Abstract
Babesia bovis, Babesia bigemina and Theileria equi are worldwide tick-borne hemoprotozoan that cause diseases characterized by fever, anemia, weight loss and abortion. A common feature of these diseases are transition from acute to chronic phases, in which parasites may persist in the host for life, and becoming a reservoir for tick transmission. The live-attenuated vaccines for B. bovis and B. bigemina are not available for worldwide use due to legal restrictions and other concerns such as potential erythrocyte antigen and pathogen contamination, and a vaccine for T. equi is not available. The use of chemotherapeutics is essential to treat and control these diseases, but several studies have shown the development of drug-resistance by these parasites, and safe and effective alternative drugs are needed. Tulathromycin, a macrolide antibiotic, has proven to be effective against a vast range of bacteria and Plasmodium yoelli, a Babesia and Theileria related intra-erythrocytic apicomplexan. Draxxin® (tulathromycin) is currently licensed to treat infections that cause respiratory diseases in cattle in several countries. In this study, the activity of Draxxin® was tested in vitro on cultured B. bovis, B. bigemina and T. equi. Addition of the drug to in vitro cultures resulted in cessation of parasite replication of the three species tested, B. bovis, B. bigemina and T. equi, with estimated IC50 of 16.7 ± 0.6 nM; 6.2 ± 0.2 nM and 2.4 ± 0.1 nM, respectively, at 72 h. Furthermore, neither parasites nor parasite DNA were detectable in cultures treated with IC100, suggesting Draxxin® is a highly effective anti-Babesia/Theileria drug. Importantly, the IC50 calculated for Draxxin® for the Babesia/Theileria parasites tested is lower that the IC50 calculated for some drugs currently in use to control these parasites. Collectively, the data strongly support in vivo testing of Draxxin® for the treatment of bovine babesiosis and equine piroplasmosis.
Keywords: Draxxin®, Tulathromycin, Babesia bovis, Babesia bigemina, Theileria equi, In vitro inhibition growth
Graphical abstract
The effect of different concentrations of Draxxin® (tulathromycin) in in vitro growth of B. bovis, B. bigemina and T. equi parasites at 72 h after drug addition to the culture.
Highlights
-
•
Chemotherapeutics are critical to treat bovine babesiosis and equine theileriosis.
-
•
Study of the activity of Draxxin® in vitro B. bovis, B. bigemina, T. equi cultures.
-
•
Addition of Draxxin® to the cultures resulted in cessation of parasite replication.
-
•
IC50 (nM) of 16.7; 6.2 and 2.4 to B. bovis, B. bigemina and T. equi, respectively.
-
•
Results suggests that Draxxin® is a highly effective anti-Babesia/Theileria drug.
1. Introduction
Chemotherapeutics are extremely important for the management of infectious diseases, especially when effective and safe vaccines are unavailable. However, the identification of safe and efficacious parasite-specific chemotherapeutics is time-consuming and expensive. Some of the disadvantages of current chemotherapeutics include increased parasite drug-resistance, the occurrence of drug-associated side-effects, such as toxicity, and the risk of contamination of the food chain (Vial and Gorenflot, 2006; Suarez and Noh, 2011). Bovine and equine piroplasmosis are tick-borne diseases present worldwide, causing significant economic losses due to decreases in meat and milk production, abortion, anemia and high mortality rate of susceptible animals in endemic areas (Uilenberg, 1995). The tick-borne apicomplexan parasites Babesia bovis and Babesia bigemina are the most prevalent Babesia species that infect cattle worldwide. The related tick-borne apicomplexan Theileria equi has significant economic consequences, especially in equine sport activities and the racing industry through restricting movement of infected horses (Wise et al., 2013). Importantly, both, cattle and horses surviving acute infections become persistent reservoirs allowing parasite transmission by ticks. Currently, there are live-attenuated vaccines available for the prevention of acute bovine babesiosis, but not for T. equi. However, these vaccines have severe limitations, such as the inability to protect against infection and are not licensed for use in all countries, including the United States of America. Available chemotherapeutics for bovine and equine piroplasmosis include imidocarb dipropionate (Imizol®, Schering-Plough Animal Health) and diminazene aceturate (Berenil®, Intervet, India Pvt. Ltd.). However, imidocarb dipropionate may have negative secondary effects in treated animals, and may result in salivation, vomiting, diarrhea and injection site inflammation (http://www.merck-animal-health-usa.com/products/130_163327/productdetails_130_163631.aspx#/product/canine/Imizol/1). Furthermore, long term use of these treatments invariably results in the emergence of drug-resistant parasites (Mosqueda et al., 2012; Hines et al., 2015).
