Abstract
Interleukin-33 (IL-33) is a member of the IL-1 family of cytokines that play a central role in the regulation of immune responses. Its release from epithelial and endothelial cells is mediated by pro-inflammatory cytokines, cell damage and by recognition of pathogen-associated molecular patterns (PAMPs). The activity of IL-33 is mediated by binding to the IL-33 receptor complex (IL-33R) and activation of NF-κB signaling via the classical MyD88/IRAK/TRAF6 module. IL-33 also induces the phosphorylation and activation of ERK1/2, JNK, p38 and PI3K/AKT signaling modules resulting in the production and release of pro-inflammatory cytokines. Aberrant signaling by IL-33 has been implicated in the pathogenesis of several acute and chronic inflammatory diseases, including asthma, atopic dermatitis, rheumatoid arthritis and ulcerative colitis among others. Considering the biomedical importance of IL-33, we developed a pathway resource of signaling events mediated by IL-33/IL-33R in this study. Using data mined from the published literature, we describe an integrated pathway reaction map of IL-33/IL-33R consisting of 681 proteins and 765 reactions. These include information pertaining to 19 physical interaction events, 740 enzyme catalysis events, 6 protein translocation events, 4 activation/inhibition events, 9 transcriptional regulators and 2492 gene regulation events. The pathway map is publicly available through NetPath (http://www.netpath.org/), a resource of human signaling pathways developed previously by our group. This resource will provide a platform to the scientific community in facilitating identification of novel therapeutic targets for diseases associated with dysregulated IL-33 signaling. Database URL: http://www.netpath.org/pathways?path_id=NetPath_120.
Electronic supplementary material
The online version of this article (10.1007/s12079-018-0464-4) contains supplementary material, which is available to authorized users.
Keywords: Immune response, Inflammation, NetSlim, Pro-inflammatory cytokine, Post-translational modifications, Protein-protein interactions
Introduction
Interleukin-33 (IL-33), also known as IL-1F11, is a member of the Interleukin-1 (IL-1) family of cytokines that play varied roles in the regulation of immune responses. It is encoded by the IL33 gene located on the human chromosome 9 (9p24.1) (Schmitz et al. 2005). IL-33 was first cloned from canine vasospastic cerebral arteries after subarachnoid hemorrhage (DVS27) and was identified as a gene encoding an unknown nuclear protein (Onda et al. 1999; Baekkevold et al. 2003). However, in 2005, Schmitz et al. for the first time identified IL-33 to be the ligand of the ST2 (IL1RL1) receptor. Through sequence-based homology analysis, they demonstrated that IL-33 was similar to IL-1 and IL-18 and therefore, exhibited similar biological functions (Schmitz et al. 2005). The structural similarity to cytokines was also confirmed by three-dimensional analysis (Lingel et al. 2009; Liu et al. 2013). IL-33 was initially found to be highly expressed in the endothelial cells of high endothelial venules (Baekkevold et al. 2003). Subsequent studies reported the constitutive expression of IL-33 in multiple cell types including the epithelial lining of the gut, gastric glands, lung, smooth muscle cells, keratinocytes, adipocytes, ovaries and in the central nervous system (Moussion et al. 2008; Carriere et al. 2007; Schmitz et al. 2005; Carlock et al. 2014; Hudson et al. 2008). The basal level of expression was reported to be further elevated by inflammatory mediators and upon tissue injury. Multiple splice forms of IL-33 have also been identified in airway epithelial cells, keratinocytes, and diverse cancer cells suggesting altered localization and activity (Tsuda et al. 2012; Hong et al. 2011; Gordon et al. 2016).
Similar to most of the IL-1 family members, IL-33 is synthesized as a full length (pro-IL-33) precursor. It consists of a non-classical nuclear localization sequence, a chromatin-binding domain at the N-terminus (Roussel et al. 2008) and a C-terminal domain with cytokine activity. The proteolytic processing of IL-33 is mediated by several proteases including calpain, elastase and cathepsin G resulting in the production of mature 18 kDa form consisting of the cytokine domain (Martin 2013; Palmer and Gabay 2011). IL-33, therefore has dual functions- acting both as a cytokine as well as a transcriptional regulator (Haraldsen et al. 2009). Although initial studies indicated that IL-33 possessed transcriptional regulatory properties modulating NF-κB activity (Carriere et al. 2007; Ali et al. 2011; Choi et al. 2012), a recent study refuted the findings by suggesting that the extracellular and not the endogenous nuclear form of IL-33 regulates protein expression in primary human endothelial cells. It is likely that the nuclear localization of IL-33 may be a mechanism to sequester/regulate its activity (Gautier et al. 2016). As a cytokine, it acts as an important mediator of the innate immune signaling mainly responsible for Th2-mediated immune responses (Hardman and Ogg 2016). The release of IL-33, both in its full length and processed forms, is mediated by several pro-inflammatory cytokines such as TNF-α, IFN-γ, IL-4 (Kopach et al. 2014; Meephansan et al. 2012; Kunisch et al. 2012; Zhao and Hu 2012), pathogen-associated molecular patterns (PAMPs) (Polumuri et al. 2012; Zhang et al. 2011), ATP (Hudson et al. 2008) and by Notch-mediated signaling (Sundlisaeter et al. 2012). However, the release is not mediated by apoptosis (Zhao and Hu 2010).
IL-33 exerts its effects through a heterodimeric receptor complex composed of IL-1 receptor-like 1 (IL1RL1) and a co-receptor, IL-1 receptor accessory protein (IL1RAcP). IL1RL1, also known as ST2, is encoded by IL1RL1 gene and is a member of Toll-like/IL-1-receptor (TLR/IL-1R) superfamily (Tominaga et al. 1991; Yanagisawa et al. 1993). It has two main splice forms resulting from the differential promoter binding - the transmembrane isoform (ST2L) which acts as the receptor for IL-33 (Schmitz et al. 2005) and the soluble isoform lacking the transmembrane domain (sST2) which acts as the decoy receptor regulating IL-33 mediated activity (Hayakawa et al. 2007; Hayakawa et al. 2016). ST2L was first identified in fibroblasts (Tominaga 1989) and is highly expressed on hematopoietic cells including mast cells (Moritz et al. 1998; Tung et al. 2014), Th2 lymphocytes (Lohning et al. 1998), macrophages (Joshi et al. 2010; Kurowska-Stolarska et al. 2009), basophils, eosinophils (Pecaric-Petkovic et al. 2009; Suzukawa et al. 2008), innate lymphoid cells including ILC1, ILC2 and ILC3 (Monticelli et al. 2011; Neill et al. 2010; Li et al. 2018)as well as in epithelial and endothelial cells (Miller et al. 2008; Sundlisaeter et al. 2012; Yagami et al. 2010). The formation of a ternary IL-33-IL1RL1-IL1RAcP complex result in the recruitment of adaptor proteins - MyD88 and IL-1R-associated kinase (IRAK) (Lingel et al. 2009; Liu et al. 2013). This complex, in turn, leads to activation of downstream mitogen-activated protein kinases (MAPK) and NF-κB through TRAF6 (Choi et al. 2009; Funakoshi-Tago et al. 2008). IL-33 also exerts its function by increasing the phosphorylation and subsequent activation of several signaling pathways includingphosphoinositide-3-kinase (PI3K)/protein kinase B (AKT), JAK2 and SYK pathways (Mun et al. 2010).Further, from our previous study, we identified IL-33 mediated regulation of phosphorylation of 672 proteins including several members of MAPK family and protein phosphatases including PTPN12 and MYPT1 in macrophages. Additionally, our analysis revealed IL-33-mediated activation of cdc42/Rho signaling, which is essential for cellular processes such as cell migration, polarity and actin cytoskeleton reorganization (Pinto et al. 2015).
