Abstract
Ezrin acts as a dynamic linkage between plasma membrane and cytoskeleton, and thus involved in many fundamental cellular functions. Yet, its potential role in human skin is virtually unknown. Here we investigate the role of Ezrin in primary skin fibroblasts, the major cells responsible extracellular matrix (ECM) production. We report that Ezrin play an important role in the maintenance of skin fibroblast size/mechanical properties and proliferation. siRNA-mediated Ezrin knockdown decreased fibroblast size and mechanical properties, and thus impaired the nuclear translocation of YAP, a protein commonly response to cell size and mechanical force. Functionally, depletion of Ezrin significantly inhibited YAP target gene expression and fibroblast proliferation. Conversely, restoration of YAP nuclear translocation by overexpression of constitutively active YAP reversed YAP target genes expression and rescued proliferation in Ezrin knockdown cells. These data reveal a novel role for Ezrin in maintenance of fibroblast size/mechanical force and regulating YAP-mediated proliferation.
Electronic supplementary material
The online version of this article (10.1007/s12079-017-0406-6) contains supplementary material, which is available to authorized users.
Keywords: Ezrin, Yap, Cell size, Mechanical properties
Introduction
Ezrin is a member of the ERM (Ezrin/radixin/moesin) family of proteins whose primary function is to organize the interface between the plasma membrane and the cytoskeleton (Sato et al., 1992; Fehon et al., 2010). In doing so, ERM proteins provide a linkage from filamentous actin in the cortex to membrane proteins on the surface of cells. By creating such a linkage, ERM proteins can perform both structural and regulatory functions in cells (Bretscher et al., 2002; Fehon et al., 2010). Like the other ERM family members, Ezrin considers a cytoskeletal linker based on their ability to interact with both the plasma membrane and filamentous actin (Arpin et al., 2011). Ezrin N-terminal region (FERM domain) binds to plasma membrane lipids and cytoplasmic tails of transmembrane proteins, while the C-terminal region binds to F-actin (Fehon et al., 2010; Neisch and Fehon, 2011). As membrane-cytoskeleton interaction is essential fundamental processes of cells, it is not surprising that Ezrin protein has been implicated in many diverse cellular functions including modulating cell morphogenesis and integrity. Under normal physiological condition, Ezrin is involved in many cellular processes, such as cell adhesion (Takeuchi et al., 1994; Pujuguet et al., 2003), migration (Crepaldi et al., 1997; Ng et al., 2001; Naba et al., 2008), determination of cell shape, cell proliferation, and morphogenesis (Crepaldi et al., 1997; Gautreau et al., 2000; Bretscher et al., 2002; Hsu et al., 2012) in response to extracellular cues. Aberrant expression of Ezrin has been shown in a variety of cancers associated with poor prognosis and metastasis; yet, the molecular mechanisms in which Ezrin participates in tumorigenesis still remain ill-defined (Clucas and Valderrama, 2014). A correlation between high expression of Ezrin and aggressive cancer behavior has been recently reported from a wide variety of cancers (Kong et al., 2013; Jin et al., 2014; Piao et al., 2015). For example, elevated Ezrin expression is common in malignant gastric tissue and is closely associated with a poor clinical outcome(Jin et al., 2012; Li et al., 2012). As such, Ezrin can be used as a biomarker of malignant conversion in gastric tissue. Although the functional details of Ezrin have been fairly well studied, we know very little about its potential role in skin biology.
Human skin is primarily composed of collagen rich extracellular matrix (ECM), which is produced by skin fibroblasts. In healthy skin, dermal fibroblasts interact with intact collagen fibrils, through integrin collagen receptors, to achieve and maintain stretched morphology. However, during aging collagen fibrils become fragmented, less dense, and disorganized (Varani et al., 2004; Fisher et al., 2009). These alterations disrupt fibroblast interactions with extracellular collagen fibrils, resulting in reduced spreading and cells size (Varani et al., 2004; Fisher et al., 2008; Fisher et al., 2009; Quan et al., 2013a; Qin et al., 2014). We have previously reported that age-related reduction of dermal fibroblast size is a prominent feature of aged human skin in vivo, and has significant impact on human skin aging (Varani et al., 2004; Fisher et al., 2009; Qin et al., 2014; Quan et al., 2015; Fisher et al., 2016). The Ezrin protein is of particular interest in this regard as it plays role in regulating cell morphology through its interaction with membrane and actin cytoskeleton. Here we report that depletion of Ezrin decreases fibroblast size/mechanical tension and impairs YAP nuclear translocation. Further investigation reveals that Ezrin depletion impairs skin fibroblast proliferation through interfering YAP nuclear translocation, a dominant regulator of cell proliferation.
Materials and methods
Materials
Dulbecco’s Modified Eagle’s Media (DMEM), trypsin solution, and penicillin/streptomycin were purchased from Life Technology (Rockville, MD, USA). Ezrin, CCN1, CCN2, and YAP antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, UAS). Phospho-YAP (Ser127) Antibody was purchased from Cell Signaling (Danvers, MA). β-actin antibody was purchased from Sigma Chemical Company (St. Louis, MO, USA). CellTracker® fluorescent dye was purchased from Molecular Probes (Eugene, OR, USA) RNeasy Mini Kit was purchased from Qiagen (Chatsworth, CA, UAS). Reagents for real-time PCR were purchased from Applied Biosystems (Foster City, CA, USA). Protein Assay Kit was purchased from Bio-Rad Laboratories (Hercules, CA). All other reagents were purchased from Sigma Chemical Company (St. Louis, MO, USA).
Cell culture
Normal adult human skin primary fibroblasts (PCS-201-012) and foreskin fibroblasts (CRL-2522) were purchased from American Type Culture Collection (Manassas, VA, USA). Cells were cultivated in DMEM at 37 °C and 5% CO2 supplemented with 10% Fetal Bovine Serum (Invitrogen, Carlsbad, CA, USA). Cells were passaged no more than nine times. Cells were plated at 70–80% confluence, and used one day after plating.
Immunofluorescence staining
Immunostaining was performed as described previously (Kong et al., 2016). Briefly, human skin fibroblasts were fixed in 2% paraformaldehyde for 30 min and then permeabilized with permeabilization buffer (0.5% Triton X-100). Cells were then pre-incubated with protein block (Biogenex Laboratories, San Ramon, CA, UAS) for 1 h at room temperature. Subsequently the cells were incubated for overnight at 4 °C with Ezrin and YAP antibodies (Santa Cruz, CA, UAS). Cells were then incubated with biotinylated antibody for 30 min, followed by incubation with avidin-labeled fluorescence dye (Invitrogen, Carlsbad, CA, USA) for 30 min. Nuclei were stained with DAPI. Specificity of staining was determined by substituting isotype-control immunoglobulin (mouse IgG2a) for the primary antibodies. No detectable staining was observed with isotype-controls (data not shown).
RNA isolation and quantitative real-time RT-PCR
Total RNA was prepared by RNeasy Mini Kit (Qiagen, Chatsworth, CA, USA) according to the manufacturer’s protocol. Total RNA (200 ng) was reverse transcribed using a Taqman reverse transcription kit (Applied Biosystems, Foster City, CA, USA). Quantitative real-time PCR was performed on a 7300 Sequence Detector (Applied Biosystems, Carlsbad, CA, USA) using Taqman Universal PCR Master Mix Reagents (Applied Biosystems, Carlsbad, CA, USA). All real-time PCR primers were purchased from RealTimePrimers.com (Elkins Park, PA, USA). Target gene mRNA expression levels were normalized to the housekeeping gene 36B4 (acidic ribosomal phosphoprotein P0)(Akamine et al., 2007) as an internal control for quantification.