Therefore, taking these considerations together, the diseases caused by these apicomplexan parasites may prove to become intractable, and new anti-parasitic drugs are urgently needed. Tulathromycin is a semi-synthetic macrolide antibiotic of the subclass triamilide (https://www.zoetisus.com/products/pages/draxxin_index/index.aspx). Importantly, a specific formulation (currently commercialized as Draxxin®) of this drug has been already approved for the treatment of respiratory disease, keratoconjunctivitis and foot rot in cattle in several countries including the US. Therefore, this drug is currently approved by the FDA and widely commercialized, greatly facilitating the process for new potential applications. A previous study demonstrated that tulathromycin has antimalarial activity in in vivo mice infected with Plasmodium yoelli (Villarino et al., 2015). Based on these preliminary observations and in the fact that Plasmodium, Theileria and Babesia are related intra-erythrocytic apicomplexan parasites, we hypothesized that Draxxin® (tulathromycin) inhibits the development of Babesia and Theileria parasites in in vitro cultures. The study presented here evaluates the use of a commercial available chemotherapeutic, Draxxin® (tulathromycin) as a drug that inhibits the in vitro growth of B. bovis, B. bigemina and T. equi.
2. Material and methods
2.1. In vitro cultivation of the B. bovis, B. bigemina and T. equi
Babesia bovis Texas strain, B. bigemina Puerto Rico strain and T. equi Florida strain cultures were grown in long-term microaerophilous stationary-phase as described by Levy and Ristic (1980), Vegas et al. (Vega et al., 1985) and Zweygarth et al. (1995), respectively. Briefly, parasites were grown in a supplemented HL-1 medium at pH 7.2, with 10% and 5% hematocrit of bovine erythrocytes (B. bovis and B. bigemina, respectively) or with 10% hematocrit equine erythrocytes (T. equi). Cultures were maintained at 37 °C in an atmosphere of 5% CO2, 5% O2 and 90% N2 and media was replaced daily.
2.2. In vitro growth inhibition assay
In vitro inhibition assays for B. bovis, B. bigemina and T. equi parasites were performed to evaluate the effect of the Draxxin® (tulathromycin). This study was performed in 96-well plates using 180 μl cultures per well containing the different Draxxin® concentration tested (1.56 nM, 3.1 nM; 6.2 nM; 12.4 nM; 18.6 nM; 24.8 nm; 37.2 nM and 49.6 nM) diluted in culture medium, with an initial percentage of parasitized erythrocytes (PPE) of 0.2% in all subcultures. The first screening was to identify if Draxxin® has an inhibitory effect on Babesia spp. and T. equi strains and calculate the 50% inhibitory concentrations (IC50). Cultures were grown in the presence and absence of Draxxin®, and non-infected erythrocytes were also kept in culture media as baseline data for flow cytometry analysis. The culture medium was replaced daily with 150 μl medium per well containing the respective Draxxin® concentration. Cultures were also monitored daily up to 72hr by flow cytometry to measure the PPE and by Giemsa-stained slides for any morphological changes.
A new set of in vitro growth inhibition assays was performed targeting a starting PPE of 1% for B. bovis and B. bigemina and 9% for T. equi. This assay was performed using of Draxxin® at IC100: B. bovis (37.2 nM), B. bigemina (12.4 nM) and T. equi (18.6 nM). Cultures grown in the absence of Draxxin® were used as control for no parasite growth inhibition, and culture of non-infected erythrocytes maintained in medium was used as negative control. The culture medium was replaced daily with 150 μl medium per well containing the respective Draxxin® concentration for a period of 72 h. After 72 h, parasites were cultivated in media (in the absence of Draxxin®), with splits every 48 h for a period of 8 days. The PPE was evaluated at 72 h and 8 days by flow cytometry.
The effect of the Imizol® (imidocarb dipropionate) was used as a positive control for the in vitro inhibition assays for B. bovis, B. bigemina and T. equi parasites, using an identical protocol as described above. The drug concentrations used were: 1 μM for B. bovis and for T. equi, and a set of different concentrations (0.001 nM, 0.01 nM; 0.1 nM; 1 nM; 10 nM; 1 μM) for the calculation of the IC50 of Imizol® for B. bigemina. The initial PPE used was 0.2% in all subcultures. Cultures were monitored daily at 72 h by flow cytometry to measure the PPE.
All experiments were carried out in triplicate for each concentration tested. The IC50 values were calculated using GraphPad Prism 7 software by fitting the PPE values (wells treated with Draxxin® at different concentration compared to the positive control well) in a nonlinear regression with a confidence interval of 95% (95% CI). Total inhibitory concentrations (IC100) was calculated as the doses of drug required to inhibit the parasite growth to an identical level as found for non-infected erythrocytes (approximately 0.1%).
2.3. Flow cytometric method for detection of parasite growth
Flow cytometric assay was performed as described by Wyatt et al. (1991). Briefly, cultures were centrifuged at 450 × g for 5 min at 4 °C. The supernatant was discarded, the cell pellet was washed twice with 150 μl of phosphate buffer saline (PBS) pH 7.2 and then, the cell pellet was resuspended in 200 μl of 25 μg/μl Hydroethidine (HE) (Invitrogen) and incubated in 5% CO2 incubator at 37 °C for 20 min in the dark. After incubation, excess of HE was washed from the cells by the addition of 200 μl of PBS followed by centrifugation at 450 × g for 5 min at 4 °C. The supernatant was discarded and the cell pellet was resuspended in 200 μl of fresh PBS. Then, resuspended cells were transferred to samples tube containing approximately 3 ml of PBS containing 0.2% sodium azide for analysis by flow cytometry using a FACSCaliber (Becton Dickinson) at a flow rate of approximately 2500 events/s with 50,000 events collected. Data was analyzed by FCS Express 3 software (De Novo Software). Stained non-infected erythrocytes were used as a negative control and gated to determine the percentage between infected and non-infected erythrocytes.
