation of stress fibers, filopodia and focal adhesions and thus contribute to invasive phenotype of transformed cells (Morrison and Cutler 1997). Owing to the significant function of mutant RAC1 as a crucial driver of cancer growth, its inhibition may have a therapeutic benefit in Ras mutant cancers. This is supported by mouse model studies where genetic ablation of RAC1 function impaired the initiation of mutant K-Ras driven lung or pancreatic cancers (Kissil et al. 2007; Heid et al. 2011).
RAS and phosphoinositide 3′-kinase (PI3K)
Cellular processes regulated by PI3-K include suppression of apoptosis, increased cell motility and invasiveness. PI3-K consists of 110kD catalytic subunit and 85kD regulatory subunit. Binding of RAS-GTP with 110kD subunit of PI3K increases its activity and concentration of 3′ phosphorylated inositol lipids. Complex sequence of events initiated by phosphatidyl inositol 3,4,5-triphosphate (PIP3), one of the PI3-K products, include activation of RAC, production of PIP2 (phosphatidyl 4,5-biphosphate) by activating PI4/PI5 kinases, uncapping of actin filaments at plus end and membrane ruffling (Ridley et al. 1992). PI3-K has been shown to suppress c-MYC-induced apoptosis by RAS. Downstream targets of serine-threonine kinase AKT (protein kinase B), the potential downstream effector of PI3-K, include pro-apoptotic protein BAD and caspase 9 (Cardone et al. 1998). Mice expressing a mutant form of PI3Ka that fails to bind RAS, were generated but could not survive to adulthood (Gupta et al. 2007; Castellano et al. 2013). Mice with defective lymphatic systems, angiogenesis and macrophage functions explain the importance of this interaction in tissue specific manner (Murillo et al. 2014). Kinase mammalian target of rapamycin (mTOR), another target of AKT, has been shown to play critical role in cell cycle progression (Yan et al. 1994).
RAS and RALGEFs
RALGDS is another potential Ras effector. It is recruited to membrane by activated Ras. Functions of RAL family proteins (RALA and RALB) have been implicated in exocytosis, endocytosis, actin organization and cell migration but the mechanism is not clearly known. Mice lacking RALGDS, one of the four RA domain-containing RALGEFs, were examined to retain viability but exhibited reduced carcinogen-induced mutant H-Ras driven skin tumour formation (Gonzalez-Garcia et al. 2005). Experiments on RNAi/ shRNA-mediated silencing of isoforms of RALGEFs, RALA and/or RALB in Ras-mutant pancreas, skin and lung organs reflect different roles for RAL GTPases in tumour initiation and progression versus maintenance, or cancer-type differences (Chien and White 2003; Lim et al. 2006; Peschard et al. 2012).
RAS and TIAM
T lymphoma invasion and metastasis protein 1 (TIAM1) has been examined as a specific GEF for RAC with RAS binding domain (Lambert et al. 2002). Involvement of TIAM1 in RAS-RAC crosstalk, RAC mediated ruffling of membrane, spreading of cell, neurotrophin induced Schwann cell migration and activation of JNK is documented (Yamauchi et al. 2005).
RAS and RASSF/NORE1
RASSF (RAS association domain family) members function as tumor suppressors in human tumors and can be silenced by DNA methylation. RASSF2, RASSF4 and RASSF5 (NORE1) interaction with K-Ras induce cell cycle arrest and promote apoptosis (Vos et al. 2003a; b). One of the isoforms, RASSF1A, forms complex with a BH-3 like protein MAP-1, promotes its binding with BAX, activates BAX and induces apoptosis (Baksh et al. 2005).
RAS, RIN and PLC
Among three isoforms of RIN, RIN1 is cytosolic while RIN2 and RIN3 are localized to endocytic vesicles. RIN1 with RA domain functions as GEF for RAB5/VPS21 like proteins and promotes RAS mediated endocytosis (Tall et al. 2001). Phospholipase C (PLC), RAS binding protein, gets activated by PDGF and EGF growth factors in a RAS and RAP1 dependent manner and has a critical role in carcinogenesis (Song et al. 2002).
RAS, AF6 and PKCζ
AF6 (Afadin) is an actin binding protein and consists of two RA domains in its N-terminus and a PDZ domain. AF6 has been examined to colocalize with tight junction protein ZO-1 and play role in the maintenance of epithelial cell-cell junctions and cell polarity (Zhadanov et al. 1999). It negatively regulates RAP1 mediated cell adhesion. PKCζ (protein kinase C-zeta) shares structural similarity with RAF and has been examined to interact directly with RAS both in vitro and in vivo (Diaz-Meco et al. 1994).
Although mutations in Ras genes, specifically K-Ras, accompany high MAPK activity in 40% of colorectal cancers, nevertheless, regulation of MAPK signaling has been shown to be preserved through wild type Ras isoforms. High activity of MAPK activates other protein kinases and gene regulatory proteins by transmitting signals downstream to it. Studies in primary colorectal cancer tissues, MAPK reporter constructs and xenograft models report high MAPK activity in tumor cells which exhibit epithelial plasticity. Such plastic tumor cells were later examined to express markers related to colon cancer stem cells (Blaj et al. 2017).
Epithelial-to-mesenchymal transition (EMT) and cancer
The process of plastic transition of epithelial and mesenchymal cells, best described as EMT, and its reverse process of mesenchymal-to-epithelial transition (MET) has been first examined in mesenchymal transformation of early epithelial cells in chick embryonic lens epithelium in 1980s (Greenburg and Hay 1982; Greenburg and Hay 1986). It is considered as important cellular mechanisms in embryogenesis, wound healing, fibrosis, chronic inflammation, embryonic stem cell differentiation, pluripotency, acquisition of cancer stem cell behavior and cancer progression (Garg 2013; Santamaria et al. 2017). Loss of adherens junctions, altered cytoskeletal organization and cell polarity, degradation of basal extracellular matrix (ECM), secretion of matrix of fibronectin and migration of cells into underlying tissues are the phenotypic changes associated with EMT (Bhatia et al. 2017). Down-regulation of epithelial cell surface marker proteins and cytoskeleton components including E-cadherin, zonula occludens (ZO)-1, claudins, occludins and cytokeratins; upregulation of mesenchymal markers including vimentin, α-smooth muscle actin, collagens and fibronectin; disruption of intercellular contacts, matrix and tissue remodeling; enhanced invasion/ migration through extracellular matrix; and apoptotic resistance are the essential features of EMT/ MET during tumor development (Lee et al. 2006; Santamaria et al. 2017).
EMT program is essentially regulated by extracellular inducers; EMT-ATFs; and downstream targets. Ras signaling pathway is orchestrated by the external inducers/ growth factors and mobilize EMT-ATFs. Snail family of zinc-finger transcription factors: Snail1 (Snail), Snail2 (Slug) and Snail3 (Smuc) bind to the promoter regions of cadherin gene (CDH1) through their carboxy-terminal zinc-finger domains and repress the expression of its protein product, E-cadherin (Garg 2013). Binding facilitates Snail1 to recruit Polycomb repressive complex 2 (PRC2) [G9a and suppressor of variegation 3–9 homologue 1 (SUV39H1), the co-repressor SIN3A, histone deacetylases 1, 2 and/or 3, and Lys-specific demethylase 1 (LSD1) methyltransferases, enhancer of zeste homologue 2 (EZH2)] and allow modifications in histone (Herranz et al. 2008). Methylation at histone 3 Lysine 9 (H3K9) and H3K27 marks repressive chromatin while methylation at H3K4 and acetylation at H3K9 mark active chromatin. Increased nuclear abundance of Snail1 and Snail2, low expression of E-cadherin and induction of EMT correlate with increased tumor metastasis in vivo (Batlle et al. 2000; Cano et al. 2000). Coexpression of mutant K-Ras and a polycomb-group repressor complex protein, Bmi1 (B lymphoma Mo-MLV insertion region 1 homologue) in human pancreatic duct-derived cells (HPNE) allows them to undergo partial EMT via upregulation of Snail and impairs the anchorage-independent growth capability of invasive cells (Chen et al. 2016).
Another family of EMT-ATF is a basic helix-loop-helix (bHLH) transcription family which consists of two transcription factors, Twist1 and Twist2. Binding of Twist1 to the promoter region of Snail2 increases its expression and induces EMT in metastatic mammary tumors. Early stage of primary breast tumor development has been studied by examining the higher expression of Twist1 in mouse models of atypical ductal hyperplasia (Hüsemann et al. 2008). Methyltransferase SET8 (mediates H4K20 monomethylation) recruited by Twist1 results in repression of E-cadherin and activation of N-cadherin (Yang et al. 2012). Twist1 along with Bmi1 regulates and represses E-cadherin and cell cycle inhibitor p16 (also known as INK4A). The role of Twist1 is well-established in cytoskeletal reorganization and cancer metastasis. It inhibits HOXD1 and RHOC (Ras homolog gene family, member C) and is regulated by microRNAs (miRs) (Clark et al. 2000; Ma et al. 2007; Chen et al. 2016).