Western analysis
Human skin fibroblasts were lysed with WCE buffer (25 mM HEPES (pH 7.2), 75 mM NaCl, 2.5 mM MgCl2, 0.2 mM EDTA, 0.1% Triton X-100, 0.5 mM DTT, 20 mM β-glycerophosphate, 0.1 mM Na3VO4, 2 μg/ml leupeptin, and 100 μg/ml PMSF). The cells were frozen/thawed/vortexed three times, and cellular proteins were collected by centrifugation at 15,000×g for 15 min. Equal amounts of proteins were resolved on 12% SDS-polyacrylamide gel (Invitrogen, Carlsbad, CA, USA) and transferred to PVDF membrane (Millipore, Bedford, MA, USA) for 4–6 h. The membranes were then blocked with 5% milk in PBST (0.1% Tween 20 in PBS) for one hour, followed by incubation with primary antibodies in PBST solution (1:200) for one hour at room temperature. The membranes were washed three times with PBST solution and incubated with appropriate secondary antibody for one hour at room temperature. After washing three times with PBST, the membranes were developed with ECF (Vistra ECF Western Blotting System, Amersham Pharmacia Biotech, Piscataway, NJ, USA) following the manufacturer’s protocol. The intensities of each band were scanned by STORM PhosphorImager (Molecular Dynamics, Sunnyvale, CA, USA). The intensities of each band were quantified using ImageQuant (GE HealthCare, Piscataway, NJ, USA) and normalized using β-actin as a loading control.
Transient transfection
Human skin fibroblasts were transiently transfected by electroporation (Amaxa, Koeln, Germany) using Human Dermal Fibroblast Nucleofector® Kit. Cells were transfected with program U-023. Transfection of Emerald Green Fluorescent Protein (EmGFP, The Vivid Colors™ ThermoFisher Waltham, MA) indicated that the transfection efficiency can be up to 80% in human primary skin fibroblasts by electroporation. Ezrin #1 siRNA was purchased from Santa Cruz Biotechnology (SC535349, Santa Cruz, CA, UAS). Ezrin #2 siRNA was purchased from Dharmacon (7430 Ezrin siRNA SMARTpool, Lafayette, CO, USA). Control siRNA (AATTGTCCGAACGTGTCACGT) were purchased from Qiagen (Chatsworth, CA, USA). Constitutively active YAP (FLAG-YAP-S127A) was kindly provided from Dr. Chen (Institute of Molecular and Cell Biology, Singapore)(Chan et al., 2011). After transfection (48 h), immunostaining was performed as described above. Total RNA and cellular protein were extracted and mRNA and protein levels were determined by real-time RT-PCR and Western analysis, respectively, as described above.
Three-dimensional (3D) collagen matrices cultures
Three-dimensional (3D) collagen gels were made following a previous publication with minor modification (Fisher et al., 2016). Briefly, early passage (<9 passages) skin fibroblasts (0.5 × 106) were mixed in 2 ml calf skin type I collagen (6 mg/ml, Elastin Products Company, Owensville, MO) and medium (pH 7.2, DMEM, 44 mM NaHCO3, 4 mM L-glutamine, 9 mM folic acid, and 1 N NaOH). The cell-collagen gel mix then plated in 35 mm bacterial culture dishes. After collagen polymerization, collagen gels were incubated with 2 ml media (DMEM, 10% FBS) at 37 °C, 5% CO2.
Crystal violet assay
The relative density of cells was determined by crystal violet assay. Briefly, the culture medium was removed from tissue culture plate, and washed gently with PBS three times, followed by adding crystal violet solution. The plate was incubated for 10 min at room temperature and washed with tap water three times by immersion in a large beaker. The plate was drained by upside down on paper towels, then added 1% SDS to solubilize the stain. The pate then agitated on orbital shaker until color is uniform with no areas of dense coloration in bottom of wells. Upon solubilization, the amount of dye taken up by the cells was quantitated in a spectrophotometer by reading absorbance of each well at 570 nm.
Atomic force microscopy (AFM) imaging
The mechanical properties of the cells were measured by AFM using previously established techniques in our laboratory with minor modifications (Quan et al., 2012). Briefly, cells were embedded in 3D type I collagen gel as described above. Cellular mechanical properties were measured in wet condition by Dimension Icon AFM system (Bruker-AXS, Santa Barbara, CA, USA). AFM was performed using PeakForce Quantitative NanoMechanics mode with a silicon AFM probe (NSC15/AIBS, MikroMasch, San Jose, CA). AFM was conducted at the Electron Microbeam Analysis Laboratory (EMAL), University of Michigan College of Engineering, and analyzed using Nanoscope Analysis software (Nanoscope Analysis v120R1sr3, Bruker-AXS, Santa Barbara, CA, USA).
Statistical analysis
Comparisons among treatment groups were made with the paired t-test (two groups) or the repeated measures of ANOVA (more than two groups). Multiple pair-wise comparisons were made with the Tukey Studentized Range test. All p values are two-tailed, and considered significant when <0.05.
Results
Ezrin knockdown decreases skin fibroblast size
