Abstract
Syringic acid (SA), a phenolic acid, has been used in Chinese and Indian medicine for treating diabetes but its role in osteogenesis has not yet been investigated. In the present study, at the molecular and cellular levels, we evaluated the effects of SA on osteoblast differentiation. At the cellular level, there was increased alkaline phosphatase (ALP) activity and calcium deposition by SA treatment in mouse mesenchymal stem cells (mMSCs). At the molecular level, SA treatment of these cells stimulated expression of Runx2, a bone transcription factor, and of osteoblast differentiation marker genes such as ALP, type I collagen, and osteocalcin. It is known that Smad7 is an antagonist of TGF-β/Smad signaling and is a negative regulator of Runx2. microRNAs (miRNAs) play a key role in the regulation of osteogenesis genes at the post-transcriptional level and studies have reported that Smad7 is one of the target genes of miR-21. We found that there was down regulation of Smad7 and up regulation of miR-21 in SA-treated mMSCs. We further identified that the 3′-untranslated region (UTR) of Smad7 was directly targeted by miR-21 in these cells. Thus, our results suggested that SA promotes osteoblast differentiation via increased expression of Runx2 by miR-21-mediated down regulation of Smad7. Hence, SA may have potential in orthopedic applications.
Keywords: Bone, miR-21, Runx2, Smad7, Syringic acid
Introduction
Bone is a dynamic tissue which undergoes formation and resorption, simultaneously. It carries out a number of important functions in the body such as support, locomotion, calcium and phosphate storage, and protection of vital organs. Bone tissue is made up of four cell types, namely, osteoblasts, osteoclasts, osteocytes, and bone lining cells. Osteoblasts make up 4–6% of bone cells and are known as bone forming cells. These cells are responsible for secreting osteoid, which results in the formation of new bone. Osteocytes are the most abundant cells found in bone tissue comprising 90–95% of the total tissue. These cells are responsible for regulating osteoblast and osteoclast function, maintaining calcium and phosphate ion homeostasis, and acting as sensory cells which regulate the loading and unloading of bone in response to stimuli (Stevens 2008; Raggatt and Partridge 2010; Boonrungsiman et al. 2012; Florencio-Silva et al. 2015; Nakashima 2016; Katsimbri 2017; Chen et al. 2017a, b; Vishal et al. 2017a; Ono and Takayanagi 2017; Boudin and Van Hul 2017).
Naturally-occurring phytochemicals have several beneficial effects on human health. Plants are the major source of flavonoids and polyphenols (Pandey and Rizvi 2009). Almost 8000 polyphenols have been reported and, of these, only 500 have been shown to be bio-active (Dudaric et al. 2015). Polyphenols help in protection of the bone by upregulation of osteoblastogenesis and downregulation of osteoclastogenesis via several mechanisms involving the action of antioxidants and osteo-immunological activity (Santangelo et al. 2007). One of the functions of polyphenols is to scavenge reactive oxygen species (ROS) and to promote osteoblast differentiation by increasing levels of Runx2, osteocalcin (OC), Wnt, and IGF-1, and also by suppressing RANKL, TRAP, and MMPs expression (Santangelo et al. 2007; Zhou et al. 2015). Defects in Runx2 cause various skeletal disorders including osteoporosis. Runx2 is one of the predominant factors in bone which is required for osteoblast differentiation (Harada et al. 1999; Komori 2010; Muthusami et al. 2011; Moorthi et al. 2013; Vimalraj et al. 2014, 2015; Vishal et al. 2017b; Selvamurugan et al. 2017; Choi et al. 2017; Ling et al. 2017).
MicroRNAs (miRNAs) are small non-coding RNAs, 22–25 nucleotides in length, that regulate gene expression at the post-transcriptional level (Vimalraj and Selvamurugan 2012; Moorthi et al. 2013; Vimalraj et al. 2014; Vimalraj and Selvamurugan 2014; Sainitya et al. 2015; Fang et al. 2015; Selvamurugan et al. 2017; Vishal et al. 2017b; Sera and zur Nieden 2017; Luo et al. 2017). A small number of studies have shown that miRNA expression can be regulated by different polyphenols (Milenkovic et al. 2012; Parasramka et al. 2012; Baselga-Escudero et al. 2014; Zhou and Lin 2014). In this study, we selected syringic acid (3,5-dimethoxy-4-hydroxybenzoic acid; SA), a phenolic acid found in olives, walnuts, and dates. It exhibits several positive effects on human health. SA has strong antioxidant, antihypertensive, anti-proliferative, anti-endotoxic, anti-cancer, hepatoprotective, and anti-hyperglycemic activities. While previous studies have established the antidiabetic properties of SA, to our knowledge, no studies have been conducted regarding its effects on osteogenesis. Hence, the aim of this study was to determine the osteogenic properties of SA and the molecular mechanisms responsible for SA-mediated osteoblast differentiation in mouse mesenchymal stem cells (mMSCs).
Materials and methods
Materials
Cell culture reagents such as DMEM, phosphate buffered saline (PBS), and trypsin were purchased from Lonza, Basel, Switzerland. The antibodies for Runx2, Smad7, HDAC4 and α-tubulin were obtained from Santa Cruz Biotechnology, Dallas, Texas, USA. Syringic acid and other chemicals for staining were obtained from Sigma (St. Louis, MI, USA). A cDNA synthesis kit was sourced from BioRad, Hercules, CA, USA. SYBR green was obtained from Clontech, Mountain view, California, USA. The primers for Runx2 and RPL13A were synthesized by Sigma, St. Louis, Missouri, USA. mMSCs (C3H10T1/2) were obtained from (NCBS, Pune, India). The cells were maintained in 10% fetal bovine serum (FBS) containing DMEM medium at 37 °C in a humidified atmosphere of 5% CO2 and 95% air.
MTT assay
A direct MTT assay was carried out to test the cytotoxicity of SA as described previously (Dhivya et al. 2015; Leena et al. 2017). mMSCs were cultured in DMEM for 2 d. The media was discarded and 200 μl of 0.05% MTT solution was added to each well. The cells were then incubated for 1 h at 37 °C. DMSO was added to solubilize the formazan crystals and the OD was measured using a spectrophotometer at 570 nm.
ALP staining
ALP activity of the differentiated mMSCs was evaluated as described previously (Vimalraj et al. 2014). The cells were then cultured for 7 d. Upon the completion of the incubation period, the cell-seeded wells were washed with 1 × PBS then fixed with 10% formalin and 5-bromo-4-chloro-3-indolyl phosphate/nitro blue tetrazolium (BCIP-NBT; Sigma-Aldrich, St. Louis, Missouri, USA) was added and incubated for 2 h. Stained cells were identified under inverted light microscopy as blue in color and were quantified at 620 nm by dissolving the stains using KOH:DMSO.
Alizarin red staining
Analysis of calcium deposits caused by differentiated and mineralized osteoblasts was performed with alizarin red staining, as described previously (Sainitya et al. 2015; Vimalraj et al. 2016; Leena et al. 2017; Saravanan et al. 2017). mMSCs were cultured for 14 d with change of fresh medium once every three days. The cells were then washed with 1 × PBS and fixed with 10% formalin for 30 min, then rehydrated with 1 mL of distilled water for 5 min. The fixed monolayer of cells was then incubated with 1% alizarin red (pH 4.2) in 2% ethanol for 10 min at room temperature. 1 mL of 70% ethanol was added and allowed to stand for 2 min. The calcified nodules stained red in colour, and micrographs were obtained by inverted light microscopy. The stained cells were also quantified at 405 nm by using 1 N acetic acid at 80 °C and neutralized with liquid ammonia.
Von Kossa staining
Analysis of calcium deposits caused by differentiated and mineralized osteoblasts was conducted by von Kossa staining, as described previously (Sowjanya et al. 2013; Kumar et al. 2014; Saravanan et al. 2015). mMSC were cultured in 6-well plates for 14 d with replenishment of fresh medium once every three days. At the end of the incubation period, cells were washed twice with 1 × PBS, fixed in 10% formalin for 10 min, and washed once with water. Water was removed, 1% silver nitrate solution was added, and the plate was exposed to UV light for 20 min and then rinsed with water. Sodium thiosulfate (5%) was added and after 5 min, the plate was rinsed in water. Calcium phosphate deposits were found as black colored spots. Image J software was used to quantify calcium phosphate deposits, and the percentage of the mineralization area was plotted.
Reverse transcriptase real-time PCR
mMSCs were cultured in 6-well plates and treated with SA at different concentrations (100, 150, and 300 μM). Total RNA was isolated using a kit from Sigma using the TRIZOL method. Reverse transcriptase was carried out using reverse transcription reagents and cDNA was prepared as described previously (Arumugam et al. 2017). qPCR was performed using Quant Studio 3 Real-Time PCR System (Life technologies, Carlsbad, California, United States) which allowed real-time quantitative detection of the PCR products by measuring the increase in SYBR green fluorescence. This was due to binding of SYBR green to double-stranded DNA. The Ct value was calculated. The relative expression of Runx2, ALP, Col1 and OC was then calculated after normalization with a house keeping gene, RPL13. The primers used for real-time PCR in the study are given in Tables 1 and 2.
Table 1.
Primers used for real time PCR in this study
| S.No. | Gene | Sequence (5′-3′) |
|---|---|---|
| 1 | Runx2 | Forward -CGGTCTCCTTCCAGGATGGT |
| Reverse -GCTTCCGTCAGCGTCAACA | ||
| 2 | RPL13AB | Forward -CCTGTTTCCGTAGCCTCATG |
| Reverse -AAGTACCAGGCAGTGACAG | ||
| 3 | ALP | Forward -TTGTGCCAGAGAAAGAGAGAGA |
| Reverse -GTTTCAGGGCATTTTTCAAGGT | ||
| 4 | OC | Forward -ATGGCTTGAAGACCGCCTAC |
| Reverse -AGGGCAGAGAGAGAGGACAG | ||
| 5 | Col-I A1 | Forward -TAACCCCCTCCCCAGCCACAAA |
| Reverse –TTCCTCTTGGCCGTGCGTCA | ||
| 6. | Osterix | Forward –ACTGGCTAGGTGGTGGTCAG |
| Reverse –GGTAGGGAGCTGGGTTAAGG |
Table 2.
