Supplemental Digital Content is available in the text
Keywords: Bile acids, bilirubin, cholestasis, critical illness, liver, nutrient restriction, sepsis
Abbreviations: ALP, alkaline phosphatase, ALT, alanine aminotransferase, AST, aspartate aminotransferase, BA, bile acids, CRP, C-reactive protein, FXR, Farnesoid-X-Receptor, gGT, gamma-glutamyltranspeptidase, ICU, intensive care unit, RXR, retinoid-X-receptor
ABSTRACT
Background and Aims:
Elevated markers of cholestasis are common in response to critical illness, and associated with adverse outcome. The role of illness duration and of nutrient restriction on underlying molecular pathways of such cholestatic responses have not been thoroughly investigated.
Methods:
In a mouse model of surgery- and sepsis-induced critical illness, molecular pathways of cholestasis were investigated up to 7 days. To assess which changes are explained by illness-induced lack of feeding, nutrient-restricted healthy mice were studied and compared with ad libitum fed healthy mice. Furthermore, serum bile acid (BA) concentrations were quantified in 1,114 human patients with either short or long intensive care unit (ICU) stay, matched for type and severity of illness, up to ICU-day-7.
Results:
In critically ill mice, either evoked by surgery or sepsis, circulating and hepatic BA-levels progressively increased with time from day-3 onward, preceded by unsuppressed or upregulated CYP7A1 and CYP27A1 protein expression. From 30 h onward, nuclear farnesoid-X-receptor-retinoid-X-receptor staining was significantly suppressed in both critically ill groups, followed from day-3 onward by decreased gene expression of the apical exporter BA-specific export pump and increased expression of basolateral exporters multidrug resistance-associated protein 3 (MRP3) and MRP4. Nutrient restriction in healthy mice only partly mirrored illness-induced alterations in circulating BA and BA-transporters, without changing nuclear receptors or synthesis markers expression. Also in human critically ill patients, serum BA increased with time in long-stay patients only, similarly for patients with or without sepsis.
Conclusions:
Circulating BA concentrations rose days after onset of sepsis- and surgery-induced, critical illness, only partially explained by lack of feeding, preceded by suppressed nuclear feedback-sensors and ongoing BA synthesis. Expression of transporters suggested ongoing reversed BA-flow toward the blood.
INTRODUCTION
Abnormal cholestatic liver test results are frequently observed in patients treated in the intensive care unit (ICU), traditionally labeled as critical illness-associated cholestatic liver dysfunction. The development of hyperbilirubinemia is associated with a more complicated course of critical illness, a longer duration of ICU stay, and a higher risk of death (1, 2). However, while increased levels of cholestatic markers can point to liver dysfunction, this may be an epiphenomenon. Vice versa, increased markers of cholestasis could also reflect an adaptive and favorable response to severe illness if increased amounts of bilirubin and bile acids (BA) in the circulation exert beneficial systemic effects (3). Such a possibility was recently suggested by findings from a large randomized controlled trial (EPaNIC) (4). The EPaNIC trial investigated the impact of delaying initiation of parenteral nutrition to beyond the first week of critical illness and found fewer infections, less organ failure, and faster recovery (4, 5). Intriguingly, throughout the 7-day intervention window, plasma bilirubin levels were significantly higher in patients who did not get parenteral nutrition, whereas biochemical markers of hepatocyte lysis and cholestasis peaked to significantly lower levels in these patients (6). These findings suggested that mild hyperbilirubinemia in response to critical illness may not necessarily reflect true cholestasis but instead could be part of the adaptive stress response.
The underlying metabolic pathways of critical illness-induced hyperbilirubinemia, and the role herein of illness duration are currently not well characterized. Although impaired bile formation/flow can be due to extrahepatic bile duct obstruction, intrahepatic nonobstructive cholestasis seems to dominate during critical illness (3). In liver biopsies harvested from prolonged critically ill patients who died in the ICU, it has been shown that expression of hepatic BA transporters is altered to promote export of bilirubin and BA back to the systemic circulation (7). Surprisingly, while circulating BA levels were 11-fold higher than normal in these patients, expression of the main BA synthesis enzyme, CYP7A1, was not suppressed. Furthermore, hepatocytic nuclear content of the Farnesoid-X-Receptor (FXR) and the Retinoid-X-Receptor (RXR), the key sensors and transcriptional regulators of BA synthesis and intrahepatic transport, were remarkably low in the patient biopsies (7). Inevitably, the results of this post-mortem study apply only to the most severe phenotype of critically ill patients. It is not clear whether such ongoing BA production and reversal of BA transport is a general phenomenon occurring in all types of critical illness. Also, although cholestatic alterations have been studied in animal models of acute inflammation and sepsis, the time course of cholestatic alterations during prolonged critical illness remains unclear (3). Furthermore, cholestatic alterations could partially be triggered by the lack of feeding, which also hallmarks critical illness. While the effect of short-term fasting on bile acid homeostasis has been investigated (8), the impact of extended nutrient restriction on the underlying molecular pathways remains poorly characterized.
This study aimed to analyze the role of illness duration and of nutrient restriction on critical illness-induced cholestatic alterations in an animal model of prolonged critical illness evoked either by surgery or sepsis. Since systemic BA are affected by both BA synthesis and intrahepatic transport, we first investigated bile acid synthesis enzymes and regulating nuclear receptors, followed by a characterization of hepatic transporters expression. To assess whether any observed changes are explained by illness-induced lack of feeding, partial nutrient restriction matching the lack of feeding with illness was studied in healthy mice over time and compared with ad libitum feeding. In addition, we examined liver histology for signs of liver damage and regeneration. As a partial human correlate, alterations over time in circulating BA levels were documented in human patients suffering from brief or prolonged critical illness either evoked by sepsis or other causes.
MATERIALS AND METHODS
Mice study
To study changes over time, 24-week-old male C57BL/6J mice (mature adult) (Janvier Labs, Le Genest-Saint-Ilse, France) were randomly allocated to “healthy control” or “critically ill” groups (Fig. 1). We used only male mice to avoid the cyclic influence of estrogens. Mice were made critically ill either by extensive surgery (“surgical critical illness”) or by extensive surgery and cecal ligation and puncture (CLP)-induced sepsis (“septic critical illness”) and sacrificed after 30 h, 3 days, 5 days, or 7 days of critical illness (Fig. 1). The Animal Ethics Committee of the KU Leuven approved the protocol for the mouse study (P134-2013). The study adheres to the European Union concerning the welfare of laboratory animals as declared in Directive 2010/63/EU.
Fig. 1.
Design of the time course mouse study and cumulative survival.
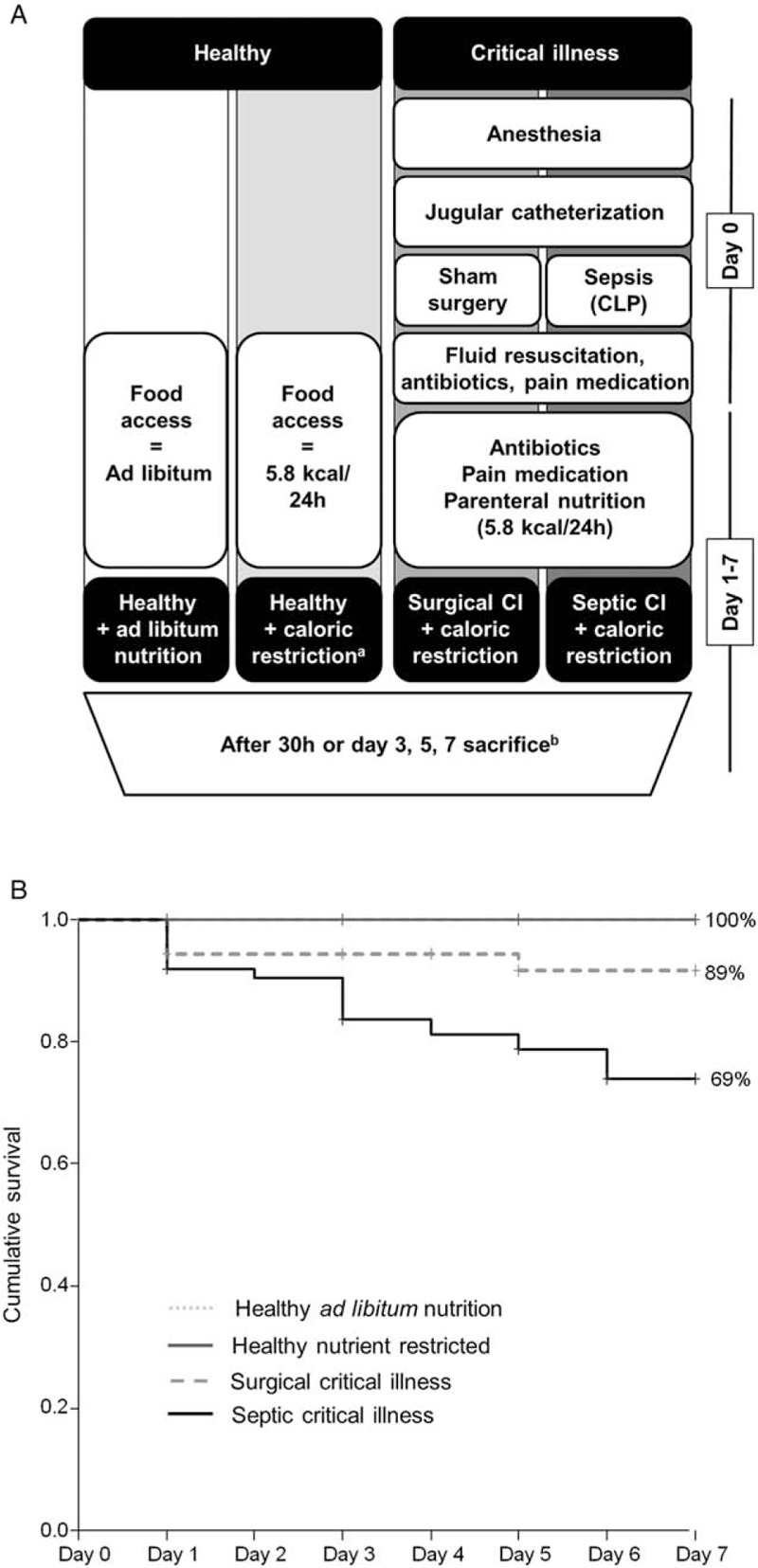
A, Experimental setup of the study groups. a: nutrient restriction in healthy animals was paired with caloric intake in critically ill animals. b: number of animals/group: Healthy ad libitum fed: 30 h: n = 15, Day-3: n = 15, Day-5: n = 15, Day-7: n = 15. Healthy caloric restricted: 30 h: n = 15, Day-3: n = 15, Day-5: n = 15, Day-7: n = 15. Surgical critical illness: 30h: n = 16, Day-3: n = 16, Day-5: n = 16, Day-7: n = 18. Septic critical illness: 30h: n = 15, Day-3: n = 16, Day-5: n = 16, Day-7: n = 15. B, Cumulative survival of the mouse study. CI indicates critical illness.