Zinc finger E-box–binding homeobox 1, Zeb1 and Zeb2 are the members of Zeb family of transcriptional repressors. They inhibit the promoter activity of CDH1 genes by binding with bipartite E-box regions of DNA that flank CDH1 gene. In nucleus, it cooperates with deacetylase sirtuin 1, modifies histone H3 and reduces binding affinity of RNA polymerase II to the promoter region of CDH1. Suppression of plakophilin-2 and ZO-3 due to ectopic expression of ZEB proteins in mammary epithelial cells is linked with the dissociation of adherens junctions and loss of epithelial phenotype. Functions of ZEB proteins in matrix remodeling mechanisms are established by increasing the expression of matrix metalloproteinases (MMPs) and inducing EMT. ZEB proteins and five members of miR-200 family form a reciprocal feedback loop, thus tightly control the plasticity of tumor cells (Burk et al. 2008; Bracken et al. 2008). Depletion of ZEB1 has been linked with suppression of stemness, colonization capacity and in particular phenotypic/metabolic plasticity of mutant K-Ras and p53 driven pancreatic tumour cells (Krebs et al. 2017).
Higher expression of LEF-1, an EMT-ATF, has been examined to promote EMT through its activation by β-catenin (Kim and Hay 2002). Another potent inducer of EMT is SOX (SRY box) transcription factor that synergizes with Snail1 or Snail2 and activate EMT (Kondoh and Kamachi 2010). FOX (Forkhead box) transcription factor is characterized to have DNA-binding forkhead domain and recently has been shown to promote EMT (Eijkelenboom and Burgering 2013). These transcription factors are regulated by signaling pathways and miRs; control the expression of each other; functionally cooperate at target genes; and drive EMT progression. In pre-clinical setting, EMT has been associated with acquisition of cellular plasticity with enhanced stemness (characterized by heterogeneity and self-renewal property); increased resistance to conventional chemotherapy; inhibition of apoptosis; accelerated tumor progression; metastasis; and recurrence.
Mechanistic regulation of epithelial plasticity through Ras activation
Receptor tyrosine kinases respond to extracellular cues and tightly control the process of EMT/MET in a spatially defined manner (Garg 2013, 2017). Mechanistic regulation of EMT in Ras driven human cancer pathogenesis due to cooperative activity of receptor tyrosine kinases/ oncogenic RAS and downstream endogenous signaling molecules is mediated by the interplay between them and miRs. MiRs are highly conserved across species in sequence and function. MiRs are cluster of small non-encoding RNA molecules of 21–23 nucleotides that serve as master regulators of many pathological and physiological processes by modulating gene expression at posttranscriptional level. MiR-134 has not only been identified to be direct target of K-Ras but also affects cell proliferation, migration, invasion and EMT in renal cell carcinoma cell lines (Liu et al. 2015). Loss of let-7 (the first known human miR) expression correlates with high K-Ras mRNA expression in colon cancer tissues (Saridaki et al. 2014). MiR-31 has been examined to be the potent enhancer of K-Ras by negatively regulating K-Ras inhibitor, RasA1, in colon and lung cancer (Kent et al. 2016). Emerging evidences identify and correlate the dysregulation/ epigenetic silencing of miR-615-5p with increased expression of RAB24; aberrant activation of RAB-RAS pathway; facilitated in vitro and in vivo hepatocellular carcinoma (HCC) growth; and metastasis (Chen et al. 2017).
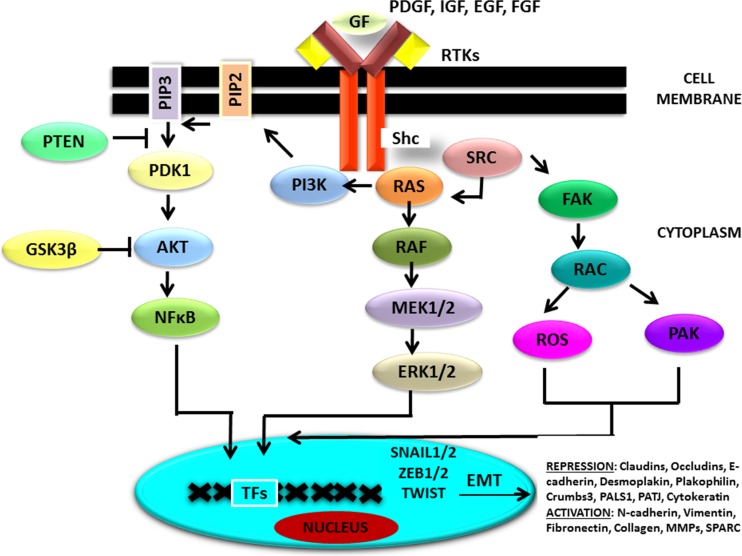
Binding of ligand [growth factors, EGF, FGF, insulin growth factor (IGF), and PDGF] to the transmembrane receptor tyrosine kinases (RTKs) brings about its autophosphorylation at tyrosine residues and activate PI3K–AKT, ERK MAPK pathways and SRC (tyrosine protein kinase) signaling (Fig. 3). Increased protein expression of RAS/PI3K/AKT related factors and reduced expression of one of the downstream targets, FoxM1 has been identified to be regulated by lower levels of miR-630 and thus affects EMT, migratory and invasive properties in gastric cancer (GC) cells (Feng et al. 2017). Functional and mutational studies validate RAF serine threonine kinases as the most potential effectors of mutant Ras-dependent cancer growth. Ligand binding or gain-of function mutations in RAS, RAF, MEK1/2 genes activate MEK-ERK module which is of principal importance in human cancer. EMT-ATFs’ reprogramming strongly correlates with ERK immunopositivity, induction of migratory phenotype of cells and poor survival of patients diagnosed with melanoma. Higher expression of ERK2 but not ERK1 has been associated with RAS-induced changes, downregulation of epithelial markers, upregulation of mesenchymal markers, increased cell migration, invasion, growth in matrigel and survival against anoikis in MCF-10A breast epithelial cells. Increased expression of miR-224 in dysplastic colorectal disease correlates with increased K-RAS activity and ERK-AKT phosphorylation (Amankwatia et al. 2015).
Fig. 3.
RAS signaling cascade and EMT: Growth factors including EGF, FGF, HGF, VEGF, PDGF, IGF act through RTKs and activate PI3K–AKT, RAS-RAF-MEK-ERK MAPK and SRC signaling cascade. RAS-RAF-MEK-ERK MAPK being the major signaling pathway promotes EMT by increasing the expression of EMT –activating transcription factors and mesenchymal proteins, repressing the expression of epithelial proteins thereby facilitates cell motility and invasion. EMT: Epithelial mesenchymal transition; EMT-ATFs: EMT-activating transcription factors; RTKs: Receptor tyrosine kinases; FGF: Fibroblast growth factor; HGF: Hepatocyte growth factor; PDGF: Platelet derived growth factor; IGF: Insulin growth factor; VEGF: Vascular endothelial growth factor; PI3K-AKT: Phosphoinositide 3’kinase- Protein kinase B; SRC: tyrosine protein kinase; RAF: Rapidly accelerated fibrosarcoma; MEK: Mitogen activated protein kinase (MAPK)/ extracellular signal regulated kinase; ERK: extracellular signal regulated kinase; MAPK: Mitogen activated protein kinase
Genome-wide analysis of FOS related antigen [FRA1 (transcription factor)] chromatin occupancy and transcriptional regulation identify EMT-related genes as a major class of direct FRA1 targets. It plays important role in mediating cross talk between oncogenic RAS-ERK and TGFβ signaling networks during tumor progression (Diesch et al. 2014). Binding of FRA1 with phosphorylated ERK2 at DEF (Docking site for ERK, F-X-F) motifs and/or D-domains (Docking domain) leads to induced EMT, anti-apoptosis, cell motility and invasiveness (Roux and Blenis 2004) (Fig. 4). MiR-221/222 regulate FRA1 expression, activate MEK, mediate reduction in E-cadherin abundance and increased expression of mesenchymal specific genes and thereby contribute to aggressive clinical behavior of basal-like breast cancers (Stinson et al. 2011). Binding of promoter region of vimentin with AP-1 factor, c-JUN and FRA1, in human breast cancer cell lines and human colon epithelial cell lines respectively contributes to the enhanced expression of vimentin in a tissue specific manner (Rinehart-Kim et al. 2000; Andreolas et al. 2008). Overexpression of RAS particularly, H-RasV12 exerts its effects by elevating FRA1 levels through the AKT, MAPK–ERK and PI3K pathway in a wide variety of tissue cell types. Caco-H1 and -H2 cells treated with FRA-1 siRNA appear to reverse mesenchymal phenotype and eliminate migrational ability. These phenotypic changes are characterized by the loss of the long protrusions of vimentin and its relocalization around the cell periphery (Andreolas et al. 2008). Post-translational modification in the form of phosphorylation of two C-terminal serine residues, Ser-252 and Ser-265 of FRA1 by RSK and ERK protein kinases results in inactivation of a C-terminal instability domain. Nevertheless, non-phosphorylated form of FRA1 promotes its ubiquitin-independent proteasomal degradation (Basbous et al. 2007). Overexpression of FRA1, its transcriptional autoregulation and posttranslational stabilization are linked with tumorigenesis and cancer progression (Casalino et al. 2003). ERK2-FRA1 pathway has been documented to regulate the expression of ZEB1/2 and thereby EMT in human breast epithelial cells (Verde et al. 2007). Madin-Darby canine kidney (MDCK) cells transformed with oncogenic K-RAS synergistically upregulate α6-integrin, αV-integrin expression, ZEB1 via activation of MAPK/ERK/FRA1 signaling cascade and Twist-related protein 1; trigger EMT; and modulate cancer cell survival and tumorigenesis (Zhang et al. 2017). Mesenchymal-like phenotypic changes in breast cancer cells mediated by H-RAS pathway is controlled by the ZEB1/miR-200c axis (Koh et al. 2015). This loop acts downstream of RAS to regulate the expression of polycomb factor, Bmi1, triggers EMT and drives tumor initiation in cancer cells (Liu et al. 2014). Large degree of heterogeneity with regard to phospho-ERK immunopositivity in melanocytic lesions containing MEK activating mutations could be due to activation of different negative feedback pathways and acquisition of bypass mechanisms in advanced cancer (Tulchinsky et al. 2014; Kim et al. 2015).