IL-33 is an essential mediator of both innate and adaptive immune responses as it induces the production and release of predominantly Th2 cytokines such as IL-4, IL-5 and IL-13 from the cells involved in innate immune signaling (Schmitz et al. 2005). IL-33 also supports Th1 immune response resulting in the release of interferon-gamma and TNF (Bourgeois et al. 2009; Smithgall et al. 2008) and IL-33-mediated activation of MAPK signaling modules contribute to the maturation of mast cells and dendritic cells (Saluja et al. 2014). Further, IL-33 has also been reported to play important role in controlling regulatory T cell accumulation and effector functions thereby mediating immunosuppression and tissue repair. The process is mediated by direct or indirect activation of type 2 innate lymphoid cells (ILC2) and polarization of macrophages (Braun et al. 2018; Cayrol and Girard 2018). Additionally, impaired IL-33 signaling is implicated in several immune-related disorders including allergy, asthma, rheumatoid arthritis, autoimmunity, organ fibrosis and cardiovascular diseases (Liew et al. 2016; Braun et al. 2018).
Although several studies have been carried out at the molecular level to characterize the IL-33 signaling pathway, the information about the IL-33 signaling pathway is scattered across the literature. To our knowledge, there are no public resources that provide a comprehensive view of the IL-33 signaling pathway data for visualization and analysis. In this study, the molecular events that occur upon IL-33 stimulation have been compiled from the available literature. The data pertaining to IL-33 mediated signaling is made available through NetPath. Our group has previously developed several such Interleukin mediated signaling pathways including IL-1, IL-2, IL-3, IL-4, IL-5, IL-6, IL-7, IL-9 and IL-11 (Balakrishnan et al. 2013; Kandasamy et al. 2010), TSLP (Zhong et al. 2014), gastrin (Subbannayya et al. 2014) and Oncostatin M (Dey et al. 2013).The comprehensive signaling pathway map developed in this study will foster further research on IL-33 mediated signaling and delineate roles of individual molecules and their modules associated with specific diseases.
Methods
Literature mining and curation of signaling events mediatedby IL-33
We carried out an extensive survey of published literature using PubMed to develop a comprehensive signaling map pertaining to IL-33/ST2 signaling. Articles were fetched using the query terms- “IL-33” OR “IL33” OR “Interleukin-33” AND “Signaling” OR “Pathway”. The research articles were further screened to assess the presence of IL-33-induced signaling events. Only those molecular events that were reported under the influence of IL-33 stimulation have been considered for further documentation based on the criteria described in NetPath (Kandasamy et al. 2010). From these research articles, information regarding protein-protein interactions (PPIs), post-translational modifications (PTMs), translocation and activation/inhibition of proteins which occur on stimulation with IL-33 in mammalian cells/cell lines were screened and manually annotated using PathBuilder annotation tool (Kandasamy et al. 2009). Additionally, gene regulation events mediated by IL-33 in mammalian cells/cell lines have also been documented. Whenever available, the information on transcription regulators were also included. Each reaction annotated in IL-33 signaling pathway was hyperlinked to the respective research article from which the data has been obtained. Additional information including details of the cell lines used in the experiment, protein site/domain involved in PPIs has also been provided. In case of PTM, site and residue information for PTM was curated. Each curated event was further subjected to quality control and internal review process followed by an external review by a Pathway Authority, an experienced scientist working in the field. All recommendations of the Pathway Authority were incorporated into the signaling pathway.
Generation of signaling pathway map
The pathway map of IL-33 mediated signaling events has been pictorially represented using PathVisio software (van Iersel et al. 2008). Further, a subset of highly confident IL-33 mediated signaling events was determined using the selection and representation criteria provided in the NetSlim database (http://www.netpath.org/netslim/criteria.html). The reactions induced by IL-33 have been arranged topologically from ligand-receptor interaction to transcriptionally regulated genes. Pathway modules such as MAPK signaling, PI3K/AKT signaling which are regulated by IL-33 have also been depicted in the pathway map. The NetSlim version of the signaling pathway map can be downloaded in various compatible file formats such as .png, .gpml and .pdf formats.
A list of protein-protein interactions across the molecules involved in IL-33 signaling was generated using STRING (http://string-db.org/) (Szklarczyk et al. 2015). The parameters used for the STRING analysis include interaction sources from experimentally derived data, text mining, co-occurrences in the literature and gene fusion events.
Results and discussion
Data integration and development of IL-33/IL-33R signaling pathway map
In the current study, 2100 research articles were screened using PubMed until November, 2017 using a number of keywords to search for articles describing IL-33-induced signaling events. Of these, 200 research articles had information pertaining to IL-33 signaling. From the screened articles, a total of 322 molecules involved in IL-33 mediated events including 19 protein-protein interactions, 740 enzyme-substrate reactions, 4 activation/inhibition reactions, and 6 protein translocation events have been documented. PPIs include 15 binary interactions and 4 protein complexes. The PTMs annotated include phosphorylation, dephosphorylation and ubiquitination. Of the 740 enzyme-substrate reactions, literature evidence was available for 672 events concerning the PTM site and residue modifications. The upstream enzymes for 4 PTM modified proteins have also been catalogued. A large majority of the enzyme-substrate reactions cataloged in this pathway are ‘indirect’ events obtained from the IL-33 regulated quantitative phosphoproteomics study published previously by our group. These include IL-33-induced phosphorylation changes in 800 sites mapping to 575 proteins. In addition, 2492 genes that were found to be differentially regulated at the transcript level upon IL-33 stimulation have also been documented (Pollheimer et al. 2013). Of these, 1433 genes were identified to be overexpressed, and 1058 were found to be downregulated. Wherever available, the information on differentially regulated genes upon IL-33 stimulation were also catalogued. To our knowledge, this is the first pathway resource cataloging IL-33 mediated such molecular reactions.
Development of IL-33 mediated signaling network map
The data pertaining to IL-33 signaling pathway is accessible in NetPath (http://www.netpath.org/pathways?path_id=NetPath_120). The pathway page also provides a short description and statistics of the molecular reactions involved in IL-33 mediated signaling. Each annotated molecule is linked to its respective NetPath molecule page which provides information based on HGNC criteria as well its association with other pathways available in NetPath. Additionally, a brief description for each type of reaction listed is included from the literature. A total of 364 molecules involved in 393 reactions are visually depicted in NetSlim. The NetSlim version map generated is provided in Fig. 1. The high confidence reaction signaling map can be downloaded from http://www.netpath.org/netslim/IL33_pathway.html. The pathway information for both the NetPath and NetSlim versions have been made available in multiple community standard data exchange formats such as Proteomics Standards Initiative for Molecular Interaction (PSI-MI), Biological PAthwayeXchange (BioPAX level 3) and Systems Biology Markup Language (SBML) and can be accessed easily.
Fig. 1.
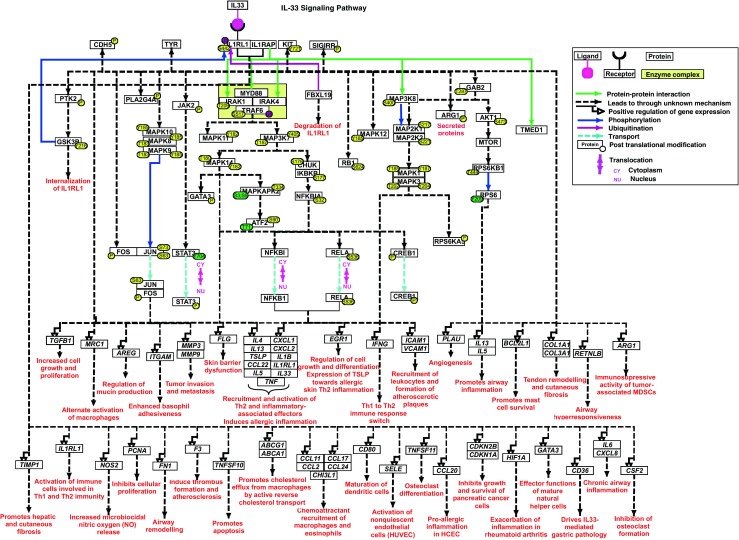
Schematic representation of IL-33 signaling. The pathway map represents ligand-receptor interactions and the downstream molecular events regulated by IL-33 including molecular association, catalysis, translocation and gene regulation events. These events are color coded as described in the pathway legend. Solid arrows indicate direct reactions and dashed arrows indicate reactions that occur through currently unknown mechanisms. Information pertaining to site and residue of post-translational modification are also included in the pathway map
Summary of IL-33 mediated signaling pathway
The dual function cytokine IL-33 is primarily localized in the nucleus where it interacts with p50 and p65 subunits in human HEK293RI cells and mouse embryonic fibroblasts (Ali et al. 2011). Upon release of IL-33 by DAMPs and PAMPs, IL-33 specifically binds to ST2L on target cells and undergoes conformational changes resulting in the recruitment of IL1RAcP forming a ternary complex. In most cell types, IL-33 signaling activates the classical MyD88/IRAK/TRAF6 module. TRAF6 further activates TAK1 (MAP3K7) which results in the activation of transcriptional regulator NF-κB by the activation of stress-activated protein kinase p38 and c-Jun N-terminal kinases (JNK). Additionally, activation of ERK signaling has also been observed in several cell types. Interestingly, in TRAF6-deficient mouse embryonic fibroblasts, ERK signaling is reported to be activated independently of TRAF6 mediated signaling (Funakoshi-Tago et al. 2008).