Both surgical and septic critically ill animals underwent extensive surgery, in which a central venous catheter was placed in the left jugular vein, as reported previously (9). Mice allocated to “surgical critical illness” received a combination of the placement of a central venous catheter, which is an extensive surgical procedure that requires prolonged anesthesia, and a sham laparotomy for which the abdomen is opened, the cecum is manipulated, but not punctured. Mice allocated to the “septic critical illness” group additionally received CLP with an 18-gauge needle to induce a polymicrobial sepsis (9). All critically ill mice received intravenous fluid resuscitation (colloids/crystalloids 1:4 at 0.3 mL/h) for 24 h, from then onward intravenous partial parenteral nutrition at 0.2 mL/h (Oliclinomel N7E, Baxter, Braine-l’Alleud, Belgium) via the central venous catheter (9). To match the human intensive care setting of reduced nutrient intake, we used mixed parenteral nutrition instead of normal chow to allow careful control of the nutritional intake. Administered nutrients were restricted in calories to 45% (5.8 kcal/24 h) of the normal daily intake to mimic illness- induced lack of feeding. Furthermore, mice received postoperative broad-spectrum antibiotics (imepenem + clilastatin) and pain medication (buprenorphine). If the central venous catheter was accidently dislocated, animals were excluded from the study. Animals were housed in individual house-made transparent swivel cages and placed in a temperature-controlled (27°C) animal cabinet with 12 h light and dark cycles (9).
Since nutrient restriction by itself can alter BA regulation, healthy mice that received restricted access to chow (ssniff R/M-H, ssniff Spezialdiäten GmbH, Soest, Germany) to match the caloric intake of the critically ill groups served as the control group (“nutrient-restricted healthy controls”). To assess the impact of prolonged nutrient restriction, nutrient-restricted animals were compared with healthy ad libitum fed animals (“healthy-fed controls”). All animals had ad libitum access to chow and water before the start of the experiment.
The study was continued until 15 surviving animals were obtained for each study group and each time point (16 groups).
Quantification of BA concentrations in mice plasma and liver
Plasma BA concentrations were measured with the use of liquid chromatography tandem-mass spectrometry as described in the online supplement, Supplemental Digital Content 1. Total BA were extracted from whole liver tissue by homogenizing 50 mg liver tissue in 75% ethanol and heated to 50°C for 2 h. The suspension was centrifuged, after which BA were quantified in the supernatant. Total hepatic BA were measured enzymatically using the enzyme cycling total BA kit (Diazyme Laboratories, Poway, Calif).
Quantification of gene and protein expression in mice liver
Total RNA was isolated from snap frozen liver tissue using Qiazol and the RNAeasy isolation kit (Qiagen, Venlo, The Netherlands). RNA was reverse transcribed and cDNA was quantified in real time as reported previously (10) with commercial TaqMan Assays (Applied Biosystems) (Sup. Table 3). For the relative expression of genes, healthy-fed control animals of the respective day were set as 1. Immunoblots with CYP7A1 (Santa Cruz Biotechnologies, Dallas, Tex) and CYP27A1 (Thermo Fisher, Aalst, Belgium) antibodies were performed as described in the online supplement, Supplemental Digital Content 1.
(Immuno)Histological analyses
Liver histological structure was analyzed on digital microscopy images of hematoxylin and eosin stained 5 μm formalin-fixed paraffin sections. Slides were scored semiquantitatively for sinusoidal dilatation, feathery appearance of cytoplasm, ballooning, and accumulation of inflammatory cells by two independent observers. Average nuclear size, as a marker of cellular stress, was quantified using an in house made automated image analyzing program (Supplemental methods).
For immunohistochemistry (IHC), paraffin sections were incubated with primary antibodies against CK7 (Abcam, Cambridge, UK) and the nuclear receptors FXR and RXR (Santa Cruz Biotechnologies) overnight at 4°C and subsequently with HRP linked secondary antibodies (Dako, Glostrup, Denmark) for 30 min and visualized with DAB (Dako). A machine learning classifier was developed to identify which of the automatically identified nuclei were stained for FXR and RXR (Supplemental methods).
Patients and controls
The human study was a secondary analysis of the randomized controlled EPaNIC trial that investigated the impact of early parenteral nutrition (11). The study protocol and consent forms were approved by the institutional ethical review boards (ML4190). The detailed study protocol and primary results have been published elsewhere (5, 6, 11). In brief, patients were randomized to receive parenteral supplementation in addition to insufficient enteral nutrition to reach their caloric goal either within 48 h (early) or after 7 days when still in ICU (late). Written informed consent was obtained from all patients or next-of-kin. To compare the course of BA over time in patients with a brief or protracted critical illness, 557 short-stay patients (≤3 days in ICU) and 557 long-stay patients (>3 days in ICU) were matched with propensity scores obtained by logistic regression (one-to-one nearest neighbor matching without replacement and with a caliper of 0.0005) for demographics, patient history markers, severity of illness scores, admission features, trial features, and nutritional intake (Sup. Fig. 1 for a complete list of matching criteria and consort diagram and Sup. Fig. 2). Based on previously published data, we calculated that to detect a difference of 0.75 uM in circulating BA with σ of 4.4 uM (effect size of 17%) with a statistical power >80% and α<0.05, a minimum of 543 patients per arm was required. To account for possible dropout because of insufficient sample availability, we matched 557 patients per group. In addition, 44 overnight fasted, volunteers matched for age, gender, and BMI were analyzed to generate healthy reference values.
Human serum analyses
For all patients (n = 1,114), total BA were quantified in serum samples collected upon admission and the third day (or last day for those patients who were discharged earlier) and additionally on day-7 in the subgroup of patients with an ICU≥7 days (n = 254). Total BA were measured using the total BA assay (NBT method, Diazyme Laboratories, Poway, Calif). Plasma concentrations of bilirubin, gamma-glutamyltranspeptidase (gGT), alkaline phosphatase (ALP), alanine aminotransferase (ALT), aspartate aminotransferase (AST), and C-reactive protein (CRP) had been measured as part of the routine laboratory tests performed in the participating ICUs.
Statistical analysis
All statistical analyses were performed with SPSS (IBM, North Castle, NY) including the R-based plugin of propensity score matching, JMP (SAS Institute, Cary, NC), MATLAB 2014b (The MathWorks, Natick, Mass) and the Gaussian Process library (12). Student t test, Mann–Whitney U tests, and Chi-square or Fisher exact tests were used as appropriate. Repeated measures ANOVA were used to analyze the time and group effect on the changes in BA of the human patients. Correlations were determined using Pearson correlation coefficient. Data are represented as either mean ± SEM or median and IQR (25th–75th percentile) as indicated in the figure legends. For all tests, a P value ≤0.05 was considered significant.
RESULTS
Bile acid synthesis and feedback regulation in critically ill mice
Cumulative mortality at 7 days was 0% in healthy nutrient-restricted and healthy ad libitum fed controls, 11% for the surgical critically ill group (P = 0.036) and 31% for the septic critically ill group (P < 0.001) (Fig. 1). Septic critically ill animals had a significantly higher mortality than surgical critically ill animals (P = 0.043).
