Abstract
Hookworms are intestinal nematode parasites that infect nearly half a billion people and are globally one of the most important contributors to iron-deficiency anemia. These parasites have significant impacts in developing children, pregnant women and working adults. Of all the soil-transmitted helminths or nematodes (STNs), hookworms are by far the most important, with disease burdens conservatively estimated at four million DALYs (Disability-Adjusted Life Years) and with productivity losses of up to US$139 billion annually. To date, mainly one drug, albendazole is used for hookworm therapy in mass drug administration, which has on average ∼80% cure rate that is lower (<40%) in some places. Given the massive numbers of people needing treatment, the threat of parasite resistance, and the inadequacy of current treatments, new and better cures against hookworms are urgently needed. Cry5B, a pore-forming protein produced by the soil bacterium Bacillus thuringiensis (Bt) has demonstrated good efficacy against Ancylostoma ceylanicum hookworm infections in hamsters. Here we broaden studies of Cry5B to include tests against infections of Ancylostoma caninum hookworms in dogs and against infections of the dominant human hookworm, Necator americanus, in hamsters. We show that Cry5B is highly effective against all hookworm parasites tested in all models. Neutralization of stomach acid improves Cry5B efficacy, which will aid in practical application of Cry5B significantly. Importantly, we also demonstrate that the anti-nematode therapeutic efficacy of Cry5B is independent of the host immune system and is not itself negated by repeated dosing. This study indicates that Bt Cry5B is a pan-hookworm anthelmintic with excellent properties for use in humans and other animals.
Keywords: Hookworm, Necator, Ancylostoma, Bacillus thuringiensis, Cry5B, Anthelmintic
Graphical abstract

Highlights
-
•
Bacillus thuringiensis (Bt) Cry5B is a new pan-hookworm cure.
-
•
Bt Cry5B is highly active against Ancylostoma caninum hookworm infections in dogs.
-
•
Bt Cry5B is highly active against Necator americanus.
-
•
Cry5B acts independent of the host immune system and directly on hookworms.
-
•
Cry5B retains full bioactivity after repeated dosing.
1. Introduction
Hookworms (Necator americanus, Ancylostoma duodenale, Ancylostoma ceylanicum) are the most important of the soil-transmitted helminths or soil-transmitted nematodes (STNs) of humans, which also include large roundworms (Ascaris lumbricoides) and whipworms (Trichuris trichiura). These parasitic intestinal nematodes are one of the most prevalent parasites globally, infecting ∼ half a billion people (Pullan et al., 2014). Infection by hookworm is associated with significant growth stunting in children, cognitive defects, anemia, malnutrition, lethargy (“germ of laziness”), reduced school attendance and educational status in children, and loss of worker productivity (Bethony et al., 2006; Bartsch et al., 2016; Loukas et al., 2016). Retrospective analyses of hookworm infections in the southern United States circa early 1900's demonstrated that children who grew up with hookworms had future income reduced ∼40% compared to their peers and that hookworms were responsible for ∼22% of the economic gap and ∼50% of the literacy gap between Northern and Southern States (Bleakley, 2007). Their impact on human health in the world today is tremendous and likely underestimated; hookworms are one of the most important parasites of our time (Bartsch et al., 2016; Loukas et al., 2016).
Current global strategy against hookworms involves mass drug administration (MDA) with single dose benzimidazoles, either albendazole or mebendazole (World Health Organization, 2013). Of these two, albendazole is clearly superior as a single dose therapy, with an average reported cure rate about 80% vs 33% for mebendazole and an average reported egg reduction rate of about 90% vs 61% for mebendazole (Moser et al., 2017). However, there are increasing reports of instances where the average cure rate is not achieved. For example, there are reports of albendazole treatment failure in Ghana, low cure rates in Lao PDR and Nigeria (respectively, 36% and 15%), and apparent loss of efficacy in a population with low hookworm burdens in Sri Lanka after years of deworming (Gunawardena et al., 2008; Soukhathammavong et al., 2012; Edelduok et al., 2013; Humphries et al., 2017). Furthermore, known benzimidazole resistance alleles exist in natural hookworm populations (e.g., in Kenya) and hookworms, with their rapid life cycle and high turnover in the environment, are extremely well suited towards the development of resistance towards benzimidazoles (Diawara et al., 2013). Indeed, resistance to benzimidazoles is widespread in veterinary medicine and has been demonstrated for numerous nematode parasites (Kaplan, 2004; Wolstenholme et al., 2004; Von Samson-Himmelstjerna et al., 2007). Although documented anthelmintic resistance to benzimidazoles has not yet been reported in human MDA, the lower-than-expected efficacy in places and the experience from veterinary medicine indicate that the need for new anthelmintics can be anticipated and is urgent.
Despite the importance of hookworms and the general recognition that current treatments are insufficient to eliminate parasites, few alternative therapies are being developed. One recently developed anthelmintic, tribendimidine, has similar average efficacy against hookworms as albendazole (Steinmann et al., 2008). Tribendimidine is a nicotinic acetylcholine receptor (nAChR) agonist (Hu et al., 2009), a class of molecules for which parasite resistance in veterinary medicine and human medicine can develop (Roepstorff et al., 1987; Bjørn et al., 1990; Reynoldson et al., 1997; Sacko et al., 1999; Kopp et al., 2007). Thus, development of additional treatment options in the pipeline are urgently needed. One such promising option is Cry5B, a Bacillus thuringiensis (Bt) three-domain crystal (Cry) protein that has been shown to be effective against A. ceylanicum hookworm infection in hamsters (Cappello et al., 2006; Hu et al., 2012; Hu et al., 2013b). Cry5B is also efficacious against Ascaris infections in pigs and against important veterinary targets in vitro (Kotze et al., 2005; Urban et al., 2013). Three-domain Bt Cry proteins are generally considered non-toxic and safe to vertebrates and are used globally to control insects that damage crops and carry disease (Betz et al., 2000; Koch et al., 2015). They are EPA/FDA approved for human ingestion and are a significant part of our food chain, e.g., >80% of all corn grown in the U.S. expresses a Bt Cry protein (Dively et al., 2016). Furthermore, mechanistic studies of Cry5B itself indicate that the obligate receptor for Cry5B in nematodes is an invertebrate-specific glycolipid (Griffitts et al. 2003, 2005). Thus, Cry5B is mechanistically safe to vertebrates, which lack the biosynthetic pathway to make such receptors. Forward genetic screens for Cry5B resistance in Caenorhabditis elegans suggest that resistance to Cry5B is less likely to develop in nematodes against Cry5B than against benzimidazoles and nAChR agonists (Hu et al., 2010b).