Fig. 4.
ERK/FRA1/EMT-ATFs pathway: Molecular mechanism of oncogenic RAS induced activation of ERK, transcription, phosphorylation and activation of FRA1 and increased expression of EMT-ATFs (ZEB and TWIST proteins) for promoting EMT, cell invasion and migration. EMT: Epithelial mesenchymal transition; EMT-ATFs: EMT-activating transcription factors; ERK: extracellular signal regulated kinase; FRA1: FOS related antigen1
ERK1 and ERK2 have been shown to induce low level of expression of TGFβ in MCF-10A cells and thus enhance autocrine TGFβ signaling. Ras signaling synergizes with TGFβ--Smad signaling and induces growth arrest, tissue fibrosis and EMT (Miyazono 2009). TGFβR functions as serine threonine kinase, gets phosphorylated and creates docking site for SH2 domain containing proteins, like PI3K, GRB2 and SOS and thus linked to the PI3K/AKT and RAS–RAF–MEK–ERK MAPK pathways. TGFβ induces the activation of RHO (by promoting GNEFs), RAC and CDC42 GTPases. The activity of two small GTPases, RAC and RHO, is stimulated by RAS via PI3K and thereby activate EMT and motility by regulating adherens junctions, focal adhesions, phosphorylation of myosin and actin stress fibers (Edme et al. 2002). Adenocarcinoma A549 cells when induced by TGF-β1 exhibit, increased expression and activity of RhoC protein; low levels of E-cadherin; high levels of vimentin; and enhanced invasive capability of the cells in vitro (Lu et al. 2016). TGF-β remarkably induces Snail expression in cooperation with RAS signals and activates EMT (Shields et al. 2013). Studies reveal the underlying mechanism and demonstrate the role of signal transducer and activator of transcription 3 (STAT3) as a mediator that synergizes TGF-β and RAS signals through enhanced Snail induction (Saitoh et al. 2016). Proteomic analysis in highly metastatic triple-negative breast cancer (TNBC) validates RAB1B, a member of the Ras oncogene family as a metastasis suppressor. Mechanistically, loss of RAB1B results in elevated expression of TGFβR1 through decreased degradation of ubiquitin; increased levels of phosphorylated SMAD3; TGF-β-induced EMT; and correlates with poor prognosis of breast cancer patients (Jiang et al. 2015). Another study identifies insulin-like growth factor binding protein-related protein 1 (IGFBP-rP1), to suppress EMT and colorectal cancer/ metastasis by repressing TGF-β-mediated EMT through the Smad signaling cascade (Zhu et al. 2015).
Hepatocyte growth factor (HGF) acts through RTK c-MET (also known as HGFR), recruits ERK MAPK pathway, allows binding of the early growth response 1 (EGR1) transcription factor to the promoter region of Snail gene, induces its expression and converts epithelial cells into migratory fibroblast-like cells. According to study by Marchetti et al. RTK- or integrin-induced AKT stimulates Snail1 expression through nuclear factor-κB (NF-κB), stabilizes Snail1 by inhibiting GSK3β (glycogen synthase kinase 3β) and stimulates its activity (Marchetti et al. 2008). Another study demonstrates the downregulation of miR-98 and miR-27b, promotion of CCL18-mediated invasion and migration and EMT via N-RAS/ERK/PI3K/NFκB/Lin28b signaling pathway in breast cancer (Lin et al. 2015). Insulin-like growth factor 1 (IGF1)-induced expression of ZEB1 and stimulation of EMT has been associated with activation of ERK-MAPK and PI3K-AKT pathways (Graham et al. 2008). ILK kinase and the ERK-MAPK pathway dependent matrix metalloproteinases, MMP2 and MMP9 mediated proteolysis, increased cell migration and EMT is linked with binding of EGF to its receptor. Human mammary epithelial cells with activated EGF receptor 2 (HER2; also known as ERBB2) show increased tumorigenic potential. Overexpression of EGF-related Cripto1 (also known as TDGF1) has been examined to induce motility and invasion of tumor cells (Ahmed et al. 2006). Platelet-derived growth factor (PDGF) in colon carcinoma cells has been shown to drive EMT by inducing dissolution of adherens junctions, repression of E-cadherin expression and nuclear localization of β-catenin (Yang et al. 2006). PDGF-related growth factor, VEGF, partly inhibits GSK3β activity, stimulates Snail and Twist expression and activates EMT in cancer development (Wanami et al. 2008).
Therapies to target RAS activation and EMT
EMT activation is presented with early local invasion followed by progression/ metastasis, imparts stemness to migratory/ circulating tumor cells (CTCs), makes them resistant to cancer therapies and contributes extremely poor prognosis to cancer cells. Since the expression of EMT marker genes is not only cell and tissue specific but is also regulated by the multiple downstream targets of RAS pathway, hence it is difficult to quantify the extent of EMT and its degree of progression. Increased resistance of mutant Ras harboring cells to ionizing radiations; its crosstalk with other protooncogenes/ downstream targets; and genetic heterogeneity of different cells and tissues make it difficult to therapeutically eliminate mutant Ras and its tumorigenic effects (Sklar 1988; McKenna et al. 1990; Grant et al. 1990; Miller et al. 1993). Since, oncogenic K-Ras is constitutively inactive but upstream stimulants can result in prolonged strong Ras activity, hence, interventions to reduce K-Ras activation in addition to targeting K-Ras downstream effectors may have important cancer-preventive value in patients with oncogenic K-Ras (Huang et al. 2014). Experimental studies in animal and human clinical trials are currently focusing on the approaches based on the use of potential inhibitors targeting (a) RAS; (b) blocking RAS membrane association; and (c) RAS downstream effectors. Among these, targeting RAS downstream effectors is the most favorable approach in the development of anti-cancer drugs to impede tumor development and progression. Despite more than three decades of efforts, none of the effective pharmacological RAS inhibitors (multiple kinase inhibitors including RAF, MEK) yet reach to clinic, however, intensive researches are on to explore potential anti-tumor therapeutics.
Blocking protein-protein interfaces of RAS-effector complexes with small molecules; targeting the RBDs of various effectors; and hyper-phosphorylation of the RAFs and SOS1 that can make components of RAS pathway unresponsive to upstream signaling, are attractive therapeutic approaches against Ras driven cancers (Simanshu et al. 2017). Ability of small-molecule pan-RAS ligands to interact with adjacent sites on the surface of oncogenic K-Ras; metabolic stability in liver microsomes; and their anti-tumor activity in xenograft mouse cancer models make them an effective therapeutic molecule in cancer treatment (Welsch et al. 2017). Expanded signaling capacity has been observed in heterocellular cancer, pancreatic ductal adenocarcinoma (PDA) [PDA is composed of mutated tumor cells, stromal fibroblasts, endothelial cells, and immune cells] where K-Ras oncogene establishes a differential reciprocal (external) signaling state in tumor cells. Reciprocal signaling employs additional kinases; doubles the number of regulated signaling nodes; regulates tumor cell proliferation/ apoptosis and increases mitochondrial capacity (Tape et al. 2016). Lack of a deep pocket (to which drugs can bind); complexity of RAS effector functions/ downstream pathways; and feedback loops/ signaling redundancy are some of the major challenges in shutting down of RAS signaling (Simanshu et al. 2017).
Owing to regulatory functions of miRs in RAS signaling induced epithelial plasticity and enhanced metastatic potential of CTCs, miR based therapeutic studies in in vitro and in vivo models hold promise for the development of personalized approaches in cancer prevention. This section provides improved understanding on the specific therapeutic mechanisms of miRs in EMT induced Ras driven human cancer (Koh et al. 2015; Kent et al. 2016). Therapies involving miRs are based on (a) local administration of antagomiRs/ antimiRs to inhibit oncogenic endogenous miRNA that show gain of functions; and (b) miRNA replacement therapy to restore the loss of function of tumor suppressor miRs. Stable expression of miRs affects the expression of target genes, reverse the plastic nature of CTCs/ EMT phenotype and sensitize the cancer cells to anti-cancer drugs (Chen et al. 2017).