To explore the potential role of Ezrin in skin fibroblasts, Ezrin expression was depleted by siRNA-mediated knockdown (Fig. 1a, and b). Interestingly, Ezrin knockdown substantially decreased skin fibroblast size (Fig. 1c and Supplementary Fig. 1a right panels). Staining cells with cytotracker® fluorescence dye further confirmed reduced cell area (Fig. 1d and Supplementary Fig. 1A upper right panels). Fibroblast area was reduced approximately 60% (Fig. 1d and Supplementary Fig. 1A). Next, skin fibroblasts were cultured in a three dimensional collagen environment, which mimic collagen-rich skin dermis. Consistent with Fig. 1d, cells shrank and decreased in size by Ezrin knockdown (Fig. 1e lower right panel). In contrast, fibroblasts in control si RNA displayed elongated and spread morphology (Fig. 1e lower left). Importantly, Ezrin knockdown significantly reduced collagen gel contraction (Fig. 1f), a reliable indicator of cell-collagen interaction. These data suggest that Ezrin regulates skin fibroblast size and mediates cell-ECM interaction.
Fig. 1.
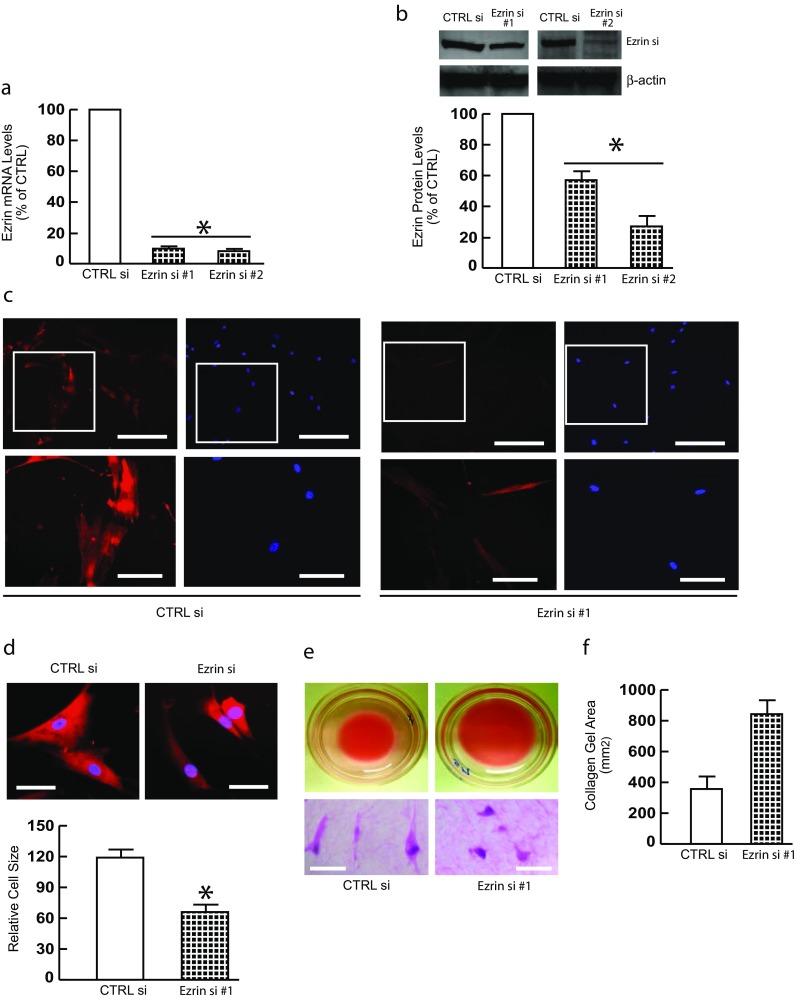
Ezrin knockdown decreases skin fibroblast size. Primary skin fibroblasts were transfected with non-specific control siRNA or Ezrin siRNAs (20 nM) for 48 h. a Ezrin mRNA levels. N = 4. b Ezrin protein levels. N = 3. c Ezrin immunostaining. Images represent four independent experiments. Bar = 100 μm. Enlargement of the boxed region is shown to the bottom (bars = 50 μm). d Cells were stained with CellTracker® fluorescent dye. Red fluorescence delineates cell cytoplasm; blue fluorescence delineates nuclei. The relative cell surface areas were quantified by ImageJ. Bars = 50 μm. N = 3. e Cells were cultured in 3D type I collagen gels (35 mm dish) as described in Methods. Collagen gels were stained with crystal violet (bottom panel). Bars = 50 μm. N = 3. f Quantification of collagen gel contraction. N = 3. mRNA levels were quantified by real-time RT-PCR. mRNA levels were normalized to mRNA for 36B4, a ribosomal protein used as an internal control for quantitation. Protein levels were determined by Western blots. Protein levels were normalized by β-actin as a loading control. Insets show representative Western blots. Data are expressed as mean±SEM, *p < 0.05 vs control si
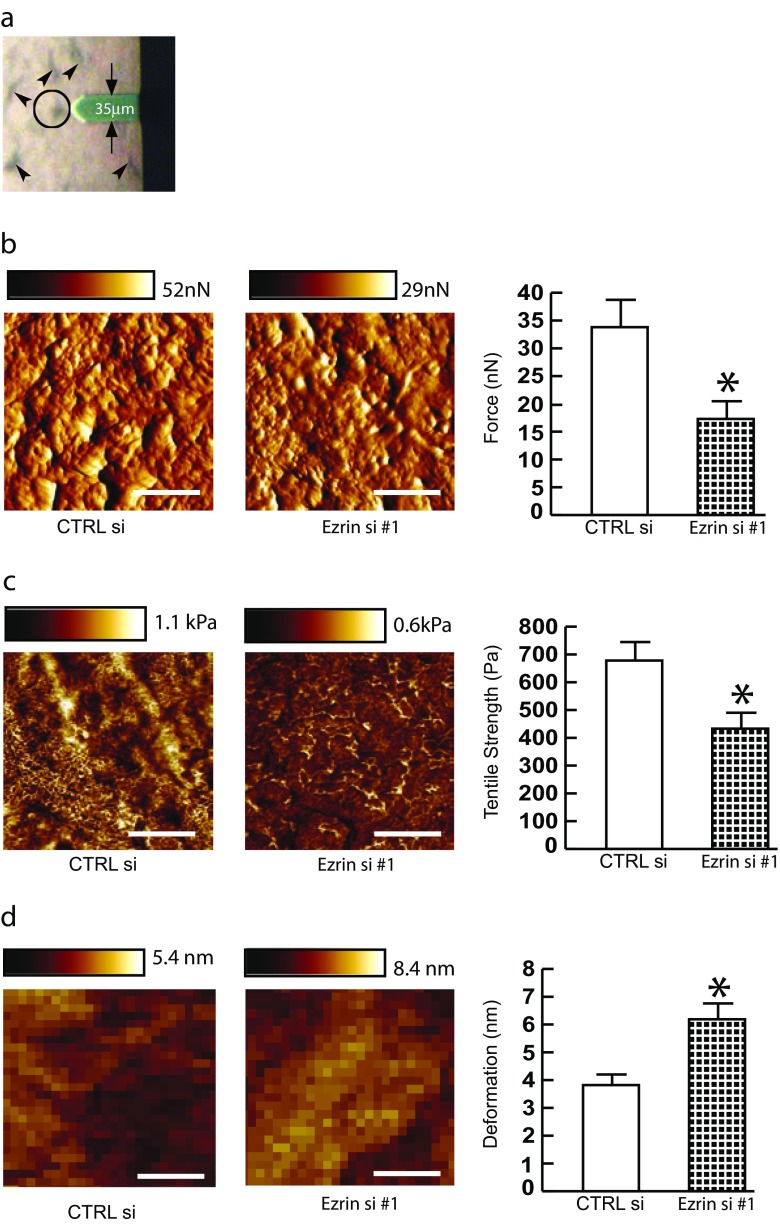
Ezrin knockdown decreases skin fibroblast mechanical properties
We next employed atomic force microscopy (AFM) to determine changes in mechanical properties associated with reduced fibroblast size. The AFM probe tip was placed directly on the surface of the central regions of the fibroblasts (Fig. 2a). Key cellular mechanical properties; traction force (Fig. 2b and Supplementary Fig. 1B) and tensile strength (Fig. 2c and Supplementary Fig. 1C) were reduced approximately 50%, while deformation was increased approximately 1.5-fold (Fig. 2d and Supplementary Fig. 1D), in Ezrin knockdown cells compared to cells transfected with control siRNA. These data suggest that Ezrin knockdown reduces cellular mechanical properties along with cell size.
Fig. 2.
Ezrin knockdown decreases skin fibroblast mechanical properties. Primary skin fibroblasts were transfected with non-specific control siRNA or Ezrin siRNAs (20 nM). Cells were then cultured in 3D type I collagen gels for 48 h as described in Methods. Mechanical properties were determined by atomic force microscopy (AFM) PeakForce Quantitative NanoMechanics mode and analyzed by Nanoscope Analysis software. a Representative bottom-view bright field image of AFM. AFM cantilever positioned on the cells are shown (black circle). Arrow heads indicate cells. b Cell traction force (nN). Representative images of traction force (left). Horizontal bars indicate traction forces scales (nN). N = 4. c Tensile strength (Pa). Representative images of traction force (left). Horizontal bars indicate tensile strength scales (Pa). N = 4. d Deformation (nm). Representative images of deformation (left). Horizontal bars indicate deformation scales (nm). N = 4. Data are expressed as mean±SEM, *p < 0.05 vs control si
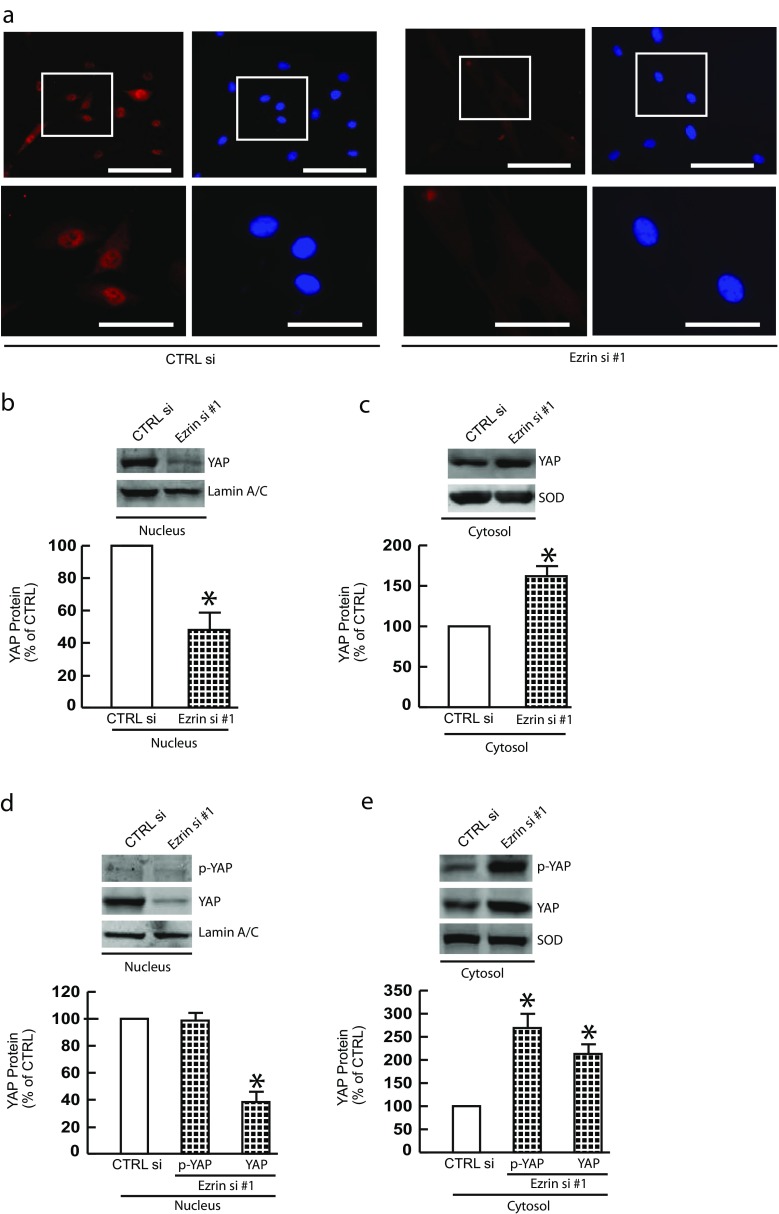
Ezrin knockdown impairs YAP nuclear translocation in skin fibroblasts
Next, we explored the impact of Ezrin-mediated cell size/mechanical properties on YAP activity, since YAP activity is primarily regulated by sub-cellular localization in response to cell size (Zhao et al., 2011), and mechanical tension (Dupont et al., 2011). Immunostaining of YAP indicate that YAP primarily localized in nuclei in control cells (Fig. 3a and Supplementary Fig. 1E left panels). In contrast, Ezrin knockdown significantly impaired YAP nuclear translocation (Fig. 3a and Supplementary Fig. 1E right panels). Western Blots on both the nuclear (Fig. 3b) and cytosolic (Fig. 3c) fractions confirmed that nuclear YAP was significantly reduced by Ezrin knockdown (Fig. 3b). We further confirmed that Ezrin knockdown resulted in cytoplasmic retention of phosphorylated YAP (Ser127) (Fig. 3e), which was unable to detect in nuclear fraction (Fig3 d). These data suggest that Ezrin knockdown impairs YAP nuclear translocation.