Primers used for detection of precursor miRNAs in real time PCR in this study
| S.No | miRNA | Sequence (5′-3′) |
|---|---|---|
| 1 | mir-30-c-1 | Forward-TGTGTAAACATCCTACAGTCTCAG |
| Reverse-GAGTAAACAACCCTCTCCCA | ||
| 2 | mir-222 | Forward -GCTCAGTAGCCAGTGTAGA |
| Reverse -AGACCCAGTACGCAGATGTA | ||
| 3 | mir-21 | Forward -GCTTATCAGACTGATGTTGACTG |
| Reverse -CAGCCCATCGACTGGTG |
Western blot analysis
Whole cell lysates were prepared using RIPA lysis buffer (Bio Basic, Markhan, Ontario, Canada) and the protein concentrations were determined by the Bradford method. The protein samples were loaded onto 12% polyacrylamide-SDS gels. After size resolution, the proteins were transferred electrophoretically to polyvinylidene difluoride membrane (PVDF) (Bio-Rad, Hercules, California, USA). After blocking in Tris-buffered saline containing 5% (w/v) non-fat dry milk, the membrane was exposed to primary antibody overnight at 4 °C. The membrane was then washed and exposed to horseradish peroxidase (HRP)-conjugated secondary antibody. Immunoreactive signals were visualized using an enhanced chemiluminescence detection kit (WESTAR Supernova; Cyanagen, Bologna, Italia) with Chemidoc instrument (Biorad, Hercules, California, USA). The blot was stripped and probed again with α-tubulin antibody for normalization.
Luciferase reporter assay
The wild-type 3′ -UTR of Smad7 (sense: 5’–AAACTAGCGCCCGCTGTTTAGACTTTAA CATAAGCTAT-3′, antisense: 3’–TTTGATCGCCGGCGACAAATCTGAAATTGTATTC GATAGATC-5′), and mutant 3’-UTR of Smad7 (sense: 5′ –AAACTAGCGCCCGCTGTTT AGACTTTAACATAATTTAT-3′, antisense: 3’–TTTGATCGCCGGCGACAAATCTGAA ATTGTATAAGATAGATC-5′) were synthesized chemically. The nucleotides which are mutated are given in bold. Primers of sense and antisense containing an internal NotI site were annealed and cloned using PmeI and XbaI restriction sites which present in the dual-luciferase miRNA expression vector pmirGLO (Promega, Madison, WI). Cloned 3’-UTRs were present in downstream of the Firefly luciferase gene. Renilla luciferase was a constitutively expressed gene which present in the pmirGLO vector system. Not1 digestion was performed to identify the clones. Cells were transiently transfected with 200 ng of the wild-type or mutant Smad7 3′ -UTR constructs along with miR-21-5p mimic at a concentration of 50 nM(HMI0371, Sigma-Aldrich, St. Louis, Missouri, USA) or control miRNA by using Lipofectamine 2000 transfection reagent (Invitrogen, Grand Island, NY). After 24 h of transfection, whole cell lysates were collected in 1× passive lysis buffer, and luciferase activity was analyzed in a SYNERGY/HTX multi-mode reader (BioTek, Winooski, VT) using a dual luciferase reporter assay kit (Promega, Madison, WI). Renilla luciferase activity was used for normalization.
Statistical analysis
Statistical analysis was carried out using Student’s t-test and one-way ANOVA. The p value of ≤0.05 was considered as significant comparing each of the group.
Results
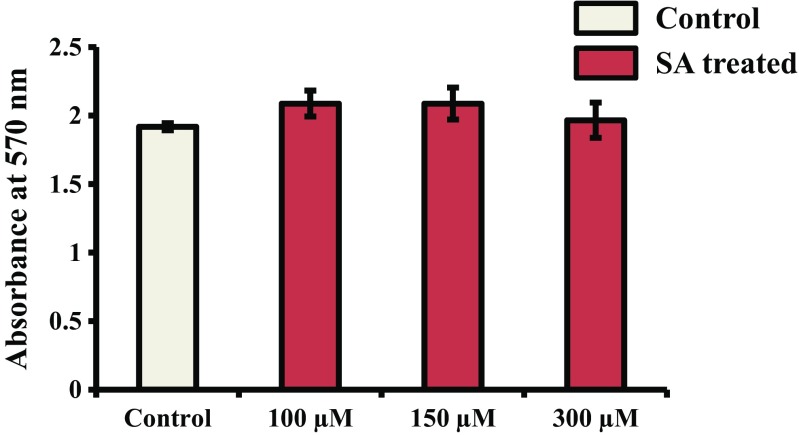
SA at three different concentrations (100, 150, and 300 μM) was used to check the cytotoxicity of SA to mMSCs. The results showed that cells treated with SA at the above concentrations had no change in OD value compared to control or untreated cells, suggesting the nontoxic nature of SA to mMSCs (Fig. 1).
Fig. 1.
Cytotoxicity assessment of SA. mMSCs were treated with SA at different concentrations (100, 150, and 300 μM) for 48 h. The MTT assay was then performed as described in the methods section
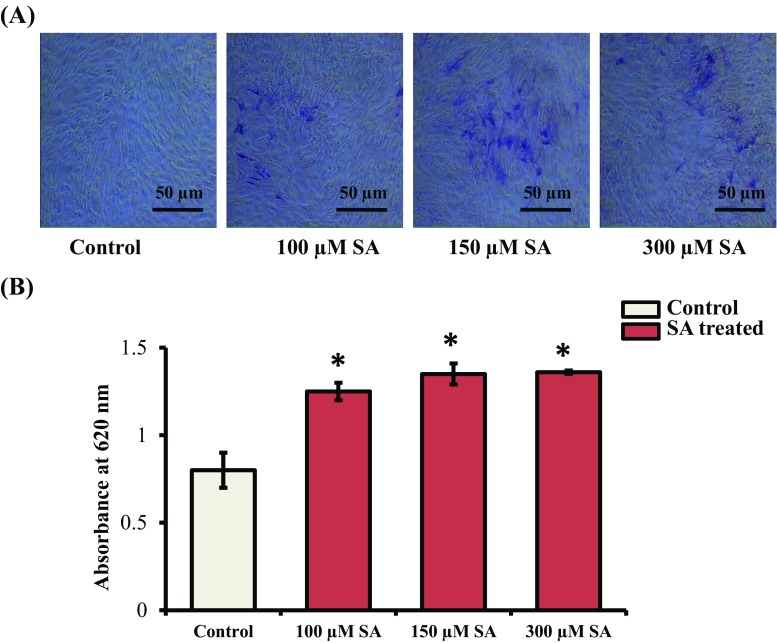
Even though the anti-diabetic properties of SA have been reported (Muthukumaran et al. 2013), its effects on osteogenesis have not been determined. To discern the effects of SA on osteoblast differentiation at the cellular level, mMSCs were treated with SA at nontoxic concentrations (100, 150, and 300 μM) for 7 d. ALP activity was determined using the BCIP-NBT assay. It was found that SA treatment at each of these concentrations increased ALP activity in mMSCs (Fig. 2A). The blue-violet staining of cells was quantified and the results showed a significant increase in ALP activity following SA treatment in these cells (Fig. 2B).
Fig. 2.
Effect of SA on ALP activity. (A) mMSCs were treated with SA at different concentrations (100, 150, and 300 μM) in DMEM media for 7 d. The cells were then stained with BCIP-NBT reagent. Images were obtained using an inverted microscope. (B) Quantification of ALP activity done by the calorimetric method at 620 nm. *indicates a significant increase compared to control (p < 0.05)
To further explore the potential nature of SA on osteoblast differentiation, Alizarin red and von Kossa staining were performed. Alizarin red staining results showed more calcium deposition when mMSCs were treated with SA for 14 d (Fig. 3A). The quantitative analysis of calcium deposits at 405 nm showed a significant increase in mineralization (Fig. 3B). von Kossa staining also revealed more accumulation of calcium after SA treatment of mMSCs (Fig. 3C). A significant increase in calcium deposits was found at concentrations of 100 and 150 μM SA in these cells (Fig. 3D). It appears that the effect of SA treatment at 150 μM was found to be maximal during osteoblast differentiation of mMSCs (Figs. 2 and 3).
Fig. 3.
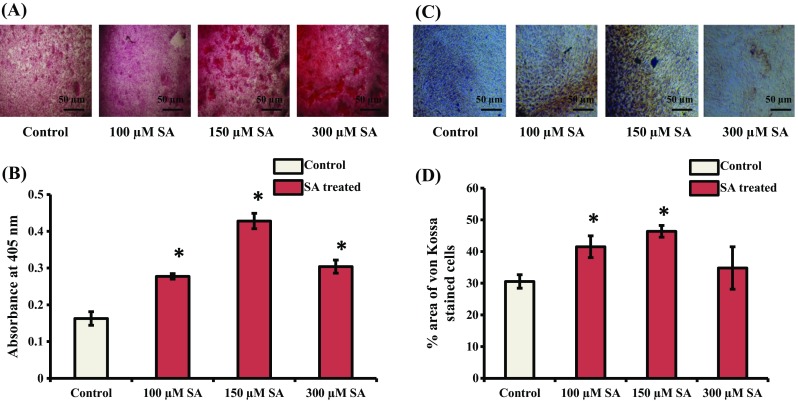
Effect of SA on Alizarin red and von Kossa staining of cells. (A) mMSCs were treated with SA at different concentrations (100, 150, and 300 μM) in DMEM media for 14 d. The cells were then subjected to Alizarin red staining. (B) Quantification analysis of cells stained using Alizarin red. (C) mMSCs were treated with SA at different concentrations (100, 150, and 300 μM) in DMEM media containing osteogenic supplements for 14 d. The cells were then subjected to von Kossa staining. (D) Calorimetric estimation of von Kossa stained cells. *indicates a significant increase compared to control (p < 0.05)
Since SA treatment of mMSCs promoted osteoblast differentiation at a cellular level (Figs. 2 and 3), we next investigated this effect at the molecular level. Osteoblast differentiation from MSCs is regulated by a complexity of transcription factors and signaling proteins, including Runx2, Osterix (Zhang 2010). Runx2 is a bone transcription factor and is essential for expression of osteoblast differentiation marker genes (Zhao et al. 2005; Javed et al. 2009; Karsenty et al. 2009; Muthusami et al. 2011; Vimalraj et al. 2014, 2015; Feng et al. 2016; Wu et al. 2017). To determine the effect of SA on Runx2 expression, mMSCs were treated with SA 150 μM for 24 h. Total RNA and protein were isolated and subjected to reverse transcriptase qPCR (RT-qPCR) and western blot analyses, respectively. The results showed that SA treatment stimulated the expression of Runx2 at the mRNA and protein levels in mMSCs (Fig. 4A and B). We noted that SA stimulated the expression of both Runx2 mRNA and protein only at the 150 μM concentration (Fig. 4).
Fig. 4.