Transfection of human GC cell line SGC 7901 with tumor suppressor miR-630 mimic has been examined to suppress EMT by regulating FoxM1 and inactivating RAS/PI3K/AKT pathway in GC cells (Feng et al. 2017). Study by Ye et al. examines the potential therapeutic implications of long noncoding RNA (lncRNA), growth arrest-specific 5 (GAS5) and its related miRs with tumor suppressor functions in osteosarcoma (Ye et al. 2017). Binding of overexpressed GAS5 to miR-221 in xenograft models has been identified to enhance the expression of aplasia RAS homologue member I (ARHI), suppress cell growth/ EMT in osteosarcoma by regulating the miR-221/ARHI pathway. MiR-615-5p functions as tumor suppressor, reduces the expression of RAB24 (RAS-related protein), suppresses EMT, adhesion and vasculogenic mimicry (VM) of HCC cells (Chen et al. 2017). Forced expression of tumor suppressor, miR-134, suppresses K-RAS-related MAPK/ERK pathway functions; reduces proliferation by triggering G1/G0 cell cycle arrest; and inhibits migration and invasion by blocking EMT in renal cell carcinoma (RCC) cell lines (Liu et al. 2015). HCC MHCC97-H and HCCLM3 cell lines transfected with let-7 g were examined to suppress EMT by downregulating K-RAS and HMGA2A. Potential therapeutic ability of overexpressed let-7 g in xenografted nude mice was analyzed using in situ hybridization (ISH). Low levels of let-7 g have been found to be significantly associated with poorer overall survival while its re-expression correlates with significantly inhibited proliferation, migration, and invasion (Chen et al. 2014). MiR-29 family members are shown to have tumor-suppressive effects in RAS activated haematopoietic, cholangiocytic and lung tumours. MiR expression suppresses the expression of proteins that are involved in the degradation of messenger RNAs with AU-rich 3′-untranslated regions, tristetraprolin (TTP), inhibits EMT and blocks metastatic spread of CTCs (Gebeshuber et al. 2009). Owing to oncogenic functions of miR-224 and its differential expression in dysplastic colorectal disease, antagomiR-mediated silencing of miR-224 in NIH3T3 cells expressing K-RAS and BRAF mutant proteins results altered cell proliferation, invasion and EMT phenotypes; and increases chemosensitivity of cells to 5-fluorouracil (Amankwatia et al. 2015).
Conclusions
RAS activation through its crosstalk leads to the uncontrolled mobilization of EMT core regulators/ inducers and thus contributes cellular plasticity to tumor cells. EMT endows primary tumor cells that shed into circulatory/ lymphatic system to transiently adapt altered morphological features which include the reduced requirement of cell-cell contact, immune evasion, enhanced proliferation and spread of tumor cells to distant organ sites. Plastic nature of tumor cells with enhanced stemness allows them to survive in an adverse environment through constant evolution. CTCs when colonized, establish pre metastatic niches; accelerate tumor progression; confer cells an advantage to survive anticancer therapeutics across diverse cancer lineages; and pose clinical challenges in cancer treatment.
Global screening and profiling of population of CTCs for quantitative identification of signature molecules which contribute to cellular plasticity by tightly coupling RAS with EMT core inducers leads to improved clinical utility for monitoring the extent of metastatic spread and tumor relapse. Posttranscriptional activation/ autoregulation and posttranslational stabilization of EMT core regulator proteins mediated by Ras oncogene-dependent accumulation through positive feedback mechanisms drastically increase their half-life. Dissecting the mechanisms that regulates the transcriptional activation and stabilization of multiple regulators/ downstream targets/ tumor promoters in response to the oncogenic Ras may help in identification of potential inhibitors that can bring about morphological transformation of tumor cells to reduce their plasticity and thus may result in complete loss of metastatic potential.
Tight regulation of epithelial plasticity by miRs in RAS driven cancer cells as evidenced by various experimental studies mentioned, encourages the use of them as potential inhibitors/ metastatic suppressors. Small size of miRs, sufficient delivery to target tumor tissues, ease of systematic delivery inside the cell cytoplasm to nucleus, lack of evidence for in vivo toxicity and no apparent loss of activity make them a valuable tool and highly promising therapeutic strategy. MiR based anticancer therapies facilitate differentiation of tumor cells to mature and committed cells, inhibit their cellular plasticity, revert or block the process of EMT, control metastatic spread and tumor recurrence. Despite extensive studies on analyzing critical functions of individual miRs, regulatory mechanisms of many of them are poorly understood. Bioinformatic tools, available online data bases and integrated analysis of multiple mRNA targets for a given miR may provide improved understanding on the regulatory interactions of miRs-mRNA specific to induction of EMT in Ras induced cancer.
Acknowledgements
One of the co-authors, KT is thankful to University Grants Commission (UGC), Govt. of India for providing research fellowship.
Compliance with ethical standards
Conflict-of-interests
Authors disclose no potential conflict-of interests.
References
- Ahmed N, Maines-Bandiera S, Quinn MA, Unger WG, Dedhar S, Auersperg N. Molecular pathways regulating EGF-induced epithelio-mesenchymal transition in human ovarian surface epithelium. Am J Phys Cell Phys. 2006;290(6):C1532–C1542. doi: 10.1152/ajpcell.00478.2005. [DOI] [PubMed] [Google Scholar]
- Akagi K, Uchibori R, Yamaguchi K, Kurosawa K, Tanaka Y, Kozu T. Characterization of a novel oncogenic K-RAS mutation in colon cancer. Biochem Biophys Res Commun. 2007;352:728–732. doi: 10.1016/j.bbrc.2006.11.091. [DOI] [PubMed] [Google Scholar]
- Amankwatia EB, Chakravarty P, Carey A, Weidlich S, Steele RJ, Munro AJ, Wolf CR, Smith G. MicroRNA-224 is associated with colorectal cancer progression and response to 5-fluorouracil-based chemotherapy by K-RAS-dependent an independent mechanisms. Br J Cancer. 2015;112(9):1480–1490. doi: 10.1038/bjc.2015.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreolas C, Kalogeropoulou M, Voulgari A, Pintzas A. Fra-1 regulates vimentin during ha-RAS-induced epithelial mesenchymal transition in human colon carcinoma cells. Int J Cancer. 2008;122:1745–1756. doi: 10.1002/ijc.23309. [DOI] [PubMed] [Google Scholar]
- Atreya CE, Corcoran RB, Kopetz S. Expanded RAS: refining the patient population. J Clin Oncol. 2015;33(7):682–685. doi: 10.1200/JCO.2014.58.9325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baksh S, Tommasi S, Fenton S, VC Y, Martins LM, Pfeifer GP, Latif F, Downward J, Neel BG. The tumor suppressor RASSF1A and MAP-1 link death receptor signaling to Bax conformational change and cell death. Mol Cell. 2005;18(6):637–650. doi: 10.1016/j.molcel.2005.05.010. [DOI] [PubMed] [Google Scholar]
- Banerjee SK, Zoubine MN, Mullick M, Weston AP, Cherian R, Campbell DR. Tumor angiogenesis in chronic pancreatitis and pancreatic adenocarcinoma: impact of K-ras mutations. Pancreas. 2000;20(3):248–255. doi: 10.1097/00006676-200004000-00005. [DOI] [PubMed] [Google Scholar]
- Basbous J, Chalbos D, Hipskind R, Jariel-Encontre I, Piechaczyk M. Ubiquitin-independent degradation of Fra-1 is antagonized by Erk1/2 pathway-mediated phosphorylation of a unique C-terminal destabilize. Mol Cell Biol. 2007;27:3936–3950. doi: 10.1128/MCB.01776-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batlle E, Sancho E, Francí C, Domínguez D, Monfar M, Baulida J, García D, Herreros A. The transcription factor snail is a repressor of E-cadherin gene expression in epithelial tumour cells. Nat Cell Biol. 2000;2:84–89. doi: 10.1038/35000034. [DOI] [PubMed] [Google Scholar]
- Bhatia S, Monkman J, Toh AKL, Nagaraj SH, Thompson EW. Targeting epithelial-mesenchymal plasticity in cancer: clinical and preclinical advances in therapy and monitoring. Biochem J. 2017;474(19):3269–3306. doi: 10.1042/BCJ20160782. [DOI] [PubMed] [Google Scholar]