Fig. 3.
Ezrin knockdown impairs YAP nuclear translocation in skin fibroblasts. Primary skin fibroblasts were transfected with non-specific control siRNA or Ezrin siRNAs (20 nM) for 48 h. a YAP immunostaining. Images represent five independent experiments. Bar = 100 μm. Enlargement of the boxed region is shown to the bottom (bar = 50 μm). b YAP protein levels in nucleus. c YAP protein levels in cytosols. d Phosphorylated YAP (Ser127) protein levels in nucleus. e Phosphorylated YAP (Ser127) protein levels in cytosols. YAP nuclear protein levels were normalized by lamin A/C as a loading control. YAP cytosol protein levels were normalized by SOD as a loading control. Protein levels were determined by Western blots. Insets show representative Western blots. N = 3. Data are expressed as mean±SEM, *p < 0.05 vs control si
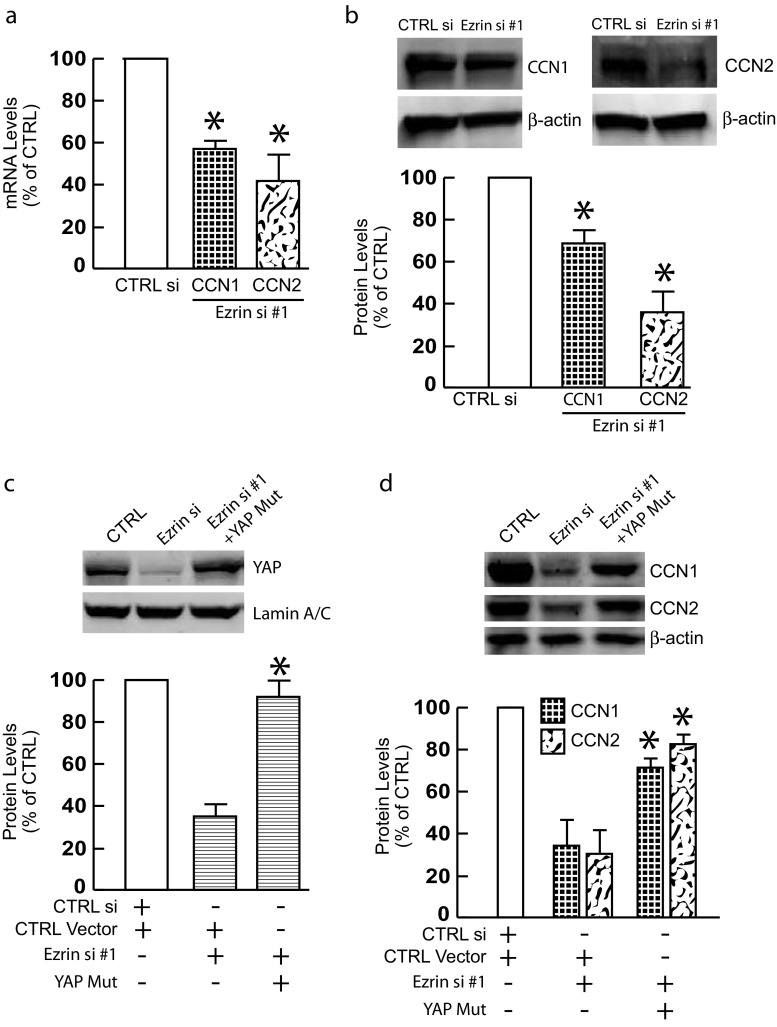
Ezrin knockdown inhibits YAP target gene expression in skin fibroblasts
Next, we examined the impact of Ezrin knockdown on YAP target gene expression. Cysteine-rich protein 61 (CCN1) and connective tissue growth factor (CCN2) are well-known transcriptional target of YAP. Indeed, Ezrin knockdown significantly downregulated both CCN1 and CCN2 mRNA (Fig. 4a) and protein (Fig. 4b and Supplementary Fig. 1F) levels. To ensure that reduced expression of CCN1 and CCN2 was caused indeed by impaired YAP activity, YAP nuclear translocation was restored by overexpression of constitutively active YAP in Ezrin knockdown cells (Fig. 4c and Supplementary Fig. 1F). Restoration of YAP nuclear translocation significantly reversed CCN1 and CCN2 expression (Fig. 4d and Supplementary Fig. 1F), suggesting YAP-dependent regulation of CCN1 and CCN2 expression in Ezrin knockdown cells.
Fig. 4.
Ezrin knockdown inhibits YAP target gene expression in skin fibroblasts. Primary skin fibroblasts were transfected with non-specific control siRNA or Ezrin siRNAs (20 nM) for 48 h. a CCN1 and CCN2 mRNA levels. N = 3. b CCN1 and CCN2 protein levels. N = 3. (c and d) Cells were transfected with non-specific control siRNA (left bar) or Ezrin siRNAs (middle bar) or Ezrin siRNAs plus constitutively active YAP (right bar) for 48 h. c YAP protein levels. N = 3. d CCN1 and CCN2 protein levels. N = 3. Protein levels were determined by Western blots. Protein levels were normalized by β-actin as a loading control. Insets show representative Western blots. Data are expressed as mean±SEM, *p < 0.05 vs control si
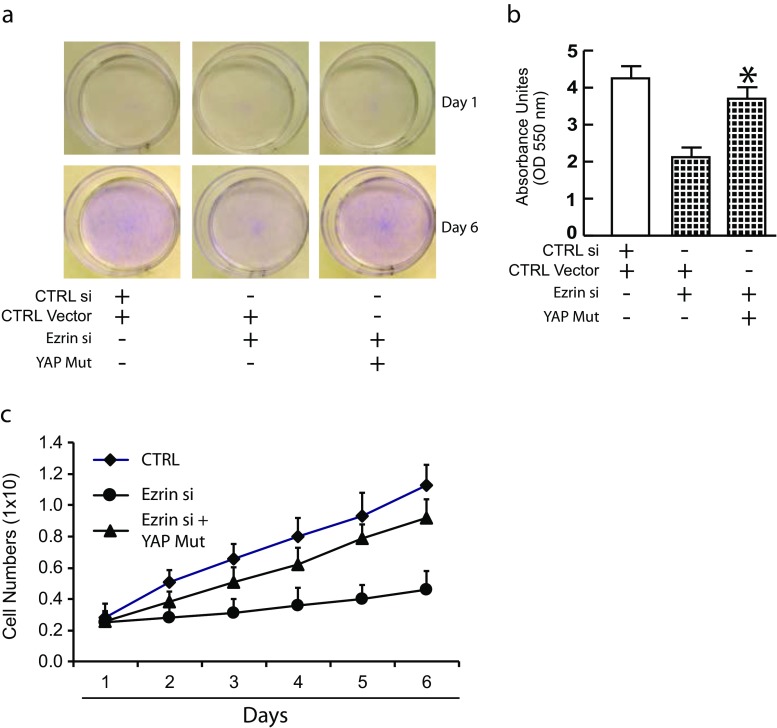
Ezrin knockdown inhibits fibroblasts proliferation via impaired YAP activity
Next, we explored the impact of Ezrin knockdown on skin fibroblast proliferation. We confirmed that Ezrin knockdown significantly reduced the density of cells confirmed by crystal violet assay (Fig. 5a middle panel and Fig. 5b middle bar). Next, we asked whether impaired YAP activity mediates Ezrin knockdown-mediated inhibition of cell proliferation. Indeed, restoration of YAP nuclear translocation reversed the density of cells (Fig. 5a right panel and Fig. 5b right bar). Consistently, Ezrin knockdown resulted in significant loss of cell proliferation, determined by cell number, and that near completely reversed by overexpression of constitutively active YAP (Fig. 5c and Supplementary Fig. 1G). These data indicate that Ezrin depletion is detrimental to fibroblast proliferation, that is mediated by impaired YAP nuclear translocation.