To be effective as a human therapeutic against this globally important parasite, it is important to establish if Cry5B is broadly active against hookworms. We therefore set out to address Cry5B efficacy against N. americanus, the dominant hookworm parasite in humans and the most difficult of the hookworms to treat, as, for example, it is more resistant to ivermectin than Ancylostoma (Behnke et al., 1993; Lawn et al., 2003; Hotez, 2011). Furthermore, we set out to test Cry5B against hookworms in non-rodent models of the human gastrointestinal tract, most notably Ancylostoma caninum infections in dogs, which are considered one of the best models for human hookworm studies. We also set out to address other key issues critical for Cry5B progression as a novel bacterial protein-based human therapy for hookworms by determining: 1) if the anthelmintic activity of Cry5B requires the immune system to expel parasites; 2) if a host response to Cry5B builds up after repeated doses that inhibits the ability of the protein to subsequently function; and 3) whether neutralization of stomach acid improves protein efficacy in order to allow for lower costs and significant reductions in dosing.
2. Materials and methods
2.1. Medium and reagents
Reagents for hookworm culture medium (HCM): RPMI 1640, Fetal Bovine Serum (FBS), Penicillin-Streptomycin and Fungizone Antimycotic were all purchased from Gibco, U.S.A. Dexamethasone 21-phosphate disodium salt (DEX) (Cat# D1159-5G) and cimetidine (Cat# C4522-5G) were purchased from Sigma-Aldrich, USA. Cry5B, Bt Cry5B spore-crystal lysate (SCL), and Bt spore lysate (SL; spores plus lysate with no Cry5B) were prepared as described before (Marroquin et al., 2000; Griffitts et al., 2001). SL alone has no impact on intestinal nematode burdens ((Hu et al., 2010a, Hu et al., 2010b); Fig. 5). DEX was used to immune suppress the hamsters for N. americanus-related experiments. The concentration of DEX in the drinking water and subcutaneously injected was 1 mg/L and 4 mg/mL, respectively. Cimetidine was prepared and dosed as described (Stepek et al., 2007). All drugs used in the in vivo canine study (maropitant, acepromazine, propofol, dexmedetomidine, atipamezole, famotidine) were purchased from the Cornell University Hospital for Animals pharmacy.
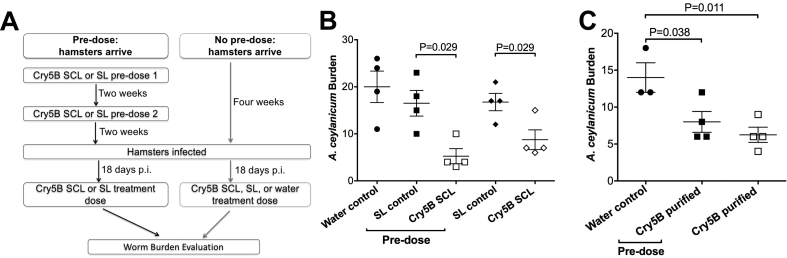
Fig. 5.
Repeated dosing with Cry5B does not abrogate efficacy. (A) Schematic of repeated dosing experiment. (B) Hookworm burdens from hamsters infected with A. ceylanicum hookworms and treated with Cry5B SCL, SL (no Cry5B), or water control. “Pre-dose” refers to hamsters treated with SCL or SL two times prior to infection and subsequent “treatment dose.” All hamsters were given a treatment dose of water, Cry5B, or SL (no Cry5B) 18 days post-infection. (C) Same experiment as in B except purified Cry5B protein was used for “pre-doses” and “treatment doses.” The dose of Cry5B used in all “pre-doses” and “treatment doses” was 10 mg/kg.
2.2. Animals and parasites
A. ceylanicum was maintained as previously reported (Hu et al., 2012). To maintain the N. americanus life cycle, hamsters were immunosuppressed with DEX, as has been previously described (Tritten et al., 2011). Three to four-week-old male Golden Syrian hamsters (HsdHan:AURA) were purchased from Envigo (U.S.A) and were infected at approximately 4–5 weeks of age with either ∼150 A. ceylanicum third-stage infectious larvae (L3i) orally or ∼400 N. americanus L3i larvae subcutaneously. Hamsters were provided with food and water (ad libitum). Ancylostoma caninum L3i were grown in charcoal culture as previously described (Lee et al., 2014). The Heligmosomoides polygyrus bakeri life cycle was maintained at the United States Department of Agriculture (USDA) as described (Camberis et al., 2003). Two purpose-bred female beagles, 6 months of age, were inoculated per os with 1000 A. caninum L3i larvae. Five-month old male and female STAT6 KO mice born and maintained on-site from breeding pairs purchased from Jackson Laboratories were inoculated with 200 H. polygyrus bakeri L3i larvae per os.
All animal experiments were carried out under protocols approved by the University of Massachusetts Medical School (hamsters), Baylor College of Medicine (hamsters), Cornell University (dogs), and USDA (mice) Animal Care and Use Committees (IACUC). All housing and care of laboratory animals used in this study conform to the NIH Guide for the Care and Use of Laboratory Animals in Research (see 18-F22) and all requirements and all regulations issued by the USDA, including regulations implementing the Animal Welfare Act (P.L. 89-544) as amended (see 18-F23).
2.3. In vitro assays
Adult N. americanus in vitro assays were carried out as described for A. ceylanicum (Hu et al. 2012, 2013a), with the exception that there were only three worms per well and that the hookworms were not separated by gender.
2.4. In vivo studies
2.4.1. A. caninum
Two purpose-bred female beagles, 6 months of age, were inoculated per os with 1000 A. caninum L3i (day 0). Only two beagles were used due to budgetary constraints for this pilot dog study. Patency of infection was confirmed on day 21 post-inoculation (p. i.) by fecal centrifugal flotation using a sugar solution with a specific gravity of 1.3. Pre-treatment hookworm burdens were determined using capsule endoscopy (Endocapsule EC-10 System, Olympus America) (Lee et al., 2014) on day 41 p. i. To enable deployment of the endoscopic capsule directly into the small intestine, dogs were briefly anesthetized. Dogs were fasted overnight, and pre-medicated with 1 mg/kg of maropitant SQ and 0.04 mg/kg acepromazine IM. A 4 mg/kg propofol bolus was administered IV to allow for intubation with a cuffed endotracheal tube. Thereafter, propofol was infused at a rate of 0.4–0.6 mg/kg IV as needed. A modified flexible gastroscope was used to deliver the endoscopic capsule into the proximal duodenum. Dogs were outfitted with a custom jacket holding the antenna pad and image recorder, and then recovered from anesthesia. An Elizabethan collar equivalent was placed around the dogs’ necks to prevent bite damage to the instruments. Dogs were regularly monitored during intestinal imaging, and recordings were stopped once the capsule was confirmed to be in the colon based on real-time images. Data were downloaded onto a laptop and reviewed by a single endoscopist.