- Blaj C, Schmidt EM, Lamprecht S, Hermeking H, Jung A, Kirchner T, Horst D. Oncogenic effects of high MAPK activity in colorectal cancer mark progenitor cells and persist irrespective of RAS mutations. Cancer Res. 2017;77(7):1763–1774. doi: 10.1158/0008-5472.CAN-16-2821. [DOI] [PubMed] [Google Scholar]
- Bos JL, Rehmann H, Wittinghofer A. GEFs and GAPs: critical elements in the control of small G proteins. Cell. 2007;129:865–877. doi: 10.1016/j.cell.2007.05.018. [DOI] [PubMed] [Google Scholar]
- Bracken CP, Gregory PA, Kolesnikoff N, Bert AG, Wang J, Shannon MF, Goodall GJ. A double-negative feedback loop between ZEB1-SIP1 and the microRNA-200 family regulates epithelial-mesenchymal transition. Cancer Res. 2008;68:7846–7854. doi: 10.1158/0008-5472.CAN-08-1942. [DOI] [PubMed] [Google Scholar]
- Brummelkamp TR, Bernards R, Agami R. Stable suppression of tumorigenicity by virus mediated RNA interference. Cancer Cell. 2002;2(3):243–247. doi: 10.1016/s1535-6108(02)00122-8. [DOI] [PubMed] [Google Scholar]
- Buday L, Downward J. Epidermal growth factor regulates p21Ras through the formation of a complex of receptor, Grb2 adapter protein, and Sos nucleotide exchange factor. Cell. 1993;73(3):611–620. doi: 10.1016/0092-8674(93)90146-h. [DOI] [PubMed] [Google Scholar]
- Burgess MR, Hwang E, Mroue R, Bielski CM, Wandler AM, Huang B, Firestone AJ, Young A, LaCap JA, Crocker L, Asthana S, Davis EM, Xu J, Akagi K, Le Beau MM, Li Q, Haley B, Stokoe D, Sampath D, Taylor BS, Evangelista M, Shannon K. KRAS allelic imbalance enhances fitness and modulates MAP kinase dependence in cancer. Cell. 2017;168(5):817–829. doi: 10.1016/j.cell.2017.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burk U, Schubert J, Wellner U, Schmalhofer O, Vincan E, Spaderna S, Brabletz T. A reciprocal repression between ZEB1 and members of the miR-200 family promotes EMT and invasion in cancer cells. EMBO Rep. 2008;9:582–589. doi: 10.1038/embor.2008.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cancer Genome Atlas Research Network. Electronic address: andrew_aguirre@dfci.harvard.edu; Cancer Genome Atlas Research Network Integrated genomic characterization of pancreatic ductal adenocarcinoma. Cancer Cell. 2017;32(2):185–203.e13. doi: 10.1016/j.ccell.2017.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cano A, Pérez-Moreno MA, Rodrigo I, Locascio A, Blanco MJ, del Barrio MG, Portillo F, Nieto MA. The transcription factor snail controls epithelial-mesenchymal transitions by repressing E-cadherin expression. Nat Cell Biol. 2000;2:76–83. doi: 10.1038/35000025. [DOI] [PubMed] [Google Scholar]
- Capon DJ, Chen EY, Levinson AD, Seeburg PH, Goeddel DV. Complete nucleotide sequences of the T24 human bladder carcinoma oncogene and its normal homologue. Nature. 1983;302(5903):33–37. doi: 10.1038/302033a0. [DOI] [PubMed] [Google Scholar]
- Cardone MH, Roy N, Stennicke HR, Salvesen GS, Franke TF, Stanbridge E, Frisch S, Reed JC. Regulation of cell death protease caspase-9 by phosphorylation. Science. 1998;282(5392):1318–1321. doi: 10.1126/science.282.5392.1318. [DOI] [PubMed] [Google Scholar]
- Casalino L, De CD, Verde P. Accumulation of Fra-1 in Ras-transformed cells depends on both transcriptional autoregulation and MEK-dependent posttranslational stabilization. Mol Cell Biol. 2003;23:4401–4415. doi: 10.1128/MCB.23.12.4401-4415.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellano E, Downward J. RAS interaction with PI3K: more than just another effector pathway. Genes Cancer. 2011;2:261–274. doi: 10.1177/1947601911408079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellano E, Sheridan C, Thin MZ, Nye E, Spencer-Dene B, Diefenbacher ME, Moore C, Kumar MS, Murillo MM, Gronroos E, Lassailly F, Stamp G, Downward J. Requirement for interaction of PI3-kinase p110a with RAS in lung tumor maintenance. Cancer Cell. 2013;24:617–630. doi: 10.1016/j.ccr.2013.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen KJ, Hou Y, Wang K, Li J, Xia Y, Yang XY, Lv G, Xing XL, Shen F. Reexpression of let-7g microRNA inhibits the proliferation and migration via K-Ras/HMGA2/snail axis in hepatocellular carcinoma. Biomed Res Int. 2014;2014:742417. doi: 10.1155/2014/742417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen SJ, Chen YT, Zeng LJ, Zhang QB, Lian GD, Li JJ, Yang KG, Huang CM, Li YQ, Chu ZH, Huang KH. Bmi1 combines with oncogenic KRAS to induce malignant transformation of human pancreatic duct cells in vitro. Tumour Biol. 2016;37(8):11299–11309. doi: 10.1007/s13277-016-4840-5. [DOI] [PubMed] [Google Scholar]
- Chen Z, Wang X, Liu R, Chen L, Yi J, Qi B, Shuang Z, Liu M, Li X, Li S, Tang H. KDM4B-mediated epigenetic silencing of miRNA-615-5p augments RAB24 to facilitate malignancy of hepatoma cells. Oncotarget. 2017;8(11):17712–17725. doi: 10.18632/oncotarget.10832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherfils J, Zeghouf M. Regulation of small GTPases by GEFs, GAPs, and GDIs. Physiol Rev. 2013;93:269–309. doi: 10.1152/physrev.00003.2012. [DOI] [PubMed] [Google Scholar]
- Chien Y, White MA. RAL GTPases are linchpin modulators of human tumour-cell proliferation and survival. EMBO Rep. 2003;4(8):800–806. doi: 10.1038/sj.embor.embor899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark EA, Golub TR, Lander ES, Hynes RO. Genomic analysis of metastasis reveals an essential role for RhoC. Nature. 2000;406:532–535. doi: 10.1038/35020106. [DOI] [PubMed] [Google Scholar]
- Clarke S. Protein-terminal isoprenylation and methylation at carboxyl cysteine residues. Annu Rev Biochem. 1992;61:355–386. doi: 10.1146/annurev.bi.61.070192.002035. [DOI] [PubMed] [Google Scholar]
- Cox AD, Der CJ. RAS history: the saga continues. Small GTPases. 2010;1(1):2–27. doi: 10.4161/sgtp.1.1.12178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox AD, Fesik SW, Kimmelman AC, Luo J, Der CJ. Drugging the undruggable RAS: mission possible? Nat Rev. 2014;13(11):828–851. doi: 10.1038/nrd4389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz-Meco MT, Lozano J, Municio MM, Berra E, Frutos S, Sanz L, Moscat J. Evidence for the in vitro and in vivo interaction of Ras with protein kinase C zet. J Biol Chem. 1994;269(50):31706–31710. [PubMed] [Google Scholar]
- Diesch J, Sanij E, Gilan O, Love C, Tran H, Fleming NI, Ellul J, Amalia M, Haviv I, Pearson RB, Tulchinsky E, Mariadason JM, Sieber OM, Hannan RD, Dhillon AS. Widespread FRA1-dependent control of mesenchymal transdifferentiation programs in colorectal cancer cells. PLoS One. 2014;9(3):e88950. doi: 10.1371/journal.pone.0088950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edkins S, O’Meara S, Parker A, Stevens C, Reis M, Jones S, Greenman C, Davies H, Dalgliesh G, Forbes S, Hunter C, Smith R, Stephens P, Goldstraw P, Nicholson A, Chan TL, Velculescu VE, Yuen ST, Leung SY, Stratton MR, Futreal PA. Recurrent KRAS codon 146 mutations in human colorectal cancer. Cancer Biol Ther. 2006;5:928–932. doi: 10.4161/cbt.5.8.3251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edme N, Downward J, Thiery JP, Boyer B. Ras induces NBT-II epithelial cell scattering through the coordinate activities of Rac and MAPK pathways. J Cell Sci. 2002;115:2591–2601. doi: 10.1242/jcs.115.12.2591. [DOI] [PubMed] [Google Scholar]
- Eijkelenboom A, Burgering BM. FOXOs: signaling integrators for homeostasis maintenance. Nat Rev Mol Cell Biol. 2013;14:83–97. doi: 10.1038/nrm3507. [DOI] [PubMed] [Google Scholar]
- Feng J, Wang X, Zhu W, Chen S, Feng C. MicroRNA-630 suppresses epithelial-to-mesenchymal transition by regulating FoxM1 in gastric cancer cells. Biochemistry (Mosc) 2017;82(6):707–714. doi: 10.1134/S0006297917060074. [DOI] [PubMed] [Google Scholar]
- Ferro E, Trabalzini L. RalGDS family members couple Ras to Ral signalling and that’s not all. Cell Signal. 2010;22:1804–1810. doi: 10.1016/j.cellsig.2010.05.010. [DOI] [PubMed] [Google Scholar]
- Garg M. Epithelial-mesenchymal transition- activating transcription factors – multifunctional regulators in cancer. World Journal of Stem Cells. 2013;5(4):188–195. doi: 10.4252/wjsc.v5.i4.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garg M. Targeting microRNAs in epithelial mesenchymal transition induced cancer stem cells: therapeutic approaches in cancer. Expert opinion in therapeutic. Targets. 2015;19(2):285–297. doi: 10.1517/14728222.2014.975794. [DOI] [PubMed] [Google Scholar]