2.4. Quantitative real-time PCR
Quantitative real-time PCR was performed to assess the copy numbers of B. bovis merozoite surface antigen (msa)-1 gene, B. bigemina limulus coagulation factor C domain protein (ccp)-3 and T. equi erythrocyte merozoite antigen (ema)-1 genes in parasites treated with IC50 and IC100 concentrations and non-treated parasites (control well) at 72 h post-addition of Draxxin®. For B. bovis the IC50 and IC100 concentrations are 16.7 nM and 37.2 nM, respectively; for B. bigemina the IC50 and IC100 concentrations are 6.2 nM and 12.4 nM, respectively; and for T. equi the IC50 and IC100 concentrations are 2.4 nM and 18.6 nM, respectively. The set of primers, PCR cycling and reaction were performed as described by Bastos et al. and Silva et al. (Bastos et al., 2013; Silva et al., 2016). Five μl of plasmid DNA (pDNA) was used for the standard curve and two μl of gDNA was used as samples. Data was analyzed by CFX Manager™ Software (Bio-Rad). Copy numbers of B. bovis msa-1, B. bigemina ccp-3 and T. equi ema-1 genes were calculated based on a standard curve (Bastos et al., 2013).
2.5. Statistical analysis
Statistical significance was determined using a two-sample t-test for differences among the treatments and one-way parametric ANOVA (GraphPad Prism 7 software). P < 0.05 were considered statistically significant. The area under the concentration (AUC) values was calculated using GraphPad Prism 7 software.
3. Results
3.1. The in vitro inhibitory effect of Draxxin® in growth of B. bovis, B. bigemina and T. equi parasites
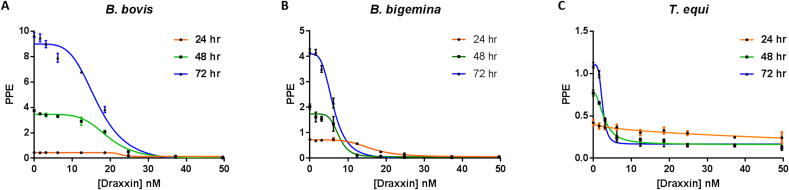
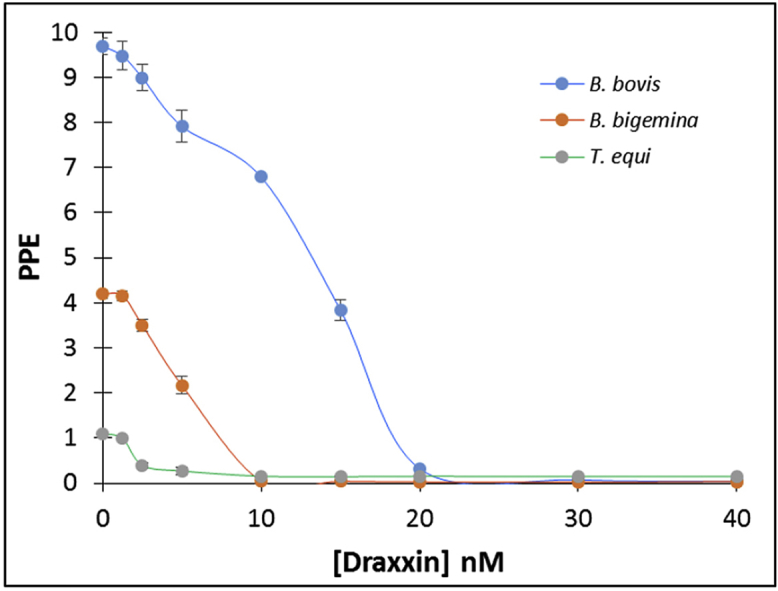
We initially tested the efficacy of Draxxin® to inhibit the in vitro growth of B. bovis, B. bigemina and T. equi parasites. The in vitro growth of those parasites was inversely related to the concentrations of Draxxin® applied, until no growth of parasites was reached (Fig. 1A–C). In addition, daily measurements of the PPE over a 72 h lapse, indicates that the drug effect is also dependent on time, with lower PPE occurring upon longer exposures to the drug (Fig. 1A–C).
Fig. 1.
Growth curve obtained at 24 h (orange line), 48 h (green line) and 72 h (blue line) after the addition of different concentrations of Draxxin® in: A) B. bovis, B) B. bigemina and C) T. equi in in vitro culture. Parasites grown without addition of Draxxin® are represented as “0”. Assay was carried out in triplicate. Error bars indicate standard error of the means for each Draxxin® concentration tested. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
The IC50 for each parasite tested were determined at 72 h after the addition of the drugs (Table 1). Overall, the growth inhibition data suggests that T. equi is the most “susceptible“ of the three parasites tested to the effect of Draxxin®, with a calculated IC50 of 2.4 nM; followed by B. bigemina (IC50 of 6.2 nM), and B. bovis (IC50 of 16.7 nM). In addition, the calculated IC100 values were 37.2 nM, 12.4 nM, and 18.6 nM for B. bovis, B. bigemina and T. equi parasites, respectively (Table 1).