IL-33 also mediates activation of other signaling modules, and these seem to be cell-type specific. In murine Th2, innate lymphoid cells, eosinophils and human endothelial cells, IL-33 results in the activation of PI-3 K/AKT/mTOR pathway (Salmond et al. 2012). In bone marrow-derived mast cells and Th2 cells, IL-33 also induces MK2/3-mediated phosphorylation of mTOR complex 1 (mTORC1), RPS6KB1 at Thr444 and Ser447. IL-33-induced IL-6 and IL-13 production strongly depend on MK2/3-mediated activation of ERK1/2 and PI3K signaling (Drube et al. 2016). In human umbilical vein endothelial cells, IL-33 through ST2/TRAF6/PI3K/Akt/eNOS signaling pathway induces the production of nitric oxide (NO) resulting in increased vascular permeability and angiogenesis (Choi et al. 2009). IL-33 has also been implicated in osteoclast cellular fates. In a study by Mun et al., it has been shown to stimulate osteoclastogenesis from CD14(+) monocytes by inducing the phosphorylation of SYK, PLCγ and GAB2 as well as enhance the expression of osteoclast differentiation factors including TRAF6, c-Fos, NFATc1 among others that are essential for osteoclast development (Mun et al. 2010). On the contrary, two studies report anti-osteoclastogenic effect of IL-33/ST2 signaling wherein IL33 was observed to increased the expression of pro-apoptotic molecules in bone-marrow derived cells (Lima et al. 2015) and repressed expression of osteoclast differentiation factors such as NFATc1 (Schulze et al. 2011). In murine embryonal fibroblasts, IL-33 activates JAK2 which in turn induces NF-κB activation; however, the activation of MAPK signaling modules including ERK, JNK and p38 remained unaffected (Funakoshi-Tago et al. 2011). In mast cells, cross-activation of c-Kit by IL-33R has been reported which results in the phosphorylation of c-Kit at Y721 as well as the phosphorylation of ERK1/2, JNK1, PKB and STAT3. Inhibiting c-Kit in mast cells results in impaired JNK1/2 and ERK1/2 activation suggesting c-Kit-mediated regulation (Drube et al. 2010). In normal epithelial and breast cancer cells JB6 Cl41, MDA-MB231 and MCF7 cells; IL-33 dose- and time-dependently increases the phosphorylation of mitogen-activated protein kinase kinase kinase 8 (MAP3K8) at S400 via ST2-COT (MAP3K8) interaction (Kim et al. 2015). In addition, the data obtained from the quantitative phosphoproteomics study carried out by our group revealed IL-33-induced changes in phosphorylation status of molecules involved in Rho-mediated signaling(Pinto et al. 2015).
In addition to the activation of various signaling modules in diverse cell types, the downstream effector proteins that are induced by IL-33 vary across cell types. IL-33 induces the activation and nuclear translocation of cytosolic NFκB1 proteins in endothelial cells and cardiac fibroblasts which results in the production and release of IL-6 and MCP-1 (Zhu and Carver 2012; Demyanets et al. 2011). In pancreatic myofibroblasts and mouse embryonic fibroblasts, IL-33 induces the expression of IL-6, IL-8, MCP-1and MCP-3 whereas in Th2 cells, IL-33 induces expression of IL-4, IL-5 and IL-13 (Funakoshi-Tago et al. 2008). In macrophages and primary human monocyte-derived macrophages, IL-33 through ERK1/2, JNK and PI3K-AKT signaling reduce the expression of ADAMTS family of metalloproteases (Ashlin et al. 2014). A subset of biological functions regulated by IL-33 is depicted in Fig. 1. A list of protein-protein interactions across the molecules identified to be involved in IL-33 signaling based on the STRING analysis tool version 10.5 is provided in Supplementary Table 1. This network would help to obtain an insight into cross-talk between multiple signaling modules such as NF-κB, ERK, p38MAPK, JAK-STAT and PLCγ.
Regulation of IL-33 dependent signaling
The regulatory mechanisms of IL-33-mediated signaling have also been annotated and depicted in the pathway map. Currently, three different modes of regulations are reported in the literature including binding by the decoy soluble ST2 receptor that abrogates IL-33 signaling. Another mechanism observed in murine lung MLE12 cells involves sequential phosphorylation and activation of FAK upon IL-33 stimulation which in turn phosphorylates GSK3B at Y216. The activated GSK3B, in turn, phosphorylates ST2L at S442 resulting in its rapid internalization and subsequent interaction between carboxyl terminus of mouse ST2L and FBXL19 an E3 ubiquitin ligase (Zhao et al. 2012). The polyubiquitinated receptor is further targeted for proteasome mediated degradation. Interestingly, interaction with the membrane protein TMED1 results in a protective effect which positively modulates cytokine production (Connolly et al. 2013). Several studies have shown that a single Ig IL-1R-related molecule (SIGIRR)/Toll IL-1R8 negatively regulates TLR-IL-1R-mediated signaling. A similar mechanism of regulation has also been observed upon IL-33 stimulation wherein the extracellular and the intracellular TIR domain of SIGIRR form a complex with ST2 in HEK293 cells stably transfected with hST2, suggesting that SIGIRR (TIR domain) inhibits IL-33-mediated signaling through its interaction with the receptor complex (Bulek et al. 2009).
Other mechanisms involved in regulating IL-33 activity include cell-type specific expression of IL-33, mediated by inflammatory cytokines such as TNF, IL-1 and IFN-γ (Meephansan et al. 2012; Kopach et al. 2014), alternate splice forms resulting in varied localization and activity (Tsuda et al. 2012; Hong et al. 2011; Gordon et al. 2016), miR-487b mediated regulation of IL-33 expression (Xiang et al. 2016; Yamazumi et al. 2016; Kearley et al. 2015; Xi et al. 2013), and proteolytic cleavage of mature form of IL-33 by proteases secreted from mast cells and neutrophils (Lefrancais et al. 2012; Lefrancais et al. 2014) and subsequent degradation by increased amount of inflammatory proteases. A recent study describes inactivation of IL-33 shortly after its release by oxidation of cysteine residues resulting in conformational change rendering it incapable to bind to the IL-33 receptor complex (Cohen et al. 2015).