Fecal egg counts were performed on day 41 p. i. using the Stoll method (Marsden, 1984) with the following modifications: 1) 4.00 g of feces were weighed out and added to the Stoll flask, which was then filled to the 60 mL mark with 0.1 N NaOH; 2) two aliquots were counted to arrive at an average egg count for each sample; and 3) corrections were made for fecal consistency by weighing a sample of the feces before and after overnight incubation at 105 °C, thus resulting in a final egg count based on dry fecal weight. On day 42 p. i. dogs were given 1.5 mg/kg famotidine PO and sedated with 375 μg/m<sup>2</sup> dexmedetomidine IM 30 min prior to gavage delivery of a 40 mg/kg Cry5B suspension. Sedation was reversed with 3750 μg/m<sup>2</sup> atipamezole IM. The dogs were fed their normal daily meal 2 h after the Cry5B treatment. Capsule endoscopy and fecal egg counts were repeated on day 43 p. i. to assess the effect of the first treatment. A second dose of 40 mg/kg Cry5B was administered on day 44 p. i. in the manner described above. A final capsule endoscopy and fecal egg count were performed post-treatment on day 49 p. i.
2.4.2. N. americanus
Infected hamsters (n = 10) were housed individually from day 20 p. i. onwards. On days 55 and 56 p. i. a fecal sample was collected from each hamster and processed as described (Hu et al. 2012, 2013b). On the basis of the fecal egg counts (FECs), N. americanus-infected hamsters were either assigned to the control or Cry5B treatment groups (roughly equal egg counts/group) and were treated per os with either water or Cry5B SCL, respectively, on day 56 p. i. Four days later, the hamsters were euthanized, and worm burdens were determined (Hu et al. 2012, 2013b).
2.4.3. A. ceylanicum
The A. ceylanicum in vivo experiments were carried out as described (Hu et al., 2012, Hu et al., 2013a, Hu et al., 2013b). Cimetidine was gavaged 15 min ahead of Cry5B administration.
2.4.4. H. polygyrus bakeri
Five-month old male and female STAT6 KO mice were inoculated per os with 200 H. polygyrus bakeri L3i. The infected mice were treated on days 7 and 9 p. i. per os with SL or Cry5B SCL and necropsied three days after the last treatment at day 12 p. i.
2.5. Statistical analyses
Prism v. 7 was used for all analyses and graphs. For all comparisons including just two groups, a one-tailed Mann-Whitney test was used. For all comparisons involving two groups relative to a control group, one-way analysis of variance (ANOVA) with Dunnett's post-test was used.
3. Results
3.1. Cry5B is broadly active against hookworms, including in dogs
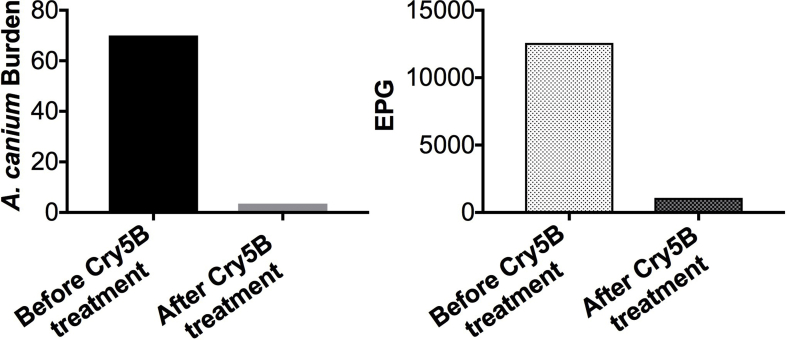
We tested the ability of Cry5B SCL to target A. caninum hookworm infections in dogs (Fig. 1). Cry5B SCL was gavaged into A. caninum-infected dogs (two doses spaced two days apart). One day after the first treatment, significant drops in hookworm burdens were seen (56% reduction based on arithmetic means; Table 1). Five days after the second treatment, Cry5B effected a near-complete elimination of hookworm infection in the dogs, with an overall 95% reduction in hookworm burdens and 91% reduction in fecal egg counts (Fig. 1 and Table 1).
Fig. 1.
Efficacy of Cry5B SCL against A. caninum infections in dogs. Two dogs were pre-treated with famotidine and dexmedetomidine, treated with Cry5B, and post-treated with atipamezole. The dose of Cry5B was 40 mg/kg on day 42 and day 44 p. i. Shown are (A) average hookworm burdens based on capsule endoscopy and (B) average fecal egg counts (eggs per gram of feces or EPG) five days after the second treatment.
Table 1.
Worm burdens and EPGs in Fig. 1.
| Worm burden |
EPG |
||||
|---|---|---|---|---|---|
| Before treatment | One day after first dose | Five days after second dose | Before treatment | Five days after second dose | |
| Dog #1 | 84 | 43 | 4 | 15750 | 1590 |
| Dog #2 | 56 | 19 | 3 | 9412 | 573 |
| Arithmetic mean (% reduction) | 70.0 | 31.0 (56%) | 3.5 (95%) | 12581.0 | 1081.5 (91%) |
| Geometric mean (% reduction) | 68.6 | 28.7 (58%) | 3.5 (95%) | 12175.4 | 954.6 (92%) |
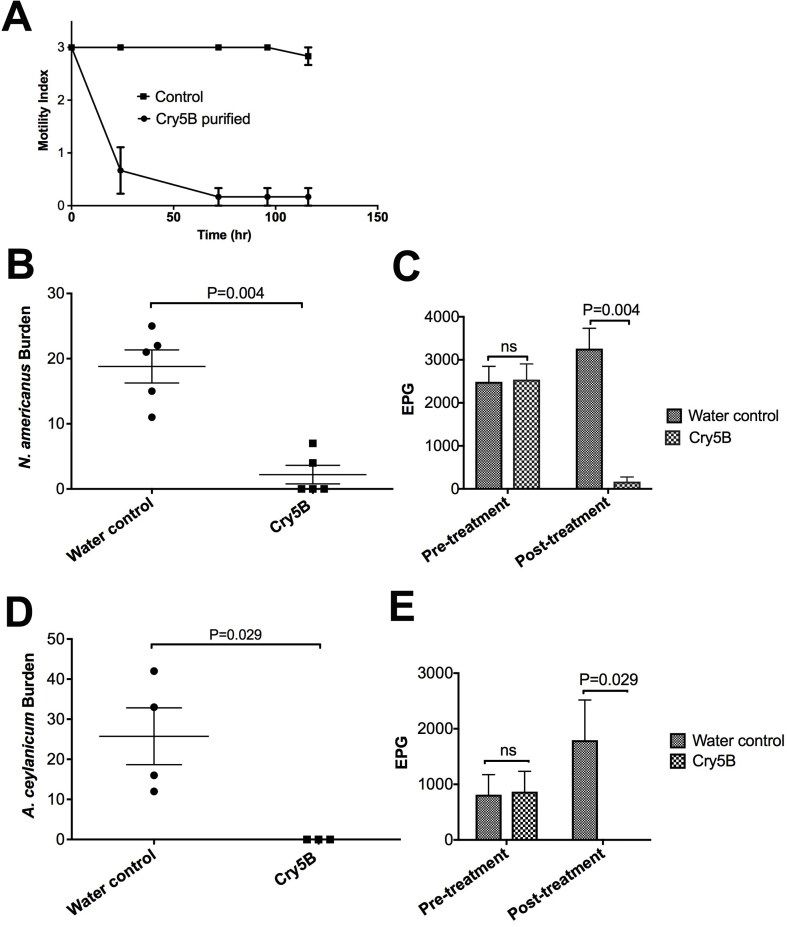
To determine if Cry5B might also be active against N. americanus, we initially tested the ability of Cry5B to intoxicate adult N. americanus hookworms in vitro. Relative to buffer-only controls, strong intoxication of N. americanus hookworms was seen with Cry5B as reflected by a significant reduction in motility index (Fig. 2A). The kinetics of N. americanus intoxication with Cry5B were similar to that of A. ceylanicum (Cappello et al., 2006).