Table 1.
IC50 and IC100 values obtained on B. bovis, B. bigemina and T. equi at 24 h, 48 h and 72 h after treatment with Draxxin®.
| Species | IC50 (nM) |
IC100 (nM) |
||||
|---|---|---|---|---|---|---|
| 24 h | 48 h | 72 h | 24 h | 48 h | 72 h | |
| B. bovis | 22.8 ± 2.0 N.A. | 19.3 ± 0.5 (18.2–20.3)a | 16.7 ± 0.6 (15.4–17.9)a | 49.6 | 37.2 | 37.2 |
| B. bigemina | 15.9 ± 0.5 (15–17.1)a | 7.6 ± 0.6 (7.2–8.9)a | 6.2 ± 0.2 (5.8–6.6)a | 37.2 | 18.6 | 12.4 |
| T. equi | N.A. N.A. | 2.9 ± 0.2 (2.6–3.3)a | 2.4 ± 0.1 (2.2–2.6)a | N.A. | 24.8 | 18.6 |
95% CI.
The Imizol® was able to completely inhibit the in vitro growth of B. bovis, B. bigemina and T. equi (Suppl. Fig. 1). We decided to estimate the Imizol® IC50 for B. bigemina (Suppl. Fig. 2 and Table 2) since, at least to our knowledge, there is no in vitro data for B. bigemina parasites. Table 2 also compares the IC50 values for the three currently used anti Babesia/Theileria drugs with Draxxin®.
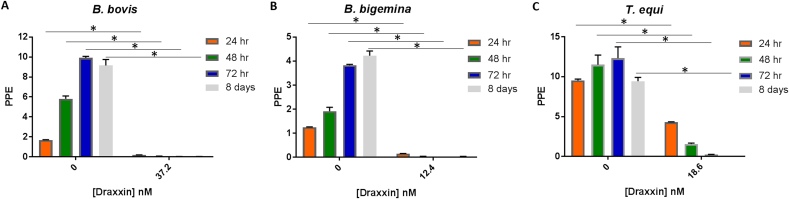
Fig. 2.
Growth curve of parasites obtained at 24 h (orange bars), 48 h (green bars) and 72 h (blue bars) after the addition of respective IC100 of Draxxin®, and 8 days (grey bars) without addition of Draxxin® in: A) B. bovis (37.2 nM), B) B. bigemina (12.4 nM) and C) T. equi (18.6 nM) in in vitro culture. PPE values are in y-axis and Draxxin® concentrations tested are in x-axis. Parasites grown without addition of Draxxin® are represented as “0”. Assay was carried out in triplicate. Error bars indicate standard error of the means for each Draxxin® concentration tested. (*) Represents p-value <0.05 indicating a statistically significant difference between media and correspondent Draxxin® using Student's t-test. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Table 2.
IC50 values obtained on B. bovis, B. bigemina and T. equi at 72 h after treatment with diminazene aceturate, imidocarb dipropionate, clofazimine and Draxxin®.
| Species | IC50 (nM) |
||
|---|---|---|---|
| B. bovis | B. bigemina | T. equi | |
| Diminazene aceturate (Rizk et al., 2015) | 400 ± 190 | 200 ± 160 | 770 ± 280 |
| Imidocarb dipropionate *(Nott et al., 1990); $(Rodriguez and Trees, 1996); #(Gopalakrishnan et al., 2016); @in this study | 8.6*$ | 0.08@ | 279# |
| Clofazimine (Tuvshintulga et al., 2016) | 4500 ± 300 | 3000 ± 200 | 290 ± 30 |
| Draxxin® | 16.7 ± 0.6 (15.4–17.9)a | 6.2 ± 0.2 (5.8–6.6)a | 2.4 ± 0.1 (2.2–2.6)a |
95% CI.
3.2. Effects of the application of Draxxin® at a IC100 doses into cultures with distinct B. bovis, B. bigemina and T. equi parasitemia levels
The performance of the drug might be affected by the existing parasite load in a given infected animal. The effects of the addition of the calculated IC100, into in vitro blood stage cultures with different starting PPE (0.2 and 1% for both Babesia parasites and 0.2 and 9% for T. equi) were also tested.