IL-33 signaling in diseases
Aberrant IL-33 signaling has been implicated in several diseases including cancer, atherosclerosis and COPD. Increased expression of IL-33 has been observed in infectious diseases, inflamed lesions of inflammatory bowel disease, allergic rhinitis and atopic dermatitis. Furthermore, elevated levels of IL-33 and IL1RL1 have been reported in the serum of patients with cardiovascular disorders, asthma and COPD suggesting their potential role as predictive biomarkers (Xia et al. 2015; Demyanets et al. 2014; Weinberg et al. 2002; Li et al. 2015). In cancer cells, IL-33 has been shown to stimulate the proliferation, tumor invasion and metastasis of colorectal cancer cells through the upregulation of matrix metalloproteinase (MMP) genes including MMP2, MMP3 and MMP9 (Liu et al. 2014). Administration of the decoy receptor sST2 negatively regulated the tumor growth and metastatic spread. Additionally, IL-33 through upregulation of MMP2 stimulates the proliferation and invasiveness of dendritic stromal cells that is abolished by the administration of sST2 (Hu et al. 2014). In contrast, however, in the case of pancreatic cancer, the antitumor activity of IL-33 has been observed. Upon stimulation, IL-33 induces downregulation of proteins involved in cellular proliferation such as CDK2, CDK4 and increases expression of pro-apoptotic molecules such as TRAIL and Bax, thereby promoting apoptosis (Fang et al. 2017). Recent evidence suggests that IL-33 modulates tumor-associated inflammatory microenvironment to restrain or promote tumorigenesis by promoting the proliferation, activation and infiltration of CD8 + T cells and NK cells via NF-κB mediated signaling resulting in the attenuation of tumor metastasis (Wasmer and Krebs 2016).
In the case of atherosclerosis, an inflammatory condition involving the vascular system, IL-33 has been shown to have a protective role by reducing atherosclerotic plaque formation (Ashlin et al. 2014; Miller et al. 2008). Similarly, a protective role of IL-33 mediated signaling pathway has also been suggested for obesity and adipose tissue-associated inflammation. IL-33 mediates downregulation of ADAMTS metalloproteases in macrophages via ERK-1/2, PI3Kγ/δ and JNK-c-Jun pathway (Ashlin et al. 2014). In endothelial cells, IL-33 through ST2/TRAF6 pathway mediates increased production of ICAM1 and VCAM as well as stimulates the production of endothelial NO. This, in turn, increases angiogenesis and vascular permeability. In pulmonary pathologies such as asthma and COPD, cigarette smoke and allergens are known to exacerbate the underlying condition by increasing the expression of IL-33 (Shang et al. 2015). IL-33, in turn, acts in a paracrine manner and enhances the expression of IL-6 and IL-8 in HBE cells and PBMCs of COPD patients via ST2/IL-1RacP pathway and MAPKs pathway (Wu et al. 2014; Shang et al. 2015). IL-33 also recruits macrophages, neutrophils and eosinophils in a paracrine manner, thereby increasing pro-inflammatory responses. In the past decade, several research groups have explored the possibility of using IL-33 as a treatment modality for reducing the development of atherosclerosis (Miller et al. 2008), obesity, clearing fungal infections and reducing the severity of experimental autoimmune uveitis in mice. Recent studies have also suggested a novel therapeutic role of IL-33 in antibacterial host defense as it enables bacterial clearance by recruiting neutrophils to the site of infection(Robinson et al. 2017; Robinson et al. 2018).
Conclusions
The availability of comprehensive signaling pathway maps helps researchers identify signaling modules that play vital roles in normal and disease physiology. To generate a comprehensive IL-33 signaling pathway map, the information pertaining tosignaling events triggered by IL-33 from literature was compiled which included data obtained from a quantitative phosphoproteomic experiment resulting in the generation of the largestsignaling network of IL-33 known till date. We anticipate that this resource will help provide more significant insights into IL-33 induced signaling mechanism and will aid in designing experiments aimed at expanding the existing knowledge of the IL-33 signaling in both normal physiology and various diseases.
Electronic supplementary material
A list of protein-protein interactions identified to be involved in IL-33 signaling based on the STRING analysis tool version 10.5. Only those protein-protein interactions that are experimentally determined with a medium confidence score threshold of 0.4 and above have been considered. (XLSX 88 kb)
Acknowledgements
We thank the Department of Biotechnology (DBT), Government of India for research support to the Institute of Bioinformatics.SMP is a recipient of INSPIRE Faculty Award from Department of Science and Technology (DST), Government of India. RR is a recipient of SERB Young Scientist award from Department of Science and Technology (DST), Government of India. JA is a recipient of Senior Research Fellowship from Council of Scientific and Industrial Research (CSIR), Government of India. OC is a recipient of INSPIRE Fellowship from the Department of Science and Technology, Government of India.
Abbreviations
- IL-1
Interleukin-1
- IL-33
Interleukin-33
- PAMPs
Pathogen-associated molecular patterns
- DAMP
Danger-associated molecular patterns
- IL-33R
IL-33 receptor complex
- NF-κB
Nuclear factor-κB
- TRAF6
TNF receptor associated factor 6
Compliance with ethical standards
Conflict of interest
The authors report no conflicts of interest.
Footnotes
Electronic supplementary material
The online version of this article (10.1007/s12079-018-0464-4) contains supplementary material, which is available to authorized users.
Contributor Information
Sneha M. Pinto, Email: sneha@yenepoya.edu.in
Yashwanth Subbannayya, Email: yashwanth@yenepoya.edu.in.
D. A. B. Rex, Email: rexprem@yenepoya.edu.in
Rajesh Raju, Email: rajrrnbt@gmail.com.
Oishi Chatterjee, Email: oishi@ibioinformatics.org.
Jayshree Advani, Email: jayshree@ibioinformatics.org.
Aneesha Radhakrishnan, Email: aneesha176.h@gmail.com.
T. S. Keshava Prasad, Email: keshav@ibioinformatics.org
Mohan R. Wani, Email: mohanwani@nccs.res.in
Akhilesh Pandey, Phone: (410)-502-6662, Email: pandey@jhmi.edu.