Fig. 2.
Efficacy of Cry5B against N. americanus and A. ceylanicum. (A) Intoxication of N. americanus adults in vitro over time at a dose of 250 μg/mL Cry5B purified protein (n = 6/dose/repeat, average of 2 repeats). (B) N. americanus hookworm burdens in placebo (water control) versus Cry5B treatment. Here and in similar figures, each dot represents hookworm burden in an individual hamster; long horizontal bar represents the average and short horizontal bars represent standard error. (C) N. americanus fecal egg counts (EPG) before treatment and after treatment with placebo (water control) or Cry5B. Here and in similar figures, each bar represents the average of all hamsters; error bar represents standard error. (D) A. ceylanicum hookworm burdens in placebo (water control) versus Cry5B treatment. (E) A. ceylanicum fecal egg counts (EPG) before treatment and after treatment with placebo (water control) or Cry5B. The dose in vivo for B-E is 40 mg/kg Cry5B, delivered as SCL.
Based on positive in vitro results, we next tested the ability of Cry5B SCL to impact N. americanus infections in vivo. We found that a single dose of Cry5B SCL delivered per os at 40 mg/kg (Cry5B content) significantly reduced hookworm burdens by 88.3% and FECs by 94.8% (Fig. 2B and C; Supplementary Table 1), with three of the five hamsters completely cured. Thus, Cry5B is active against the dominant human hookworm species. For comparison, we tested Cry5B SCL at the same dose (40 mg/kg per os) in A. ceylanicum-infected hamsters. As shown in Fig. 2D and E, this dose of Cry5B resulted in a complete cure of A. ceylanicum hookworms in hamsters (100% elimination of parasites).
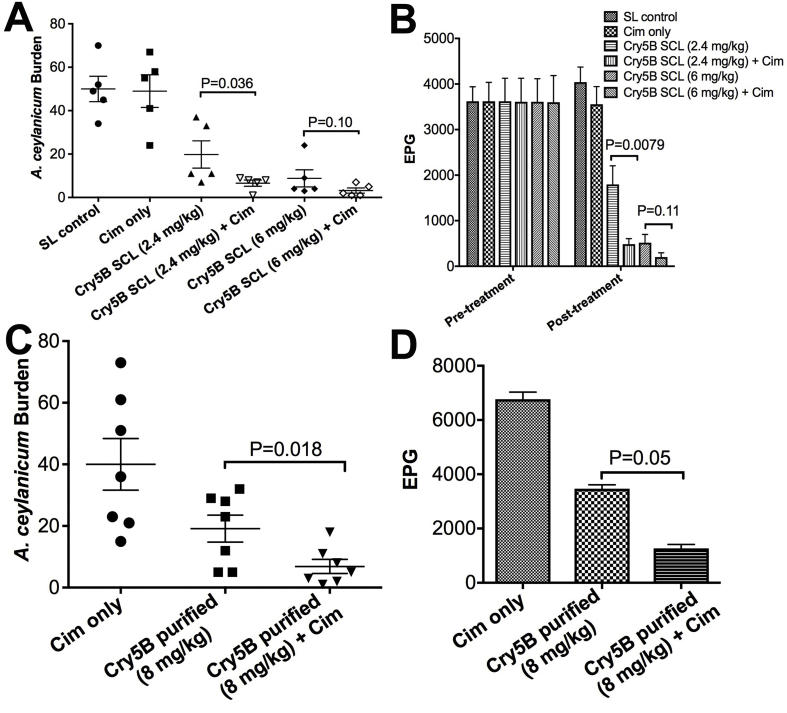
The dosing used in the above studies is low on a molar level-- e.g., 40 mg/kg Cry5B is ∼300 nmol/kg, which is equivalent to 80 μg/kg albendazole (a dose of albendazole that has no effect on hookworm burdens in hamsters; unpublished observations). Thus, Cry5B is far more potent than albendazole on a molecule by molecule basis. However, we recognize that 40 mg/kg Cry5B is a significant dose by mass. Since >99.9% of Cry5B is rapidly degraded in stomach acid (Hu et al., 2010a), we hypothesized that protection from stomach acid should improve efficacy. Previous attempts to protect Cry5B from stomach acid using nanoparticles failed, likely due to slow release kinetics (Wu et al., 2015). To circumvent the challenges with formulation strategies, we tested whether direct inhibition of stomach acid proteolysis would improve Cry5B efficacy. We pre-treated A. ceylanicum hookworm-infected hamsters with cimetidine, a histamine H2 receptor antagonist that inhibits stomach acid production (and thus pepsin activity) and pepsin secretion (Burland et al., 1975; Schlippert, 1978). Fifteen minutes following cimetidine treatment, the hamsters were given Cry5B SCL at two different doses. Control groups included no pre-treatment with cimetidine or cimetidine alone. Whereas cimetidine alone had no impact on hookworm burdens, pre-gavage with cimetidine improved Cry5B SCL efficacy (Fig. 3A and B; Supplementary Table 1), which was reproducible (data not shown). Similar results were found using purified Cry5B protein (Fig. 3C and D, Supplementary Table 1).
Fig. 3.
Inhibition of pepsin via cimetidine improves Cry5B efficacy. (A) A. ceylanicum hookworm burdens in hamsters treated with spore lysate (SL), cimetidine alone (Cim only), or Cry5B SCL at the indicated dose without or with (+Cim) a dose of cimetidine that inhibits pepsin activity 15 min prior to SCL treatment. (B) Corresponding fecal egg counts (EPG) for experiment in A before treatment and after treatment. (C, D) Same experiment as in (A, B) except using purified Cry5B.