- Garg M. Epithelial, mesenchymal and hybrid epithelial/ mesenchymal phenotypes and their clinical relevance in cancer metastasis. Expert Rev Mol Med. 2017;19:e3. doi: 10.1017/erm.2017.6. [DOI] [PubMed] [Google Scholar]
- Gebeshuber CA, Zatloukal K, Martinez J. miR-29a suppresses tristetraprolin, which is a regulator of epithelial polarity and metastasis. EMBO Rep. 2009;10(4):400–405. doi: 10.1038/embor.2009.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Garcia A, Pritchard CA, Paterson HF, Mavria G, Stamp G, Marshall CJ. RALGDS is required for tumor formation in a model of skin carcinogenesis. Cancer Cell. 2005;7:219–226. doi: 10.1016/j.ccr.2005.01.029. [DOI] [PubMed] [Google Scholar]
- Graham TR, Zhau HE, Odero-Marah VA, Osunkoya AO, Kimbro KS, Tighiouart M, Liu T, Simons JW, O'Regan RM. Insulin-like growth factor-independent up-regulation of ZEB1 drives epithelial-to-mesenchymal transition in human prostate cancer cells. Cancer Res. 2008;68(7):2479–2488. doi: 10.1158/0008-5472.CAN-07-2559. [DOI] [PubMed] [Google Scholar]
- Grant ML, Bruton RK, Byrd PJ, Gallimore PH, Steele JC, Taylor AM, Grand RJ. Sensitivity to ionising radiation of transformed human cells containing mutant Ras genes. Oncogene. 1990;5(8):1159–1164. [PubMed] [Google Scholar]
- Greenburg G, Hay ED. Epithelia suspended in collagen gels can lose polarity and express characteristics of migrating mesenchymal cells. J Cell Biol. 1982;95(1):333–339. doi: 10.1083/jcb.95.1.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenburg G, Hay ED. Cytodifferentiation and tissue phenotype change during transformation of embryonic lens epithelium to mesenchyme-like cells in vitro. Dev Biol. 1986;115(2):363–379. doi: 10.1016/0012-1606(86)90256-3. [DOI] [PubMed] [Google Scholar]
- Gupta S, Ramjaun AR, Haiko P, Wang Y, Warne PH, Nicke B, Nye E, Stamp G, Alitalo K, Downward J. Binding of ras to phosphoinositide 3-kinase p110alpha is required for ras-driven tumorigenesis in mice. Cell. 2007;129:957–968. doi: 10.1016/j.cell.2007.03.051. [DOI] [PubMed] [Google Scholar]
- Heid I, Lubeseder-Martellato C, Sipos B, Mazur PK, Lesina M, Schmid RM, Siveke JT. Early requirement of RAC1 in a mouse model of pancreatic cancer. Gastroenterology. 2011;141:719–730.e7. doi: 10.1053/j.gastro.2011.04.043. [DOI] [PubMed] [Google Scholar]
- Herranz N, Pasini D, Díaz VM, Francí C, Gutierrez A, Dave N, Escrivà M, Hernandez-Muñoz I, Di Croce L, Helin K, García de Herreros A, Peiró S. Polycomb complex 2 is required for E-cadherin repression by the Snail1 transcription factor. Mol Cell Biol. 2008;28:4772–4781. doi: 10.1128/MCB.00323-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobbs G, Der C, Rossman K. RAS isoforms and mutations in cancer at a glance. J Cell Sci. 2016;129(7):1287–1292. doi: 10.1242/jcs.182873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H, Daniluk J, Liu Y, Chu J, Li Z, Ji B, Logsdon CD. Oncogenic K-Ras requires activation for enhanced activity. Oncogene. 2014;33(4):532–535. doi: 10.1038/onc.2012.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hüsemann Y, Geigl JB, Schubert F, Musiani P, Meyer M, Burghart E, Forni G, Eils R, Fehm T, Riethmüller G, Klein CA. Systemic spread is an early step in breast cancer. Cancer Cell. 2008;13:58–68. doi: 10.1016/j.ccr.2007.12.003. [DOI] [PubMed] [Google Scholar]
- Jiang HL, Sun HF, Gao SP, Li LD, Hu X, Wu J, Jin W. Loss of RAB1B promotes triple-negative breast cancer metastasis by activating TGF-β/SMAD signaling. Oncotarget. 2015;6(18):16352–16365. doi: 10.18632/oncotarget.3877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanda M, Matthaei H, Wu J, Hong SM, Yu J, Borges M, Hruban RH, Maitra A, Kinzler K, Vogelstein B, Goggins M. Presence of somatic mutations in most early-stage pancreatic intraepithelial neoplasia. Gastroenterology. 2012;142(4):730–733. doi: 10.1053/j.gastro.2011.12.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan DR, Morrison DK, Wong G, McCormick F, Williams LT. PDGF beta-receptor stimulates tyrosine phosphorylation of GAP and association of GAP with a signaling complex. Cell. 1990;61:125–133. doi: 10.1016/0092-8674(90)90220-9. [DOI] [PubMed] [Google Scholar]
- Karaguni IM, Herter P, Debruyne P, Chtarbova S, Kasprzynski A, Herbrand U, Ahmadian MR, Glüsenkamp KH, Winde G, Mareel M, Möröy T, Müller O. The new sulindac derivative IND 12 reverses RAS-induced cell transformation. Cancer Res. 2002;62:1718–1723. [PubMed] [Google Scholar]
- Kato K, Cox AD, Hisaka MM, Graham SM, Buss JE, Der CJ. Isoprenoid addition to Ras protein is the critical modification for its membrane association and transforming activity. Proc Natl Acad Sci U S A. 1992;89(14):6403–6407. doi: 10.1073/pnas.89.14.6403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kent OA, Mendell JT, Rottapel R. Transcriptional regulation of miR-31 by oncogenic K-RAS mediates metastatic phenotypes by repressing RASA1. Mol Cancer Res. 2016;14:267–277. doi: 10.1158/1541-7786.MCR-15-0456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim KLZ, Hay ED. Direct evidence for a role of β-catenin/LEF-1 signaling pathway in induction of EMT. Cell Biol Int. 2002;26:463–476. doi: 10.1006/cbir.2002.0901. [DOI] [PubMed] [Google Scholar]
- Kim RK, Suh Y, Yoo KC, Cui YH, Kim H, Kim MJ, Gyu Kim I, Lee SJ. Activation of K-RAS promotes the mesenchymal features of basal-type breast cancer. Exp Mol Med. 2015;47:e137. doi: 10.1038/emm.2014.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kissil JL, Walmsley MJ, Hanlon L, Haigis KM, Bender Kim CF, Sweet-Cordero A, Eckman MS, Tuveson DA, Capobianco AJ, Tybulewicz VL, Jacks T. Requirement for RAC1 in a KRAS induced lung cancer in the mouse. Cancer Res. 2007;67:8089–8094. doi: 10.1158/0008-5472.CAN-07-2300. [DOI] [PubMed] [Google Scholar]
- Koh M, Woo Y, Valiathan RR, Jung HY, Park SY, Kim YN, Kim HR, Fridman R, Moon A. Discoidin domain receptor 1 is a novel transcriptional target of ZEB1 in breast epithelial cells undergoing H-Ras-induced epithelial to mesenchymal transition. Int J Cancer. 2015;136(6):508–520. doi: 10.1002/ijc.29154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolch W, Heidecker G, Lloyd P, Rapp UR. Raf-1 protein kinase is required for growth of induced NIH/3T3 cells. Nature. 1991;349(6308):426–428. doi: 10.1038/349426a0. [DOI] [PubMed] [Google Scholar]
- Kondoh H, Kamachi Y. SOX-partner code for cell specification: regulatory target selection and underlying molecular mechanisms. Int J Biochem Cell Biol. 2010;42:391–399. doi: 10.1016/j.biocel.2009.09.003. [DOI] [PubMed] [Google Scholar]
- Krebs AM, Mitschke J, LasierraLosada M, Schmalhofer O, Boerries M, Busch H, Boettcher M, Mougiakakos D, Reichardt W, Bronsert P, Brunton VG, Pilarsky C, Winkler TH, Brabletz S, Stemmler MP, Brabletz T. The EMT-activator Zeb1 is a key factor for cell plasticity and promotes metastasis in pancreatic cancer. Nat Cell Biol. 2017;19(5):518–529. doi: 10.1038/ncb3513. [DOI] [PubMed] [Google Scholar]
- Lake D, Correa SA, Muller J. Negative feedback regulation of the ERK1/2 MAPK pathway. Cell Mol Life Sci. 2016;73:4397–4413. doi: 10.1007/s00018-016-2297-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert JM, Lambert QT, Reuther GW, Malliri A, Siderovski DP, Sondek J, Collard JG, Der CJ. Tiam1 mediates Ras activation of Rac by a PI(3)K-independent mechanism. Nat Cell Biol. 2002;4(8):621–625. doi: 10.1038/ncb833. [DOI] [PubMed] [Google Scholar]
- Lange-Carter CA, Johnson GL. Ras-dependent growth factor regulation of MEK kinase in PC12 cells. Science. 1994;265(5177):1458–1461. doi: 10.1126/science.8073291. [DOI] [PubMed] [Google Scholar]
- Lee JM, Dedhar S, Kalluri R, Thompson EW. The epithelial-mesenchymal transition: new insights in signaling, development, and disease. J Cell Biol. 2006;172(7):973–981. doi: 10.1083/jcb.200601018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim KH, Counter CM. Reduction in the requirement of oncogenic RAS signaling to activation of PI3K/AKT pathway during tumor maintenance. Cancer Cell. 2005;8(5):381–392. doi: 10.1016/j.ccr.2005.10.014. [DOI] [PubMed] [Google Scholar]