References
- Ali S, Mohs A, Thomas M, Klare J, Ross R, Schmitz ML, Martin MU. The dual function cytokine IL-33 interacts with the transcription factor NF-kappaB to dampen NF-kappaB-stimulated gene transcription. J Immunol. 2011;187:1609–1616. doi: 10.4049/jimmunol.1003080. [DOI] [PubMed] [Google Scholar]
- Ashlin TG, Buckley ML, Salter RC, Johnson JL, Kwan AP, Ramji DP. The anti-atherogenic cytokine interleukin-33 inhibits the expression of a disintegrin and metalloproteinase with thrombospondin motifs-1, −4 and −5 in human macrophages: requirement of extracellular signal-regulated kinase, c-Jun N-terminal kinase and phosphoinositide 3-kinase signaling pathways. Int J Biochem Cell Biol. 2014;46:113–123. doi: 10.1016/j.biocel.2013.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baekkevold ES, Roussigne M, Yamanaka T, Johansen FE, Jahnsen FL, Amalric F, Brandtzaeg P, Erard M, Haraldsen G, Girard JP. Molecular characterization of NF-HEV, a nuclear factor preferentially expressed in human high endothelial venules. Am J Pathol. 2003;163:69–79. doi: 10.1016/S0002-9440(10)63631-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balakrishnan L, Soman S, Patil YB, Advani J, Thomas JK, Desai DV, Kulkarni-Kale U, Harsha HC, Prasad TS, Raju R, Pandey A, Dimitriadis E, Chatterjee A. IL-11/IL11RA receptor mediated signaling: a web accessible knowledgebase. Cell Commun Adhes. 2013;20:81–86. doi: 10.3109/15419061.2013.791683. [DOI] [PubMed] [Google Scholar]
- Bourgeois E, Van LP, Samson M, Diem S, Barra A, Roga S, Gombert JM, Schneider E, Dy M, Gourdy P, Girard JP, Herbelin A. The pro-Th2 cytokine IL-33 directly interacts with invariant NKT and NK cells to induce IFN-gamma production. Eur J Immunol. 2009;39:1046–1055. doi: 10.1002/eji.200838575. [DOI] [PubMed] [Google Scholar]
- Braun H, Afonina IS, Mueller C, Beyaert R. Dichotomous function of IL-33 in health and disease: from biology to clinical implications. Biochem Pharmacol. 2018;148:238–252. doi: 10.1016/j.bcp.2018.01.010. [DOI] [PubMed] [Google Scholar]
- Bulek K, Swaidani S, Qin J, Lu Y, Gulen MF, Herjan T, Min B, Kastelein RA, Aronica M, Kosz-Vnenchak M, Li X. The essential role of single Ig IL-1 receptor-related molecule/toll IL-1R8 in regulation of Th2 immune response. J Immunol. 2009;182:2601–2609. doi: 10.4049/jimmunol.0802729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlock CI, Wu J, Zhou C, Tatum K, Adams HP, Tan F, Lou Y. Unique temporal and spatial expression patterns of IL-33 in ovaries during ovulation and estrous cycle are associated with ovarian tissue homeostasis. J Immunol. 2014;193:161–169. doi: 10.4049/jimmunol.1400381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carriere V, Roussel L, Ortega N, Lacorre DA, Americh L, Aguilar L, Bouche G, Girard JP. IL-33, the IL-1-like cytokine ligand for ST2 receptor, is a chromatin-associated nuclear factor in vivo. Proc Natl Acad Sci U S A. 2007;104:282–287. doi: 10.1073/pnas.0606854104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cayrol C, Girard JP. Interleukin-33 (IL-33): a nuclear cytokine from the IL-1 family. Immunol Rev. 2018;281:154–168. doi: 10.1111/imr.12619. [DOI] [PubMed] [Google Scholar]
- Choi YS, Choi HJ, Min JK, Pyun BJ, Maeng YS, Park H, Kim J, Kim YM, Kwon YG. Interleukin-33 induces angiogenesis and vascular permeability through ST2/TRAF6-mediated endothelial nitric oxide production. Blood. 2009;114:3117–3126. doi: 10.1182/blood-2009-02-203372. [DOI] [PubMed] [Google Scholar]
- Choi YS, Park JA, Kim J, Rho SS, Park H, Kim YM, Kwon YG. Nuclear IL-33 is a transcriptional regulator of NF-kappaB p65 and induces endothelial cell activation. Biochem Biophys Res Commun. 2012;421:305–311. doi: 10.1016/j.bbrc.2012.04.005. [DOI] [PubMed] [Google Scholar]
- Cohen ES, Scott IC, Majithiya JB, Rapley L, Kemp BP, England E, Rees DG, Overed-Sayer CL, Woods J, Bond NJ, Veyssier CS, Embrey KJ, Sims DA, Snaith MR, Vousden KA, Strain MD, Chan DT, Carmen S, Huntington CE, Flavell L, Xu J, Popovic B, Brightling CE, Vaughan TJ, Butler R, Lowe DC, Higazi DR, Corkill DJ, May RD, Sleeman MA, Mustelin T. Oxidation of the alarmin IL-33 regulates ST2-dependent inflammation. Nat Commun. 2015;6:8327. doi: 10.1038/ncomms9327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connolly DJ, O'Neill LA, McGettrick AF. The GOLD domain-containing protein TMED1 is involved in interleukin-33 signaling. J Biol Chem. 2013;288:5616–5623. doi: 10.1074/jbc.M112.403899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demyanets S, Konya V, Kastl SP, Kaun C, Rauscher S, Niessner A, Pentz R, Pfaffenberger S, Rychli K, Lemberger CE, de Martin R, Heinemann A, Huk I, Groger M, Maurer G, Huber K, Wojta J. Interleukin-33 induces expression of adhesion molecules and inflammatory activation in human endothelial cells and in human atherosclerotic plaques. Arterioscler Thromb Vasc Biol. 2011;31:2080–2089. doi: 10.1161/ATVBAHA.111.231431. [DOI] [PubMed] [Google Scholar]
- Demyanets S, Speidl WS, Tentzeris I, Jarai R, Katsaros KM, Farhan S, Krychtiuk KA, Wonnerth A, Weiss TW, Huber K, Wojta J. Soluble ST2 and interleukin-33 levels in coronary artery disease: relation to disease activity and adverse outcome. PLoS One. 2014;9:e95055. doi: 10.1371/journal.pone.0095055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dey G, Radhakrishnan A, Syed N, Thomas JK, Nadig A, Srikumar K, Mathur PP, Pandey A, Lin SK, Raju R, Prasad TS. Signaling network of Oncostatin M pathway. J Cell Commun Signal. 2013;7:103–108. doi: 10.1007/s12079-012-0186-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drube S, Heink S, Walter S, Lohn T, Grusser M, Gerbaulet A, Berod L, Schons J, Dudeck A, Freitag J, Grotha S, Reich D, Rudeschko O, Norgauer J, Hartmann K, Roers A, Kamradt T. The receptor tyrosine kinase c-kit controls IL-33 receptor signaling in mast cells. Blood. 2010;115:3899–3906. doi: 10.1182/blood-2009-10-247411. [DOI] [PubMed] [Google Scholar]
- Drube S, Kraft F, Dudeck J, Muller AL, Weber F, Gopfert C, Meininger I, Beyer M, Irmler I, Hafner N, Schutz D, Stumm R, Yakovleva T, Gaestel M, Dudeck A, Kamradt T. MK2/3 are pivotal for IL-33-induced and mast cell-dependent leukocyte recruitment and the resulting skin inflammation. J Immunol. 2016;197:3662–3668. doi: 10.4049/jimmunol.1600658. [DOI] [PubMed] [Google Scholar]
- Fang Y, Zhao L, Xiao H, Cook KM, Bai Q, Herrick EJ, Chen X, Qin C, Zhu Z, Wakefield MR, Nicholl MB. IL-33 acts as a foe to MIA PaCa-2 pancreatic cancer. Med Oncol. 2017;34:23. doi: 10.1007/s12032-016-0880-3. [DOI] [PubMed] [Google Scholar]
- Funakoshi-Tago M, Tago K, Hayakawa M, Tominaga S, Ohshio T, Sonoda Y, Kasahara T. TRAF6 is a critical signal transducer in IL-33 signaling pathway. Cell Signal. 2008;20:1679–1686. doi: 10.1016/j.cellsig.2008.05.013. [DOI] [PubMed] [Google Scholar]
- Funakoshi-Tago M, Tago K, Sato Y, Tominaga S, Kasahara T. JAK2 is an important signal transducer in IL-33-induced NF-kappaB activation. Cell Signal. 2011;23:363–370. doi: 10.1016/j.cellsig.2010.10.006. [DOI] [PubMed] [Google Scholar]