3.2. Cry5B anthelmintic activity does not require the Th2 immune response
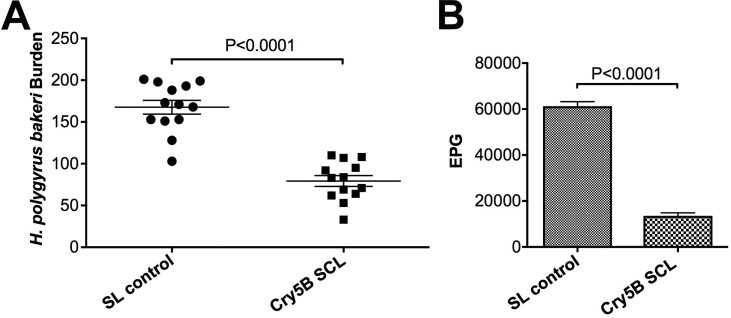
Since Cry proteins have never been used therapeutically in mammals before, it was unclear how the host immune system modulates Cry5B activity. Because Cry5B is able to largely clear N. americanus infections in hamsters immunosuppressed with DEX, these data suggest that Cry5B does not require an intact host immune system to act as an anthelmintic. To independently confirm this result, we tested the ability of Cry5B to act on the parasitic nematode H. polygyrus bakeri in mice for which the STAT6 gene has been knocked out. STAT6−/− mice lack a strong Th2 response, which plays a central role in host-mediated intestinal nematode expulsion (Maizels and Holland, 1998; Finkelman et al., 2004; Bao and Reinhardt, 2015). We found that Cry5B treatment in STAT6−/− mice (2 × 40 mg/kg) infected with H. polygyrus bakeri resulted in a significant reduction in worm burdens (53% reduction) and fecal egg counts (78% reduction; Fig. 4A and B; Supplementary Table 1), consistent with previously published effects of Cry5B on H. polygyrus bakeri infections in immunocompetent mice (Hu et al., 2010a). Taken together, our data demonstrate that Cry5B has a direct effect on the parasite and does not require an intact immune system to function as an anthelmintic.
Fig. 4.
Cry5B is efficacious in the absence of a Th2 immune response. (A) H. polygyrus bakeri worm burdens in placebo (SL control) versus Cry5B SCL treatment in STAT6−/− mice. (B) H. polygyrus bakeri fecal egg counts in placebo (SL control) versus Cry5B SCL treatment in STAT6−/− mice. The Cry5B dose is 2 × 40 mg/kg. For comparison, a single 100 mg/kg dose reduced H. polygyrus bakeri burdens 67% in immunocompetent mice (Hu et al., 2010a).
3.3. Repeated dosing does not abrogate Cry5B efficacy
Conversely, it is important to establish whether there is a host response that might weaken Cry5B activity over time. As Cry proteins have never been used therapeutically, it was unclear whether repeated Cry5B dosing might result in a host response that would neutralize its anthelmintic effects. We set up a repeated dosing experiment to address this question (Fig. 5A). We pre-treated uninfected hamsters with two Cry5B SCL doses spaced two weeks apart. Two weeks after the second “pre-dose,” hamsters were infected with A. ceylanicum hookworms. Eighteen days after infection, these same hamsters were then given a third “treatment dose” of Cry5B SCL to test its efficacy in hamsters with prior Cry5B SCL dosing. The placebo control for this group of hamsters was a second set of hamsters treated before and after infection with the same timing except we administered SL lacking Cry5B instead of SCL with Cry5B. The efficacy of these treatment groups was then compared to three other sets of hamsters that were not given pre-doses but were infected at the same time as the previous two groups. These latter three sets of hamsters were then given a treatment dose of Cry5B SCL, SL (no Cry5B), or water at the same time as the pre-dosed groups.
The results are shown in Fig. 5B and Supplementary Table 1. Treatment with SL, whether given as part of a pre-treatment regimen or not, had no impact on hookworm burdens relative to water control. Conversely, treatment with Cry5B was equally efficacious whether given after a “pre-dose” regimen or not. Thus, repeated dosing with Cry5B had no impact on its ability to effect a cure on a subsequent dose. In addition, there was no apparent toxicity (e.g., no diarrhea, inappetence, agitation) associated with these repeated treatments. These results were reproducible using purified Cry5B protein (Fig. 5C; Supplementary Table 1).
4. Discussion
Here we demonstrate that the promising new anthelmintic, Cry5B, is effective against both genera of human hookworms. Hookworms are the single-most important intestinal nematode parasite in terms of morbidity, DALYs, and priority for treatment. Hookworms, unlike whipworms and large roundworms, are comprised of multiple species within two different genera, each of which can have different susceptibilities to anthelmintics. Notably, Necator is generally much less susceptible to ivermectin than Ancylostoma and hence ivermectin is not an approved drug for MDA to control intestinal nematodes (Naquira et al., 1989; Behnke et al., 1993; Richards et al., 1995; Lawn et al., 2003). In these experiments, we also found that Cry5B efficacy was aided by protection from stomach degradation by pepsin using the proton pump inhibitor cimetidine.
Cry5B is also highly active against A. caninum hookworm infections in dogs. The dog GI tract is an excellent model for the human GI tract, and hookworm infection in dogs is one of the best models for hookworm infection in humans (Hotez et al., 1996; Fujiwara et al., 2006). We similarly found in a prior study that Cry5B was efficacious against Ascaris in pigs (Urban et al., 2013). Taken together and given that Cry proteins are generally safe and that the Cry5B receptor is an invertebrate-specific molecule, our data predict that Cry5B will safely cure hookworms and Ascaris in humans. Our pilot dog study highlights the need for the development of an enteric-protected formulation and further dose-response and dose-timing studies in this system. We don't know, for example, what would have happened at lower doses or if only a single dose had been used. For example, a 56% drop in hookworm burdens was seen one day after the first dose, before a second dose was then given the following day with 95% clearance of hookworms in this preliminary study (multiple doses of anthelmintics over the course of a few days are not uncommon for treatment of intestinal helminths in dogs (Roberson and Burke, 1982)). An additional challenge in dogs is that, in general, they have slower gastric emptying in the fed state and faster small intestinal transit than humans (Dressman, 1986), which respectively would lead to more degradation of Cry5B in the stomach and a relatively short contact time between Cry5B and hookworms residing in the small intestine. Based on our results here, expanded dosing studies in dogs and improved enteric formulations are recommended for future studies.
We also addressed two important questions related to Cry5B activity and the host. First, we demonstrated that Cry5B efficacy does not require an intact immune system or a functional Th2 response in the host. Cry5B is efficacious against N. americanus in hamsters treated with dexamethasone, even though corticosteroid therapy inhibits the hamster immune system from expelling intestinal parasites naturally. Furthermore, Cry5B is active in STAT6−/− mice that lack the Th2 response, which plays a major role in parasite expulsion. Taken together, these data indicate that Cry5B anthelmintic activity is due to direct action against the parasites in vivo.