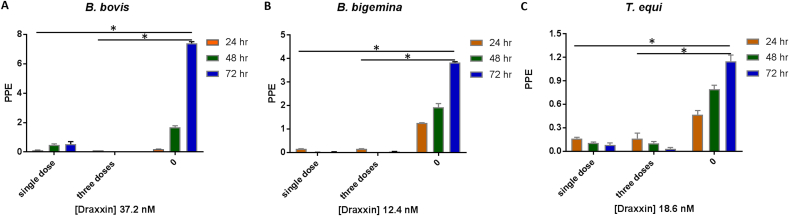
Parasites were not able to growth in the cultures with the addition of 37.2 nM, 12.4 nM and 18.6 nM of Draxxin® in B. bovis, B. bigemina and T. equi cultures, respectively (Fig. 2A–C) regardless of their initial PPE. In addition, no parasites were detected at 8 days after treatment with these doses (P < 0.05) (Fig. 2A–C), suggesting the lack of Draxxin®-resistant parasites. An additional follow up study was performed where cultures were treated with a single IC100 dose (time 0). No parasite growth was evident in the following 72 h, which was the full duration of the study (Fig. 3A–C). In case of T. equi parasites, the lack of growth was observed at 72 h after treatment (P < 0.05) but the parasite growth was significantly decreased at 24 h and 48 h after treatment (P < 0.05).
Fig. 3.
Growth curve of parasites obtained at 24 h (orange bars), 48 h (green bars) and 72 h (blue bars) after the addition of respective IC100 of Draxxin® on a single dose (0 h) or three doses (over 72 h) in: A) B. bovis (37.2 nM), B) B. bigemina (12.4 nM) and C) T. equi (18.6 nM) in in vitro culture. PPE values are in y-axis and Draxxin® concentrations tested are in x-axis. Parasites grown without addition of Draxxin® are represented as “0”. Assay was carried out in triplicate. Error bars indicate standard error of the means for each Draxxin® concentration tested. (*) Represents p-value <0.05 indicating a statistically significant difference between media and correspondent Draxxin® using Student's t-test. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
The AUC estimated values for B. bovis, B. bigemina and T. equi cultures at IC100 values and after 72 h with Draxxin® was 720 ng*hr/μl, 1680 ng*hr/μl and 1080 ng*hr/μl, respectively.
3.3. Quantitation of parasites using qPCR and calculation of IC50 and IC100 concentration of Draxxin® for B. bovis, B. bigemina and T. equi
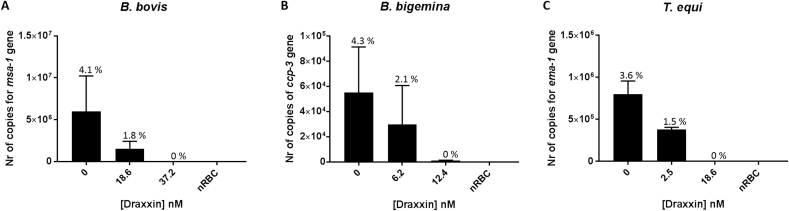
Cultured parasites were treated with either IC50 and IC100 doses of Draxxin® for 72 h. Total gDNA was extracted from all cultures at 72 h, and analyzed by qPCR. While quantitative PCR detected parasite DNA in all samples treated with IC50, no gDNA was detected from the samples collected from cultures treated with IC100 doses for B. bovis and T. equi. However although qPCR detected 0.17% of total parasites in B. bigemina gDNA samples derived from parasites treated with IC100, no parasites were detected by FACS. The PPE of B. bovis, B. bigemina and T. equi cultures in the absence of Draxxin®, IC50 and IC100, respectively, are shown in Fig. 4-C. The results are consistent with full abrogation of parasite growth at 72 h, when the cultures are treated with an IC100 for the three parasites tested.
Fig. 4.
Number of copies obtained from the quantitative PCR at IC50 and IC100 and non-treated control well (parasites grown without the addition of Draxxin®, represented as “0”). A) B. bovis, B) B. bigemina and C) T. equi. On the top of each bar is presented the PPE quantified. Assay was carried out in triplicate, and samples analyzed individually after 72 h in culture. Error bars indicate the standard error of the means for each sample analyzed. (*) Represents p-value <0.05 indicating a statistically significant difference between media and correspondent Draxxin® using one-way ANOVA.
4. Discussion
Treatment of acute bovine babesiosis and equine theileriosis is achieved by using chemotherapeutics, for example, imidocarb dipropionate. However, studies have shown emerging resistance to imidocarb dipropionate by these parasites which leads to the need of new, safe and effective drugs (Mosqueda et al., 2012; Hines et al., 2015). This study demonstrates inhibition of the in vitro growth of B. bovis, B. bigemina and T. equi parasites by the commercially available antibiotic Draxxin® at relatively low concentrations (nM). Interestingly, B. bovis parasites require 3 times more Draxxin® to fully inhibit the parasite growth, with an IC50 which is approximately 3 times higher than the IC50 of B. bigemina, and approximately 7 times higher than the IC50 of T. equi. The reasons for increased IC50 in B. bovis remain unknown, but could be related to the mechanism(s) of drug action. It was shown that macrolide antibiotics binds with the 23S prokaryotic rRNA (Schlunzen et al., 2001; Hansen et al., 2002; Andersen et al., 2012). It is likely that the antibiotic interferes with the protein synthesis machinery of the plastid organelle present in most apicomplexan parasites. This organelle is the result of an ancient symbiotic association of the ancestor of apicomplexans with a red algae containing a primary photosynthetic endosymbiont, and its function is essential for the survival of the parasites, thus is a possible drug target (Wiesner et al., 2008). It is possible that the differences in the Draxxin® IC50 values among the distinct parasites may be related to sequence variation occurring in the targeted 23S rRNA present in the plastid organelles (Hansen et al., 2002; Wiesner et al., 2008). Or alternatively, to other undefined pathways related to the mechanisms of action that can be due to a single factor or a combination of several factors. These include a distinct rate of degradation or elimination of the drug by the parasites, the accessibility of the drug to the actual target molecule(s), interaction of the drug with distinct competing molecules in the parasites, etc.