Plasma total BA concentrations in both surgical critically ill mice and septic critically ill mice were comparable at baseline to those in healthy nutrient-restricted controls and increased over time both in critically ill animals and in healthy nutrient-restricted controls when compared with healthy-fed controls (Fig. 2A). While the increase in plasma total BA concentrations was mainly due to a rise in conjugated BA in surgical critically ill mice and healthy nutrient-restricted mice, septic critically ill mice displayed an overall elevation in unconjugated BA (Sup. Table 5). This coincided with a decrease in mRNA expression of the conjugating enzymes BACS and BAAT in septic, but not in surgical critically ill mice (Sup. Fig. 3, E and F). In critically ill animals, BACS expression correlated inversely with both interleukin 1β (IL-1β) (R = −0.232, P = 0.01) and tumor necrosis factor α (TNFα) (R = −0.238, P = 0.009). Indeed, hepatic gene expression of cytokines TNFα and IL1β was only increased in the septic critically ill group (Sup. Fig. 4). Both critically ill groups showed signs of impaired secondary BA transformation, as the main secondary BA (deoxycholic acid) decreased over time in surgical and septic critically ill animals, but not in healthy nutrient-restricted and healthy-fed animals. In contrast to plasma BA concentrations, intrahepatic BA content was significantly increased to a similar extent in both critically ill groups from day-3 onward, while there was no effect of restricted nutrient intake in healthy mice on hepatic BA content (Fig. 2B).
Fig. 2.
Bile acid concentrations and synthesis enzymes.
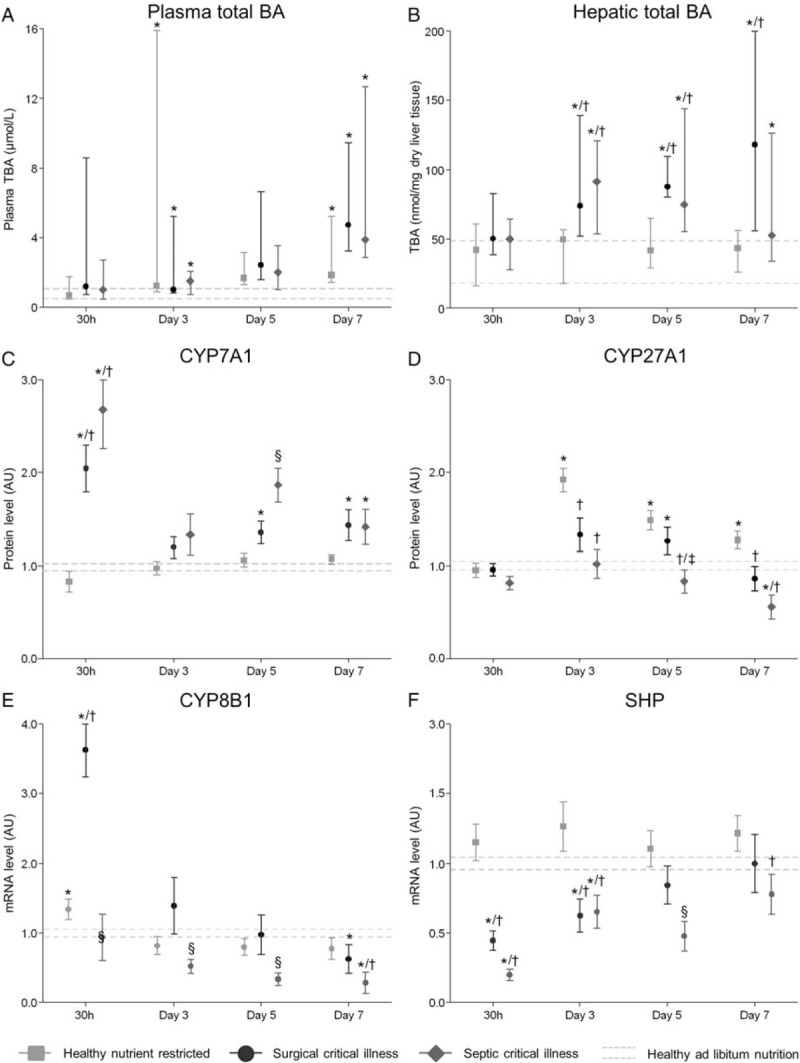
A, Total plasma bile acid pool in umol/L. This value represents the sum of UDCA, g-UDCA, t-UDCA, CA, g-CA, t-CA, CDCA, g-CDCA, t-CDCA, DCA, g-DCA, t-DCA, and LCA. Number of animals/group: Healthy ad libitum fed: 30 h: n = 11, Day-3: n = 15, Day-5: n = 9, Day-7: n = 12. Healthy caloric restricted: 30 h: n = 10, Day-3: n = 8, Day-5: n = 9, Day-7: n = 8. Surgical critical illness: 30 h: n = 14, Day-3: n = 11, Day-5: n = 10, Day-7: n = 8. Septic critical illness: 30 h: n = 14, Day-3: n = 12, Day-5: n = 10, Day-7: n = 11. B, Hepatic bile acid content in nmol/mg dry liver tissue. C, Relative protein expression of the rate limiting enzyme in bile acid synthesis CYP7A1. D, Relative protein expression of the key enzyme in the alternative BA secretion pathway CYP27A1. E, Relative gene expression of CYP8A1. F, Relative gene expression of SHP. A and B, Data are represented as median with IQR (25th—75th percentiles). The median and IQR of the healthy controls of all days are shown with the gray dashed lines. C–F, Data are represented as mean ± SEM. The mean ± SEM of the healthy-fed controls as average over all days are shown with the gray dashed lines. ∗P≤0.05 compared with healthy-fed controls, †P≤0.05 compared with healthy nutrient-restricted animals, §P≤0.05 compared with healthy-fed controls, healthy nutrient-restricted animals, and surgical critical illness. Number of animals/group B to F as stated in Figure 1.
Despite elevated intrahepatic BA levels, protein content of CYP7A1, the rate limiting enzyme in BA synthesis, was maintained and even acutely elevated in both critically ill groups compared with healthy nutrient-restricted mice. Protein content of CYP27A1, the key enzyme in the alternative BA synthetic pathway, was higher in healthy nutrient-restricted animals compared with both critically ill groups. However, compared with healthy-fed mice, levels were maintained or increased in healthy nutrient-restricted animals and in both critically ill groups, except for a downregulation on day-7 in the septic critically ill group (Fig. 2, C and D). Gene expression of these enzymes was acutely downregulated at 30 h in both critically ill groups, but normalized thereafter (Sup. Fig. 3, A and B). CYP8B1 gene expression displayed an acute increase at 30 h in the surgical critically ill group, but was downregulated thereafter in both critically ill groups (Fig. 2E). Gene expression of CYP7A1, CYP27A1, and CYP8B1 did not differ between healthy nutrient-restricted animals and healthy ad libitum fed mice.
The maintained to high protein content of enzymes that play a key role in BA synthesis in the critically ill mice was in line with an acute reduction in the gene expression of small heterodimer partner (SHP), a protein that suppresses BA synthesis (Fig. 2F). The synthesis of BA is normally mainly regulated by a network of nuclear receptors, of which the FXR-RXR heterodimer is considered the most important BA sensor and regulator. Remarkably, despite elevated intrahepatic BA levels compared with healthy nutrient-restricted controls, FXR and RXR immunostaining was clearly decreased in the hepatocytic nuclei of both critically ill groups already in the acute phase of critical illness (Fig. 3). Nutrient restriction in healthy mice did not affect nuclear FXR and RXR immunostaining. Gene expression of RXR and FXR was generally unaffected by critical illness and nutrient-restriction (Sup. Fig. 3, C and D).
Fig. 3.
Nuclear receptors.
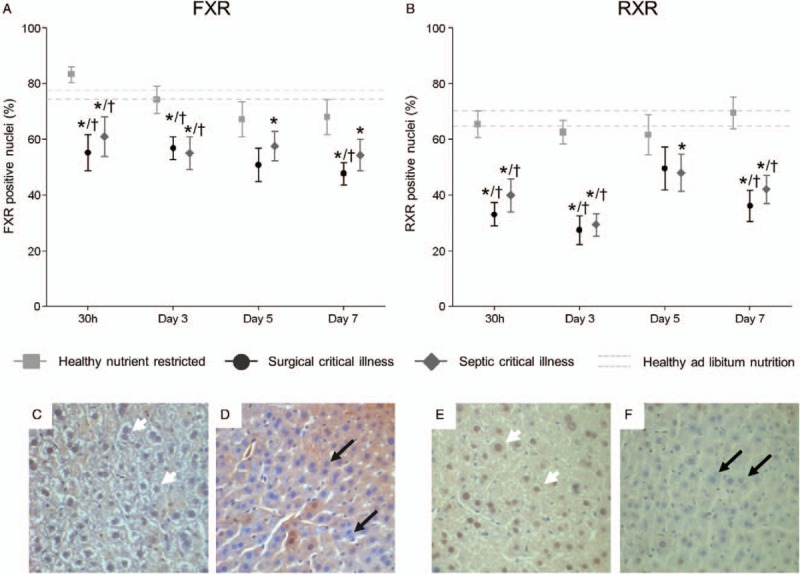
A and B, Immunostaining of FXR and RXR. Images were analyzed with an in-house made program in which nuclei were identified and assigned to be stained or unstained using a threshold, resulting in a percentage of stained nuclei. C and E, Representative IHC staining of a healthy ad libitum fed animal (day 3). D and F, Representative IHC staining of a septic critically ill animal (day 3). Nuclei with brown color, indicated by white arrowheads, indicate presence of the respective receptor (that accumulates in the nuclei). Blue nuclei, marked with black arrows, indicate absence of the receptor. FXR indicates farnesoid-X-receptor; RXR, retinoid-X-receptor. Data are represented as mean ± SEM. Data are represented as mean ± SEM. The mean ± SEM of the healthy-fed controls as average over all days are shown with the gray dashed lines. ∗P≤0.05 compared with healthy-fed controls, †P≤0.05 compared with healthy nutrient-restricted animals. Number of animals/group as stated in Figure 1.