Second, we demonstrated that repeated dosing of Cry5B over time does not lead to its loss of activity or to host toxicity. Our experimental design for this test used three oral doses in less than six weeks, which is more frequent than MDA for STNs, which typically involves dosing 1-2 times per year. Despite the fact that Cry proteins are used in massive quantities around the world in agriculture and vector control, and are a prominent part of our food chain, Cry proteins have not been used as an ingested therapeutic, e.g., to control intestinal nematodes in mammals. We note that although the vast majority of safety studies show no concerns with Bt Cry proteins and Bt Cry proteins have an excellent safety record in our food supply, a few previous studies showing immunogenetic effects in rodents have been reported (Hammond et al., 2013). We did not directly test for similar immunogenetic effects here, although the data in Fig. 5 indicate that a localized immune response to neutralize the protein in the intestine is not apparent. In addition, there is some question as to the relevance of these previous studies showing immunogenetic effects (e.g., intranasal route of administration, lack of reproducibility, lack of proper controls, presence of mycotoxins, and high dosing), and there are numerous other studies that contradict these immunogenic findings (Hammond et al., 2013; Reiner et al., 2014; Tan et al., 2016; Sánchez and Parrott, 2017).
In summary, our data here indicate that Cry5B, a novel mechanism-of-action oral, protein anthelmintic, broadly cures hookworms upon repeated dosing in a wide range of hosts, with or without a robust immune system, and will likely be effective in humans.
Acknowledgements
Funding: This research was supported by (1) the National Institutes of Health/National Institute of Allergy and Infectious Diseases grant 5R01AI056189, (2) Agriculture and Food Research Initiative Competitive Grant no. 2015-11323 from the USDA National Institute of Food and Agriculture, and (3) Bill & Melinda Gates FoundationBill & Melinda Gates Foundation Grant no. OPP1067992 all to R.V.A. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.ijpddr.2018.05.001.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- Bao K., Reinhardt R.L. The differential expression of IL-4 and IL-13 and its impact on type-2 immunity. Cytokine. 2015;75:25–37. doi: 10.1016/j.cyto.2015.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartsch S.M., Hotez P.J., Asti L., Zapf K.M., Bottazzi M.E., Diemert D.J., Lee B.Y. The global economic and health burden of human hookworm infection. PLoS Negl Trop Dis. 2016;10:e0004922. doi: 10.1371/journal.pntd.0004922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behnke J.M., Rose R., Garside P. Sensitivity to ivermectin and pyrantel of Ancylostoma ceylanicum and Necator americanus. Int. J. Parasitol. 1993;23:945–952. doi: 10.1016/0020-7519(93)90061-3. [DOI] [PubMed] [Google Scholar]
- Bethony J., Brooker S., Albonico M., Geiger S.M., Loukas A., Diemert D., Hotez P.J. Soil-transmitted helminth infections: ascariasis, trichuriasis, and hookworm. Lancet. 2006;367:1521–1532. doi: 10.1016/S0140-6736(06)68653-4. [DOI] [PubMed] [Google Scholar]
- Betz F.S., Hammond B.G., Fuchs R.L. Safety and advantages of Bacillus thuringiensis-protected plants to control insect pests. Regul. Toxicol. Pharmacol. 2000;32:156–173. doi: 10.1006/rtph.2000.1426. [DOI] [PubMed] [Google Scholar]
- Bjørn H., Roepstorff A., Waller P.J., Nansen P. Resistance to levamisole and cross-resistance between pyrantel and levamisole in Oesophagostomum quadrispinulatum and Oesophagostomum dentatum of pigs. Vet. Parasitol. 1990;37:21–30. doi: 10.1016/0304-4017(90)90022-4. [DOI] [PubMed] [Google Scholar]
- Bleakley H. Disease and development: evidence from hookworm eradication in the american south. Q. J. Econ. 2007;122:73–117. doi: 10.1162/qjec.121.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burland W.L., Duncan W.A., Hesselbo T., Mills J.G., Sharpe P.C., Haggie S.J., Wyllie J.H. Pharmacological evaluation of cimetidine, a new histamine H2-receptor antagonist, in healthy man. Br. J. Clin. Pharmacol. 1975;2:481–486. doi: 10.1111/j.1365-2125.1975.tb00564.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camberis M., Le Gros G., Urban J., Jr. Animal model of Nippostrongylus brasiliensis and Heligmosomoides polygyrus. Curr Protoc Immunol Chapter 19:Unit. 2003;19.12 doi: 10.1002/0471142735.im1912s55. [DOI] [PubMed] [Google Scholar]
- Cappello M., Bungiro R.D., Harrison L.M., Bischof L.J., Griffitts J.S., Barrows B.D., Aroian R.V. A purified Bacillus thuringiensis crystal protein with therapeutic activity against the hookworm parasite Ancylostoma ceylanicum. Proc. Natl. Acad. Sci. U. S. A. 2006;103:15154–15159. doi: 10.1073/pnas.0607002103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diawara A., Halpenny C.M., Churcher T.S., Mwandawiro C., Kihara J., Kaplan R.M., Streit T.G., Idaghdour Y., Scott M.E., Basáñez M.-G. Association between response to albendazole treatment and β-tubulin genotype frequencies in soil-transmitted helminths. PLoS Negl Trop Dis. 2013;7:e2247. doi: 10.1371/journal.pntd.0002247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dively G.P., Venugopal P.D., Finkenbinder C. Field-evolved resistance in corn earworm to Cry proteins expressed by transgenic sweet corn. PLoS One. 2016;11:e0169115. doi: 10.1371/journal.pone.0169115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dressman J.B. Comparison of canine and human gastrointestinal physiology. Pharm. Res. (N. Y.) 1986;3:123–131. doi: 10.1023/A:1016353705970. [DOI] [PubMed] [Google Scholar]
- Edelduok E.G., Eke F.N., Evelyn N.E., Atama C.I., Eyo J.E. Efficacy of a single dose albendazole chemotherapy on human intestinal helminthiasis among school children in selected rural tropical communities. Ann. Trop. Med. Publ. Health. 2013;6:413. [Google Scholar]
- Finkelman F.D., Shea-Donohue T., Morris S.C., Gildea L., Strait R., Madden K.B., Schopf L., Urban J.F., Jr. Interleukin-4- and interleukin-13-mediated host protection against intestinal nematode parasites. Immunol. Rev. 2004;201:139–155. doi: 10.1111/j.0105-2896.2004.00192.x. [DOI] [PubMed] [Google Scholar]