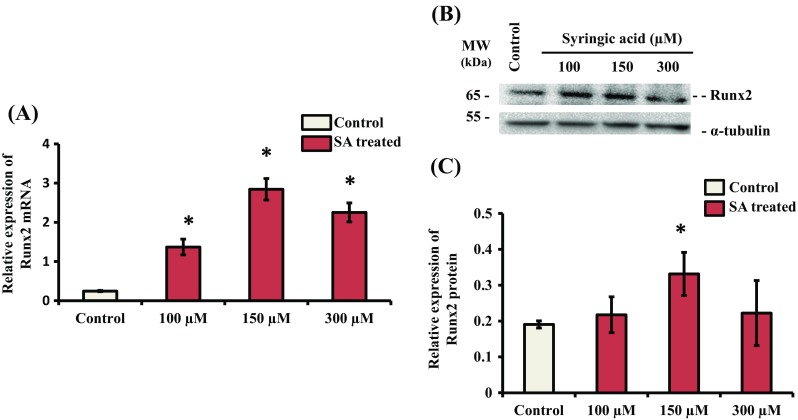
Effect of SA on the expression of Runx2. mMSCs were treated with SA at different concentrations (100, 150 and 300 μM) in DMEM media for 24 h. (A) Total RNA was isolated, and cDNA was synthesized and subjected to RT-qPCR analysis using Runx2 gene primers. RPL13A was used as an internal control for normalization. *indicates a significant increase compared to control (p < 0.05). (B) Whole cell lysates were prepared and subjected to western blot analysis using Runx2 antibody. α-tubulin was used as an internal control for normalization. (C) Relative quantification analysis of biological triplicate samples. *indicates a significant increase compared to control (p < 0.05)
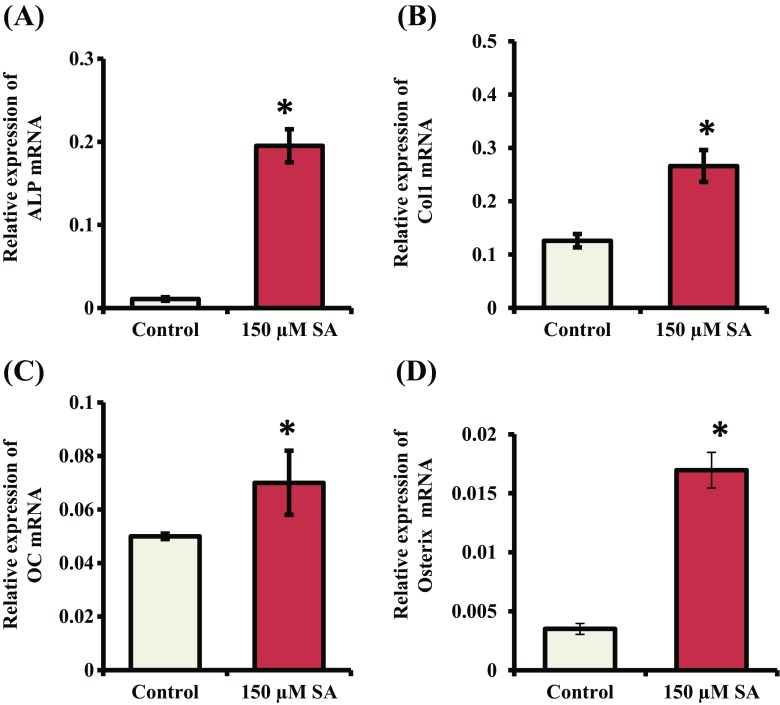
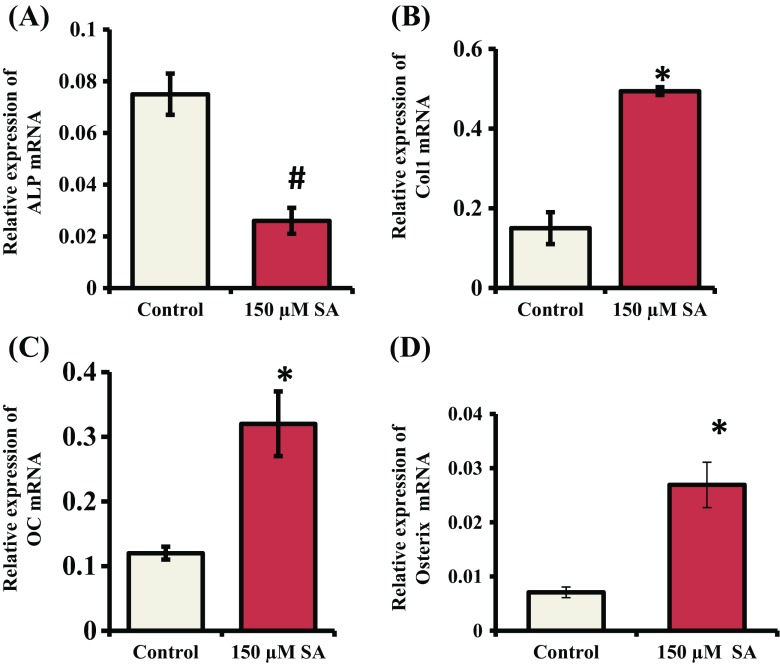
Since Runx2 contributes a role in increasing expression of osteoblast differentiation marker genes (ALP, Col-I, OC) and SA treatment stimulated the expression of Runx2 maximally at 150 μM concentration in mMSCs (Fig. 4), we next determined the effects of SA on the expression of osteoblast marker genes. mMSCs were treated with SA at 150 μM for 7 and 14 d. Total RNA was isolated and subjected to RT-qPCR analysis. mRNA expression of ALP, Col-I and OC was significantly increased when mMSCs were treated with SA for 7 d (Fig. 5A, B and C). After 14 d of SA treatment, mRNA expression of Col-I and OC in mMSCs was significantly increased (Fig. 6A, B and C). Since Osterix is a downstream gene of Runx2 and is also involved in osteoblast differentiation (Zhang 2010), we determined its expression. The result showed that SA treatment for 7 and 14 d stimulated the expression of Osterix at the mRNA level in mMSCs (Figs. 5D and 6D).
Fig. 5.
Effect of SA on the expression of osteoblast differentiation marker genes and Osterix. mMSCs were treated with SA at 150 μM in DMEM media for 7 d. Total RNA was isolated and subjected to RT-qPCR analysis using primers for (A) ALP, (B) Col-I, (C) OC and (D) Osterix genes. RPL13A was used as an internal control for normalization. *indicates a significant increase compared to control (p < 0.05)
Fig. 6.
Effect of SA on the expression of osteoblast differentiation marker genes and Osterix. mMSCs were treated with SA at 150 μM in DMEM media for 14 d. Total RNA was isolated and subjected to RT-qPCR analysis using the primers for (A) ALP, (B) Col-I, (C) OC and (D) Osterix genes. RPL13A was used as an internal control for normalization. *indicates a significant increase compared to control (p < 0.05)
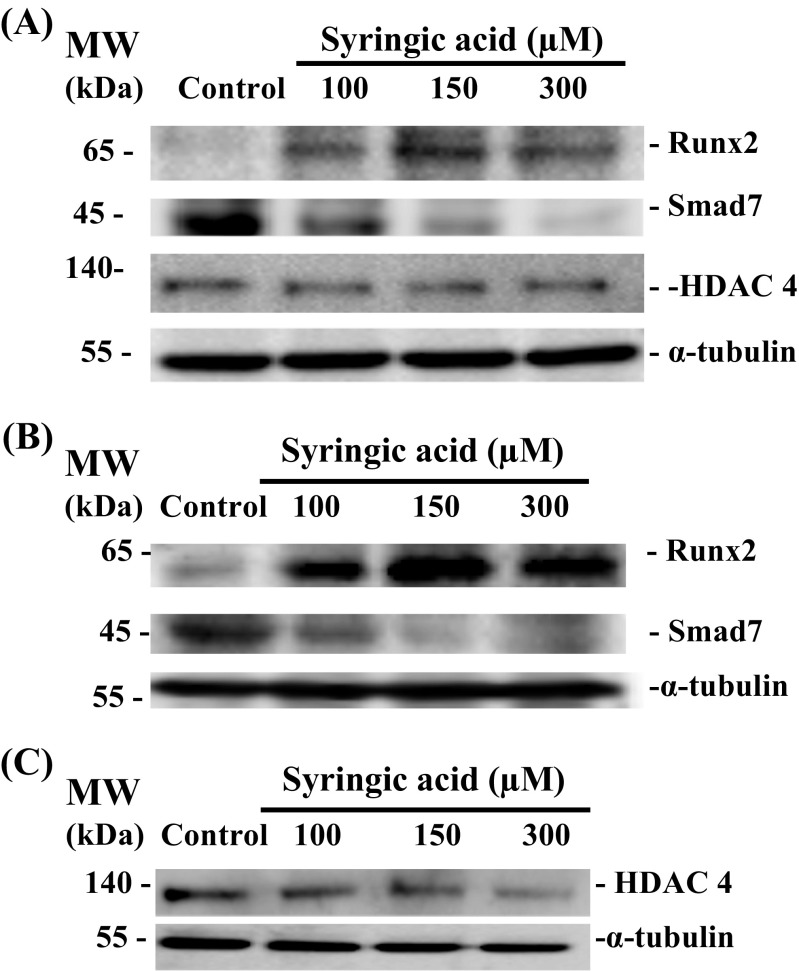
It was evident from our results that SA stimulated expression of Runx2 (Fig. 4) and this might be responsible for the promotion of osteoblast differentiation (Figs. 4, 5, and 6). There are reports indicating that Smad7, a co-repressor for Runx2, inhibits osteoblast differentiation (Valcourt et al. 2002; Eliseev et al. 2006; Iwai et al. 2008; Yano et al. 2012; Jia et al. 2014; Selvamurugan et al. 2017; Vishal et al. 2017b). Inhibition of Smad7 during osteoblast differentiation promoted Runx2 expression (Li et al. 2014; Vishal et al. 2017b; Wei et al. 2017; Selvamurugan et al. 2017). HDAC4 is another co-repressor that has been shown to negatively regulate Runx2 expression/activity (Vega et al. 2004; Westendorf 2006; Jeon et al. 2006; Karsenty et al. 2009; Shimizu et al. 2010; Shimizu et al. 2014; Wang et al. 2014a, b; Bradley et al. 2015; Carpio and Westendorf 2016; Vishal et al. 2017a, b; Carpio et al. 2017; Xu et al. 2017). Hence, we next determined whether SA treatment involves any changes associated with expression of Smad7 and HDAC4 during osteoblast differentiation (Fig. 7). mMSCs were treated with SA at different concentrations for 7 d and 14 d. Whole cell lysates were prepared and subjected to western blot analysis. We identified up- and down regulation of Runx2 and Smad7 proteins, respectively, by SA treatment after 7 d (Fig. 7A) and 14 d (Fig. 7B) treatments of mMSCs. The effect of SA treatment at 150 μM was found to be maximal during osteoblast differentiation of mMSCs (Figs. 2 and 3) and at this concentration, there was no change in HDAC4 protein level when cells were treated with SA for 7 (Fig. 7A) and 14 d (Fig. 7C).
Fig. 7.