Expression of hepatic bile acids transporters in critically ill mice
In normal, healthy conditions, BA are taken up from the intestine in the portal circulation and enter the hepatocyte via the basolateral situated influx pumps Na+-taurocholate cotransporting polypeptide (NTCP) and several isoforms of the organic anion transporting polypeptide (OATP) family. Compared with nutrient-restricted healthy controls, gene expression of NTCP and OATP's was acutely downregulated in both critically ill groups (Fig. 4, A and B). In the prolonged phase of critical illness, at day-7, gene expression of NTCP normalized, but OATP1a1 and OATP1b2 mRNA expression remained lower (Fig. 4, A and B and Sup. Fig. 5A). Nutrient restriction in healthy mice only partly mimicked alterations in hepatic BA uptake transporter expression observed in critically ill animals (Fig. 4 and Sup. Fig. 5).
Fig. 4.
Gene expression of hepatic bile acid transporters.
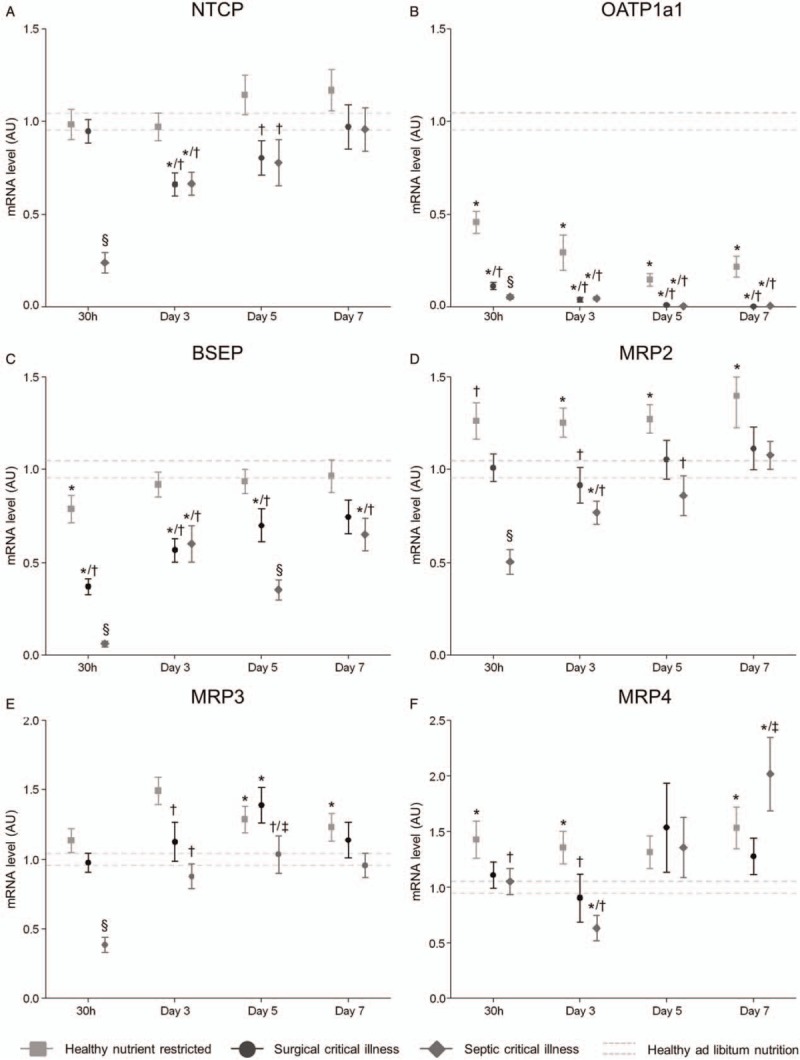
Relative gene expression of hepatic basolateral influx pumps (NTCP, OATP), canalicular export transporters (BSEP, MRP2), and basolateral efflux pumps (MRP3, MRP4) of healthy and critically ill animals at different time points. Data are represented as mean ± SEM. The mean ± SEM of the healthy-fed controls as average over all days are shown with the gray dashed lines. ∗P≤0.05 compared with healthy-fed controls, †P≤0.05 compared with healthy nutrient-restricted animals, ‡P≤0.05 compared with surgical critical illness, §P≤0.05 compared with healthy-fed controls, healthy nutrient-restricted animals, and surgical critical illness. Number of animals/group as stated in Figure 1. BSEP indicates bile acid-specific export pump; MRP, multidrug resistance-associated protein; NTCP, Na+-taurocholate co-transporting polypeptide; OATP, organic anion transporting polypeptide.
BA are normally excreted toward the bile canaliculi via the BA-specific export pump (BSEP) and the less specific multidrug resistance-associated protein 2 (MRP2). In addition to BA export, the multidrug resistance protein 2 (MDR2) adds phospholipids and the anion exchanger 2 (AE2) adds bicarbonate to the excreted bile. In both critically ill groups, mRNA of BSEP was acutely downregulated as compared with healthy nutrient-restricted controls and healthy-fed animals. During the course of critical illness, mRNA of BSEP increased, but remained significantly lower compared with healthy nutrient restricted animals (Fig. 4C). Only in septic critically ill mice, MRP2 mRNA levels were acutely decreased compared with healthy-fed and healthy-nutrient restricted animals, but increased to normal levels thereafter. Healthy nutrient-restricted animals had higher MRP2 mRNA levels than healthy-fed controls at all time-points (Fig. 4D). In contrast, expression of the phospholipid export pump MDR2 acutely increased and maintained thereafter at normal levels in surgical critically ill animals and healthy nutrient-restricted mice as compared with healthy-fed controls. Septic critically ill mice showed an overall decreased expression of MDR2. Overall, AE2 mRNA was maintained in both critically ill groups compared with nutrient-restricted animals, except for a transient decrease on day-3, and no effect of nutrient restriction was observed in healthy mice (Sup Fig. 5, B and C).
As an alternative to the normal canalicular export, BA can also be excreted toward the systemic circulation via the basolateral located efflux transporters multidrug resistance-associated protein 3 and 4 (MRP3, MRP4) and the Organic Solute Transporter (OST). MRP3, MRP4, and OSTβ mRNA was comparable to normal healthy-fed animals in the acute first days of critical illness, except for a transient decrease on day-1 (MRP3) and day 3 (MRP4) in the septic critically ill mice, and on day 1 (OSTβ) for the surgical critically ill mice. Expression of MRP3, MRP4, and OSTβ increased over time in both critically ill groups, an increase that was also observed in healthy-nutrient-restricted animals (Fig. 4, E and F and Sup Fig. 5D).
Macroscopic and microscopic characteristics of liver damage and regeneration in critically ill mice
The total wet liver weight decreased from day-3 onward in both critically ill groups and in nutrient-restricted animals, but this was most pronounced in the surgical critically ill animals. This was in line with the change in total body weight during the study period. In contrast, total dry weight of the liver was not affected by critical illness or nutrient restriction (Sup. Fig. 6). The basic microscopic structure was analyzed for signs of hemodynamic impairment, inflammation, and cell damage. We observed significantly more sinusoidal dilatation, indicating hypoperfusion, in both critically ill groups compared with healthy nutrient-restricted or fed controls (Fig. 5A–C and Sup. Fig. 7A). No difference in sinusoidal dilatation was observed between healthy-fed and healthy nutrient-restricted mice. Feathery appearance of hepatocyte cytoplasm can indicate the presence of intracellular glycogen storages. Compared with healthy nutrient-restricted animals, both critically ill groups showed a trend toward less feathery appearance, which was much more pronounced compared with healthy-fed animals (Fig. 5, D–F and Sup. Fig. 7B). Monocyte infiltration was rarely observed (data not shown). No overt signs of cell stress or cell damage, quantified by nuclear size and cellular ballooning, were observed in critically ill animals (data not shown).
Fig. 5.
Representative histological images of healthy ad libitum fed and critically ill animals.