- Fujiwara R.T., Geiger S.M., Bethony J., Mendez S. Comparative immunology of human and animal models of hookworm infection. Parasite Immunol. 2006;28:285–293. doi: 10.1111/j.1365-3024.2006.00821.x. [DOI] [PubMed] [Google Scholar]
- Griffitts J.S., Haslam S.M., Yang T., Garczynski S.F., Mulloy B., Morris H., Cremer P.S., Dell A., Adang M.J., Aroian R.V. Glycolipids as receptors for Bacillus thuringiensis crystal toxin. Science. 2005;307:922–925. doi: 10.1126/science.1104444. [DOI] [PubMed] [Google Scholar]
- Griffitts J.S., Huffman D.L., Whitacre J.L., Barrows B.D., Marroquin L.D., Müller R., Brown J.R., Hennet T., Esko J.D., Aroian R.V. Resistance to a bacterial toxin is mediated by removal of a conserved glycosylation pathway required for toxin-host interactions. J. Biol. Chem. 2003;278:45594–45602. doi: 10.1074/jbc.M308142200. [DOI] [PubMed] [Google Scholar]
- Griffitts J.S., Whitacre J.L., Stevens D.E., Aroian R.V. Bt toxin resistance from loss of a putative carbohydrate-modifying enzyme. Science. 2001;293:860–864. doi: 10.1126/science.1062441. [DOI] [PubMed] [Google Scholar]
- Gunawardena N.K., Amarasekera N.D.D.M., Pathmeswaran A., de Silva N.R. Effect of repeated mass chemotherapy for filariasis control on soil-transmitted helminth infections in Sri Lanka. Ceylon Med. J. 2008;53:13–16. doi: 10.4038/cmj.v53i1.220. [DOI] [PubMed] [Google Scholar]
- Hammond B., Kough J., Herouet-Guicheney C., Jez J.M. ILSI international food biotechnology committee task force on use of mammalian toxicology studies in safety assessment of GM foods. Toxicological evaluation of proteins introduced into food crops. Crit Rev Toxicol. 2013;43(Suppl. 2):25–42. doi: 10.3109/10408444.2013.842956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotez P.J. Nelson Textbook of Pediatrics. Elsevier; 2011. Hookworms (necator americanus and ancylostoma spp; pp. 1218–1221. e2. [Google Scholar]
- Hotez P.J., Hawdon J.M., Cappello M., Jones B.F., Ghosh K., Volvovitz F., Xiao S.H. Molecular approaches to vaccinating against hookworm disease. Pediatr. Res. 1996;40:515–521. doi: 10.1203/00006450-199610000-00001. [DOI] [PubMed] [Google Scholar]
- Humphries D., Nguyen S., Kumar S., Quagraine J.E., Otchere J., Harrison L.M., Wilson M., Cappello M. Effectiveness of albendazole for hookworm varies widely by community and correlates with nutritional factors: a cross-sectional study of school-age children in Ghana. Am. J. Trop. Med. Hyg. 2017;96:347–354. doi: 10.4269/ajtmh.16-0682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Y., Ellis B.L., Yiu Y.Y., Miller M.M., Urban J.F., Shi L.Z., Aroian R.V. An extensive comparison of the effect of anthelmintic classes on diverse nematodes. PLoS One. 2013;8:e70702. doi: 10.1371/journal.pone.0070702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Y., Miller M.M., Derman A.I., Ellis B.L., Monnerat R.G., Pogliano J., Aroian R.V. Bacillus subtilis strain engineered for treatment of soil-transmitted helminth diseases. Appl. Environ. Microbiol. 2013;79:5527–5532. doi: 10.1128/AEM.01854-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Y., Georghiou S.B., Kelleher A.J., Aroian R.V. Bacillus thuringiensis Cry5B protein is highly efficacious as a single-dose therapy against an intestinal roundworm infection in mice. PLoS Negl Trop Dis. 2010;4:e614. doi: 10.1371/journal.pntd.0000614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Y., Platzer E.G., Bellier A., Aroian R.V. Discovery of a highly synergistic anthelmintic combination that shows mutual hypersusceptibility. Proc. Natl. Acad. Sci. U. S. A. 2010;107:5955–5960. doi: 10.1073/pnas.0912327107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Y., Xiao S.-H., Aroian R.V. The new anthelmintic tribendimidine is an L-type (levamisole and pyrantel) nicotinic acetylcholine receptor agonist. PLoS Negl Trop Dis. 2009;3:e499. doi: 10.1371/journal.pntd.0000499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Y., Zhan B., Keegan B., Yiu Y.Y., Miller M.M., Jones K., Aroian R.V. Mechanistic and single-dose in vivo therapeutic studies of Cry5B anthelmintic action against hookworms. PLoS Negl Trop Dis. 2012;6:e1900. doi: 10.1371/journal.pntd.0001900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan R.M. Drug resistance in nematodes of veterinary importance: a status report. Trends Parasitol. 2004;20:477–481. doi: 10.1016/j.pt.2004.08.001. [DOI] [PubMed] [Google Scholar]
- Koch M.S., Ward J.M., Levine S.L., Baum J.A., Vicini J.L., Hammond B.G. The food and environmental safety of Bt crops. Front. Plant Sci. 2015;6:283. doi: 10.3389/fpls.2015.00283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopp S.R., Kotze A.C., McCarthy J.S., Coleman G.T. High-level pyrantel resistance in the hookworm Ancylostoma caninum. Vet. Parasitol. 2007;143:299–304. doi: 10.1016/j.vetpar.2006.08.036. [DOI] [PubMed] [Google Scholar]
- Kotze A.C., O'Grady J., Gough J.M., Pearson R., Bagnall N.H., Kemp D.H., Akhurst R.J. Toxicity of Bacillus thuringiensis to parasitic and free-living life-stages of nematode parasites of livestock. Int. J. Parasitol. 2005;35:1013–1022. doi: 10.1016/j.ijpara.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Lawn S.D., Grant A.D., Wright S.G. Case reports: acute hookworm infection: an unusual cause of profuse watery diarrhoea in returned travellers. Trans. R. Soc. Trop. Med. Hyg. 2003;97:414–415. doi: 10.1016/s0035-9203(03)90073-3. [DOI] [PubMed] [Google Scholar]
- Lee A.C.Y., Hostetler J.A., Bowman D.D. Assessing the speed of kill of hookworms, Ancylostoma caninum, by Advantage Multi® for Dogs using endoscopic methods. Vet. Parasitol. 2014;204:402–406. doi: 10.1016/j.vetpar.2014.05.028. [DOI] [PubMed] [Google Scholar]
- Loukas A., Hotez P.J., Diemert D., Yazdanbakhsh M., McCarthy J.S., Correa-Oliveira R., Croese J., Bethony J.M. Hookworm infection. Nat Rev Dis Primers. 2016;2:16088. doi: 10.1038/nrdp.2016.88. [DOI] [PubMed] [Google Scholar]
- Maizels R.M., Holland M.J. Parasite immunity: pathways for expelling intestinal helminths. Curr. Biol. 1998;8:R711–R714. doi: 10.1016/s0960-9822(98)70455-5. [DOI] [PubMed] [Google Scholar]