- Gautier V, Cayrol C, Farache D, Roga S, Monsarrat B, Burlet-Schiltz O, Gonzalez de Peredo A, Girard JP. Extracellular IL-33 cytokine, but not endogenous nuclear IL-33, regulates protein expression in endothelial cells. Sci Rep. 2016;6:34255. doi: 10.1038/srep34255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon ED, Simpson LJ, Rios CL, Ringel L, Lachowicz-Scroggins ME, Peters MC, Wesolowska-Andersen A, Gonzalez JR, MacLeod HJ, Christian LS, Yuan S, Barry L, Woodruff PG, Ansel KM, Nocka K, Seibold MA, Fahy JV. Alternative splicing of interleukin-33 and type 2 inflammation in asthma. Proc Natl Acad Sci U S A. 2016;113:8765–8770. doi: 10.1073/pnas.1601914113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haraldsen G, Balogh J, Pollheimer J, Sponheim J, Kuchler AM. Interleukin-33 - cytokine of dual function or novel alarmin? Trends Immunol. 2009;30:227–233. doi: 10.1016/j.it.2009.03.003. [DOI] [PubMed] [Google Scholar]
- Hardman C, Ogg G. Interleukin-33, friend and foe in type-2 immune responses. Curr Opin Immunol. 2016;42:16–24. doi: 10.1016/j.coi.2016.05.004. [DOI] [PubMed] [Google Scholar]
- Hayakawa H, Hayakawa M, Kume A, Tominaga S. Soluble ST2 blocks interleukin-33 signaling in allergic airway inflammation. J Biol Chem. 2007;282:26369–26380. doi: 10.1074/jbc.M704916200. [DOI] [PubMed] [Google Scholar]
- Hayakawa H, Hayakawa M, Tominaga SI. Soluble ST2 suppresses the effect of interleukin-33 on lung type 2 innate lymphoid cells. Biochem Biophys Rep. 2016;5:401–407. doi: 10.1016/j.bbrep.2016.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong J, Bae S, Jhun H, Lee S, Choi J, Kang T, Kwak A, Hong K, Kim E, Jo S, Kim S. Identification of constitutively active interleukin 33 (IL-33) splice variant. J Biol Chem. 2011;286:20078–20086. doi: 10.1074/jbc.M111.219089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu WT, Li MQ, Liu W, Jin LP, Li DJ, Zhu XY. IL-33 enhances proliferation and invasiveness of decidual stromal cells by up-regulation of CCL2/CCR2 via NF-kappaB and ERK1/2 signaling. Mol Hum Reprod. 2014;20:358–372. doi: 10.1093/molehr/gat094. [DOI] [PubMed] [Google Scholar]
- Hudson CA, Christophi GP, Gruber RC, Wilmore JR, Lawrence DA, Massa PT. Induction of IL-33 expression and activity in central nervous system glia. J Leukoc Biol. 2008;84:631–643. doi: 10.1189/jlb.1207830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi AD, Oak SR, Hartigan AJ, Finn WG, Kunkel SL, Duffy KE, Das A, Hogaboam CM. Interleukin-33 contributes to both M1 and M2 chemokine marker expression in human macrophages. BMC Immunol. 2010;11:52. doi: 10.1186/1471-2172-11-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandasamy K, Keerthikumar S, Raju R, Keshava Prasad TS, Ramachandra YL, Mohan S, Pandey A. PathBuilder--open source software for annotating and developing pathway resources. Bioinformatics. 2009;25:2860–2862. doi: 10.1093/bioinformatics/btp453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandasamy K, Mohan SS, Raju R, Keerthikumar S, Kumar GS, Venugopal AK, Telikicherla D, Navarro JD, Mathivanan S, Pecquet C, Gollapudi SK, Tattikota SG, Mohan S, Padhukasahasram H, Subbannayya Y, Goel R, Jacob HK, Zhong J, Sekhar R, Nanjappa V, Balakrishnan L, Subbaiah R, Ramachandra YL, Rahiman BA, Prasad TS, Lin JX, Houtman JC, Desiderio S, Renauld JC, Constantinescu SN, Ohara O, Hirano T, Kubo M, Singh S, Khatri P, Draghici S, Bader GD, Sander C, Leonard WJ, Pandey A. NetPath: a public resource of curated signal transduction pathways. Genome Biol. 2010;11:R3. doi: 10.1186/gb-2010-11-1-r3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kearley J, Silver JS, Sanden C, Liu Z, Berlin AA, White N, Mori M, Pham TH, Ward CK, Criner GJ, Marchetti N, Mustelin T, Erjefalt JS, Kolbeck R, Humbles AA. Cigarette smoke silences innate lymphoid cell function and facilitates an exacerbated type I interleukin-33-dependent response to infection. Immunity. 2015;42:566–579. doi: 10.1016/j.immuni.2015.02.011. [DOI] [PubMed] [Google Scholar]
- Kim JY, Lim SC, Kim G, Yun HJ, Ahn SG, Choi HS. Interleukin-33/ST2 axis promotes epithelial cell transformation and breast tumorigenesis via upregulation of COT activity. Oncogene. 2015;34:4928–4938. doi: 10.1038/onc.2014.418. [DOI] [PubMed] [Google Scholar]
- Kopach P, Lockatell V, Pickering EM, Haskell RE, Anderson RD, Hasday JD, Todd NW, Luzina IG, Atamas SP. IFN-gamma directly controls IL-33 protein level through a STAT1- and LMP2-dependent mechanism. J Biol Chem. 2014;289:11829–11843. doi: 10.1074/jbc.M113.534396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunisch E, Chakilam S, Gandesiri M, Kinne RW. IL-33 regulates TNF-alpha dependent effects in synovial fibroblasts. Int J Mol Med. 2012;29:530–540. doi: 10.3892/ijmm.2012.883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurowska-Stolarska M, Stolarski B, Kewin P, Murphy G, Corrigan CJ, Ying S, Pitman N, Mirchandani A, Rana B, van Rooijen N, Shepherd M, McSharry C, McInnes IB, Xu D, Liew FY. IL-33 amplifies the polarization of alternatively activated macrophages that contribute to airway inflammation. J Immunol. 2009;183:6469–6477. doi: 10.4049/jimmunol.0901575. [DOI] [PubMed] [Google Scholar]
- Lefrancais E, Roga S, Gautier V, Gonzalez-de-Peredo A, Monsarrat B, Girard JP, Cayrol C. IL-33 is processed into mature bioactive forms by neutrophil elastase and cathepsin G. Proc Natl Acad Sci U S A. 2012;109:1673–1678. doi: 10.1073/pnas.1115884109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefrancais E, Duval A, Mirey E, Roga S, Espinosa E, Cayrol C, Girard JP. Central domain of IL-33 is cleaved by mast cell proteases for potent activation of group-2 innate lymphoid cells. Proc Natl Acad Sci U S A. 2014;111:15502–15507. doi: 10.1073/pnas.1410700111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li R, Yang G, Yang R, Peng X, Li J. Interleukin-33 and receptor ST2 as indicators in patients with asthma: a meta-analysis. Int J Clin Exp Med. 2015;8:14935–14943. [PMC free article] [PubMed] [Google Scholar]
- Li Q, Li D, Zhang X, Wan Q, Zhang W, Zheng M, Zou L, Elly C, Lee JH, Liu YC. E3 ligase VHL promotes group 2 innate lymphoid cell maturation and function via glycolysis inhibition and induction of Interleukin-33 receptor. Immunity. 2018;48(258–270):e255. doi: 10.1016/j.immuni.2017.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liew FY, Girard JP, Turnquist HR. Interleukin-33 in health and disease. Nat Rev Immunol. 2016;16:676–689. doi: 10.1038/nri.2016.95. [DOI] [PubMed] [Google Scholar]
- Lima IL, Macari S, Madeira MF, Rodrigues LF, Colavite PM, Garlet GP, Soriani FM, Teixeira MM, Fukada SY, Silva TA. Osteoprotective effects of IL-33/ST2 link to osteoclast apoptosis. Am J Pathol. 2015;185:3338–3348. doi: 10.1016/j.ajpath.2015.08.013. [DOI] [PubMed] [Google Scholar]