Fig. 5.
Ezrin knockdown inhibits fibroblasts proliferation via impaired YAP activity. Cells were transfected with non-specific control siRNA or Ezrin siRNAs or Ezrin siRNAs plus constitutively active YAP for two days. a Cells were harvested two days after transfection and 2 × 105 cells were cultured in 60 mm plates for one day (top panel) and 6 days (bottom panel) followed by stain with crystal violet blue dye to visualize cells. N = 4. b To quantify crystal violet, methanol was added to solubilize the dye, followed by reading optical density (O.D.540). Results are expressed as the mean ± SEM, N = 4, *p < 0.05 vs control si. c Cells were harvested two days after transfection and 2.5 × 105 cells were cultured in 60 mm plates. Cells harvested at indicated days and counted. Data are expressed as mean±SEM. N = 4
Ezrin regulates cell size/mechanical properties and YAP-dependent proliferation in neonatal foreskin human fibroblasts
Next, we employed neonatal foreskin dermal fibroblasts to confirm above observations, which were generated using adult dermal fibroblasts. Ezrin knockdown (Fig. 6a and b) in neonatal foreskin dermal fibroblasts resulted in substantial reduction of cell size (Fig. 6c), reduced mechanical properties (Fig. 6d and Fig. 6e), impaired YAP nuclear translocation (Fig. 6f), reduced YAP target gene expression (Fig. 6g). Importantly, reduced YAP target gene expression (Fig. 6g) and proliferation (Fig. 6h) were significantly restored by expression of constitutively active YAP. These data demonstrate that Ezrin regulates cell size/mechanical properties and YAP-dependent proliferation in neonatal foreskin human fibroblasts, as observed in adult dermal fibroblasts.
Fig. 6.
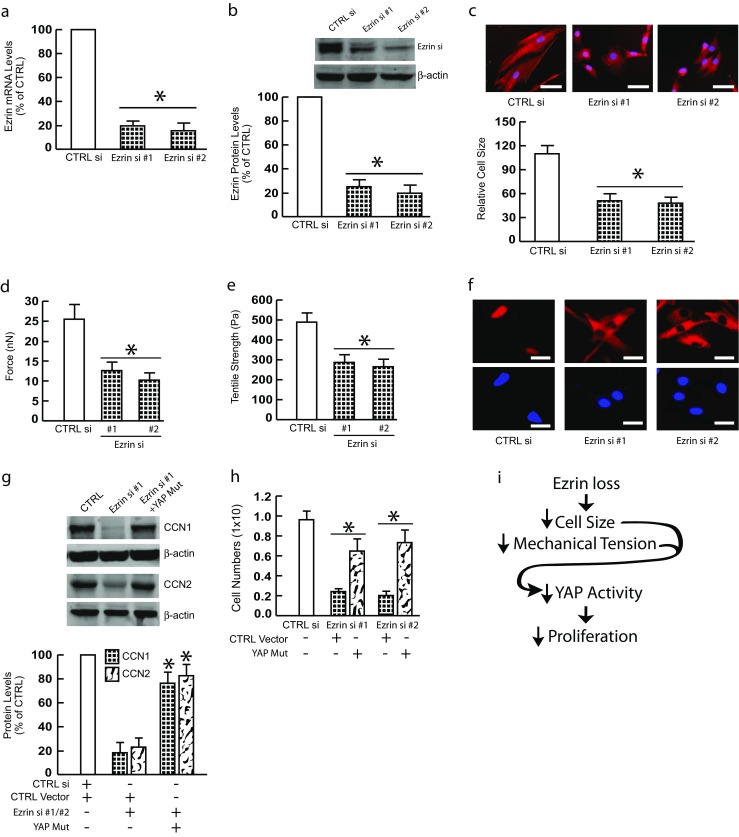
Ezrin regulates cell size/mechanical properties and YAP-dependent proliferation in neonatal foreskin human fibroblasts. Neonatal foreskin human fibroblasts were transfected with non-specific control siRNA or Ezrin siRNAs (20 nM) for 48 h. a Ezrin mRNA levels were reduced by Ezrin siRNAs. N = 4. b Ezrin protein levels were reduced by Ezrin siRNAs. N = 3. c Cell size was reduced by Ezrin siRNAs. Cells were stained with CellTracker® fluorescent dye. Red fluorescence delineates cell cytoplasm; blue fluorescence delineates nuclei. The relative cell surface areas were quantified by ImageJ. Bars = 50 μm. N = 3. d Cell traction force (nN) was reduced by Ezrin siRNAs. N = 4. e Cell tensile strength (Pa) was reduced by Ezrin siRNAs. N = 4. Mechanical properties were determined by atomic force microscopy (AFM) PeakForce Quantitative NanoMechanics mode and analyzed by Nanoscope Analysis software. f Impaired YAP nuclear translocation was determined by immunostaining. Images represent three independent experiments. Blue fluorescence delineates nuclei. Bar = 50 μm. g Restoration of YAP nuclear translocation reversed YAP target gene expression. Cells were transfected with non-specific control siRNA or Ezrin siRNAs or Ezrin siRNAs plus constitutively active YAP for 48 h. CCN1 and CCN2 protein levels were determined by Western blots. Protein levels were normalized by β-actin as a loading control. Insets show representative Western blots. N = 3. h Ezrin knockdown inhibits fibroblasts proliferation via impaired YAP activity. Cells were transfected with non-specific control siRNA or Ezrin siRNAs or Ezrin siRNAs plus constitutively active YAP for two days. Cells were harvested two days after transfection and 2.5 × 105 cells were cultured in 60 mm plates for six days. Cells were than harvested and counted. All data are expressed as mean±SEM, *p < 0.05 vs control. i Scheme summarizing the role of Ezrin in regulation of skin fibroblast size/mechanical properties and YAP-mediated proliferation (see Discussion for details)
Discussion
Emerging evidence indicates that cell size and mechanical force, which derive from plasma membrane-actin cytoskeleton assembly, play critical roles in diverse cellular biological processes, such as proliferation, differentiation, signal transduction, and gene expression (Butcher et al., 2009; Mammoto and Ingber, 2009; Mammoto et al., 2012). The impact of fibroblast, the major ECM producing cells in skin, size and mechanical force on skin biology is relatively unexplored. Here we investigated the importance of Ezrin, an actin-cytoskeleton linker protein, in regulation of skin fibroblast size/mechanical properties and function by manipulating levels of Ezrin. Our results indicate that Ezrin depletion profoundly impairs fibroblast size/mechanical force, which leads to YAP-dependent impairment of proliferation (Fig. 5d). As the role of Ezrin in human skin is largely unknown, our data suggest that Ezrin functions as an important protein in maintenance of fibroblast size/mechanical force and proliferation.