Effect of SA on the expression of Smad7 protein. mMSCs were treated with SA at different concentrations (100, 150, and 300 μM) in DMEM media for (A) 7 d and (B) and (C) 14 d. Whole cell lysates were prepared and subjected to western blot analysis using antibodies against Smad7, HDAC4, Runx2 and α-tubulin. α-tubulin was used as an internal control for normalization
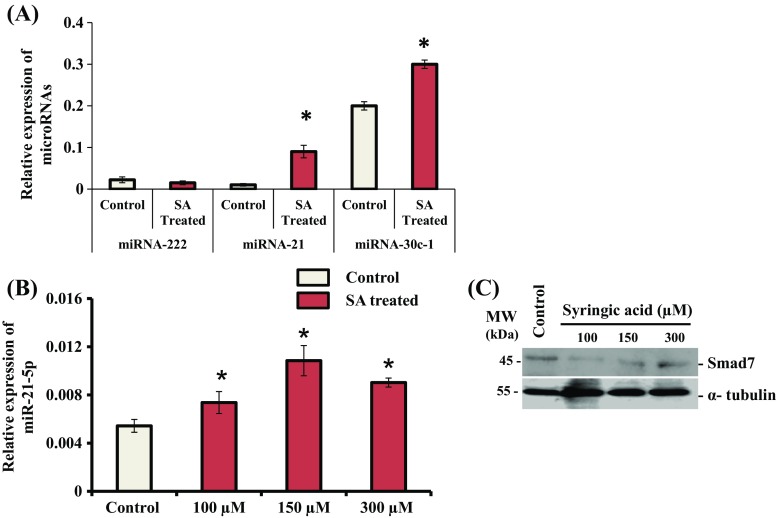
To identify the molecular mechanisms responsible for down regulation of Smad7 by SA-treatment in mMSCs, we determined the expression of miRNAs. Three miRNAs namely miR-222, miR-21, and miR-30c (Moorthi et al. 2013; Li et al. 2013; Lin et al. 2014; Yan et al. 2016a; Yoshizuka et al. 2016; Selvamurugan et al. 2017), which are known to target Runx2 corepressors, were selected for determining their precursor expression. Cells were treated with SA at 150 μM for 14 d. Total RNA was isolated and subjected to RT-qPCR analysis for determination of expression of mir-222, mir-21 and mir-30c-1 in these cells. The result showed that SA treatment stimulated the expression of mir-21 and mir-30c-1 in mMSCs (Fig. 8A). There was greater stimulation of mir-21 by SA treatment for 14 d in these cells. We also found a significant increase in the expression of mir-21 by SA treatment for 24 h in mMSCs using RT-qPCR analysis (Fig. 8B) and this correlated with down regulation of Smad7 protein in these cells (Fig. 8C).
Fig. 8.
Effect of SA on the expression of miRNAs. (A) mMSCs were treated with SA at 150 uM in DMEM media for 14 d. Total RNA was isolated, and cDNA was synthesized and subjected to qPCR analysis using the primers for mir-222, mir-21 and mir-30-c-1. (B) mMSCs were treated with SA at different concentrations (100, 150 and 300 uM) in DMEM media for 24 h. Total RNA was isolated, and cDNA was synthesized and subjected to qPCR analysis using the primers for mir-21. RPL13A was as used as an internal control for normalization. *indicates a significant increase compared to control (p < 0.05). (C) mMSCs were treated with SA at different concentrations (100, 150, and 300 μM) in DMEM media for 24 h. Whole cell lysates were prepared and subjected to western blot analysis using Smad7 and α-tubulin antibodies. α-tubulin was used as an internal control for normalization
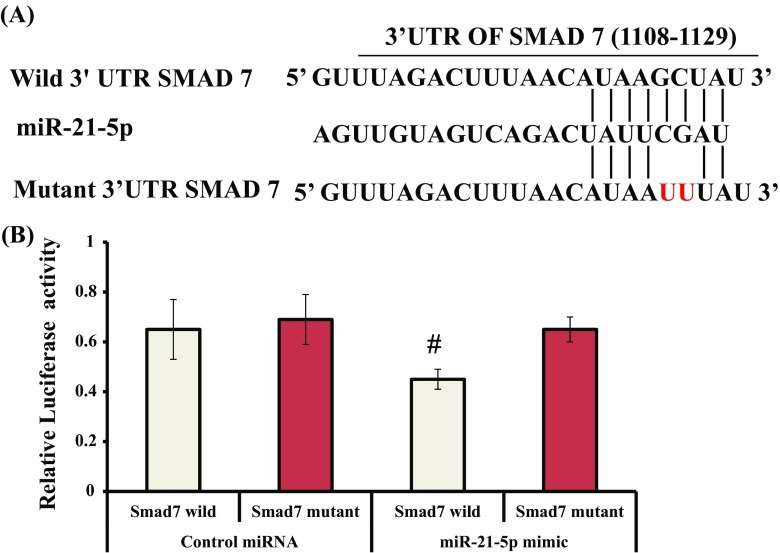
To identify that Smad7 is a direct target of miR-21-5p, a dual luciferase reporter assay system as reported earlier was used in the study (Vimalraj et al. 2014, 2017b). A region in the Smad7 3’UTR (1108–1129) was predicted to be the target of miR-21-5p using bioinformatics tool (Fig. 9A). The pmirGLO constructs containing the wild or mutant Smad7 3′-UTR downstream to the firefly luciferase reporter gene were transiently co-transfected along with control miRNA or miR-21-5p mimic in mMSCs. After 24 h, the luciferase activities of firefly and Renilla were measured. There was a significant decrease in luciferase activity when mMSCs were transfected with the pmirGLO containing Smad7 wild-type 3′-UTR along with the miR-21-5p mimic, whereas control miRNA had no significant effect on luciferase activity (Figs. 9 and 10). The mutant 3′-UTR of Smad7 was not significantly affected after transfection with the control miRNA or miR-21-5p, indicating the specificity of the 3′-UTR of Smad7 to miR-21-5p in these cells. Thus, this experiment provided conclusive evidence that miR-21-5p directly targets the 3′-UTR of the Smad7 gene.
Fig. 9.
(A) A region of wild or mutant Smad7 3’ UTR binding site (1108–1129) with the seed sequence of miR-21-5p. (B) miR-21 directly targets Smad7. mMSCs were transiently transfected with the pmirGLO construct containing the wild-type or mutant Smad7 3′-UTR along with control miRNA or miR-21-5p. After 24 h, cell lysates were prepared and relative luciferase activity was calculated after normalization of firefly luciferase activity with Renilla luciferase activity. # indicates significant decrease compared to control miRNA (p < 0.05)
Fig. 10.
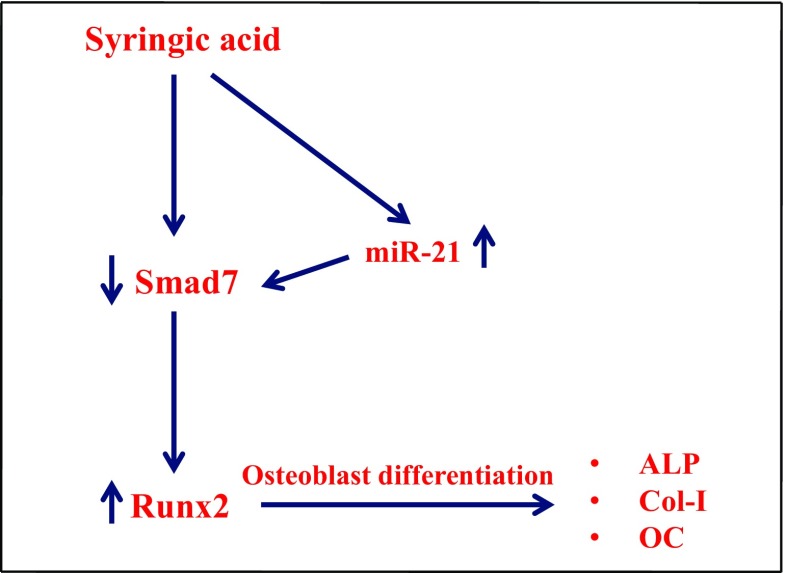
Schematic diagram of SA action on mMSCs in osteoblast differentiation. SA treatment upregulated the expression of miR-21, and the increased miR-21 targeted Smad7 expression, resulting in increased Runx2 expression, thus leading to expression of osteoblast differentiation marker genes such as ALP, Col-I, and OC in mMSCs
Discussion
Osteoblast activity can be promoted by several drugs to treat skeletal disorders such as osteoporosis, but these drugs have potential harmful effects on human health due to their toxicity (Wissing 2015). Plant-derived polyphenols help in minimizing the toxicity to bone health (Pandey and Rizvi 2009; Tou 2015). It has been reported that SA exhibits anti-diabetic activity and has been used to treat diabetes in Chinese and Indian medicine (Wei et al. 2012a, b; Muthukumaran et al. 2013). Nontoxic behavior of SA is necessary for any type of cellular and molecular studies in mammalian cell lines. Hence, the toxicity of SA has been studied based on detection of blue-colored formazan by calorimetric methods using MTT reagent: the results demonstrated its nontoxic effects on mMSCs (Fig. 1). Even though a number of polyphenols have been reported for anti-diabetic, anti-oxidant and anti-cancerous activities, few have been tested for their activity on osteogenicity (Tseng et al. 2011; Gu et al. 2012; Al-Obaidi et al. 2014; Xiao et al. 2014; Fan et al. 2015; Du et al. 2017). Flavonoids such as Fisetin showed increased expression of Runx2 and inhibited osteoclast differentiation (Leotoing et al. 2014). Flavonoids from Tridax procumbens directly promoted osteoblast differentiation by suppressing RANKL-induced differentiation of osetoclasts (Al Mamun et al. 2015). In our study, we reported that SA treatment promoted osteoblast differentiation at the cellular level, as evidenced by elevated ALP activity and increased accumulation of calcium deposits in mMSCs (Figs. 2 and 3).
Runx2 is a bone transcription factor which is essential for the promotion of expression of osteoblast differentiation marker genes such as ALP, Col-I and OC (Zhao et al. 2005; Javed et al. 2009; Karsenty et al. 2009; Muthusami et al. 2011; Vimalraj et al. 2014, 2015; Feng et al. 2016; Wu et al. 2017). A defect in the expression of Runx2 leads to skeletal abnormalities associated with bone diseases (Lou et al. 2008; Cardoso et al. 2010; Shibata et al. 2015; Sun et al. 2016; Zhang et al. 2017; Bir et al. 2017; Chen et al. 2017a, b; Zeng et al. 2017; Jung et al. 2017). The analysis of osteoblast differentiation at the molecular level by the SA treatment in mMSCs showed the stimulation of Runx2 expression in these cells (Fig. 4). Others, such as resveratrol, hyperoside, baicalin, kaempferol, linarin, and formononetin, also promoted high levels of Runx2 mRNA expression in osteoblastic cells (Tseng et al. 2011; Guo et al. 2011; Guo et al. 2012; Zhang et al. 2014; Li et al. 2016; Singh et al. 2017; Gaoli et al. 2017; Wu et al. 2017). Upon treatment with flavonoids, Runx2 levels increased, which promoted the expression of osteogenic genes in cells. Quercitin, a flavonoid, stimulated ALP, OC, OPN, BMP-2, BSP expression in bone marrow-derived mesenchymal stem cells (Kim et al. 2006; La et al. 2013; Zhou and Lin 2014). In our study, the stimulation of Runx2 expression by SA treatment in mMSCs correlated with the expression of ALP, Col-1, and OC at 7 d (Fig. 5) and at 14 d (Fig. 6).