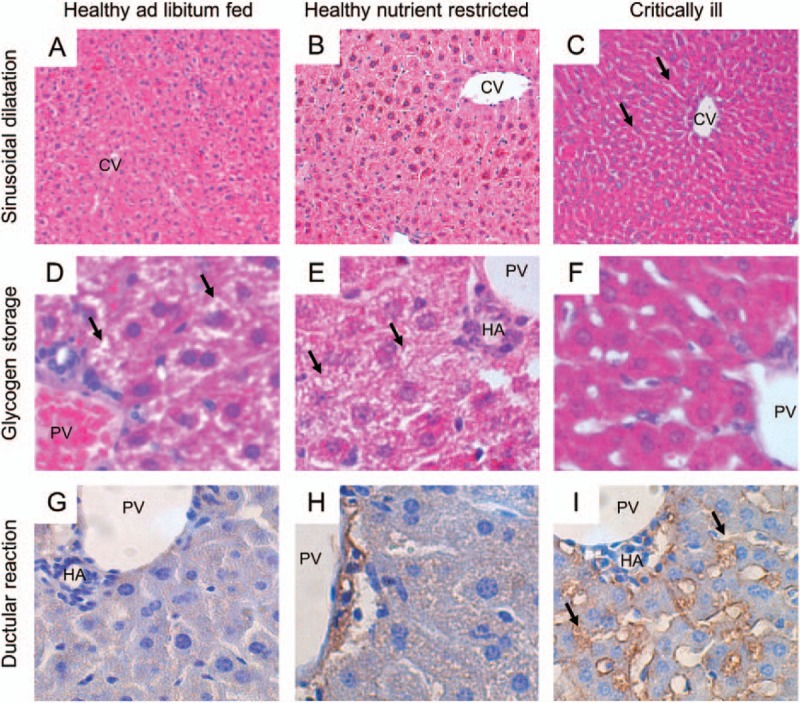
A–C, ×100 H&E-staining showing increased dilatation of sinusoids in critically ill mice (C), but not in ad libitum healthy-fed control animals (A) and healthy nutrient-restricted animals (B). D–F: ×400 H&E-staining, showing normal glycogen storages (black arrows) in ad libitum healthy-fed control mice (D), a mild decrease in healthy nutrient-restricted animals (E) and an extensive decrease in glycogen storages in critically ill animals (F). G–I, ×400 CK7-staining, showing increase in ductular reaction (black arrows) in critically ill animals (I) compared with healthy-fed control mice (G) and to a lesser extent in healthy nutrient-restricted animals (H). All images reflect animals sacrificed at day 3. Both septic and surgical critically ill animals showed similar histological changes, and are shown as one “critically ill group.” Semiquantitative scoring of these features is shown in SFig. 7. CV indicates central vein; HA, hepatic artery; PV, portal vein. Number of animals/group as stated in Figure 1.
CK-7 staining, indicating ductular reaction, was increased in both critically ill groups compared with healthy nutrient-restricted controls from day-3 onward, and was much more pronounced compared with healthy-fed animals from day-5 onward (Fig. 5, G–I and Sup. Fig. 7C). This was in line with the gene expression of augmenter of liver regeneration (ALR) and hepatic growth factor (HGF), which are involved in cell growth and liver regeneration. In both critically ill groups, mRNA expression of ALR and HGF acutely increased, but then decreased again to or below levels observed in healthy nutrient-restricted and healthy-fed animals (Sup. Fig. 8, A and C). Expression of epidermal growth factor, also involved in hepatocyte growth and liver regeneration, was similarly elevated in both critically ill groups and healthy nutrient-restricted animals from 30 h onward (Sup. Fig. 8B). In contrast, mRNA expression of transforming growth factor β, a potent growth inhibitor, remained low in surgical critically ill animals, but increased during the course of septic critical illness, except for a transient decrease on day 3 (Sup. Fig. 8D).
Human study: serum bile acid concentrations and other cholestatic markers in short- and long-stay critically ill patients evoked by sepsis or other causes
We studied a mixed ICU population of 1,114 patients with either short (≤3 days) or long (>3 days) ICU stay. With this population, we addressed whether admission values and early course of circulating BA levels differ between short- and long-stay critically ill patients.
Short-stay (n = 557) and long-stay (n = 557) patients were well matched for baseline risk factors and admission severity of illness markers and nutritional intake (Table 1 and Sup. Fig. 2). Although ICU and in hospital mortality was similar, the outcome factors indicating the degree of complicated ICU stay (new infections, requirement of hemodynamic support and renal replacement therapy, duration of hospital stay) were significantly better in short-stay than in long-stay patients (Table 1).
Table 1.
Baseline and outcome characteristics for the propensity matched short stay (≤3 days) and long stay (>3 days) patients
| Short stay (n = 557) | Long stay (n = 557) | P-value | |
| Baseline characteristics | |||
| Gender—male (n, %) | 364 (50.6%) | 355 (49.6%) | 0.6 |
| Age—years (median + IQR) | 65.1 ± 13.8 | 64.8 ± 14.4 | 0.7 |
| BMI—kg/m2 (median + IQR) | 25.8 [22.8–28.8] | 25.8 [23.0–29.1] | 0.6 |
| History diabetes (n, %) | 96 (17.2%) | 101 (18.1%) | 0.7 |
| History malignancy (n, %) | 91 (16.3%) | 102 (18.3%) | 0.4 |
| Pre-admission dialysis (n, %) | 6 (1.1%) | 5 (0.9%) | 0.8 |
| Emergency admission (n, %) | 188 (33.8%) | 198 (35.5%) | 0.5 |
| Apache-II score admission (median + IQR) | 21.1 ± 9.4 | 21.0 ± 8.9 | 0.8 |
| SOFA score 1st ICU day (median + IQR) | 7.7 ± 2.0 | 7.6 ± 2.0 | 0.5 |
| Randomization early parenteral administration (n, %) | 281 (50.4%) | 279 (50.1%) | 0.9 |
| Diagnostic category (n, %) | 0.9 | ||
| Complicated abdominal/pelvic surgery | 30 (5.4%) | 30 (5.4%) | |
| Other | 13 (2.3%) | 15 (2.7%) | |
| Cardiac surgery | 386 (69.3%) | 382 (68.6%) | |
| Cardiovascular disease | 2 (0.4%) | 1 (0.2%) | |
| Gastroenterological or hepatic disease | 7 (1.3%) | 10 (1.8%) | |
| Hematological or oncological disease | 2 (0.4%) | 2 (0.4%) | |
| Metabolic disorder | 0 (0%) | 0 (0%) | |
| Neurological disease | 0 (0%) | 0 (0%) | |
| Complicated neurosurgery | 12 (2.2%) | 15 (2.7%) | |
| Neurological presentation of medical disease | 2 (0.4%) | 6 (1.1%) | |
| Renal disease | 2 (0.4%) | 0 (0%) | |
| Respiratory disease | 12 (2.2%) | 13 (2.3%) | |
| Complicated pulmonary or esophageal surgery | 13 (2.3%) | 14 (2.5%) | |
| Transplantation | 46 (8.3%) | 36 (6.5%) | |
| Trauma, burns or reconstructive surgery | 15 (2.7%) | 18 (3.2%) | |
| Complicated vascular surgery | 15 (2.7%) | 15 (2.7%) | |
| Sepsis (n, %) | 71 (12.7%) | 79 (14.2%) | 0.5 |
| Infection (n, %) | 77 (13.8%) | 88 (15.8%) | 0.4 |
| CRP 1st day—mg/L (median + IQR) | 56.40 [36–84] | 59.60 [34–88] | 0.4 |
| blood glucose admission—mg/dL (median + IQR) | 139 [112–167] | 136 [112–161] | 0.3 |
| Outcome characteristics | |||
| ICU mortality (n, %) | 19 (3.4%) | 21 (3.8%) | 0.7 |
| ICU length of stay (median + IQR) | 2 [1–3] | 6 [4–12] | <0.001 |
| Hospital mortality (n, %) | 39 (7.0%) | 42 (7.5%) | 0.7 |
| Hospital length of stay (median + IQR) | 11 [8–18] | 20 [13–32] | <0.001 |
| New infection (n, %) | 7 (1.3%) | 236 (42.4%) | <0.001 |
| Ventilator support needed (n, %) | 538 (96.6%) | 540 (96.6%) | 0.7 |
| Hemodynamic support needed (n, %) | 437 (78.5%) | 492 (88.3%) | <0.001 |
| Renal replacement therapy needed (n, %) | 11 (2.0%) | 41 (7.4%) | <0.001 |
| Other liver parameters (median + IQR) | |||
| Bilirubin max 1st week—mg/dL | 0.85 [0.59–1.33] | 1.04 [0.74–1.68] | <0.001 |
| ALT max 1st week—IU/L | 19 [12–42] | 21 [14–40.75] | 0.2 |
| AST max 1st week—IU/L | 46 [32–89] | 50 [32–80] | 0.7 |
| GGT max 1st week—IU/L | 25 [14–56] | 39 [21–81] | <0.001 |
| ALP max 1st week—IU/L | 119 [86–167.25] | 159.50 [111–261] | <0.001 |
Data are represented as median with IQR (25th—75th percentiles) or number + percentage as appropriate.