- Marroquin L.D., Elyassnia D., Griffitts J.S., Feitelson J.S., Aroian R.V. Bacillus thuringiensis (Bt) toxin susceptibility and isolation of resistance mutants in the nematode Caenorhabditis elegans. Genetics. 2000;155:1693–1699. doi: 10.1093/genetics/155.4.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsden P.D. Clinicai parasitology. In: Beaver P.C., Jung R.C., Cupp E.W., editors. 1984. Rev Soc Bras Med Trop 17. ninth ed. Lea and Febiger; Philadelphia: 1984. 219–219. [Google Scholar]
- Moser W., Schindler C., Keiser J. Efficacy of recommended drugs against soil transmitted helminths: systematic review and network meta-analysis. BMJ. 2017;358:j4307. doi: 10.1136/bmj.j4307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naquira C., Jimenez G., Guerra J.G., Bernal R., Nalin D.R., Neu D., Aziz M. Ivermectin for human strongyloidiasis and other intestinal helminths. Am. J. Trop. Med. Hyg. 1989;40:304–309. doi: 10.4269/ajtmh.1989.40.304. [DOI] [PubMed] [Google Scholar]
- Pullan R.L., Smith J.L., Jasrasaria R., Brooker S.J. Global numbers of infection and disease burden of soil transmitted helminth infections in 2010. Parasit Vectors. 2014;7:37. doi: 10.1186/1756-3305-7-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiner D., Lee R.-Y., Dekan G., Epstein M.M. No adjuvant effect of Bacillus thuringiensis-maize on allergic responses in mice. PLoS One. 2014;9:e103979. doi: 10.1371/journal.pone.0103979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynoldson J.A., Behnke J.M., Pallant L.J., Macnish M.G., Gilbert F., Giles S., Spargo R.J., Thompson R.C. Failure of pyrantel in treatment of human hookworm infections (Ancylostoma duodenale) in the Kimberley region of north west Australia. Acta Trop. 1997;68:301–312. doi: 10.1016/s0001-706x(97)00106-x. [DOI] [PubMed] [Google Scholar]
- Richards J.C., Behnke J.M., Duce I.R. In vitro studies on the relative sensitivity to ivermectin of Necator americanus and Ancylostoma ceylanicum. Int. J. Parasitol. 1995;25:1185–1191. doi: 10.1016/0020-7519(95)00036-2. [DOI] [PubMed] [Google Scholar]
- Roberson E.L., Burke T.M. Evaluation of granulated fenbendazole as a treatment for helminth infections in dogs. J. Am. Vet. Med. Assoc. 1982;180:53–55. [PubMed] [Google Scholar]
- Roepstorff A., Bjørn H., Nansen P. Resistance of Oesophagostomum spp. in pigs to pyrantel citrate. Vet. Parasitol. 1987;24:229–239. doi: 10.1016/0304-4017(87)90044-6. [DOI] [PubMed] [Google Scholar]
- Sacko M., De Clercq D., Behnke J.M., Gilbert F.S., Dorny P., Vercruysse J. Comparison of the efficacy of mebendazole, albendazole and pyrantel in treatment of human hookworm infections in the southern region of Mali, West Africa. Trans. R. Soc. Trop. Med. Hyg. 1999;93:195–203. doi: 10.1016/s0035-9203(99)90306-1. [DOI] [PubMed] [Google Scholar]
- Sánchez M.A., Parrott W.A. Characterization of scientific studies usually cited as evidence of adverse effects of GM food/feed. Plant Biotechnol. J. 2017;15:1227–1234. doi: 10.1111/pbi.12798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlippert W. Cimetidine. H2-receptor blockade in gastrointestinal disease. Arch. Intern. Med. 1978;138:1257–1260. doi: 10.1001/archinte.138.8.1257. [DOI] [PubMed] [Google Scholar]
- Soukhathammavong P.A., Sayasone S., Phongluxa K., Xayaseng V., Utzinger J., Vounatsou P., Hatz C., Akkhavong K., Keiser J., Odermatt P. Low efficacy of single-dose albendazole and mebendazole against hookworm and effect on concomitant helminth infection in Lao PDR. PLoS Negl Trop Dis. 2012;6:e1417. doi: 10.1371/journal.pntd.0001417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinmann P., Zhou X.-N., Du Z.-W., Jiang J.-Y., Xiao S.-H., Wu Z.-X., Zhou H., Utzinger J. Tribendimidine and albendazole for treating soil-transmitted helminths, Strongyloides stercoralis and Taenia spp.: open-label randomized trial. PLoS Negl Trop Dis. 2008;2:e322. doi: 10.1371/journal.pntd.0000322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stepek G., Lowe A.E., Buttle D.J., Duce I.R., Behnke J.M. Anthelmintic action of plant cysteine proteinases against the rodent stomach nematode, Protospirura muricola, in vitro and in vivo. Parasitology. 2007;134:103–112. doi: 10.1017/S0031182006001302. [DOI] [PubMed] [Google Scholar]
- Tan X., Zhou X., Tang Y., Lv J., Zhang L., Sun L., Yang Y., Miao Y., Jiang H., Chen G. Immunotoxicological evaluation of genetically modified rice expressing Cry1Ab/Ac protein (TT51-1) by a 6-month feeding study on cynomolgus monkeys. PLoS One. 2016;11:e0163879. doi: 10.1371/journal.pone.0163879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tritten L., Silbereisen A., Keiser J. In vitro and in vivo efficacy of Monepantel (AAD 1566) against laboratory models of human intestinal nematode infections. PLoS Negl Trop Dis. 2011;5:e1457. doi: 10.1371/journal.pntd.0001457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urban J.F., Jr., Hu Y., Miller M.M., Scheib U., Yiu Y.Y., Aroian R.V. Bacillus thuringiensis-derived Cry5B has potent anthelmintic activity against Ascaris suum. PLoS Negl Trop Dis. 2013;7:e2263. doi: 10.1371/journal.pntd.0002263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Samson-Himmelstjerna G., Blackhall W.J., McCarthy J.S., Skuce P.J. Single nucleotide polymorphism (SNP) markers for benzimidazole resistance in veterinary nematodes. Parasitology. 2007;134:1077–1086. doi: 10.1017/S0031182007000054. [DOI] [PubMed] [Google Scholar]
- Wolstenholme A.J., Fairweather I., Prichard R., von Samson-Himmelstjerna G., Sangster N.C. Drug resistance in veterinary helminths. Trends Parasitol. 2004;20:469–476. doi: 10.1016/j.pt.2004.07.010. [DOI] [PubMed] [Google Scholar]
- World Health Organization . 2013. Assessing the Efficacy of Anthelminthic Drugs against Schistosomiasis and Soil-transmitted Helminthiases.http://apps.who.int/iris/bitstream/10665/79019/1/9789241564557_eng.pdf [Google Scholar]
- Wu C.-C., Hu Y., Miller M., Aroian R.V., Sailor M.J. Protection and delivery of anthelmintic protein Cry5B to nematodes using mesoporous silicon particles. ACS Nano. 2015;9:6158–6167. doi: 10.1021/acsnano.5b01426. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.