- Lingel A, Weiss TM, Niebuhr M, Pan B, Appleton BA, Wiesmann C, Bazan JF, Fairbrother WJ. Structure of IL-33 and its interaction with the ST2 and IL-1RAcP receptors--insight into heterotrimeric IL-1 signaling complexes. Structure. 2009;17:1398–1410. doi: 10.1016/j.str.2009.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Hammel M, He Y, Tainer JA, Jeng US, Zhang L, Wang S, Wang X. Structural insights into the interaction of IL-33 with its receptors. Proc Natl Acad Sci U S A. 2013;110:14918–14923. doi: 10.1073/pnas.1308651110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Zhu L, Lu X, Bian H, Wu X, Yang W, Qin Q. IL-33/ST2 pathway contributes to metastasis of human colorectal cancer. Biochem Biophys Res Commun. 2014;453:486–492. doi: 10.1016/j.bbrc.2014.09.106. [DOI] [PubMed] [Google Scholar]
- Lohning M, Stroehmann A, Coyle AJ, Grogan JL, Lin S, Gutierrez-Ramos JC, Levinson D, Radbruch A, Kamradt T. T1/ST2 is preferentially expressed on murine Th2 cells, independent of interleukin 4, interleukin 5, and interleukin 10, and important for Th2 effector function. Proc Natl Acad Sci U S A. 1998;95:6930–6935. doi: 10.1073/pnas.95.12.6930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin MU. Special aspects of interleukin-33 and the IL-33 receptor complex. Semin Immunol. 2013;25:449–457. doi: 10.1016/j.smim.2013.10.006. [DOI] [PubMed] [Google Scholar]
- Meephansan J, Tsuda H, Komine M, Tominaga S, Ohtsuki M. Regulation of IL-33 expression by IFN-gamma and tumor necrosis factor-alpha in normal human epidermal keratinocytes. J Invest Dermatol. 2012;132:2593–2600. doi: 10.1038/jid.2012.185. [DOI] [PubMed] [Google Scholar]
- Miller AM, Xu D, Asquith DL, Denby L, Li Y, Sattar N, Baker AH, McInnes IB, Liew FY. IL-33 reduces the development of atherosclerosis. J Exp Med. 2008;205:339–346. doi: 10.1084/jem.20071868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monticelli LA, Sonnenberg GF, Abt MC, Alenghat T, Ziegler CG, Doering TA, Angelosanto JM, Laidlaw BJ, Yang CY, Sathaliyawala T, Kubota M, Turner D, Diamond JM, Goldrath AW, Farber DL, Collman RG, Wherry EJ, Artis D. Innate lymphoid cells promote lung-tissue homeostasis after infection with influenza virus. Nat Immunol. 2011;12:1045–1054. doi: 10.1038/ni.2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moritz DR, Rodewald HR, Gheyselinck J, Klemenz R. The IL-1 receptor-related T1 antigen is expressed on immature and mature mast cells and on fetal blood mast cell progenitors. J Immunol. 1998;161:4866–4874. [PubMed] [Google Scholar]
- Moussion C, Ortega N, Girard JP. The IL-1-like cytokine IL-33 is constitutively expressed in the nucleus of endothelial cells and epithelial cells in vivo: a novel 'alarmin'? PLoS One. 2008;3:e3331. doi: 10.1371/journal.pone.0003331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mun SH, Ko NY, Kim HS, Kim JW, Kim do K, Kim AR, Lee SH, Kim YG, Lee CK, Kim BK, Beaven MA, Kim YM, Choi WS. Interleukin-33 stimulates formation of functional osteoclasts from human CD14(+) monocytes. Cell Mol Life Sci. 2010;67:3883–3892. doi: 10.1007/s00018-010-0410-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neill DR, Wong SH, Bellosi A, Flynn RJ, Daly M, Langford TK, Bucks C, Kane CM, Fallon PG, Pannell R, Jolin HE, McKenzie AN. Nuocytes represent a new innate effector leukocyte that mediates type-2 immunity. Nature. 2010;464:1367–1370. doi: 10.1038/nature08900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onda H, Kasuya H, Takakura K, Hori T, Imaizumi T, Takeuchi T, Inoue I, Takeda J. Identification of genes differentially expressed in canine vasospastic cerebral arteries after subarachnoid hemorrhage. J Cereb Blood Flow Metab. 1999;19:1279–1288. doi: 10.1097/00004647-199911000-00013. [DOI] [PubMed] [Google Scholar]
- Palmer G, Gabay C. Interleukin-33 biology with potential insights into human diseases. Nat Rev Rheumatol. 2011;7:321–329. doi: 10.1038/nrrheum.2011.53. [DOI] [PubMed] [Google Scholar]
- Pecaric-Petkovic T, Didichenko SA, Kaempfer S, Spiegl N, Dahinden CA. Human basophils and eosinophils are the direct target leukocytes of the novel IL-1 family member IL-33. Blood. 2009;113:1526–1534. doi: 10.1182/blood-2008-05-157818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto SM, Nirujogi RS, Rojas PL, Patil AH, Manda SS, Subbannayya Y, Roa JC, Chatterjee A, Prasad TS, Pandey A. Quantitative phosphoproteomic analysis of IL-33-mediated signaling. Proteomics. 2015;15:532–544. doi: 10.1002/pmic.201400303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollheimer J, Bodin J, Sundnes O, Edelmann RJ, Skanland SS, Sponheim J, Brox MJ, Sundlisaeter E, Loos T, Vatn M, Kasprzycka M, Wang J, Kuchler AM, Tasken K, Haraldsen G, Hol J. Interleukin-33 drives a proinflammatory endothelial activation that selectively targets nonquiescent cells. Arterioscler Thromb Vasc Biol. 2013;33:e47–e55. doi: 10.1161/ATVBAHA.112.253427. [DOI] [PubMed] [Google Scholar]
- Polumuri SK, Jayakar GG, Shirey KA, Roberts ZJ, Perkins DJ, Pitha PM, Vogel SN. Transcriptional regulation of murine IL-33 by TLR and non-TLR agonists. J Immunol. 2012;189:50–60. doi: 10.4049/jimmunol.1003554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson KM, Ramanan K, Clay ME, McHugh KJ, Rich HE, Alcorn JF (2017) Novel protective mechanism for interleukin-33 at the mucosal barrier during influenza-associated bacterial superinfection. Mucosal Immunol [DOI] [PMC free article] [PubMed]
- Robinson KM, Ramanan K, Clay ME, McHugh KJ, Rich HE, Alcorn JF. Novel protective mechanism for interleukin-33 at the mucosal barrier during influenza-associated bacterial superinfection. Mucosal Immunol. 2018;11:199–208. doi: 10.1038/mi.2017.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roussel L, Erard M, Cayrol C, Girard JP. Molecular mimicry between IL-33 and KSHV for attachment to chromatin through the H2A-H2B acidic pocket. EMBO Rep. 2008;9:1006–1012. doi: 10.1038/embor.2008.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmond RJ, Mirchandani AS, Besnard AG, Bain CC, Thomson NC, Liew FY. IL-33 induces innate lymphoid cell-mediated airway inflammation by activating mammalian target of rapamycin. J Allergy Clin Immunol. 2012;130(1159–1166):e1156. doi: 10.1016/j.jaci.2012.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saluja R, Hawro T, Eberle J, Church MK, Maurer M. Interleukin-33 promotes the proliferation of mouse mast cells through ST2/MyD88 and p38 MAPK-dependent and kit-independent pathways. J Biol Regul Homeost Agents. 2014;28:575–585. [PubMed] [Google Scholar]
- Schmitz J, Owyang A, Oldham E, Song Y, Murphy E, McClanahan TK, Zurawski G, Moshrefi M, Qin J, Li X, Gorman DM, Bazan JF, Kastelein RA. IL-33, an interleukin-1-like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T helper type 2-associated cytokines. Immunity. 2005;23:479–490. doi: 10.1016/j.immuni.2005.09.015. [DOI] [PubMed] [Google Scholar]
- Schulze J, Bickert T, Beil FT, Zaiss MM, Albers J, Wintges K, Streichert T, Klaetschke K, Keller J, Hissnauer TN, Spiro AS, Gessner A, Schett G, Amling M, McKenzie AN, Horst AK, Schinke T. Interleukin-33 is expressed in differentiated osteoblasts and blocks osteoclast formation from bone marrow precursor cells. J Bone Miner Res. 2011;26:704–717. doi: 10.1002/jbmr.269. [DOI] [PubMed] [Google Scholar]
- Shang J, Zhao J, Wu X, Xu Y, Xie J. Interleukin-33 promotes inflammatory cytokine production in chronic airway inflammation. Biochem Cell Biol. 2015;93:359–366. doi: 10.1139/bcb-2014-0163. [DOI] [PubMed] [Google Scholar]