CRP indicates C-reactive protein; ICU, intensive care unit.
Admission serum BA concentrations were significantly higher (P = 0.001) in short-stay patients compared with long-stay patients (Fig. 6A). However, the progression of BA over time differed significantly between the two groups: where BA gradually increased in long-stay patients, they decreased over time in short-stay patients (P = 0.001) (Fig. 6A). In patients who stayed in the ICU for at least 7 days, circulating BA increased further (Fig. 6B). In both short-stay and long-stay patients, a similar course in BA values was observed for patients with and without sepsis at ICU admission (data not shown).
Fig. 6.
Serum total bile acid concentrations of 1,114 critically ill patients with either short- or long-stay in ICU, matched for baseline risk factors.
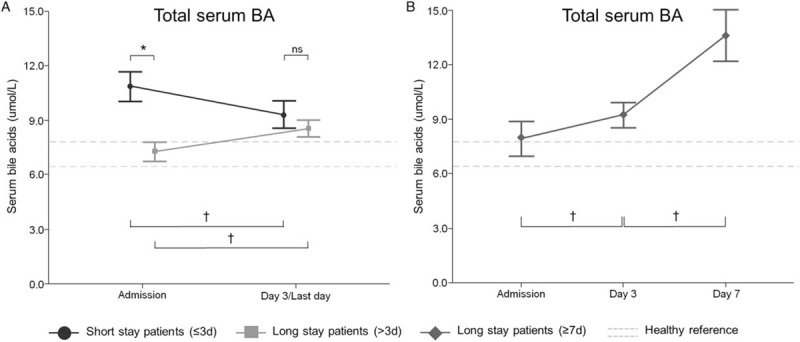
Left panel: black dots represent values of patients with an ICU stay ≤3 days (n = 557), whereas gray squares are presenting values from patients with an ICU stay >3 days (n = 557). Right panels: dark gray diamonds represent data from patients with an ICU ≥7 days (n = 254). Data are represented as mean ± SEM. Dashed gray lines show the mean ± SEM of healthy control subjects (n = 44) for total bile acids. ∗indicates P≤0.05 between patients with a length of stay ≤3 days and a length of stay >3 days. †indicates P≤0.05 between to sequential time points within a specific group. ICU indicates intensive care unit; ns, non-significant.
During the first week in ICU, plasma bilirubin and the other markers of cholestasis, GGT, and ALP peaked significantly lower in short-stay than in long-stay patients, whereas markers of hepatocyte lysis, ALT and AST were not different (Table 1).
DISCUSSION
We demonstrated in both mice and humans with sepsis and other types of critical illness a progressive increase in total BA over time in ICU. Despite increased circulating and hepatic BA levels, BA synthesis was maintained in both surgical and septic critically ill animals. The nuclear RXR-FXR system, which senses and controls BA synthesis, appeared to be switched off already in the early phase of critical illness. In addition, analysis of expression of BA transporters clearly suggested activation of alternative BA flow toward the systemic circulation during critical illness. No microscopic signs of hepatocellular stress or damage were observed, while signs of hypoperfusion and of ductular regeneration were clearly present during critical illness. Also, healthy nutrient-restricted animals showed higher circulating BA levels, and revealed similar hepatocellular transport alterations and histological alterations when compared with healthy ad libitum fed controls. In humans, while BA decreased after admission in short-stay patients, levels of BA increased with time in long-stay patients, this both in septic and in non-septic patients.
In our validated mouse model of acute and prolonged critical illness (9), we documented in detail the time course of circulating and hepatic BA levels, key enzymes in BA synthesis and regulation and BA transporters during critical illness of surgical and combined surgical and septic origin. Both circulating and intrahepatic BA increased over time in critically ill animals. However, conjugation of BA appeared to be lost in septic critical illness while still intact in surgical critical illness. Conversely, in a detailed analysis of the BA pool of critical ill patients, intact conjugation has been documented during septic shock and in postoperative patients and appears to remain intact (13). Surprisingly, even while intrahepatic and circulating BA increased above healthy reference values in critically ill animals, overall protein expression of rate-limiting enzymes involved in BA production was maintained or high. In parallel, gene expression of SHP, the main suppressor of CYP7A1 transcription, was low throughout critical illness. Similar changes have been reported to occur in non-surviving critically ill patients (7). Also, mainly primary BA were increased in the circulation, whereas circulating secondary BA decreased, suggesting that resorption and recirculation in the intestine is reduced.
This study indicates that an at least partial suppression of the negative feedback system, which regulates BA production, occurs early during both surgical and septic critical illness. The central cellular regulators in BA feedback are the nuclear receptors FXR and its obligatory heterodimer partner RXR. We observed a distinct reduction of FXR and RXR in the hepatocytic nucleus, where they normally exert their regulatory function via direct binding to DNA. As the nuclear decrease of FXR and RXR was already clearly evident on the first day of critical illness, this might explain the lack of suppression of BA synthesis. This observation was in line with in vitro studies showing a rapid reduction in nuclear abundance of RXR upon LPS or IL1β treatment with a concurrently transient appearance in the cytosolic compartment (14, 15). Also gene expression of nuclear receptors has shown to be decreased after LPS and cytokine challenge (16, 17). In our experiments, gene expression of RXR and FXR were not or only slightly affected by critical illness. Whether cytokines played an import role in our experiment is unclear, as we only observed increase in the hepatic TNFα and IL1β gene expression in septic critical illness but not in surgical critically ill mice. Also, we did not measure circulating cytokines. However, the observation that both surgical and septic critically ill animals overall displayed very similar changes in BA synthesis and feedback mechanisms suggests that these alterations are part of a general stress-induced response. Also in a human study on post-mortem liver biopsies of a mixed medical-surgical ICU population of which 50% was diagnosed with sepsis, markers of cholestasis were not associated with markers of inflammation (7).
In addition to the loss of feedback regulation, alterations in hepatic BA transporter expression also appear to contribute to the elevated circulating BA levels. First, the basolateral uptake transporters NTCP and OATPs were decreased. The marked downregulated expression of the canalicular export pump BSEP in both acute and protracted critical illness suggests a decrease in BA excretion toward the canalicular system and intestine. If BA secretion is reduced via the BSEP export pump, also bile flow through the other export transporters might be hampered. The observation that both AE2 and MDR2 expression were not decreased during surgical critical illness, suggests that protective mechanisms remain intact. Indeed, the canalicular transporters AE2 and MDR2 normally excrete a protective HCO3--film and phospholipids to protect the canalicular membrane and biliary epithelium against the potentially toxic biliary content. However, as MDR2 expression was overall decreased in septic critically ill mice, it suggests that this protective layer might be comprised during severe sepsis. Basolateral efflux pumps MRP3 and MRP4 were expressed at normal or high levels during the course of critical illness, implying that the alternative export route toward the systemic circulation was maintained. This was in line with previous studies of obstructive cholestasis (18), endotoxemia (19), and lethal critical illness (7), in which the basolateral efflux transporters are normal or even elevated in response to the induced cholestatic state. As we did not measure BA flux, we could not draw firm conclusions on the altered direction of the BA flow.
Despite elevated BA levels, no overt histological sings of liver injury were observed. Nevertheless, markers of liver growth and regeneration were acutely upregulated. Also, from day 3 onward, CK-7 staining increased in critical animals, indicating an increase in ductular reaction, which might indicate the formation of new bile canaliculi to facilitate canalicular bile flow (20, 21).
Importantly, the observed cholestatic alterations in critically ill animals were in part mimicked by a similar degree of nutrient restriction in healthy animals. The observed increase in circulating BA in prolonged nutrient-restriction was surprising, as one would rather expect a reduction due to reduced BA re-uptake in the intestine. Such a decrease in circulating BA was indeed observed in humans with life-style-induced weight loss (22). However, reduced intestinal BA re-uptake might also have reduced the expression of intestinal FGF15/19, a potent stimulator of bile acid homeostasis (23). In addition, the changes in circulating BA could be explained by variation in diurnal rhythm and timing of nutritional intake (8, 24). However, the increase in BA corresponded to an increased expression of basolateral export pumps. In contrast to circulating BA, nutrient restriction did not alter hepatic bile acid content. Markers of BA synthesis and of the RXR-FXR-feedback system were also not affected by nutrient restriction in healthy animals. This suggests that only a small part of the cholestatic alterations observed in critically ill patients, in particular the reversed BA transport, could be due to restricted nutrient intake. Also in human patients, reduced nutritional intake by withholding parenteral nutrition and tolerating a large caloric deficit during the first week in the ICU has shown to increase circulating bilirubin levels, similarly in septic and non-septic patients (6).