Regulation of cellular size and force is a highly complex process involving the coordinated assembly of a large number of cytoskeletal proteins and plasma membrane. There many types of interactions between the cytoskeleton and the plasma membrane. Such membrane-cytoskeleton interactions are mediated and modulated by cytoskeleton linker proteins, which intimately bind to plasma membrane and actin cytoskeleton. Now it is well established that Ezrin functions as a molecular connection between the plasma membrane and the underlying actin-cytoskeleton. These data suggest that Ezrin may play role in maintenance of cell shape and morphological integrity by organizing actin filaments and regulating cortical stiffness. Our data are in agreement with and complementary to the previous reports demonstrating that Ezrin acts as a cross-linker of the membrane-cytoskeleton interface, and thus its disruption alters cell size and mechanical force. In addition to their role in binding filamentous actin, Ezrin performs regulatory function through its ability to bind transmembrane proteins and link them to downstream signaling components. It is becoming increasingly apparent that Ezrin protein is able to bind to many receptors at the plasma membrane, such as CD44, EGFR, and HGFR (Bretscher et al., 2002; Fehon et al., 2010; Clucas and Valderrama, 2014). There is also evidence that the Ezrin protein plays an important role in cell–cell and cell–matrix interaction through interactions with cadherin complexes and integrins (Hiscox and Jiang, 1999; Pujuguet et al., 2003; Jung and McCarty, 2012). Ezrin interaction with integrins is of particular interest, as integrins play a pivotal role in maintaining cell size and mechanical force through its interaction with ECM and intracellular actin cytoskeleton. Cells maintain normal cell size and mechanical force through interaction with surrounding ECM via cell surface integrin receptors, which function as a physical link between ECM microenvironment and intracellular actin cytoskeleton, thereby functioning as mechanoreceptors for maintaining normal cell size and mechanical force.
From a biomechanical perspective, Ezrin has been show to modulate the plasma membrane tension though direct linkage between the plasma membrane and the actin cytoskeleton (Larson et al., 2010; Rouven Bruckner et al., 2015). Overexpression of an active Ezrin mutant increases membrane tension (Liu et al., 2012), and conversely overexpressing a dominant negative form of Ezrin to disrupt Ezrin decreases membrane tension (Krieg et al., 2008). Accumulating evidence indicates that cellular mechanical force regulate essential cell functions (Butcher et al., 2009; Mammoto and Ingber, 2009; Mammoto et al., 2012). Cellular mechanical force largely relies on interactions of cellular actin cytoskeleton cells with surrounding ECM through integrins. Our data demonstrate that depletion of Ezrin significantly reduces cellular mechanical properties in conjunction with reduced cell size, suggesting an essential role of Ezrin in maintenance of normal mechanical force. Ezrin might serves as a critical nexus between the extracellular environment/cell membrane and the underlying cytoskeleton and cytoplasm. Further study related Ezrin regulation of the mechanical properties is likely to reveal more diverse functions for Ezrin and may provide insight into the novel role of Ezrin in organization and control cell shape and morphology.
Our data indicate that depletion of Ezrin impairs skin fibroblast proliferation through inhibition of YAP nuclear translocation, indicating that Ezrin is involved in modulating the YAP pathway. YAP nuclear translocation is primarily regulated by sub-cellular localization. Hitherto, to our knowledge, no study tried to elucidate the connection between Ezrin and YAP in regulating cell proliferation. YAP pathway has been shown to be one of the most intriguing signaling pathways that plays a pivotal role in regulating multiple cellular functions including proliferation, apoptosis, regeneration and organ size control (Harvey and Hariharan, 2012; Moroishi et al., 2015; Plouffe et al., 2015). Meantime, the complexity of YAP nuclear translocation has expanded considerably in recent years, as it is regulated by non-canonical hippo pathway, such as mechanical force. It is unknown how Ezrin regulates YAP nuclear translocation, as observed in current study. It is conceivable that Ezrin-mediated alteration of cell size and mechanical force might have a significant impact on YAP activity, since YAP activity is largely regulated by cell size and mechanical force through undefined mechanism. Integration of this information into a comprehensive role of Ezrin will increase our understanding that Ezrin is not only involved in cytoskeletal organization but also participate in signal-transduction pathways. Clearly, further studies are needed to elucidate the molecular details in which Ezrin is involved in diverse signal-transduction pathways.
In human skin dermis, fibroblasts are surrounded by a collagen-rich ECM microenvironment. During aging collagen fibrils become fragmented, less dense, and disorganized (Varani et al., 2004; Fisher et al., 2008; Fisher et al., 2009; Qin et al., 2014). These alterations not only disrupt fibroblast interaction with extracellular collagen fibrils, but also intracellular organization of actin cytoskeleton, resulting in reduced spreading and cells size (Fisher et al., 2008; Fisher et al., 2009; Quan et al., 2013a; Qin et al., 2014). We have previously reported that age-related reduction of skin fibroblast size is a prominent feature of aged human skin in vivo, and has significant impact on human skin aging (Varani et al., 2004; Fisher et al., 2008; Quan et al., 2015). For example, reduced skin fibroblast size impairs TGF-β signaling, which contributes to reduced collagen biosynthesis (Quan and Fisher, 2015; Fisher et al., 2016); increases multiple matrix metalloproteinases (MMPs), which contribute to collagen fibrils fragmentation. (Fisher et al., 2009; Quan et al., 2013a; Qin et al., 2014). In this regard, it is of interest to investigate the role of Ezrin in skin connective tissue aging with respect to its interaction with collagen binding integrins.
We recently reported that enhancing cell size and mechanical properties are able to activate aged quiescent fibroblasts to a more “youthful” state in aged human skin in vivo (Wang et al., 2007; Quan et al., 2013b). Injection of dermal filler (cross-linked hyaluronic acid) into the skin of individuals over 70 years of age stimulates fibroblast proliferation, expands vasculature, and increases epidermal thickness. This stimulation is associated with localized increase of cell size and mechanical forces. Thus, fibroblasts in aged human skin retain their capacity for functional activation, which can be restored by enhancing size and mechanical support. These findings attract our attention that Ezrin might function as an important linker protein in mediating cell size and mechanical force in activation of quiescent fibroblasts in aged human skin.
In summary, depletion of Ezrin decreases fibroblast size/mechanical tension and impairs YAP-dependent proliferation. Our data reveal that Ezrin is an important protein that maintains fibroblast size/mechanical force and proliferation through YAP pathway.
Electronic supplementary material
Ezrin siRNA #2 reduces cell size/mechanical properties and impairs YAP-dependent proliferation in primary human skin fibroblasts. Primary human skin fibroblasts were transfected with non-specific control siRNA or Ezrin siRNA #2 (20 nM) for 48 h. (A) Cell size was reduced by Ezrin siRNA #2. Cells were stained with CellTracker® fluorescent dye. Red fluorescence delineates cell cytoplasm; blue fluorescence delineates nuclei. The relative cell surface areas were quantified by ImageJ. Bars = 50 μm. N = 3. (B) Cell traction force (nN) was reduced by Ezrin siRNA #2. N = 3. (C) Cell tensile strength (Pa) was reduced by Ezrin siRNA #2. N = 3. (D) Cell deformation was increased by Ezrin siRNA #2. N = 3. Mechanical properties were determined by atomic force microscopy (AFM) PeakForce Quantitative NanoMechanics mode and analyzed by Nanoscope Analysis software. (E) Impaired YAP nuclear translocation was determined by immunostaining. Images represent three independent experiments. Blue fluorescence delineates nuclei. Bar = 50 μm. (F) Restoration of YAP nuclear translocation reversed YAP target gene expression. Cells were transfected with non-specific control siRNA or Ezrin siRNAs or Ezrin siRNAs plus constitutively active YAP for two days. CCN1 and CCN2 protein levels were determined by Western blots. Protein levels were normalized by β-actin as a loading control. Insets show representative Western blots. N = 3. (G) Ezrin knockdown inhibits fibroblasts proliferation via impaired YAP activity. Cells were transfected with non-specific control siRNA or Ezrin siRNAs or Ezrin siRNAs plus constitutively active YAP for two days. Cells were harvested two days after transfection and 2.5 × 105 cells were cultured in 60 mm plates. Cells were harvested at indicated days and counted. Data are expressed as mean±SEM, *p < 0.05 vs control. (PDF 59 kb)
Acknowledgements
Y Yan is supported by Milstein Medical Asian American Partnership Foundation (2015 Fellowship Award in Skin Disease).