- Lim KH, O'Hayer K, Adam SJ, Kendall SD, Campbell PM, Der CJ, Counter CM. Divergent roles for RALA and RALB in malignant growth of human pancreatic carcinoma cells. Curr Biol. 2006;16:2385–2394. doi: 10.1016/j.cub.2006.10.023. [DOI] [PubMed] [Google Scholar]
- Lin SR, Tsai JH, Yang YC, Lee SC. Mutations of K-RAS oncogene in human adrenal tumours in Taiwan. Br J Cancer. 1998;77:1060–1065. doi: 10.1038/bjc.1998.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin X, Chen L, Yao Y, Zhao R, Cui X, Chen J, Hou K, Zhang M, Su F, Chen J, Song E. CCL18-mediated down-regulation of miR98 and miR27b promotes breast cancer metastasis. Oncotarget. 2015;6(24):20485–20499. doi: 10.18632/oncotarget.4107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Sánchez-Tilló E, Lu X, Huang L, Clem B, Telang S, Jenson AB, Cuatrecasas M, Chesney J, Postigo A, Dean DC. The ZEB1 transcription factor acts in a negative feedback loop with miR200 downstream of Ras and Rb1 to regulate Bmi1 expression. J Biol Chem. 2014;289(7):4116–4125. doi: 10.1074/jbc.M113.533505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Zhang M, Qian J, Bao M, Meng X, Zhang S, Zhang L, Zhao R, Li S, Cao Q, Li P, Ju X, Lu Q, Li J, Shao P, Qin C, Yin C. miR-134 functions as a tumor suppressor in cell proliferation and epithelial-to-mesenchymal transition by targeting K-RAS in renal cell carcinoma cells. DNA Cell Biol. 2015;34(6):429–436. doi: 10.1089/dna.2014.2629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu X, Guo H, Chen X, Xiao J, Zou Y, Wang W, Chen Q. Effect of RhoC on the epithelial-mesenchymal transition process induced by TGF-β1 in lung adenocarcinoma cells. Oncol Rep. 2016;36(6):3105–3112. doi: 10.3892/or.2016.5146. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Lusk JB, Lam VY, Tolwinski NS. Epidermal growth factor pathway signaling in drosophila embryogenesis: tools for understanding cancer. Cancers (Basel) 2017;9(2):Pii: E16. doi: 10.3390/cancers9020016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma L, Teruya-Feldstein J, Weinberg RA. Tumour invasion and metastasis initiated by microRNA-10b in breast cancer. Nature. 2007;449:682–688. doi: 10.1038/nature06174. [DOI] [PubMed] [Google Scholar]
- Marchetti A, Colletti M, Cozzolino AM, Steindler C, Lunadei M, Mancone C, Tripodi M. RK5/MAPK is activated by TGFβ in hepatocytes and required for the GSK-3β-mediated snail protein stabilization. Cell Signal. 2008;20(11):2113–2118. doi: 10.1016/j.cellsig.2008.08.002. [DOI] [PubMed] [Google Scholar]
- Maruta H, Burgess AW. Regulation of the Ras signaling network. BioEssays. 1996;16(7):139–180. doi: 10.1002/bies.950160708. [DOI] [PubMed] [Google Scholar]
- McKay MM, Morrison DK. Integrating signals from RTKs to ERK/MAPK. Oncogene. 2007;26(22):3113–3121. doi: 10.1038/sj.onc.1210394. [DOI] [PubMed] [Google Scholar]
- McKenna WG, Weiss MC, Endlich B, Ling CC, Bakanauskas VJ, Kelsten ML, Muschel RJ. Synergistic effect of the v-myc oncogene with H-RAS on radioresistance. Cancer Res. 1990;50(1):97–102. [PubMed] [Google Scholar]
- Medarde AF, Santos E. Ras in cancer and developmental diseases. Genes and. Cancer. 2011;2(3):344–335. doi: 10.1177/1947601911411084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller AC, Kariko K, Myers CE, Clark EP, Samid D. Increased radioresistance of EJ Ras-transformed human osteosarcoma cells and its modulation by lovastatin, an inhibitor of p21 Ras isoprenylation. Int J Cancer. 1993;53(3):302–307. doi: 10.1002/ijc.2910530222. [DOI] [PubMed] [Google Scholar]
- Miyakura Y, Sugano K, Fukayama N, Konishi F, Nagai H. Concurrent mutations of K-RAS oncogene at codons 12 and 22 in colon cancer. Jpn J Clin Oncol. 2002;32:219–221. doi: 10.1093/jjco/hyf043. [DOI] [PubMed] [Google Scholar]
- Miyazono K. Transforming growth factor-beta signaling in epithelial-mesenchymal transition and progression of cancer. Proc Jpn Acad Ser B Phys Biol Sci. 2009;85(8):314–323. doi: 10.2183/pjab.85.314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison DK, Cutler RE. The complexity of Raf-1 regulation. Curr Opin Cell Biol. 1997;9(2):174–179. doi: 10.1016/s0955-0674(97)80060-9. [DOI] [PubMed] [Google Scholar]
- Murillo MM, Zelenay S, Nye E, Castellano E, Lassailly F, Stamp G, Downward J. RAS interaction with PI3K p110a is required for tumorinducedangiogenesis. J Clin Invest. 2014;124:3601–3611. doi: 10.1172/JCI74134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naguib A, Wilson CH, Adams DJ, Arends MJ. Activation of K-RAS by co-mutation of codons 19 and 20 is transforming. J Mol Signal. 2011;6:2. doi: 10.1186/1750-2187-6-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oktay M, Wary KK, Dans M, Birge RB, Giancotti FG. Integrin-mediated activation of focal adhesion kinase is required for signaling to Jun NH2-terminal kinase and progression through the G1 phase of the cell cycle. J Cell Biol. 1999;145(7):1461–1469. doi: 10.1083/jcb.145.7.1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouerhani S, Bougatef K, Soltani I, Elgaaied IBA, Abbes S, Menif S. The prevalence and prognostic significance of K-RAS mutation in bladder cancer, chronic myeloid leukemia and colorectal cancer. Mol Biol Rep. 2013;40:4109–4114. doi: 10.1007/s11033-013-2512-8. [DOI] [PubMed] [Google Scholar]
- Peschard P, McCarthy A, Leblanc-Dominguez V, Yeo M, Guichard S, Stamp G, Marshall CJ. Genetic deletion of RALA and RALB small GTPases reveals redundant functions in development and tumorigenesis. Curr Biol. 2012;22:2063–2068. doi: 10.1016/j.cub.2012.09.013. [DOI] [PubMed] [Google Scholar]
- Prior I, Lewis P, Mattos C. A comprehensive survey of Ras mutations in cancer. Cancer Res. 2012;72(10):2457–2467. doi: 10.1158/0008-5472.CAN-11-2612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi L, Sun B, Liu Z, Cheng R, Li Y, Zhao X. Wnt3a expression is associated with epithelial-mesenchymal transition and promotes colon cancer progression. J Exp Clin Cancer Res. 2014;33(1):10. doi: 10.1186/s13046-014-0107-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridley AJ, Paterson HF, Johnston CL, Diekmann D, Hall A. The small GTP-binding protein rac regulates growth factor-induced membrane ruffling. Cell. 1992;70(3):401–410. doi: 10.1016/0092-8674(92)90164-8. [DOI] [PubMed] [Google Scholar]
- Rinehart-Kim J, Johnston M, Birrer M, Bos T. Alterations in the gene expression profile of MCF-7 breast tumor cells in response to c-Jun. Int J Cancer. 2000;88:180–190. doi: 10.1002/1097-0215(20001015)88:2<180::aid-ijc6>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- Roux PP, Blenis J. ERK and p38 MAPK-activated protein kinases: a family of protein kinases with diverse biological functions. Microbiol Mol Biol Rev. 2004;68:320–344. doi: 10.1128/MMBR.68.2.320-344.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozengurt E. Mitogenic signaling pathways induced by G protein-coupled receptors. J Cell Physiol. 2007;213(3):589–602. doi: 10.1002/jcp.21246. [DOI] [PubMed] [Google Scholar]
- Saitoh M, Endo K, Furuya S, Minami M, Fukasawa A, Imamura T, Miyazawa K. STAT3 integrates cooperative Ras and TGF-β signals that induce snail expression. Oncogene. 2016;35(8):1049–1057. doi: 10.1038/onc.2015.161. [DOI] [PubMed] [Google Scholar]
- Santamaria PG, Moreno-Bueno G, Portillo F, Cano A. EMT: present and future in clinical oncology. Mol Oncol. 2017;11(7):718–738. doi: 10.1002/1878-0261.12091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saridaki Z, Weidhaas JB, Lenz HJ, Laurent-Puig P, Jacobs B, de Schutter J, de Roock W, Salzman DW, Zhang W, Yang D, Pilati C, Bouché O, Piessevaux H, Tejpar S. A let-7 microRNA-binding site polymorphism in K-RAS predicts improved outcome in patients with metastatic colorectal cancer treated with salvage cetuximab/panitumumab monotherapy. Clin Cancer Res. 2014;20:4499–4510. doi: 10.1158/1078-0432.CCR-14-0348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaap D, van der Wal J, Howe LR, Marshall CJ, van Blitterswijk WJ. A dominant-negative mutant of raf blocks mitogen-activated protein kinase activation by growth factors and oncogenic p21Ras. J Biol Chem. 1993;268(27):20232–20236. [PubMed] [Google Scholar]