We further dissected the molecular mechanisms responsible for SA-stimulated osteoblast differentiation in mMSCs. Flavonoids are known to stimulate osteoblast genes via various signaling cascades. Isoflavones (Syringetin, Genistein, Linarin, hesperetin and sulfuretin) have been shown to promote osteoblast differentiation via upregulation/activation of BMP2/SMAD5/Akt/Runx2 pathways (Hsu et al. 2009; Trzeciakiewicz et al. 2010; Dai et al. 2013; Li et al. 2016; Auh et al. 2016). The TGF-β/BMP signaling pathway involves the activation of type I and type II serine threonine kinase receptors, which phosphorylate intracellular Smad proteins. Receptor or regulatory Smads, such as Smad-1, −2, −3, −5, and −8, bind to Smad-4 (common or co-Smad). They undergo nuclear translocation and initiate the transcription of target genes (Selvamurugan et al. 2007; Horbelt et al. 2012; Chen et al. 2012; Crane and Cao 2014; Arumugam et al. 2017; Selvamurugan et al. 2017). This pathway in turn can be affected by a negative feedback loop controlled by inhibitory Smads such as Smad-6 and -7 (Ishida et al. 2000; Yan et al. 2009; Kamiya et al. 2010; Yano et al. 2012; Yan et al. 2016b). Runx2 corepressors such as HDAC4, Smurf1, and Smad7 that negatively regulate osteoblast differentiation (Zhao et al. 2004; Hug 2004; Shimizu et al. 2010; Obri et al. 2014; Li et al. 2014; Shimazu et al. 2016; Vishal et al. 2016; Vishal et al., 2017a, b; Wei et al. 2017) were identified. The expression of Smad7 as one of the Runx2 corepressors was found to be decreased by the SA treatment in mMSCs (Fig. 7), which suggested the involvement of the TGF-β/BMP signaling pathway for SA-stimulated osteoblast differentiation in these cells.
Several miRNAs have been identified as positive (miR-20a, 27, 29c, 196a, 210, 29-b, 15b, 433-3p, 215, 335-5p, 378, 2861) and negative (miR-23a, 26a,100, 103a, 133, 138, 132, 135, 182, 204, 211, 335,637) regulators (Lian et al. 2012; Wei et al. 2012a, b; Zheng et al. 2012; Kim et al. 2012; Vimalraj and Selvamurugan 2012; Vimalraj and Selvamurugan 2015; Moorthi et al. 2013; Hu et al. 2015; Zuo et al. 2015; Tang et al. 2017) during skeletal development, indicating that miRNAs act as regulators of osteogenesis. The reasons for selecting three miRNAs in this study were as follows: expression of osteoblast marker genes can be upregulated by down regulation of miR-222 (Yoshizuka et al. 2016), and miR-21 and miR-30c-1 have been reported to target Smad7 and HDAC4, respectively. The expression levels of both mir-21 and mir-30c-1 were stimulated by the SA treatment in mMSCs (Fig. 8A) but the fold change was higher with the expression of mir-21 (Fig. 8B) with down regulation of Smad7 expression (Fig. 8C). Smad7 was also identified as a direct target of miR-21 using the luciferase reporter system in mMSCs (Fig. 9). There are evidences indicating that miR-21 targets Smad7 in several types of cells (Liu et al. 2010; Lin et al. 2014; Wang et al. 2014a, b; McClelland et al. 2015; Yang et al. 2016; Selvamurugan et al. 2017). We recently reported that the targeting of Smad7 by miR-590-5p resulted in stabilization of Runx2 during osteoblast differentiation (Vishal et al. 2017b). Thus, the decreased expression levels of Smad7 by miR-21 could be correlated with increased expression/stabilization of Runx2 in mMSCs by SA treatment. Therefore, it is more likely that, as a result of SA treatment, there was increased expression of miR-21 which may inhibit Smad7 expression so that expression of Runx2 increased, which led to increased osteoblast differentiation (Fig. 10).
Overall, based on our results, we suggest that SA is potentially involved in the promotion of mMSCs into osteoblasts, and hence it may be considered as an osteogenic compound to treat various bone and bone-related diseases in the future.
Acknowledgements
This work was supported in part by the Department of Biotechnology, India (Grant Nos. BT/PR7792/MED/30/950/2013 and BT/PR15014/BRB/10/1481/2016 to N. S). We thank Dr. N. Subbarayan for advice on statistical analyses.
Abbreviations
- ALP
Alkaline phosphatase
- COL-I
Type I Collagen-I
- DMEM
Dulbecco’s modified eagle’s medium
- HDAC4
Histone deacetylases
- MMPs
Matrix metalloproteinases
- mMSC
mouse Mesenchymal Stem Cell
- MTT
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- OC
Osteocalcin
- PBS
Phosphate-buffered saline
- RANKL
Receptor activator of nuclear factor kappa-B ligand
- ROS
Reactive oxygen species
- SA
Syringic acid
- TGF-β
Transforming growth factor- β
- TRAP
Tartrate-resistant acid phosphatase
References
- Al Mamun MA, Hosen MJ, Islam K, Khatun A, Alam MM, Al-Bari MAA (2015) Tridax procumbens flavonoids promote osteoblast differentiation and bone formation. Biol Res 48(65) [DOI] [PMC free article] [PubMed] [Retracted]
- Al-Obaidi MM, Al-Bayaty FH, Al Batran R, Hussaini J, Khor GH (2014) Impact of ellagic acid in bone formation after tooth extraction: an experimental study on diabetic rats. Sci World J: 10.1155/2014/908098 [DOI] [PMC free article] [PubMed]
- Arumugam B, Vairamani M, Partridge NC, Selvamurugan N (2017) Characterization of Runx2 phosphorylation sites required for TGF-β1-mediated stimulation of matrix Metalloproteinase-13 expression in osteoblastic cells. J Cell Physiol 10.1002/jcp.25964 [DOI] [PubMed]
- Auh QS, Park KR, Yun HM, Lim HC, Kim GH, Lee DS, Kim YC, Oh H, Kim EC. Sulfuretin promotes osteoblastic differentiation in primary cultured osteoblasts and in vivo bone healing. Oncotarget. 2016;7(48):78320. doi: 10.18632/oncotarget.12460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baselga-Escudero L, Blade C, Ribas-Latre R, Casanova E, Susarez M, Torres JL, Salvado MJ, Arola L, Arola-Arnal A. Resveratrol and EGCG bind directly and distinctively to miR-33a and miR-122 and modulate divergently their levels in hepatic cells. Nucleic Acids Res. 2014;42:882–892. doi: 10.1093/nar/gkt1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bir FD, Dinçkan N, Güven Y, Baş F, Altunoglu U, Kuvvetli SS, Poyrazoglu S, Toksoy G, Kayserili H, Uyguner ZO. Cleidocranial dysplasia: clinical, endocrinologic and molecular findings in 15 patients from 11 families. Eur J Med Genet. 2017;60(3):163–168. doi: 10.1016/j.ejmg.2016.12.007. [DOI] [PubMed] [Google Scholar]
- Boonrungsiman S, Gentleman E, Carzaniga R, Evans ND, McComb DW, Porter AE, Stevens MM. The role of intracellular calcium phosphate in osteoblast-mediated bone apatite formation. Proc Natl Acad Sci USA. 2012;109(35):14170–14175. doi: 10.1073/pnas.1208916109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boudin E, Van Hul W. Mechanisms in endocrinology: genetics of human bone formation. Eur J Endocrinol. 2017;177(2):R69–R83. doi: 10.1530/EJE-16-0990. [DOI] [PubMed] [Google Scholar]
- Bradley EW, Carpio LR, Van Wijnen AJ, McGee-Lawrence ME, Westendorf JJ. Histone deacetylases in bone development and skeletal disorders. Physiol Rev. 2015;95(4):1359–1381. doi: 10.1152/physrev.00004.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardoso BM, Dupont J, Castanhinha S, Ejarque-Albuquerque M, Pereira S, Miltenberger-Miltenyi G, Oliveira G. Cleidocranial dysplasia with severe parietal bone dysplasia: a new (p. Val124Serfs) RUNX2 mutation. Clin Dysmorphol. 2010;19(3):150–152. doi: 10.1097/MCD.0b013e32833593a1. [DOI] [PubMed] [Google Scholar]
- Carpio LR, Westendorf JJ. Histone deacetylases in cartilage homeostasis and osteoarthritis. Curr Rheumatol Rep. 2016;18(8):1–9. doi: 10.1007/s11926-016-0602-z. [DOI] [PubMed] [Google Scholar]
- Carpio LR, Bradley EW, Westendorf JJ. Histone deacetylase 3 suppresses Erk phosphorylation and matrix metalloproteinase (Mmp)-13 activity in chondrocytes. Connect Tissue Res. 2017;58(1):27–36. doi: 10.1080/03008207.2016.1236088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G, Deng C, Li YP. TGF-β and BMP signaling in osteoblast differentiation and bone formation. Int J Biol Sci. 2012;8(2):272. doi: 10.7150/ijbs.2929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q, Liu C, Chen J, Xiong F, Wu B. A novel, complex RUNX2 gene mutation causes cleidocranial dysplasia. Bmc Med Genet. 2017;18(1):13. doi: 10.1186/s12881-017-0375-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Wang Z, Duan N, Zhu G, Schwarz EM, Xie C. Osteoblast–osteoclast interactions. Connect Tissue Res. 2017;23:1–9. doi: 10.1080/03008207.2017.1290085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi YH, Han Y, Jin SW, Lee GH, Kim GS, Lee DY, Chung YC, Lee KY, Jeong HG (2017) Pseudoshikonin I enhances osteoblast differentiation by stimulating Runx2 and Osterix. J Cell Biochem10.1002/jcb.26238 [DOI] [PubMed]
- Crane JL, Cao X. Bone marrow mesenchymal stem cells and TGF-β signaling in bone remodeling. J Clin Invest. 2014;124(2):466. doi: 10.1172/JCI70050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai J, Li Y, Zhou H, Chen J, Chen M, Xiao Z. Genistein promotion of osteogenic differentiation through BMP2/SMAD5/RUNX2 signaling. Int J Biol Sci. 2013;9(10):1089–1098. doi: 10.7150/ijbs.7367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhivya S, Saravanan S, Sastry TP, Selvamurugan N. Nanohydroxyapatite-reinforced chitosan composite hydrogel for bone tissue repair in vitro and in vivo. J Nanobiotechnol. 2015;13(1):40. doi: 10.1186/s12951-015-0099-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du K, Li Z, Fang X, Cao T, Xu Y. Ferulic acid promotes osteogenesis of bone marrow-derived mesenchymal stem cells by inhibiting MicroRNA-340 to induce β-catenin expression through hypoxia. Eur J Cell Biol. 2017;S0171-9335(17):30102–30104. doi: 10.1016/j.ejcb.2017.07.002. [DOI] [PubMed] [Google Scholar]