Funding information
This work was supported by a grant from the NIH (AG019364 to T Quan).
Abbreviations
- CCN1
Cysteine-rich protein 61
- CCN2
Connective tissue growth factor
- ECM
Extracellular matrix
- YAP
Yes-associated protein
Footnotes
Chunji Quan and Yan Yan contributed equally to this work.
Electronic supplementary material
The online version of this article (10.1007/s12079-017-0406-6) contains supplementary material, which is available to authorized users.
Contributor Information
Zhenhua Lin, Email: zhlin720@ybu.edu.cn.
Taihao Quan, Email: thquan@umich.edu.
References
- Akamine R, Yamamoto T, Watanabe M, Yamazaki N, Kataoka M, Ishikawa M, Ooie T, Baba Y, Shinohara Y. Usefulness of the 5′ region of the cDNA encoding acidic ribosomal phosphoprotein P0 conserved among rats, mice, and humans as a standard probe for gene expression analysis in different tissues and animal species. J Biochem Biophys Methods. 2007;70:481–486. doi: 10.1016/j.jbbm.2006.11.008. [DOI] [PubMed] [Google Scholar]
- Arpin M, Chirivino D, Naba A, Zwaenepoel I. Emerging role for ERM proteins in cell adhesion and migration. Cell Adhes Migr. 2011;5:199–206. doi: 10.4161/cam.5.2.15081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bretscher A, Edwards K, Fehon RG. ERM proteins and merlin: integrators at the cell cortex. Nat Rev Mol Cell Biol. 2002;3:586–599. doi: 10.1038/nrm882. [DOI] [PubMed] [Google Scholar]
- Butcher DT, Alliston T, Weaver VM. A tense situation: forcing tumour progression. Nat Rev Cancer. 2009;9:108–122. doi: 10.1038/nrc2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan SW, Lim CJ, Chong YF, Pobbati AV, Huang C, Hong W. Hippo pathway-independent restriction of TAZ and YAP by angiomotin. J Biol Chem. 2011;286:7018–7026. doi: 10.1074/jbc.C110.212621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clucas J, Valderrama F. ERM proteins in cancer progression. J Cell Sci. 2014;127:267–275. doi: 10.1242/jcs.133108. [DOI] [PubMed] [Google Scholar]
- Crepaldi T, Gautreau A, Comoglio PM, Louvard D, Arpin M. Ezrin is an effector of hepatocyte growth factor-mediated migration and morphogenesis in epithelial cells. J Cell Biol. 1997;138:423–434. doi: 10.1083/jcb.138.2.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupont S, Morsut L, Aragona M, Enzo E, Giulitti S, Cordenonsi M, Zanconato F, Le Digabel J, Forcato M, Bicciato S, et al. Role of YAP/TAZ in mechanotransduction. Nature. 2011;474:179–183. doi: 10.1038/nature10137. [DOI] [PubMed] [Google Scholar]
- Fehon RG, McClatchey AI, Bretscher A. Organizing the cell cortex: the role of ERM proteins. Nat Rev Mol Cell Biol. 2010;11:276–287. doi: 10.1038/nrm2866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher GJ, Quan T, Purohit T, Shao Y, Cho MK, He T, Varani J, Kang S, Voorhees JJ. Collagen fragmentation promotes oxidative stress and elevates matrix metalloproteinase-1 in fibroblasts in aged human skin. Am J Pathol. 2009;174:101–114. doi: 10.2353/ajpath.2009.080599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher GJ, Shao Y, He T, Qin Z, Perry D, Voorhees JJ, Quan T. Reduction of fibroblast size/mechanical force down-regulates TGF-beta type II receptor: implications for human skin aging. Aging Cell. 2016;15:67–76. doi: 10.1111/acel.12410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher GJ, Varani J, Voorhees JJ. Looking older: fibroblast collapse and therapeutic implications. Arch Dermatol. 2008;144:666–672. doi: 10.1001/archderm.144.5.666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautreau A, Louvard D, Arpin M. Morphogenic effects of ezrin require a phosphorylation-induced transition from oligomers to monomers at the plasma membrane. J Cell Biol. 2000;150:193–203. doi: 10.1083/jcb.150.1.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey KF, Hariharan IK. The hippo pathway. Cold Spring Harb Perspect Biol. 2012;4:a011288. doi: 10.1101/cshperspect.a011288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiscox S, Jiang WG. Ezrin regulates cell-cell and cell-matrix adhesion, a possible role with E-cadherin/beta-catenin. J Cell Sci. 1999;112(Pt 18):3081–3090. doi: 10.1242/jcs.112.18.3081. [DOI] [PubMed] [Google Scholar]
- Hsu YY, Shi GY, Kuo CH, Liu SL, Wu CM, Ma CY, Lin FY, Yang HY, Wu HL. Thrombomodulin is an ezrin-interacting protein that controls epithelial morphology and promotes collective cell migration. FASEB J. 2012;26:3440–3452. doi: 10.1096/fj.12-204917. [DOI] [PubMed] [Google Scholar]
- Jin J, Jin T, Quan M, Piao Y, Lin Z. Ezrin overexpression predicts the poor prognosis of gastric adenocarcinoma. Diagn Pathol. 2012;7:135. doi: 10.1186/1746-1596-7-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin T, Jin J, Li X, Zhang S, Choi YH, Piao Y, Shen X, Lin Z. Prognostic implications of ezrin and phosphorylated ezrin expression in non-small cell lung cancer. BMC Cancer. 2014;14:191. doi: 10.1186/1471-2407-14-191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung Y, McCarty JH. Band 4.1 proteins regulate integrin-dependent cell spreading. Biochem Biophys Res Commun. 2012;426:578–584. doi: 10.1016/j.bbrc.2012.08.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong J, Di C, Piao J, Sun J, Han L, Chen L, Yan G, Lin Z. Ezrin contributes to cervical cancer progression through induction of epithelial-mesenchymal transition. Oncotarget. 2016;7:19631–19642. doi: 10.18632/oncotarget.7779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong J, Li Y, Liu S, Jin H, Shang Y, Quan C, Li Y, Lin Z. High expression of ezrin predicts poor prognosis in uterine cervical cancer. BMC Cancer. 2013;13:520. doi: 10.1186/1471-2407-13-520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krieg M, Helenius J, Heisenberg CP, Muller DJ. A bond for a lifetime: employing membrane nanotubes from living cells to determine receptor-ligand kinetics. Angew Chem Int Ed Engl. 2008;47:9775–9777. doi: 10.1002/anie.200803552. [DOI] [PubMed] [Google Scholar]
- Larson SM, Lee HJ, Hung PH, Matthews LM, Robinson DN, Evans JP. Cortical mechanics and meiosis II completion in mammalian oocytes are mediated by myosin-II and Ezrin-radixin-Moesin (ERM) proteins. Mol Biol Cell. 2010;21:3182–3192. doi: 10.1091/mbc.e10-01-0066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Gao H, Xu H, Wang X, Pan Y, Hao F, Qiu X, Stoecker M, Wang E, Wang E. Expression of ezrin correlates with malignant phenotype of lung cancer, and in vitro knockdown of ezrin reverses the aggressive biological behavior of lung cancer cells. Tumour Biol. 2012;33:1493–1504. doi: 10.1007/s13277-012-0400-9. [DOI] [PubMed] [Google Scholar]
- Liu Y, Belkina NV, Park C, Nambiar R, Loughhead SM, Patino-Lopez G, Ben-Aissa K, Hao JJ, Kruhlak MJ, Qi H, et al. Constitutively active ezrin increases membrane tension, slows migration, and impedes endothelial transmigration of lymphocytes in vivo in mice. Blood. 2012;119:445–453. doi: 10.1182/blood-2011-07-368860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mammoto A, Ingber DE. Cytoskeletal control of growth and cell fate switching. Curr Opin Cell Biol. 2009;21:864–870. doi: 10.1016/j.ceb.2009.08.001. [DOI] [PubMed] [Google Scholar]