Despite the association of critical illness-induced cholestasis with poor outcomes, it remains unclear whether hyperbilirubinemia during critical illness is causally linked with adverse outcome. In fact, an association between mild hyperbilirubinemia and better clinical outcome has also been described (6). Notably, the current study demonstrated a more pronounced increase in circulating total BA in long-stay human patients only, whereas BA levels in short-stay patients decreased over time. In addition, in our animal study, we observed very similar changes in markers of BA synthesis, feedback receptors, and transport in both surgery-induced critical illness and in the more severe sepsis-induced critical illness, without overt histological sings of liver injury. Combined, these findings suggest that a maintained BA synthesis together with a reversed BA transport back toward the systemic circulation during critical illness might be a general and possibly beneficial process during critical illness to increase the circulating availability of bilirubin and BA. Moreover, BA or BA kinetics could potentially be useful as disease severity marker as it appears to reflect the underlying molecular alterations in the liver. Indeed, total bile acids predicted 28-day mortality independently of sex, age, serum bilirubin, and severity of illness (13). Undeniably, BA have a more broad role than merely to function in dietary lipid digestion and absorption, as they have shown to impact metabolism via affecting lipid and cholesterol regulation, glucose homeostasis, energy expenditure, and inflammation (25). While BA are currently explored as promising new drug targets for metabolic diseases, their role and regulation in the course of critical illness is not well investigated (26, 27). In addition, a mild elevation in circulating bilirubin, although known as a marker of liver dysfunction, may exert protective organ protective properties (28, 29). Indeed, via a positive feed-forward mechanism, even small amounts of bilirubin may exert known anti-inflammatory and anti-oxidative effects (30). Bilirubin has shown to be protective in various animal models including sepsis, by reducing intracellular metabolic and oxidative stress (29–32).
Alternatively, a diverted bile acid transport could be interpreted as a protective attempt of the hepatocyte to avoid excessively high intracellular levels of BA. High intracellular BA levels may cause cellular stress and cell death (33, 34). We observed significantly more ductular reaction in prolonged surgical and septic critically ill animals, suggesting increasing need for cellular regeneration. Also, higher peak ALP and GGT in human patients with prolonged ICU stay might indicate that ongoing critical illness could be harmful for the liver micro-environment. Indeed, elevated liver parameters might reflect ongoing hepatocyte injury that can progress in severe secondary liver pathology (35). Upregulating basolateral export transporters facilitates excretion of toxic compounds and could therefore be a protective response, as is seen in animal models of obstructive cholestasis (36). Disruption of the transport machinery leads to decreased excretion of waste products and hepatocellular accumulation of xenobiotics (37). Also absence of nuclear receptor FXR or RXR protects animals against cholestatic liver injury by altering BA synthesis and changing the hepatic transporter profile toward the systemic circulation (38–40). In contrast, while FXR absence might be protective in initial cholestatic disease, prolonged FXR absence is associated with the development of cell loss, lobular inflammation, and steatosis (41).
With this study, we have generated important new insights into the time course of cholestatic alterations induced by both septic and non-septic critical illnesses, both in the acute and prolonged phase of critical illness. However, an important limitation of the current study is its observational design. Importantly, our data indicate that these mouse models can be used to further investigate underlying mechanisms that are relevant for the human condition. Additional intervention studies or transgenic/knockout animal models will be required to provide detailed information on the exact—potential beneficial—role of the observed alterations in bile acid synthesis, transport, and nuclear feedback regulation. Importantly, although mice models are frequently used in critical care research, translation to the human setting should be done with caution, as the murine metabolic response to severe stress and illness can be quite different (42, 43). In our model of critical illness, animals were fluid-resuscitated and received antibiotics, pain medication, and partial nutritional support, but were not mechanically ventilated or hemodynamically supported with vasopressors. Also, although mature adult mice were used, we only studied male mice without visible comorbidities, which decreases generalizability to the human setting. Circulating BA levels increased much quicker in critically ill humans compared with mice, possibly also explained by a more prolonged disease onset in humans before admission to the ICU, whereas animals were healthy up until the start of the experiment. Additionally, of the studied human ICU patients, 69% were admitted to the ICU after cardiac surgery, which might prevent extrapolation of our findings to more mixed patient populations, although similar findings were observed in patients with and without sepsis.
In conclusion, in critically ill mice, hepatic and circulating BA levels increased already at an early stage of critical illness. In critical illness, whether evoked by surgery or sepsis, BA synthesis enzymes CYP7A1 and CYP27A1 were maintained or elevated, accompanied by a decrease in SHP. These changes were preceded by nuclear scarcity of the BA sensing nuclear receptors FXR and RXR. Apparently, already in the acute phase of critical illness, hepatocytes appear to rapidly switch off the BA sensors and regulating mechanisms. Simultaneously, the BA transport machinery in the hepatocyte appeared to be altered, with less expression of basolateral influx and canalicular efflux transporters and maintained or increased expression of basolateral efflux pumps. This suggests an overall BA transport toward the systemic circulation. These alterations were in part mimicked by nutrient restriction during health, which induced a similar increase as critical illness in circulating but not hepatic BA, corresponding to an increased expression of basolateral export pumps only and unaltered markers of BA synthesis and of the RXR-FXR-feedback system. These findings thus suggest a partial role of illness-induced lack of feeding in critical illness-induced cholestatic alterations. The increase in circulating BA over time was also observed in human ICU patients. Remarkably, patients with an early increase and higher admission BA were those who needed a shorter ICU stay. However, whether increased hepatic and circulating BA availability reflects a biochemical epiphenomenon or indicates an early adaptive and beneficial response to critical illness remains to be investigated.
Supplementary Material
Footnotes
This work is supported by Methusalem Program of the Flemish Government (GVdB and LL) via the KU Leuven University (METH/08/07); by an ERC Advanced grant (AdvG-2012-321670) to GVdB from the Ideas Program of the European Union 7th framework program.
The authors report no conflicts of interest.
REFERENCES
- 1.Thomson SJ, Cowan ML, Johnston I, Musa S, Grounds M, Rahman TM. ‘Liver function tests’ on the intensive care unit: a prospective, observational study. Intensive Care Med 35 8: 1406–1411, 2009. [DOI] [PubMed] [Google Scholar]
- 2.Kramer L, Jordan B, Druml W, Bauer P, Metnitz PG. Austrian Epidemiologic Study on Intensive Care ASG. Incidence and prognosis of early hepatic dysfunction in critically ill patients—a prospective multicenter study. Crit Care Med 35 4: 1099–1104, 2007. [DOI] [PubMed] [Google Scholar]
- 3.Jenniskens M, Langouche L, Vanwijngaerden YM, Mesotten D, Van den Berghe G. Cholestatic liver (dys) function during sepsis and other critical illnesses. Intensive Care Med 42 1: 16–27, 2016. [DOI] [PubMed] [Google Scholar]
- 4.Casaer MP, Mesotten D, Hermans G, Wouters PJ, Schetz M, Meyfroidt G, Van Cromphaut S, Ingels C, Meersseman P, Muller J, et al. Early versus late parenteral nutrition in critically ill adults. N Engl J Med 365 6: 506–517, 2011. [DOI] [PubMed] [Google Scholar]
- 5.Hermans G, Casaer MP, Clerckx B, Güiza F, Vanhullebusch T, Derde S, Meersseman P, Derese I, Mesotten D, Wouters PJ, et al. Effect of tolerating macronutrient deficit on the development of intensive-care unit acquired weakness: a subanalysis of the EPaNIC trial. Lancet Respir Med 1 8: 621–629, 2013. [DOI] [PubMed] [Google Scholar]
- 6.Vanwijngaerden YM, Langouche L, Brunner R, Debaveye Y, Gielen M, Casaer M, Liddle C, Coulter S, Wouters PJ, Wilmer A, et al. Withholding parenteral nutrition during critical illness increases plasma bilirubin but lowers the incidence of biliary sludge. Hepatology 60: 202–210, 2013. [DOI] [PubMed] [Google Scholar]