- Smithgall MD, Comeau MR, Yoon BR, Kaufman D, Armitage R, Smith DE. IL-33 amplifies both Th1- and Th2-type responses through its activity on human basophils, allergen-reactive Th2 cells, iNKT and NK cells. Int Immunol. 2008;20:1019–1030. doi: 10.1093/intimm/dxn060. [DOI] [PubMed] [Google Scholar]
- Subbannayya Y, Anuja K, Advani J, Ojha UK, Nanjappa V, George B, Sonawane A, Kumar RV, Ramaswamy G, Pandey A, Somani BL, Raju R. A network map of the gastrin signaling pathway. J Cell Commun Signal. 2014;8:165–170. doi: 10.1007/s12079-014-0224-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundlisaeter E, Edelmann RJ, Hol J, Sponheim J, Kuchler AM, Weiss M, Udalova IA, Midwood KS, Kasprzycka M, Haraldsen G. The alarmin IL-33 is a notch target in quiescent endothelial cells. Am J Pathol. 2012;181:1099–1111. doi: 10.1016/j.ajpath.2012.06.003. [DOI] [PubMed] [Google Scholar]
- Suzukawa M, Iikura M, Koketsu R, Nagase H, Tamura C, Komiya A, Nakae S, Matsushima K, Ohta K, Yamamoto K, Yamaguchi M. An IL-1 cytokine member, IL-33, induces human basophil activation via its ST2 receptor. J Immunol. 2008;181:5981–5989. doi: 10.4049/jimmunol.181.9.5981. [DOI] [PubMed] [Google Scholar]
- Szklarczyk D, Franceschini A, Wyder S, Forslund K, Heller D, Huerta-Cepas J, Simonovic M, Roth A, Santos A, Tsafou KP, Kuhn M, Bork P, Jensen LJ, von Mering C. STRING v10: protein-protein interaction networks, integrated over the tree of life. Nucleic Acids Res. 2015;43:D447–D452. doi: 10.1093/nar/gku1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tominaga S. A putative protein of a growth specific cDNA from BALB/c-3T3 cells is highly similar to the extracellular portion of mouse interleukin 1 receptor. FEBS Lett. 1989;258:301–304. doi: 10.1016/0014-5793(89)81679-5. [DOI] [PubMed] [Google Scholar]
- Tominaga S, Jenkins NA, Gilbert DJ, Copeland NG, Tetsuka T. Molecular cloning of the murine ST2 gene. Characterization and chromosomal mapping. Biochim Biophys Acta. 1991;1090:1–8. doi: 10.1016/0167-4781(91)90029-L. [DOI] [PubMed] [Google Scholar]
- Tsuda H, Komine M, Karakawa M, Etoh T, Tominaga S, Ohtsuki M. Novel splice variants of IL-33: differential expression in normal and transformed cells. J Invest Dermatol. 2012;132:2661–2664. doi: 10.1038/jid.2012.180. [DOI] [PubMed] [Google Scholar]
- Tung HY, Plunkett B, Huang SK, Zhou Y. Murine mast cells secrete and respond to interleukin-33. J Interf Cytokine Res. 2014;34:141–147. doi: 10.1089/jir.2012.0066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Iersel MP, Kelder T, Pico AR, Hanspers K, Coort S, Conklin BR, Evelo C. Presenting and exploring biological pathways with PathVisio. BMC Bioinformatics. 2008;9:399. doi: 10.1186/1471-2105-9-399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasmer MH, Krebs P. The role of IL-33-dependent inflammation in the tumor microenvironment. Front Immunol. 2016;7:682. doi: 10.3389/fimmu.2016.00682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg EO, Shimpo M, De Keulenaer GW, MacGillivray C, Tominaga S, Solomon SD, Rouleau JL, Lee RT. Expression and regulation of ST2, an interleukin-1 receptor family member, in cardiomyocytes and myocardial infarction. Circulation. 2002;106:2961–2966. doi: 10.1161/01.CIR.0000038705.69871.D9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H, Yang S, Wu X, Zhao J, Ning Q, Xu Y, Xie J. Interleukin-33/ST2 signaling promotes production of interleukin-6 and interleukin-8 in systemic inflammation in cigarette smoke-induced chronic obstructive pulmonary disease mice. Biochem Biophys Res Commun. 2014;450:110–116. doi: 10.1016/j.bbrc.2014.05.073. [DOI] [PubMed] [Google Scholar]
- Xi S, Xu H, Shan J, Tao Y, Hong JA, Inchauste S, Zhang M, Kunst TF, Mercedes L, Schrump DS. Cigarette smoke mediates epigenetic repression of miR-487b during pulmonary carcinogenesis. J Clin Invest. 2013;123:1241–1261. doi: 10.1172/JCI61271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia J, Zhao J, Shang J, Li M, Zeng Z, Wang J, Xu Y, Xie J. Increased IL-33 expression in chronic obstructive pulmonary disease. Am J Physiol Lung Cell Mol Physiol. 2015;308:L619–L627. doi: 10.1152/ajplung.00305.2014. [DOI] [PubMed] [Google Scholar]
- Xiang Y, Eyers F, Herbert C, Tay HL, Foster PS, Yang M. MicroRNA-487b is a negative regulator of macrophage activation by targeting IL-33 production. J Immunol. 2016;196:3421–3428. doi: 10.4049/jimmunol.1502081. [DOI] [PubMed] [Google Scholar]
- Yagami A, Orihara K, Morita H, Futamura K, Hashimoto N, Matsumoto K, Saito H, Matsuda A. IL-33 mediates inflammatory responses in human lung tissue cells. J Immunol. 2010;185:5743–5750. doi: 10.4049/jimmunol.0903818. [DOI] [PubMed] [Google Scholar]
- Yamazumi Y, Sasaki O, Imamura M, Oda T, Ohno Y, Shiozaki-Sato Y, Nagai S, Suyama S, Kamoshida Y, Funato K, Yasui T, Kikutani H, Yamamoto K, Dohi M, Koyasu S, Akiyama T. The RNA binding protein Mex-3B is required for IL-33 induction in the development of allergic airway inflammation. Cell Rep. 2016;16:2456–2471. doi: 10.1016/j.celrep.2016.07.062. [DOI] [PubMed] [Google Scholar]
- Yanagisawa K, Takagi T, Tsukamoto T, Tetsuka T, Tominaga S. Presence of a novel primary response gene ST2L, encoding a product highly similar to the interleukin 1 receptor type 1. FEBS Lett. 1993;318:83–87. doi: 10.1016/0014-5793(93)81333-U. [DOI] [PubMed] [Google Scholar]
- Zhang L, Lu R, Zhao G, Pflugfelder SC, Li DQ. TLR-mediated induction of pro-allergic cytokine IL-33 in ocular mucosal epithelium. Int J Biochem Cell Biol. 2011;43:1383–1391. doi: 10.1016/j.biocel.2011.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao W, Hu Z. The enigmatic processing and secretion of interleukin-33. Cell Mol Immunol. 2010;7:260–262. doi: 10.1038/cmi.2010.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao WH, Hu ZQ. Up-regulation of IL-33 expression in various types of murine cells by IL-3 and IL-4. Cytokine. 2012;58:267–273. doi: 10.1016/j.cyto.2012.01.019. [DOI] [PubMed] [Google Scholar]
- Zhao J, Wei J, Mialki RK, Mallampalli DF, Chen BB, Coon T, Zou C, Mallampalli RK, Zhao Y. F-box protein FBXL19-mediated ubiquitination and degradation of the receptor for IL-33 limits pulmonary inflammation. Nat Immunol. 2012;13:651–658. doi: 10.1038/ni.2341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong J, Sharma J, Raju R, Palapetta SM, Prasad TS, Huang TC, Yoda A, Tyner JW, van Bodegom D, Weinstock DM, Ziegler SF, Pandey A (2014) TSLP signaling pathway map: a platform for analysis of TSLP-mediated signaling. Database (Oxford) 2014:bau007 [DOI] [PMC free article] [PubMed]
- Zhu J, Carver W. Effects of interleukin-33 on cardiac fibroblast gene expression and activity. Cytokine. 2012;58:368–379. doi: 10.1016/j.cyto.2012.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
A list of protein-protein interactions identified to be involved in IL-33 signaling based on the STRING analysis tool version 10.5. Only those protein-protein interactions that are experimentally determined with a medium confidence score threshold of 0.4 and above have been considered. (XLSX 88 kb)