- Shields MA, Ebine K, Sahai V, Kumar K, Siddiqui K, Hwang RF, Grippo PJ, Munshi HG (2013). Snail cooperates with KrasG12D to promote pancreatic fibrosis. Mol Cancer Res 2013 11(9):1078–1087 [DOI] [PMC free article] [PubMed]
- Simanshu DK, Nissley DV, McCormick F. RAS proteins and their regulators in human disease. Cell. 2017;170(1):17–33. doi: 10.1016/j.cell.2017.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh A, Greninger P, Rhodes D, Koopman L, Violette S, Bardeesy N, Settleman J. A gene expression signature associated with “K-RAS addiction” reveals regulators of EMT and tumor cell survival. Cancer Cell. 2009;15(6):489–500. doi: 10.1016/j.ccr.2009.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sklar MD. The Ras oncogenes increase the intrinsic resistance of NIH 3T3 cells to ionizing radiation. Science. 1988;239(4840):645–647. doi: 10.1126/science.3277276. [DOI] [PubMed] [Google Scholar]
- Smith G, Bounds R, Wolf H, Steele RJC, Carey FA, Wolf CR. Activating K-RAS mutations outwith ‘hotspot’ codons in sporadic colorectal tumours – implications for personalised cancer medicine. Br J Cancer. 2010;102:693–703. doi: 10.1038/sj.bjc.6605534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song C, Satoh T, Edamatsu H, Wu D, Tadano M, Gao X, Kataoka T. Differential roles of Ras and Rap1 in growth factor-dependent activation of phospholipase C epsilon. Oncogene. 2002;21(53):8105–8113. doi: 10.1038/sj.onc.1206003. [DOI] [PubMed] [Google Scholar]
- Stephen AG, Esposito D, Bagni RK, McCormick F. Dragging RAS back in the ring. Cancer Cell. 2002;25:272–281. doi: 10.1016/j.ccr.2014.02.017. [DOI] [PubMed] [Google Scholar]
- Stinson S, Lackner MR, Adai AT, Yu N, Kim HJ, O'Brien C, Spoerke J, Jhunjhunwala S, Boyd Z, Januario T, Newman RJ, Yue P, Bourgon R, Modrusan Z, Stern HM, Warming S, de Sauvage FJ, Amler L, Yeh RF, Dornan D. miR-221/222 targeting of trichorhinophalangeal 1 (TRPS1) promotes epithelial-to-mesenchymal transition in breast cancer. Sci Signal. 2011;l4(186):pt5. doi: 10.1126/scisignal.2002258. [DOI] [PubMed] [Google Scholar]
- Tall GG, Barbieri MA, Stahl PD, Horazdovsky BF. Ras-activated endocytosis is mediated by the Rab5 guanine nucleotide exchange activity of RIN1. Dev Cell. 2001;1(1):73–82. doi: 10.1016/s1534-5807(01)00008-9. [DOI] [PubMed] [Google Scholar]
- Tape CJ, Ling S, Dimitriadi M, McMahon KM, Worboys JD, Leong HS, Norrie IC, Miller CJ, Poulogiannis G, Lauffenburger DA, Jørgensen C. Oncogenic KRAS regulates tumor cell signaling via stromal reciprocation. Cell. 2016;165(4):910–920. doi: 10.1016/j.cell.2016.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tothova Z, Ebert BL. Doubling down on mutant RAS can MEK or break leukemia. Cell. 2017;168(5):749–750. doi: 10.1016/j.cell.2017.02.013. [DOI] [PubMed] [Google Scholar]
- Trahey M, McCormick F. A cytoplasmic protein stimulates normal N-RASp21 GTPase, but does not affect oncogenic mutants. Science. 1987;238(4826):542–545. doi: 10.1126/science.2821624. [DOI] [PubMed] [Google Scholar]
- Tulchinsky E, Pringle JH, Caramel J, Ansieau S. Plasticity of melanoma and EMT-TF reprogramming. Oncotarget. 2014;5(1):1–2. doi: 10.18632/oncotarget.1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varras MN, Koffa M, Koumantakis E, Ergazaki M, Protopapa E, Michalas S, Spandidos DA. Ras gene mutations in human endometrial carcinoma. Oncology. 1996;53(6):505–510. doi: 10.1159/000227627. [DOI] [PubMed] [Google Scholar]
- Verde P, Casalino L, Talotta F, Yaniv M, Weitzman JB. Deciphering AP-1 function in tumorigenesis: fra-ternizing on target promoters. Cell Cycle. 2007;6(21):2633–2639. doi: 10.4161/cc.6.21.4850. [DOI] [PubMed] [Google Scholar]
- Vos MD, Ellis CA, Elam C, Ulku AS, Taylor BJ, Clark GJ. RASSF2 is a novel K-RAS-specific effector and potential tumor suppressor. J Biol Chem. 2003;278(30):28045–28051. doi: 10.1074/jbc.M300554200. [DOI] [PubMed] [Google Scholar]
- Vos MD, Martinez A, Ellis CA, Vallecorsa T, Clark GJ. The proapoptotic Ras effector Nore1 may serve as a Ras-regulated tumor suppressor in the lung. J Biol Chem. 2003;278(24):21938–21943. doi: 10.1074/jbc.M211019200. [DOI] [PubMed] [Google Scholar]
- Wanami LS, Chen HY, Peiró S, García de Herreros A, Bachelder RE. Vascular endothelial growth factor-a stimulates snail expression in breast tumor cells: implications for tumor progression. Exp Cell Res. 2008;314(13):2448–2453. doi: 10.1016/j.yexcr.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsch ME, Kaplan A, Chambers JM, Stokes ME, Bos PH, Zask A, Zhang Y, Sanchez-Martin M, Badgley MA, Huang CS, Tran TH, Akkiraju H, Brown LM, Nandakumar R, Cremers S, Yang WS, Tong L, Olive KP, Ferrando A, Stockwell BR. Multivalent small-molecule pan-RAS inhibitors. Cell. 2017;168(5):878–889.e29. doi: 10.1016/j.cell.2017.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whyte DB, Kirschmeier P, Hockenberry TN, Nunez-Oliva I, James L, Catino JJ, Bishop WR, Pai JK. K- and N-RAS are geranylgeranylated in cells treated with farnesyl protein transferase inhibitors. J Biol Chem. 1997;272:14459–14464. doi: 10.1074/jbc.272.22.14459. [DOI] [PubMed] [Google Scholar]
- Yamauchi J, Miyamoto Y, Tanoue A, Shooter EM, Chan JR. Ras activation of a Rac1 exchange factor, Tiam1, mediates neurotrophin-3- induced Schwann cell migration. Proc Natl Acad Sci U S A. 2005;102(41):14889–14894. doi: 10.1073/pnas.0507125102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan M, Dai T, Deak JC, Kyriakis JM, Zon LI, Woodgett JR, Templeton DJ. Activation of stress-activated protein kinase by MEKK1 phosphorylation of its activator SEK1. Nature. 1994;372(6508):798–800. doi: 10.1038/372798a0. [DOI] [PubMed] [Google Scholar]
- Yang L, Lin C, Liu ZR. p68 RNA helicase mediates PDGF-induced epithelial mesenchymal transition by displacing Axin from beta-catenin. Cell. 2006;127(1):139–155. doi: 10.1016/j.cell.2006.08.036. [DOI] [PubMed] [Google Scholar]
- Yang F, Sun L, Li Q, Han X, Lei L, Zhang H, Shang Y. SET8 promotes epithelial-mesenchymal transition and confers TWIST dual transcriptional activities. EMBO J. 2012;31:110–123. doi: 10.1038/emboj.2011.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye K, Wang S, Zhang H, Han H, Ma B, Nan W (2017) Long noncoding RNA GAS5 suppresses cell growth and epithelial-mesenchymal transition in osteosarcoma by regulating the miR-221/ARHI pathway. J Cell Biochem. 10.1002/jcb.26145 [DOI] [PubMed]
- Zhadanov AB, Provance DW, Jr, Speer CA, Coffin JD, Goss D, Blixt JA, Reichert CM, Mercer JA. Absence of the tight junctional protein AF-6 disrupts epithelial cell–cell junctions and cell polarity during mouse development. Curr Biol. 1999;l9(16):880–888. doi: 10.1016/s0960-9822(99)80392-3. [DOI] [PubMed] [Google Scholar]
- Zhang C, Spevak W, Zhang Y, Burton EA, Ma Y, Habets G, Zhang J, Lin J, Ewing T, Matusow B, Tsang G, Marimuthu A, Cho H, Wu G, Wang W, Fong D, Nguyen H, Shi S, Womack P, Nespi M, Shellooe R, Carias H, Powell B, Light E, Sanftner L, Walters J, Tsai J, West BL, Visor G, Rezaei H, Lin PS, Nolop K, Ibrahim PN, Hirth P, Bollag G. RAF inhibitors that evade paradoxical MAPK pathway activation. Nature. 2015;526:583–586. doi: 10.1038/nature14982. [DOI] [PubMed] [Google Scholar]
- Zhang K, Myllymäki SM, Gao P, Devarajan R, Kytölä V, Nykter M, Wei GH, Manninen A. Oncogenic K-Ras upregulates ITGA6 expression via FOSL1 to induce anoikis resistance and synergizes with αV-class integrins to promote EMT. Oncogene. 2017;36(41):5681–5694. doi: 10.1038/onc.2017.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Prakash P, Liang H, Cho KJ, Gorfe AA, Hancock JF. Lipid-sorting specificity encoded in K-Ras membrane anchor regulates signal output. Cell. 2017;168:239–251. doi: 10.1016/j.cell.2016.11.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu S, Zhang J, Xu F, Xu E, Ruan W, Ma Y, Huang Q, Lai M. IGFBP-rP1 suppresses epithelial-mesenchymal transition and metastasis in colorectal cancer. Cell Death Dis. 2015;6:e1695. doi: 10.1038/cddis.2015.59. [DOI] [PMC free article] [PubMed] [Google Scholar]