- 7.Vanwijngaerden YM, Wauters J, Langouche L, Vander Perre S, Liddle C, Coulter S, Vanderborght S, Roskams T, Wilmer A, Van den Berghe G, et al. Critical illness evokes elevated circulating bile acids related to altered hepatic transporter and nuclear receptor expression. Hepatology 54 5: 1741–1752, 2011. [DOI] [PubMed] [Google Scholar]
- 8.Zhang YK, Guo GL, Klaassen CD. Diurnal variations of mouse plasma and hepatic bile acid concentrations as well as expression of biosynthetic enzymes and transporters. PLoS One 6 2:e16683, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Derde S, Thiessen S, Goossens C, Dufour T, Van den Berghe G, Langouche L. Use of a central venous line for fluids, drugs and nutrient administration in a mouse model of critical illness. J Vis Exp 123, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Langouche L, Vanhorebeek I, Vlasselaers D, Vander Perre S, Wouters PJ, Skogstrand K, Hansen TK, Van den Berghe G. Intensive insulin therapy protects the endothelium of critically ill patients. J Clin Invest 115 8: 2277–2286, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Casaer MP, Hermans G, Wilmer A, Van den Berghe G. Impact of early parenteral nutrition completing enteral nutrition in adult critically ill patients (EPaNIC trial): a study protocol and statistical analysis plan for a randomized controlled trial. Trials 12:21, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rasmussen C WC: Documentation for GPML Matlab Code, availible at: http://www.gaussianprocess.org/gpml/code/ Accessed July 2017. [Google Scholar]
- 13.Horvatits T, Drolz A, Rutter K, Roedl K, Langouche L, Van den Berghe G, Fauler G, Meyer B, Hulsmann M, Heinz G, et al. Circulating bile acids predict outcome in critically ill patients. Ann Intensive Care 7 1:48, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ghose R, Zimmerman TL, Thevananther S, Karpen SJ. Endotoxin leads to rapid subcellular re-localization of hepatic RXRalpha: a novel mechanism for reduced hepatic gene expression in inflammation. Nucl Recept 2 1:4, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zimmerman TL, Thevananther S, Ghose R, Burns AR, Karpen SJ. Nuclear export of retinoid X receptor alpha in response to interleukin-1beta-mediated cell signaling: roles for JNK and SER260. J Biol Chem 281 22: 15434–15440, 2006. [DOI] [PubMed] [Google Scholar]
- 16.Kim MS, Shigenaga J, Moser A, Feingold K, Grunfeld C. Repression of farnesoid X receptor during the acute phase response. J Biol Chem 278 11: 8988–8995, 2003. [DOI] [PubMed] [Google Scholar]
- 17.Geier A, Dietrich CG, Voigt S, Kim S-K, Gerloff T, Kullak-Ublick GA, Lorenzen J, Matern S, Gartung C. Effects of proinflammatory cytokines on rat organic anion transporters during toxic liver injury and cholestasis. Hepatology 38 2: 345–354, 2003. [DOI] [PubMed] [Google Scholar]
- 18.Soroka CJ, Lee JM, Azzaroli F, Boyer JL. Cellular localization and up-regulation of multidrug resistance-associated protein 3 in hepatocytes and cholangiocytes during obstructive cholestasis in rat liver. Hepatology 33 4: 783–791, 2001. [DOI] [PubMed] [Google Scholar]
- 19.Cherrington NJ, Slitt AL, Li N, Klaassen CD. Lipopolysaccharide-mediated regulation of hepatic transporter mRNA levels in rats. Drug Metab Dispos 32 7: 734–741, 2004. [DOI] [PubMed] [Google Scholar]
- 20.Hirata K, Ikeda S, Honma T, Mitaka T, Furuhata T, Katsuramaki T, Hata F, Mukaiya M. Sepsis and cholestasis: basic findings in the sinusoid and bile canaliculus. J Hepatobiliary Pancreat Surg 8 1: 20–26, 2001. [DOI] [PubMed] [Google Scholar]
- 21.Lefkowitch JH. Bile ductular cholestasis: an ominous histopathologic sign related to sepsis and “cholangitis lenta”. Hum Pathol 13 1: 19–24, 1982. [DOI] [PubMed] [Google Scholar]
- 22.Biemann R, Penner M, Borucki K, Westphal S, Luley C, Ronicke R, Biemann K, Weikert C, Lux A, Goncharenko N, et al. Serum bile acids and GLP-1 decrease following telemetric induced weight loss: results of a randomized controlled trial. Sci Rep 6:30173, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Inagaki T, Choi M, Moschetta A, Peng L, Cummins CL, McDonald JG, Luo G, Jones SA, Goodwin B, Richardson JA, et al. Fibroblast growth factor 15 functions as an enterohepatic signal to regulate bile acid homeostasis. Cell Metab 2 4: 217–225, 2005. [DOI] [PubMed] [Google Scholar]
- 24.Setchell KD, Lawson AM, Blackstock EJ, Murphy GM. Diurnal changes in serum unconjugated bile acids in normal man. Gut 23 8: 637–642, 1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Keitel V, Kubitz R, Haussinger D. Endocrine and paracrine role of bile acids. World J Gastroenterol 14 37: 5620–5629, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thomas C, Pellicciari R, Pruzanski M, Auwerx J, Schoonjans K. Targeting bile-acid signalling for metabolic diseases. Nat Rev Drug Discov 7 8: 678–693, 2008. [DOI] [PubMed] [Google Scholar]
- 27.Schaap FG, Trauner M, Jansen PLM. Bile acid receptors as targets for drug development. Nat Rev Gastroenterol Hepatol 11 1: 55–67, 2014. [DOI] [PubMed] [Google Scholar]
- 28.Boon AC, Lam AK, Gopalan V, Benzie IF, Briskey D, Coombes JS, Fassett RG, Bulmer AC. Endogenously elevated bilirubin modulates kidney function and protects from circulating oxidative stress in a rat model of adenine-induced kidney failure. Sci Rep 5:15482, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maruhashi T, Soga J, Fujimura N, Idei N, Mikami S, Iwamoto Y, Kajikawa M, Matsumoto T, Kihara Y, Chayama K, et al. Hyperbilirubinemia, augmentation of endothelial function, and decrease in oxidative stress in Gilbert syndrome. Circulation 126 5: 598–603, 2012. [DOI] [PubMed] [Google Scholar]
- 30.Baranano DE, Rao M, Ferris CD, Snyder SH. Biliverdin reductase: a major physiologic cytoprotectant. Proc Natl Acad Sci U S A 99 25: 16093–16098, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Castilho ÁF, Aveleira CA, Leal EC, Simões NF, Fernandes CR, Meirinhos RI, Baptista FI, Ambrósio AF. Heme oxygenase-1 protects retinal endothelial cells against high glucose- and oxidative/nitrosative stress-induced toxicity. PLoS One 7 8:e42428, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zelenka J, Muchova L, Zelenkova M, Vanova K, Vreman HJ, Wong RJ, Vitek L. Intracellular accumulation of bilirubin as a defense mechanism against increased oxidative stress. Biochimie 94 8: 1821–1827, 2012. [DOI] [PubMed] [Google Scholar]
- 33.Schoemaker MH, Conde de la Rosa L, Buist-Homan M, Vrenken TE, Havinga R, Poelstra K, Haisma HJ, Jansen PL, Moshage H. Tauroursodeoxycholic acid protects rat hepatocytes from bile acid-induced apoptosis via activation of survival pathways. Hepatology 39 6: 1563–1573, 2004. [DOI] [PubMed] [Google Scholar]
- 34.Woolbright BL, Dorko K, Antoine DJ, Clarke JI, Gholami P, Li F, Kumer SC, Schmitt TM, Forster J, Fan F, et al. Bile acid-induced necrosis in primary human hepatocytes and in patients with obstructive cholestasis. Toxicol Appl Pharmacol 283 3: 168–177, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Leonhardt S, Veltzke-Schlieker W, Adler A, Schott E, Hetzer R, Schaffartzik W, Tryba M, Neuhaus P, Seehofer D. Trigger mechanisms of secondary sclerosing cholangitis in critically ill patients. Crit Care 19:131, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Donner MG, Keppler D. Up-regulation of basolateral multidrug resistance protein 3 (Mrp3) in cholestatic rat liver. Hepatology 34 2: 351–359, 2001. [DOI] [PubMed] [Google Scholar]
- 37.Recknagel P, Gonnert FA, Westermann M, Lambeck S, Lupp A, Rudiger A, Dyson A, Carré JE, Kortgen A, Krafft C, et al. Liver dysfunction and phosphatidylinositol-3-kinase signalling in early sepsis: experimental studies in rodent models of peritonitis. PLoS Med 9 11:e1001338, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wagner M, Fickert P, Zollner G, Fuchsbichler A, Silbert D, Tsybrovskyy O, Zatloukal K, Guo GL, Schuetz JD, Gonzalez FJ, et al. Role of farnesoid X receptor in determining hepatic ABC transporter expression and liver injury in bile duct-ligated mice. Gastroenterology 125 3: 825–838, 2003. [DOI] [PubMed] [Google Scholar]
- 39.Stedman C, Liddle C, Coulter S, Sonoda J, Alvarez JG, Evans RM, Downes M. Benefit of farnesoid X receptor inhibition in obstructive cholestasis. Proc Natl Acad Sci U S A 103 30: 11323–11328, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gyamfi MA, Wan YJ. Mechanisms of resistance of hepatocyte retinoid X receptor alpha-null mice to WY-14,643-induced hepatocyte proliferation and cholestasis. J Biol Chem 284 14: 9321–9330, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bjursell M, Wedin M, Admyre T, Hermansson M, Bottcher G, Goransson M, Linden D, Bamberg K, Oscarsson J, Bohlooly YM. Ageing Fxr deficient mice develop increased energy expenditure, improved glucose control and liver damage resembling NASH. PLoS One 8 5:e64721, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Esmon CT. Why do animal models (sometimes) fail to mimic human sepsis? Crit Care Med 32 5 suppl:S219–S222, 2004. [DOI] [PubMed] [Google Scholar]
- 43.Granger JI, Ratti PL, Datta SC, Raymond RM, Opp MR. Sepsis-induced morbidity in mice: effects on body temperature, body weight, cage activity, social behavior and cytokines in brain. Psychoneuroendocrinology 38 7: 1047–1057, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