- Dudaric L, Fuzinac-Smojver A, Muhvic D, Giacometti J. The role of polyphenols on bone metabolism in osteoporosis. Food Res Int. 2015;77:290–298. [Google Scholar]
- Eliseev RA, Schwarz EM, Zuscik MJ, O'keefe RJ, Drissi H, Rosier RN. Smad7 mediates inhibition of Saos2 osteosarcoma cell differentiation by NFκB. Exp Cell Res. 2006;312(1):40–50. doi: 10.1016/j.yexcr.2005.09.016. [DOI] [PubMed] [Google Scholar]
- Fan JZ, Yang X, Bi ZG. The effects of 6-gingerol on proliferation, differentiation, and maturation of osteoblast-like MG-63 cells. Braz J Med Biol Res. 2015;48(7):637–643. doi: 10.1590/1414-431X20154494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang S, Deng Y, Gu P, Fan X. MicroRNAs regulate bone development and regeneration. Int J Mol Sci. 2015;8:227–253. doi: 10.3390/ijms16048227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng Y, Su L, Zhong X, Guohong W, Xiao H, Li Y, Xiu L. Exendin-4 promotes proliferation and differentiation of MC3T3-E1 osteoblasts by MAPKs activation. J Mol Endocrinol. 2016;56(3):189–199. doi: 10.1530/JME-15-0264. [DOI] [PubMed] [Google Scholar]
- Florencio-Silva R, Sasso GR, Sasso-Cerri E, Simoes MJ, Cerri PS (2015) Biology of bone tissue: structure, function, and factors that influence bone cells. Biomed Res Int. 10.1155/2015/421746 [DOI] [PMC free article] [PubMed]
- Gaoli X, Yi L, Lili W, Qiutao S, Guang H, Zhiyuan G (2017) Effect of naringin combined with bone morphogenetic protein-2 on the proliferation and differentiation of MC3T3-E1 cells. W China J Stomatol 1;35(3):275 [DOI] [PMC free article] [PubMed]
- Gu Q, Cai Y, Huang C, Shi Q, Yang H. Curcumin increases rat mesenchymal stem cell osteoblast differentiation but inhibits adipocyte differentiation. Pharmacogn Mag. 2012;8(31):202. doi: 10.4103/0973-1296.99285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo AJ, Choi RC, Cheung AW, Chen AP, Xu SL, Dong TT, Chen JJ, Tsim KW. Baicalin, a flavone, induces the differentiation of cultured osteoblasts an action via the Wnt/β-catenin signaling pathway. J Biol Chem. 2011;286:27882–27893. doi: 10.1074/jbc.M111.236281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo AJ, Choi RC, Zheng KY, Chen VP, Dong TT, Wang ZT, Vollmer G, Lau DT, Tsim KW. Kaempferol as a flavonoid induces osteoblastic differentiation via estrogen receptor signaling. Chin Med. 2012;7(1):10. doi: 10.1186/1749-8546-7-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada H, Tagashira S, Fujiwara M, Ogawa S, Katsumata T, Yamaguchi A, Komori T, Nakatsuka M. Cbfa1 isoforms exert functional differences in osteoblast differentiation. J Biol Chem. 1999;274(11):6972–6978. doi: 10.1074/jbc.274.11.6972. [DOI] [PubMed] [Google Scholar]
- Horbelt D, Denkis A, Knaus P. A portrait of transforming growth factor β superfamily signalling: background matters. Int J Biochem Cell B. 2012;44(3):469–474. doi: 10.1016/j.biocel.2011.12.013. [DOI] [PubMed] [Google Scholar]
- Hsu YL, Liang HL, Hung CH, Kuo PL. Syringetin, a flavonoid derivative in grape and wine, induces human osteoblast differentiation through bone morphogenetic protein-2/extracellular signal-regulated kinase 1/2 pathway. Mol Nutr Food Res. 2009;53(11):1452–1461. doi: 10.1002/mnfr.200800483. [DOI] [PubMed] [Google Scholar]
- Hu Z, Wang Y, Sun Z, Wang H, Zhou H, Zhang L, Zhang S, Cao X. miRNA-132-3p inhibits osteoblast differentiation by targeting Ep300 in simulated microgravity. Sci Rep. 2015;5:18655. doi: 10.1038/srep18655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hug BA. HDAC4: a corepressor controlling bone development. Cell. 2004;119(4):448–449. doi: 10.1016/j.cell.2004.10.023. [DOI] [PubMed] [Google Scholar]
- Ishida W, Hamamoto T, Kusanagi K, Yagi K, Kawabata M, Takehara K, Sampath TK, Kato M, Miyazono K. Smad6 is a Smad1/5-induced Smad inhibitor characterization of bone morphogenetic protein-responsive element in the mouse Smad6 promoter. J Biol Chem. 2000;275(9):6075–6079. doi: 10.1074/jbc.275.9.6075. [DOI] [PubMed] [Google Scholar]
- Iwai T, Murai J, Yoshikawa H, Tsumaki N. Smad7 inhibits chondrocyte differentiation at multiple steps during endochondral bone formation and down-regulates p38 MAPK pathways. J Biol Chem. 2008;283:27154–27164. doi: 10.1074/jbc.M801175200. [DOI] [PubMed] [Google Scholar]
- Javed A, Afzal F, Bae JS, Gutierrez S, Zaidi K, Pratap J, Van Wijnen AJ, Stein JL, Stein GS, Lian JB. Specific residues of RUNX2 are obligatory for formation of BMP2-induced RUNX2-SMAD complex to promote osteoblast differentiation. Cells Tissues Organs. 2009;189(1–4):133–137. doi: 10.1159/000151719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeon EJ, Lee KY, Choi NS, Lee MH, Kim HN, Jin YH, Ryoo HM, Choi JY, Yoshida M, Nishino N, Oh BC. Bone morphogenetic protein-2 stimulates Runx2 acetylation. J Biol Chem. 2006;281(24):16502–16511. doi: 10.1074/jbc.M512494200. [DOI] [PubMed] [Google Scholar]
- Jia J, Feng X, Xu W, Yang S, Zhang Q, Liu X, Feng Y, Dai Z. MiR-17-5p modulates osteoblastic differentiation and cell proliferation by targeting SMAD7 in non-traumatic osteonecrosis. Exp Mol Med. 2014;46(7):e107. doi: 10.1038/emm.2014.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung YJ, Bae HS, Ryoo HM, Baek SH (2017) A novel RUNX2 mutation in exon 8, G462X, in a patient with Cleidocranial dysplasia. J Cell Biochem 10.1002/jcb.26283 [DOI] [PubMed]
- Kamiya Y, Miyazono K, Miyazawa K. Smad7 inhibits transforming growth factor-β family type I receptors through two distinct modes of interaction. J Bio Chem. 2010;285(40):30804–30813. doi: 10.1074/jbc.M110.166140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karsenty G, Kronenberg HM, Settembre C (2009) Genetic control of bone formation. Annu Rev Cell Dev Biol:629–648 [DOI] [PubMed]
- Katsimbri P (2017) The biology of normal bone remodelling. Eur J Cancer Care 10.1111/ecc.12740 [DOI] [PubMed]
- Kim YJ, Bae YC, Suh KT, Jung JS. Quercetin, a flavonoid, inhibits proliferation and increases osteogenic differentiation in human adipose stromal cells. Biochem Pharmacol. 2006;72(10):1268–1278. doi: 10.1016/j.bcp.2006.08.021. [DOI] [PubMed] [Google Scholar]
- Kim KM, Park SJ, Jung SH, Kim EJ, Jogeswar G, Ajita J, Rhee Y, Kim CH, Lim SK. miR-182 is a negative regulator of osteoblast proliferation, differentiation, and skeletogenesis through targeting FoxO1. J Bone Miner Res. 2012;27(8):1669–1679. doi: 10.1002/jbmr.1604. [DOI] [PubMed] [Google Scholar]
- Komori T (2010) Regulation of osteoblast differentiation by Runx2. Adv Exp Med Biol 658:43–49 [DOI] [PubMed]
- Kumar JP, Lakshmi L, Jyothsna V, Balaji DR, Saravanan S, Moorthi A, Selvamurugan N. Synthesis and characterization of diopside particles and their suitability along with chitosan matrix for bone tissue engineering in vitro and in vivo. J Biomed Nanotechnol. 2014;10(6):970–981. doi: 10.1166/jbn.2014.1808. [DOI] [PubMed] [Google Scholar]
- La S, Messer JG, Hopkins RG, Kipp DE (2013) Quercetin protects osteoblast development in the presence of oxidative stress in fetal rat calvaria cells. FASEB J:862–818
- Leena RS, Vairamani M, Selvamurugan N. Alginate/gelatin scaffolds incorporated with Silibinin-loaded chitosan nanoparticles for bone formation in vitro. Colloids Surf B. 2017;158:308–318. doi: 10.1016/j.colsurfb.2017.06.048. [DOI] [PubMed] [Google Scholar]
- Leotoing L, Davicco MJ, Lebecque P, Wittrant Y, Coxam V. The flavonoid fisetin promotes osteoblasts differentiation through Runx2 transcriptional activity. Mol Nutr Food Res. 2014;58:1239–1248. doi: 10.1002/mnfr.201300836. [DOI] [PubMed] [Google Scholar]
- Li Q, Zhang D, Wang Y (2013) MiR-21/Smad 7 signaling determines TGF-β1-induced CAF formation. Sci Rep 3(2038) [DOI] [PMC free article] [PubMed]
- Li N, Lee WY, Lin SE, Ni M, Zhang T, Huang XR, Lan HY, Li G. Partial loss of Smad7 function impairs bone remodeling, osteogenesis and enhances osteoclastogenesis in mice. Bone. 2014;67:46–55. doi: 10.1016/j.bone.2014.06.033. [DOI] [PubMed] [Google Scholar]
- Li J, Hao L, Wu J, Zhang J, Su J. Linarin promotes osteogenic differentiation by activating the BMP-2/RUNX2 pathway via protein kinase a signaling. Int J Mol Med. 2016;37(4):901–910. doi: 10.3892/ijmm.2016.2490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lian JB, Stein GS, Van Wijnen AJ, Stein JL, Hassan MQ, Gaur T, Zhang Y. MicroRNA control of bone formation and homeostasis. Nat Rev Endocrinol. 2012;8(4):212–227. doi: 10.1038/nrendo.2011.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin L, Gan H, Zhang H, Tang W, Sun Y, Tang X, Kong D, Zhou J, Wang Y, Zhu Y. MicroRNA-21 inhibits SMAD7 expression through a target sequence in the 3'untranslated region and inhibits proliferation of renal tubular epithelial cells. Mol Med Rep. 2014;10(2):707–712. doi: 10.3892/mmr.2014.2312. [DOI] [PubMed] [Google Scholar]