In this study, we addressed the possible existence of Draxxin® resistant parasites by testing for parasite DNA in long term cultures treated with IC100 concentrations of the drug for 8 days. Flow cytometric analysis performed on the 8th day and negligible amounts of parasite DNA as detected by quantitative PCR supports the conclusion that no parasites survived Draxxin® treatment.
In addition, application of IC100 was able to completely abrogate the growth of the three parasite species tested, independent of the starting PPE. Most persistently infected animals in the field maintain PPE levels that are undetectable by light microscopy. However, acute infections, and outbreaks caused by highly virulent strains, or infections affecting immunocompromised animals, may result in much elevated parasite loads. Having a drug that is effective against high PPEs of acute infections is critical.
Importantly, in all three species tested, the in vitro IC50 calculated for Draxxin® is at least six fold lower than the values calculated for diminazene aceturate (Rizk et al., 2015) which is currently approved for use in cattle and equine treatment of babesiosis/piroplasmosis. In addition, the IC50 calculated for Draxxin® is significantly lower (100 fold) than the IC50 calculated for clofazimine, a drug that demonstrated inhibitory effect in in vitro, but remains unlicensed for in vivo use (Tuvshintulga et al., 2016).
In addition, the IC50 value for T. equi for Draxxin® is one fold lower than the IC50 values for imidocarb dipropionate (Gopalakrishnan et al., 2016). Also, the imidocarb dipropionate IC50 value calculated for B. bigemina using an in vitro assay and reported for the first time in this study, is lower than the value calculated for B. bovis. The B. bovis IC50 for Draxxin® is two times higher than the value previously calculated for imidocarb dipropionate (Nott et al., 1990; Rodriguez and Trees, 1996). Together, these data suggest that the active principle of Draxxin® might be considered a reasonable option for treatment of cattle and horses infected with Babesia and Theileria parasites, respectively, especially since resistance to imidocarb dipropionate in T. equi has been reported recently (Hines et al., 2015). However effective and safe dosage regimens remains to be established.
Draxxin® is approved by Food and Drug Administration and the European Medicine Agency for use in veterinary species. The antibacterial effect has been well-known and study in beef and dairy calves, and in swine. The in vitro data estimated for the AUC versus time curve of Draxxin® in our study, was comparable to the AUC reported for cattle treated with the FDA-approved label dose (2.5 mg/kg) for treating pneumonia (Nowakowski et al., 2004). This suggests that the currently approved dose can be also be applicable for the treatment of babesiosis and theileriosis in large animals.
To our knowledge, this study showed for the first time the inhibitory effects of Draxxin® in Babesia spp. and T. equi parasites. The data demonstrates the potential for the development of Draxxin® as a novel pharmacological intervention against Babesia/Theileria spp. parasites. Furthermore, the results of this study permit us to speculate that other commercially available macrolides for treating pneumonia in cattle (gamithromycin, tilosin and tilmicosin) would also be active against B. bovis, B. bigemina and T. equi.
In conclusion, Draxxin® showed a full inhibitory effect on the in vitro growth of B. bovis, B. bigemina and T. equi. Draxxin® is commercially available currently, and approved for use in beef and dairy cattle. Future studies will evaluate the in vivo efficacy of the FDA-approved dosage regimen for the treatment of B. bovis, B. bigemina infection in cattle and T. equi in horses. The process for its worldwide approval for the treatment of these parasitic diseases can be highly facilitated, considering that the human, food and animal safety of the FDA-approved dosage regimen is well established.
Authors’ contributions
MGS, NV and CES designed the study. MGS performed the in vitro studies and statistical analysis. MGS, NV, DK and CES wrote the manuscript. All authors read and approved the final version of the manuscript.
Conflicts of interest
The authors declare no competing interests.
Acknowledgements
The authors would like to acknowledge Paul Lacy for his help providing the in vitro B. bovis and bigemina cultures and cultures supplies, Jacob Laughery for providing uninfected bovine erythrocytes and serum, Edith Orozco for her help with flow cytometry readings and qPCR, Fernanda Gimenez for her scientific input in the effect of tulathromycin in T. equi, Lowell Kappemeyer for providing the initial in vitro T. equi cultures and the uninfected equine erythrocytes, Megan Blauert for animal handling and blood draw. This work was supported by the USDA-ARS CRIS Project No. 5348-32000-028-00D.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.ijpddr.2018.04.004.
Contributor Information
Marta G. Silva, Email: marta_silva@wsu.edu.
Nicolas F. Villarino, Email: nicolas.villarino@wsu.edu.
Donald P. Knowles, Email: dknowles@wsu.edu.
Carlos E. Suarez, Email: suarez@wsu.edu.