- Mammoto A, Mammoto T, Ingber DE. Mechanosensitive mechanisms in transcriptional regulation. J Cell Sci. 2012;125:3061–3073. doi: 10.1242/jcs.093005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moroishi T, Hansen CG, Guan KL. The emerging roles of YAP and TAZ in cancer. Nat Rev Cancer. 2015;15:73–79. doi: 10.1038/nrc3876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naba A, Reverdy C, Louvard D, Arpin M. Spatial recruitment and activation of the Fes kinase by ezrin promotes HGF-induced cell scattering. EMBO J. 2008;27:38–50. doi: 10.1038/sj.emboj.7601943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neisch AL, Fehon RG. Ezrin, radixin and Moesin: key regulators of membrane-cortex interactions and signaling. Curr Opin Cell Biol. 2011;23:377–382. doi: 10.1016/j.ceb.2011.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng T, Parsons M, Hughes WE, Monypenny J, Zicha D, Gautreau A, Arpin M, Gschmeissner S, Verveer PJ, Bastiaens PI, et al. Ezrin is a downstream effector of trafficking PKC-integrin complexes involved in the control of cell motility. EMBO J. 2001;20:2723–2741. doi: 10.1093/emboj/20.11.2723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piao J, Liu S, Xu Y, Wang C, Lin Z, Qin Y, Liu S. Ezrin protein overexpression predicts the poor prognosis of pancreatic ductal adenocarcinomas. Exp Mol Pathol. 2015;98:1–6. doi: 10.1016/j.yexmp.2014.11.003. [DOI] [PubMed] [Google Scholar]
- Plouffe SW, Hong AW, Guan KL. Disease implications of the hippo/YAP pathway. Trends Mol Med. 2015;21:212–222. doi: 10.1016/j.molmed.2015.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pujuguet P, Del Maestro L, Gautreau A, Louvard D, Arpin M. Ezrin regulates E-cadherin-dependent adherens junction assembly through Rac1 activation. Mol Biol Cell. 2003;14:2181–2191. doi: 10.1091/mbc.e02-07-0410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin Z, Voorhees JJ, Fisher GJ, Quan T. Age-associated reduction of cellular spreading/mechanical force up-regulates matrix metalloproteinase-1 expression and collagen fibril fragmentation via c-Jun/AP-1 in human dermal fibroblasts. Aging Cell. 2014;13:1028–1037. doi: 10.1111/acel.12265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quan C, Cho MK, Perry D, Quan T. Age-associated reduction of cell spreading induces mitochondrial DNA common deletion by oxidative stress in human skin dermal fibroblasts: implication for human skin connective tissue aging. J Biomed Sci. 2015;22:62. doi: 10.1186/s12929-015-0167-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quan T, Fisher GJ. Role of age-associated alterations of the dermal extracellular matrix microenvironment in human skin aging: a mini-review. Gerontology. 2015;61:427–434. doi: 10.1159/000371708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quan T, Little E, Quan H, Qin Z, Voorhees JJ, Fisher GJ. Elevated matrix metalloproteinases and collagen fragmentation in photodamaged human skin: impact of altered extracellular matrix microenvironment on dermal fibroblast function. J Invest Dermatol. 2013;133:1362–1366. doi: 10.1038/jid.2012.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quan T, Qin Z, Voorhees JJ, Fisher GJ. Cysteine-rich protein 61 (CCN1) mediates replicative senescence-associated aberrant collagen homeostasis in human skin fibroblasts. J Cell Biochem. 2012;113:3011–3018. doi: 10.1002/jcb.24179. [DOI] [PubMed] [Google Scholar]
- Quan T, Wang F, Shao Y, Rittie L, Xia W, Orringer JS, Voorhees JJ, Fisher GJ. Enhancing structural support of the dermal microenvironment activates fibroblasts, endothelial cells, and keratinocytes in aged human skin in vivo. J Invest Dermatol. 2013;133:658–667. doi: 10.1038/jid.2012.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouven Bruckner B, Pietuch A, Nehls S, Rother J, Janshoff A. Ezrin is a major regulator of membrane tension in epithelial cells. Sci Rep. 2015;5:14700. doi: 10.1038/srep14700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato N, Funayama N, Nagafuchi A, Yonemura S, Tsukita S, Tsukita S. A gene family consisting of ezrin, radixin and moesin. Its specific localization at actin filament/plasma membrane association sites. J Cell Sci. 1992;103(Pt 1):131–143. doi: 10.1242/jcs.103.1.131. [DOI] [PubMed] [Google Scholar]
- Takeuchi K, Sato N, Kasahara H, Funayama N, Nagafuchi A, Yonemura S, Tsukita S, Tsukita S. Perturbation of cell adhesion and microvilli formation by antisense oligonucleotides to ERM family members. J Cell Biol. 1994;125:1371–1384. doi: 10.1083/jcb.125.6.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varani J, Schuger L, Dame MK, Leonard C, Fligiel SE, Kang S, Fisher GJ, Voorhees JJ. Reduced fibroblast interaction with intact collagen as a mechanism for depressed collagen synthesis in photodamaged skin. J Invest Dermatol. 2004;122:1471–1479. doi: 10.1111/j.0022-202X.2004.22614.x. [DOI] [PubMed] [Google Scholar]
- Wang F, Garza LA, Kang S, Varani J, Orringer JS, Fisher GJ, Voorhees JJ. In vivo stimulation of de novo collagen production caused by cross-linked hyaluronic acid dermal filler injections in photodamaged human skin. Arch Dermatol. 2007;143:155–163. doi: 10.1001/archderm.143.2.155. [DOI] [PubMed] [Google Scholar]
- Zhao B, Tumaneng K, Guan KL. The hippo pathway in organ size control, tissue regeneration and stem cell self-renewal. Nat Cell Biol. 2011;13:877–883. doi: 10.1038/ncb2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Ezrin siRNA #2 reduces cell size/mechanical properties and impairs YAP-dependent proliferation in primary human skin fibroblasts. Primary human skin fibroblasts were transfected with non-specific control siRNA or Ezrin siRNA #2 (20 nM) for 48 h. (A) Cell size was reduced by Ezrin siRNA #2. Cells were stained with CellTracker® fluorescent dye. Red fluorescence delineates cell cytoplasm; blue fluorescence delineates nuclei. The relative cell surface areas were quantified by ImageJ. Bars = 50 μm. N = 3. (B) Cell traction force (nN) was reduced by Ezrin siRNA #2. N = 3. (C) Cell tensile strength (Pa) was reduced by Ezrin siRNA #2. N = 3. (D) Cell deformation was increased by Ezrin siRNA #2. N = 3. Mechanical properties were determined by atomic force microscopy (AFM) PeakForce Quantitative NanoMechanics mode and analyzed by Nanoscope Analysis software. (E) Impaired YAP nuclear translocation was determined by immunostaining. Images represent three independent experiments. Blue fluorescence delineates nuclei. Bar = 50 μm. (F) Restoration of YAP nuclear translocation reversed YAP target gene expression. Cells were transfected with non-specific control siRNA or Ezrin siRNAs or Ezrin siRNAs plus constitutively active YAP for two days. CCN1 and CCN2 protein levels were determined by Western blots. Protein levels were normalized by β-actin as a loading control. Insets show representative Western blots. N = 3. (G) Ezrin knockdown inhibits fibroblasts proliferation via impaired YAP activity. Cells were transfected with non-specific control siRNA or Ezrin siRNAs or Ezrin siRNAs plus constitutively active YAP for two days. Cells were harvested two days after transfection and 2.5 × 105 cells were cultured in 60 mm plates. Cells were harvested at indicated days and counted. Data are expressed as mean±SEM, *p < 0.05 vs control. (PDF 59 kb)