- Ling M, Huang P, Islam S, Heruth DP, Li X, Zhang LQ, Li DY, Hu Z, Ye SQ. Epigenetic regulation of Runx2 transcription and osteoblast differentiation by nicotinamide phosphoribosyltransferase. Cell Biosci. 2017;7(1):27. doi: 10.1186/s13578-017-0154-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu G, Friggeri A, Yang Y, Milosevic J, Ding Q, Thannickal VJ, Kaminski N, Abraham E. miR-21 mediates fibrogenic activation of pulmonary fibroblasts and lung fibrosis. J Exp Med. 2010;207(8):1589–1597. doi: 10.1084/jem.20100035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lou Y, Javed A, Hussain S, Colby J, Frederick D, Pratap J, Xie R, Gaur T, Van Wijnen AJ, Jones SN, Stein GS. A Runx2 threshold for the cleidocranial dysplasia phenotype. Hum Mol Genet. 2008;18(3):556–568. doi: 10.1093/hmg/ddn383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo H, Gao H, Liu F, Qiu B. Regulation of Runx2 by microRNA-9 and microRNA-10 modulates the osteogenic differentiation of mesenchymal stem cells. Int J Mol Med. 2017;39(4):1046–1052. doi: 10.3892/ijmm.2017.2918. [DOI] [PubMed] [Google Scholar]
- McClelland AD, Herman-Edelstein M, Komers R, Jha JC, Winbanks CE, Hagiwara S, Gregorevic P, Kantharidis P, Cooper ME. miR-21 promotes renal fibrosis in diabetic nephreopathy by targeting PTEN and SMAD7. Clin Sci. 2015;129(12):1237–1249. doi: 10.1042/CS20150427. [DOI] [PubMed] [Google Scholar]
- Milenkovic D, Deval C, Gouranton E, Landrier JF, Scalbert A, Morand C, Mazur A (2012) Modulation of miRNA expression by dietary polyphenols in apoE deficient mice: a new mechanism of the action of polyphenols. PLoS One 7:e29837 [DOI] [PMC free article] [PubMed]
- Moorthi A, Vimalraj S, Chaudhary A, He Z, Partridge NC, Selvamurugan N. Expression of MicroRNA-30c and its target genes by nano-bioglass ceramic-treatment in human osteoblastic cells. Int J Biol Macromol. 2013;56:181. doi: 10.1016/j.ijbiomac.2013.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthukumaran J, Srinivasan S, Venkatesan RS, Ramachandran V, Muruganathan U. Syringic acid, a novel natural phenolic acid, normalizes hyperglycemia with special reference to glycoprotein components in experimental diabetic rats. J Acute Dis. 2013;2(4):304–309. [Google Scholar]
- Muthusami S, Senthilkumar K, Vignesh C, Ilangovan R, Stanley J, Selvamurugan N, Srinivasan N. Effects of Cissus Quadrangularis on the proliferation, differentiation and matrix mineralization of human osteoblast like SaOS-2 cells. J Cell Biochem. 2011;112(4):1035–1045. doi: 10.1002/jcb.23016. [DOI] [PubMed] [Google Scholar]
- Nakashima T. Bone homeostasis and Mechano biology. Clin Calcium. 2016;26(12):1685–1695. [PubMed] [Google Scholar]
- Obri A, Makinistoglu MP, Zhang H, Karsenty G. HDAC4 integrates PTH and sympathetic signaling in osteoblasts. J Cell Biol. 2014;205(6):771–780. doi: 10.1083/jcb.201403138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono T, Takayanagi H. Osteoimmunology in bone fracture healing. Curr Osteo Rep. 2017;15(4):367–375. doi: 10.1007/s11914-017-0381-0. [DOI] [PubMed] [Google Scholar]
- Pandey KB, Rizvi SI. Plant polyphenols as dietary antioxidants in human health and disease. Oxidative Med Cell Longev. 2009;2:270–278. doi: 10.4161/oxim.2.5.9498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parasramka MA, Ho E, Williams DE, Dashwood RH. MicroRNAs, diet, and cancer: new mechanistic insights on the epigenetic actions of phytochemicals. Mol Carcinog. 2012;5:213–230. doi: 10.1002/mc.20822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raggatt LJ, Partridge NC. Cellular and molecular mechanisms of bone remodeling. J Biol Chem. 2010;285(33):25103–25108. doi: 10.1074/jbc.R109.041087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sainitya R, Sriram M, Kalyanaraman V, Dhivya S, Saravanan S, Vairamani M, Sastry TP, Selvamurugan N. Scaffolds containing chitosan/carboxymethyl cellulose/mesoporous wollastonite for bone tissue engineering. Int J Biol Macromol. 2015;80:481–488. doi: 10.1016/j.ijbiomac.2015.07.016. [DOI] [PubMed] [Google Scholar]
- Santangelo C, Vari R, Scazzocchio B, Di Benedetto R, Filesi C, Masella R. Polyphenols, intracellular signalling and inflammation. Ann I Super Sanita. 2007;43:394. [PubMed] [Google Scholar]
- Saravanan S, Vimalraj S, Vairamani M, Selvamurugan N. Role of mesoporous wollastonite (calcium silicate) in mesenchymal stem cell proliferation and osteoblast differentiation: a cellular and molecular study. J Biomed Nanotechnol. 2015;11(7):1124–1138. doi: 10.1166/jbn.2015.2057. [DOI] [PubMed] [Google Scholar]
- Saravanan S, Chawla A, Vairamani M, Sastry TP, Subramanian KS, Selvamurugan N. Scaffolds containing chitosan, gelatin and graphene oxide for bone tissue regeneration in vitro and in vivo. Int J Biol Macromol. 2017;8130(16):32105–32105. doi: 10.1016/j.ijbiomac.2017.01.034. [DOI] [PubMed] [Google Scholar]
- Selvamurugan N, Kwok S, Vasilov A, Jefcoat SC, Partridge NC. Effects of BMP-2 and pulsed electromagnetic field (PEMF) on rat primary osteoblastic cell proliferation and gene expression. J Orthop Res. 2007;25(9):1213–1220. doi: 10.1002/jor.20409. [DOI] [PubMed] [Google Scholar]
- Selvamurugan N, He Z, Rifkin D, Dabovic B, Partridge NC (2017) Pulsed electromagnetic field regulates MicroRNA 21 expression to activate TGF-β signaling in human bone marrow stromal cells to enhance osteoblast differentiation. Stem Cells Int 10.1155/2017/2450327 [DOI] [PMC free article] [PubMed]
- Sera SR, zur Nieden NI. MicroRNA regulation of skeletal development. Curr Osteoporos Rep. 2017;20:1–4. doi: 10.1007/s11914-017-0379-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata A, Machida J, Yamaguchi S, Kimura M, Tatematsu T, Miyachi H, Matsushita M, Kitoh H, Ishiguro N, Nakayama A, Higashi Y. Characterisation of novel RUNX2 mutation with alanine tract expansion from Japanese cleidocranial dysplasia patient. Mutagenesis. 2015;31(1):61–67. doi: 10.1093/mutage/gev057. [DOI] [PubMed] [Google Scholar]
- Shimazu J, Wei J, Karsenty G. Smurf1 inhibits osteoblast differentiation, bone formation, and glucose homeostasis through serine 148. Cell Rep. 2016;15(1):27–35. doi: 10.1016/j.celrep.2016.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu E, Selvamurugan N, Westendorf JJ, Olson EN, Partridge.NC (2010) HDAC4 represses matrix metalloproteinase-13 transcription in osteoblastic cells, and parathyroid hormone controls this repression. J Biol Chem 285(13)9616–9626 [DOI] [PMC free article] [PubMed]
- Shimizu E, Nakatani T, He Z, Partridge NC. Parathyroid hormone regulates histone deacetylase (HDAC) 4 through protein kinase A-mediated phosphorylation and dephosphorylation in osteoblastic cells. J Biol Chem. 2014;289(31):21340–21350. doi: 10.1074/jbc.M114.550699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh KB, Dixit M, Dev K, Maurya R, Singh D. Formononetin, a methoxy isoflavone, enhances bone regeneration in a mouse model of cortical bone defect. Br J Nutr. 2017;117(11):1511–1522. doi: 10.1017/S0007114517001556. [DOI] [PubMed] [Google Scholar]
- Sowjanya JA, Singh J, Mohita T, Sarvanan S, Moorthi A, Srinivasan N, Selvamurugan N. Biocomposite scaffolds containing chitosan/alginate/nano-silica for bone tissue engineering. Colloids Surf B. 2013;109:294–300. doi: 10.1016/j.colsurfb.2013.04.006. [DOI] [PubMed] [Google Scholar]
- Stevens MM. Biomaterials for bone tissue engineering. Mater Today. 2008;11:18–25. [Google Scholar]
- Sun X, Wang X, Zhang C, Liu Y, Yang X, Yan W, Liu Z, Wang Y, Zheng S. RUNX2 mutation impairs bone remodelling of dental follicle cells and periodontal ligament cells in patients with cleidocranial dysplasia. Mutagenesis. 2016;31(6):677–685. doi: 10.1093/mutage/gew039. [DOI] [PubMed] [Google Scholar]
- Tang X, Lin J, Wang G, Lu J. MicroRNA-433-3p promotes osteoblast differentiation through targeting DKK1 expression. PloS One. 2017;12(6):e0179860. doi: 10.1371/journal.pone.0179860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tou JC. Resveratrol supplementation affects bone acquisition and osteoporosis: pre-clinical evidence toward translational diet therapy. BBA-Mol Basis Dis. 2015;1852(6):1186–1194. doi: 10.1016/j.bbadis.2014.10.003. [DOI] [PubMed] [Google Scholar]
- Trzeciakiewicz A, Habauzit V, Mercier S, Lebecque P, Davicco MJ, Coxam V, Demigne C, Horcajada MN. Hesperetin stimulates differentiation of primary rat osteoblasts involving the BMP signalling pathway. J Nutr Biochem. 2010;21(5):424–431. doi: 10.1016/j.jnutbio.2009.01.017. [DOI] [PubMed] [Google Scholar]
- Tseng PC, Hou SM, Chen RJ, Peng HW, Hsieh CF, Kuo ML, Yen ML. Resveratrol promotes osteogenesis of human mesenchymal stem cells by upregulating RUNX2 gene expression via the SIRT1/FOXO3A axis. J Bone Miner Res. 2011;26:2552–2563. doi: 10.1002/jbmr.460. [DOI] [PubMed] [Google Scholar]
- Valcourt U, Gouttenoire J, Moustakas A, Herbage D, Mallein-Gerin F. Functions of transforming growth factor-β family type I receptors and Smad proteins in the hypertrophic maturation and osteoblastic differentiation of chondrocytes. J Biol Chem. 2002;277(37):33545–33558. doi: 10.1074/jbc.M202086200. [DOI] [PubMed] [Google Scholar]