Appendix A. Supplementary data
The following are the supplementary data related to this article:
Suppl. Fig. 1.
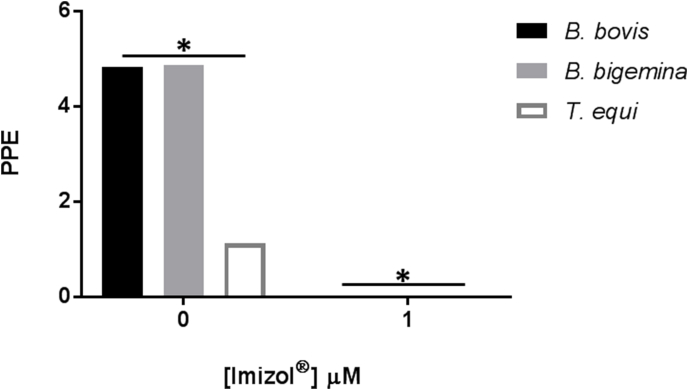
Growth curve of parasites obtained at 72 h after the addition of Imizol® at 1 μM in in vitro culture. Parasites grown without addition of Imizol® are represented as “0”. Assay was carried out in triplicate. Error bars indicate standard error of the means for each sample tested. (*) Represents p-value <0.05 indicating a statistically significant difference between media and Imizol® using Student's t-test.
Suppl. Fig. 2.
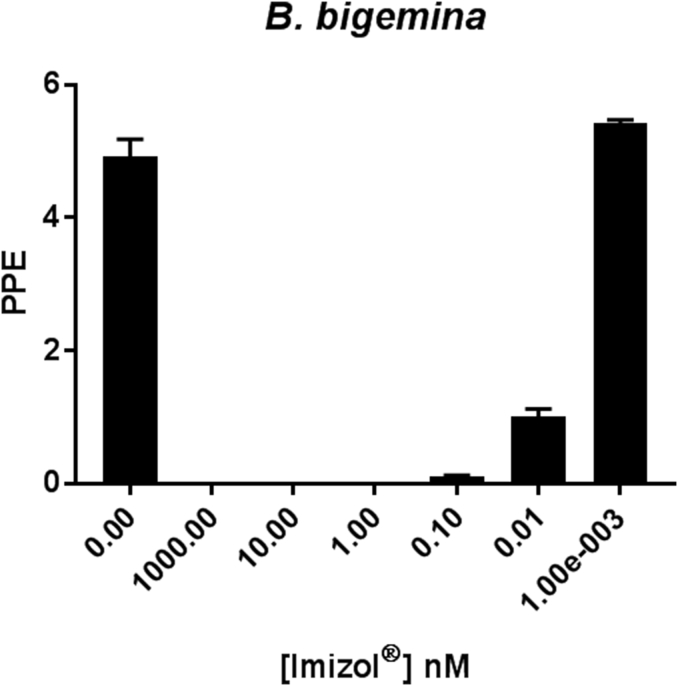
Growth curve obtained at 72 h after the addition of different concentrations of Imizol® in B. bigemina in in vitro culture. Parasites grown without addition of Imizol® are represented as “0”. Assay was carried out in triplicate. Error bars indicate standard error of the means for each sample tested.
References
- Andersen N.M., Poehlsgaard J., Warrass R., Douthwaite S. Inhibition of protein synthesis on the ribosome by tildipirosin compared with other veterinary macrolides. Antimicrob. Agents Chemother. 2012;56:6033–6036. doi: 10.1128/AAC.01250-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastos R.G., Suarez C.E., Laughery J.M., Johnson W.C., Ueti M.W., Knowles D.P. Differential expression of three members of the multidomain adhesion CCp family in Babesia bigemina, Babesia bovis and Theileria equi. PLoS One. 2013;8 doi: 10.1371/journal.pone.0067765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gopalakrishnan A., Maji C., Dahiya R.K., Suthar A., Kumar R., Gupta A.K., Dimri U., Kumar S. In vitro growth inhibitory efficacy of some target specific novel drug molecules against Theileria equi. Vet. Parasitol. 2016;217:1–6. doi: 10.1016/j.vetpar.2015.12.024. [DOI] [PubMed] [Google Scholar]
- Hansen J.L., Ippolito J.A., Ban N., Nissen P., Moore P.B., Steitz T.A. The structures of four macrolide antibiotics bound to the large ribosomal subunit. Mol. Cell. 2002;10:117–128. doi: 10.1016/s1097-2765(02)00570-1. [DOI] [PubMed] [Google Scholar]
- Hines S.A., Ramsay J.D., Kappmeyer L.S., Lau A.O., Ojo K.K., Van Voorhis W.C., Knowles D.P., Mealey R.H. Theileria equi isolates vary in susceptibility to imidocarb dipropionate but demonstrate uniform in vitro susceptibility to a bumped kinase inhibitor. Parasites Vectors. 2015;8:33. doi: 10.1186/s13071-014-0611-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy M.G., Ristic M. Babesia bovis: continuous cultivation in a microaerophilous stationary phase culture. Science. 1980;207:1218–1220. doi: 10.1126/science.7355284. [DOI] [PubMed] [Google Scholar]
- Mosqueda J., Olvera-Ramirez A., Aguilar-Tipacamu G., Canto G.J. Current advances in detection and treatment of babesiosis. Curr. Med. Chem. 2012;19:1504–1518. doi: 10.2174/092986712799828355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nott S.E., O'Sullivan W.J., Gero A.M., Bagnara A.S. Routine screening for potential babesicides using cultures of Babesia bovis. Int. J. Parasitol. 1990;20:797–802. doi: 10.1016/0020-7519(90)90014-e. [DOI] [PubMed] [Google Scholar]
- Nowakowski M.A., Inskeep P.B., Risk J.E., Skogerboe T.L., Benchaoui H.A., Meinert T.R., Sherington J., Sunderland S.J. Pharmacokinetics and lung tissue concentrations of tulathromycin, a new triamilide antibiotic, in cattle. Vet. Therapeut.: Res. Appl. Vet. Med. 2004;5:60–74. [PubMed] [Google Scholar]
- Rizk M.A., El-Sayed S.A., Terkawi M.A., Youssef M.A., El Said el Sel S., Elsayed G., El-Khodery S., El-Ashker M., Elsify A., Omar M., Salama A., Yokoyama N., Igarashi I. Optimization of a fluorescence-based assay for large-scale drug screening against Babesia and Theileria parasites. PLoS One. 2015;10 doi: 10.1371/journal.pone.0125276. e0125276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez R.I., Trees A.J. In vitro responsiveness of Babesia bovis to imidocarb dipropionate and the selection of a drug-adapted line. Vet. Parasitol. 1996;62:35–41. doi: 10.1016/0304-4017(95)00850-0. [DOI] [PubMed] [Google Scholar]
- Schlunzen F., Zarivach R., Harms J., Bashan A., Tocilj A., Albrecht R., Yonath A., Franceschi F. Structural basis for the interaction of antibiotics with the peptidyl transferase centre in eubacteria. Nature. 2001;413:814–821. doi: 10.1038/35101544. [DOI] [PubMed] [Google Scholar]
- Silva M.G., Knowles D.P., Suarez C.E. Identification of interchangeable cross-species function of elongation factor-1 alpha promoters in Babesia bigemina and Babesia bovis. Parasites Vectors. 2016;9:576. doi: 10.1186/s13071-016-1859-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suarez C.E., Noh S. Emerging perspectives in the research of bovine babesiosis and anaplasmosis. Vet. Parasitol. 2011;180:109–125. doi: 10.1016/j.vetpar.2011.05.032. [DOI] [PubMed] [Google Scholar]
- Tuvshintulga B., AbouLaila M., Davaasuren B., Ishiyama A., Sivakumar T., Yokoyama N., Iwatsuki M., Otoguro K., Omura S., Igarashi I. Clofazimine inhibits the growth of Babesia and Theileria parasites in vitro and in vivo. Antimicrob. Agents Chemother. 2016;60:2739–2746. doi: 10.1128/AAC.01614-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uilenberg G. International collaborative research: significance of tick-borne hemoparasitic diseases to world animal health. Vet. Parasitol. 1995;57:19–41. doi: 10.1016/0304-4017(94)03107-8. [DOI] [PubMed] [Google Scholar]
- Vega C.A., Buening G.M., Green T.J., Carson C.A. In vitro cultivation of Babesia bigemina. Am. J. Vet. Res. 1985;46:416–420. [PubMed] [Google Scholar]
- Vial H.J., Gorenflot A. Chemotherapy against babesiosis. Vet. Parasitol. 2006;138:147–160. doi: 10.1016/j.vetpar.2006.01.048. [DOI] [PubMed] [Google Scholar]
- Villarino N., Denny J.E., Schmidt N.W. Antimalarial activity of tulathromycin in a murine model of malaria. Antimicrob. Agents Chemother. 2015;59:3672–3674. doi: 10.1128/AAC.02858-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiesner J., Reichenberg A., Heinrich S., Schlitzer M., Jomaa H. The plastid-like organelle of apicomplexan parasites as drug target. Curr. Pharmaceut. Des. 2008;14:855–871. doi: 10.2174/138161208784041105. [DOI] [PubMed] [Google Scholar]
- Wise L.N., Kappmeyer L.S., Mealey R.H., Knowles D.P. Review of equine piroplasmosis. J. Vet. Intern. Med. 2013;27:1334–1346. doi: 10.1111/jvim.12168. [DOI] [PubMed] [Google Scholar]
- Wyatt C.R., Goff W., Davis W.C. A flow cytometric method for assessing viability of intraerythrocytic hemoparasites. J. Immunol. Meth. 1991;140:23–30. doi: 10.1016/0022-1759(91)90122-v. [DOI] [PubMed] [Google Scholar]
- Zweygarth E., Just M.C., de Waal D.T. Continuous in vitro cultivation of erythrocytic stages of Babesia equi. Parasitol. Res. 1995;81:355–358. doi: 10.1007/BF00931544. [DOI] [PubMed] [Google Scholar]