- Vimalraj S, Selvamurugan N. MicroRNAs: synthesis, gene regulation and osteoblast differentiation. Curr Issues Mol Biol. 2012;15(1):7–18. [PubMed] [Google Scholar]
- Vimalraj S, Selvamurugan N. MicroRNAs expression and their regulatory networks during mesenchymal stem cells differentiation toward osteoblasts. Int J Biol Macromol. 2014;66:194–202. doi: 10.1016/j.ijbiomac.2014.02.030. [DOI] [PubMed] [Google Scholar]
- Vimalraj S, Selvamurugan N. Regulation of proliferation and apoptosis in human osteoblastic cells by microRNA-15b. Int J Biol Macromol. 2015;79:490–497. doi: 10.1016/j.ijbiomac.2015.05.017. [DOI] [PubMed] [Google Scholar]
- Vimalraj S, Partridge NC, Selvamurugan N. A positive role of MicroRNA-15b on regulation of osteoblast differentiation. J Cell Physiol. 2014;229(9):1236–1244. doi: 10.1002/jcp.24557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vimalraj S, Arumugam B, Miranda PJ, Selvamurugan N. Runx2: structure, function, and phosphorylation in osteoblast differentiation. Int J Biol Macromol. 2015;78:202–208. doi: 10.1016/j.ijbiomac.2015.04.008. [DOI] [PubMed] [Google Scholar]
- Vimalraj S, Saravanan S, Vairamani M, Gopalakrishnan C, Sastry TP, Selvamurugan N. A combinatorial effect of carboxymethyl cellulose based scaffold and microRNA-15b on osteoblast differentiation. Int J Biol Macromol. 2016;93:1457–1464. doi: 10.1016/j.ijbiomac.2015.12.083. [DOI] [PubMed] [Google Scholar]
- Vishal M, Ajeetha R, Keerthana R, Selvamurugan N. Regulation of Runx2 by histone deacetylases in bone. Curr Protein and Pept Sci. 2016;17(4):343–351. doi: 10.2174/1389203716666150623104017. [DOI] [PubMed] [Google Scholar]
- Vishal M, Swetha R, Thejaswini G, Arumugam B, Selvamurugan N. Role of Runx2 in breast cancer-mediated bone metastasis. Int J Biol Macromol. 2017;99:608–614. doi: 10.1016/j.ijbiomac.2017.03.021. [DOI] [PubMed] [Google Scholar]
- Vishal M, Vimalraj S, Ajeetha R, Gokulnath M, Keerthana R, He Z, Partridge NC, Selvamurugan N. MicroRNA-590-5p stabilizes Runx2 by targeting Smad7 during osteoblast differentiation. J Cell Physiol. 2017;232(2):371–380. doi: 10.1002/jcp.25434. [DOI] [PubMed] [Google Scholar]
- Wang JY, Gao YB, Zhang N, Zou DW, Wang P, Zhu ZY, Li JY, Zhou SN, Wang SC, Wang YY, Yang JK. miR-21 overexpression enhances TGF-β1-induced epithelial-to-mesenchymal transition by target smad7 and aggravates renal damage in diabetic nephropathy. Mol Cell Endocrinol. 2014;392(1):163–172. doi: 10.1016/j.mce.2014.05.018. [DOI] [PubMed] [Google Scholar]
- Wang Z, Qin G, Zhao TC. HDAC4: mechanism of regulation and biological functions. Epigenomics-UK. 2014;6(1):139–150. doi: 10.2217/epi.13.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei J, Shi Y, Zheng L, Zhou B, Inose H, Wang J, Guo XE, Grosschedl R, Karsenty G. miR-34s inhibit osteoblast proliferation and differentiation in the mouse by targeting SATB2. J Cell Biol. 2012;197(4):509–521. doi: 10.1083/jcb.201201057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei X, Chen D, Yi Y, Qi H, Gao X, Fang H, Gu Q, Wang L, Gu L. Syringic acid extracted from Herba dendrobii prevents diabetic cataract pathogenesis by inhibiting aldose reductase activity. Evid-Based Compl Alt. 2012;29:2012. doi: 10.1155/2012/426537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei B, Wei W, Zhao B, Guo X, Liu S. Long non-coding RNA HOTAIR inhibits miR-17-5p to regulate osteogenic differentiation and proliferation in non-traumatic osteonecrosis of femoral head. PLoS One. 2017;12(2):e0169097. doi: 10.1371/journal.pone.0169097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westendorf JJ. Transcriptional co-repressors of Runx2. J Cell Biochem. 2006;98(1):54–64. doi: 10.1002/jcb.20805. [DOI] [PubMed] [Google Scholar]
- Wissing MD. Chemotherapy-and irradiation-induced bone loss in adults with solid tumors. Curr Osteoporos Rep. 2015;13:140–145. doi: 10.1007/s11914-015-0266-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu T, Shu T, Kang L, Wu J, Xing J, Lu Z, Chen S, Lv J. Icaritin, a novel plant-derived osteoinductive agent, enhances the osteogenic differentiation of human bone marrow-and human adipose tissue-derived mesenchymal stem cells. Int J Mol Med. 2017;39(4):984–992. doi: 10.3892/ijmm.2017.2906. [DOI] [PubMed] [Google Scholar]
- Xiao HH, Gao QG, Zhang Y, Wong KC, Dai Y, Yao XS, Wong MS. Vanillic acid exerts oestrogen-like activities in osteoblast-like UMR 106 cells through MAP kinase (MEK/ERK)-mediated ER signaling pathway. J Steroid Biochem. 2014;144:382–391. doi: 10.1016/j.jsbmb.2014.08.002. [DOI] [PubMed] [Google Scholar]
- Xu D, Gao Y, Hu N, Wu L, Chen Q. miR-365 ameliorates dexamethasone-induced suppression of osteogenesis in MC3T3-E1 cells by targeting HDAC4. Int J Mol Sci. 2017;18(5):977. doi: 10.3390/ijms18050977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan X, Liu Z, Chen Y. Regulation of TGF-β signaling by Smad7. Acta Biochim Biophys Sin. 2009;41(4):263–272. doi: 10.1093/abbs/gmp018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan J, Guo D, Yang S, Sun H, Wu B, Zhou D. Inhibition of miR-222-3p activity promoted osteogenic differentiation of hBMSCs by regulating Smad5-RUNX2 signal axis. Biochem Biophys Res Commun. 2016;470(3):498–503. doi: 10.1016/j.bbrc.2016.01.133. [DOI] [PubMed] [Google Scholar]
- Yan X, Liao H, Cheng M, Shi X, Lin X, Feng XH, Chen YG. Smad7 protein interacts with receptor-regulated smads (r-smads) to inhibit transforming growth factor-β (tgf-β)/smad signaling. J Biol Chem. 2016;291(1):382–392. doi: 10.1074/jbc.M115.694281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang F, Wang Y, Xue J, Ma Q, Zhang J, Chen YF, Shang ZZ, Li QQ, Zhang SL, Zhao L. Effect of Corilagin on the miR-21/smad7/ERK signaling pathway in a schistosomiasis-induced hepatic fibrosis mouse model. Parasitol Int. 2016;65(4):308–315. doi: 10.1016/j.parint.2016.03.001. [DOI] [PubMed] [Google Scholar]
- Yano M, Inoue Y, Tobimatsu T, Hendy G, Canaff L, Sugimoto T, Seino S, Kaji H. Smad7 inhibits differentiation and mineralization of mouse osteoblastic cells. Endocr J. 2012;59(8):653–662. doi: 10.1507/endocrj.ej12-0022. [DOI] [PubMed] [Google Scholar]
- Yoshizuka M, Nakasa T, Kawanishi Y, Hachisuka S, Furuta T, Miyaki S, Adachi N, Ochi M. Inhibition of microRNA-222 expression accelerates bone healing with enhancement of osteogenesis, chondrogenesis, and angiogenesis in a rat refractory fracture model. J Orthop Sci. 2016;21(6):852–858. doi: 10.1016/j.jos.2016.07.021. [DOI] [PubMed] [Google Scholar]
- Zeng L, Wei J, Han D, Liu H, Liu Y, Zhao N, Sun S, Wang Y, Feng H (2017) Functional analysis of novel RUNX2 mutations in cleidocranial dysplasia. Mutagenesis 10.1093/mutage/gex012 [DOI] [PubMed]
- Zhang C. Transcriptional regulation of bone formation by the osteoblast-specific transcription factor Osx. J Orthop Surg Res. 2010;5:37. doi: 10.1186/1749-799X-5-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang N, Ying MD, Wu YP, Zhou ZH, Ye ZM, Li H, Lin DS. Hyperoside, a flavonoid compound, inhibits proliferation and stimulates osteogenic differentiation of human osteosarcoma cells. PLoS One. 2014;9:e98973. doi: 10.1371/journal.pone.0098973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Liu Y, Wang X, Sun X, Zhang C, Zheng S. Analysis of novel RUNX2 mutations in Chinese patients with cleidocranial dysplasia. PLoS One. 2017;12(7):e0181653. doi: 10.1371/journal.pone.0181653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao M, Qiao M, Harris SE, Oyajobi BO, Mundy GR, Chen D (2004) Smurf1 inhibits osteoblast differentiation and bone formation in vitro and in vivo. J Biol Chem (13):279, 12854–12279 [DOI] [PMC free article] [PubMed]
- Zhao Z, Zhao M, Xiao G, Franceschi RT. Gene transfer of the Runx2 transcription factor enhances osteogenic activity of bone marrow stromal cells in vitro and in vivo. Mol Ther. 2005;12:247–253. doi: 10.1016/j.ymthe.2005.03.009. [DOI] [PubMed] [Google Scholar]
- Zheng Y, Qu X, Li H, Huang S, Wang S, Xu Q, Lin R, Han Q, Li J, Zhao RC. MicroRNA-100 regulates osteogenic differentiation of human adipose-derived mesenchymal stem cells by targeting BMPR2. FEBS Lett. 2012;586(16):2375–2381. doi: 10.1016/j.febslet.2012.05.049. [DOI] [PubMed] [Google Scholar]
- Zhou C, Lin Y. Osteogenic differentiation of adipose-derived stem cells promoted by quercetin. Cell Prolif. 2014;47(2):124–132. doi: 10.1111/cpr.12097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Wu Y, Jiang X, Zhang X, Xia L, Lin K, Xu Y. The effect of quercetin on the osteogenesic differentiation and angiogenic factor expression of bone marrow-derived mesenchymal stem cells. PLoS One. 2015;10:e0129605. doi: 10.1371/journal.pone.0129605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo B, Zhu J, Li J, Wang C, Zhao X, Cai G, Li Z, Peng J, Wang P, Shen C, Huang Y. microRNA-103a functions as a mechanosensitive microRNA to inhibit bone formation through targeting Runx2. J Bone Miner Res. 2015;30(2):330–345. doi: 10.1002/jbmr.2352. [DOI] [PubMed] [Google Scholar]