Abstract
Purpose of review
Hypovitaminosis C and vitamin C deficiency are very common in critically ill patients due to increased needs and decreased intake. Because vitamin C has pleiotropic functions, deficiency can aggravate the severity of illness and hamper recovery.
Recent findings
Vitamin C is a key circulating antioxidant with anti-inflammatory and immune-supporting effects, and a cofactor for important mono and dioxygenase enzymes. An increasing number of preclinical studies in trauma, ischemia/reperfusion, and sepsis models show that vitamin C administered at pharmacological doses attenuates oxidative stress and inflammation, and restores endothelial and organ function. Older studies showed less organ dysfunction when vitamin C was administered in repletion dose (2–3 g intravenous vitamin C/day). Recent small controlled studies using pharmacological doses (6–16 g/day) suggest that vitamin C reduces vasopressor support and organ dysfunction, and may even decrease mortality.
Summary
A short course of intravenous vitamin C in pharmacological dose seems a promising, well tolerated, and cheap adjuvant therapy to modulate the overwhelming oxidative stress in severe sepsis, trauma, and reperfusion after ischemia. Large randomized controlled trials are necessary to provide more evidence before wide-scale implementation can be recommended.
Keywords: antioxidant, critical care, multiple organ dysfunction, oxidative stress, vitamin C
INTRODUCTION
Many of us will associate vitamin C deficiency with sailors dying from scurvy during the ‘Age of Exploration.’ However, it has now become evident that vitamin C deficiency is certainly not a thing of the past, but develops during extreme circumstances. In fact, deficiency (normal plasma vitamin C levels ≥23 μmol/l) is frequently encountered in critically ill patients [1▪]. A recent study found that 88% of the septic patients had hypovitaminosis C (i.e. <23 μmol/l), whereas 38% had scurvy levels (i.e. <11 μmol/l) [2▪▪]. The physiologic importance of vitamin can be observed in pond turtles, which possess particularly high concentrations of vitamin C in the brain. These animals exhibit a high tolerance for oxygen depletion during diving, and vitamin C may help to prevent oxidative damage to neurons during re-oxygenation following a hypoxic period [1▪]. In analogy to these freshwater turtles [3], evidence suggests that vitamin C could protect patients against overwhelming oxidative stress, and that early administration might improve outcome [4].
Box 1.
no caption available
WHY IS VITAMIN C DEFICIENT?
The acute vitamin C deficiency is probably caused by increased metabolic consumption due to critically illness-induced oxidative stress with reduced recycling of dehydroascorbic acid (DHA), oxidized vitamin C. Most animals are capable of synthesizing vitamin C from glucose-6-phosphate, and their endogenous synthesis increases during stress [5]. However, humans have lost the ability to synthesize vitamin C due to mutations in the L-gulono-γ-lacton oxidase which encodes the terminal step of the vitamin C synthesis. During normal circumstances, dietary intake is sufficient to keep vitamin C levels within normal range. However, during critical illness, increased demands may lead to insufficiency. Lowest vitamin C levels are seen in patients with multiple organ failure [6], and plasma vitamin C levels are inversely related to sequential organ failure assessment (SOFA) scores and mortality [7]. Because vitamin C has pleiotropic functions, deficiency can aggravate the severity of illness and hamper recovery. Therefore repletion, and perhaps pharmacological dosing of vitamin C, could be an effective adjuvant therapy in conditions with excessive oxidative stress. Under these circumstances, it is essential that vitamin C is administered intravenously, because during critical illness, enteral uptake is both unpredictable and limited. In this narrative review, we will summarize the current knowledge of the pivotal role of vitamin C in critically ill patients. To meet this objective, we will discuss the clinical and preclinical studies published in the past 5 years investigating repletion and pharmacological dosing of intravenous vitamin C as adjuvant therapy in trauma, ischemia/reperfusion injury, and sepsis.
PATHOPHYSIOLOGY
In multiple critical conditions, such as trauma, ischemia/reperfusion injury, and sepsis, oxidative stress plays a crucial role. The original insult induces a progressive inflammatory response by activation of nuclear kappa-B with release of cytokines and production of multiple reactive oxygen species (ROS) by various inter-related pathways [8,9] (Fig. 1) and leading to uncoupling of mitochondrial phosphorylation, activation of nicotinamide adenine dinucleotide phosphate oxidase, lipoxygenase, cyclooxygenase and inducible nitric oxidase (iNOS), and oxidation of catecholamines. The abundance of ROS, especially when insufficiently opposed by antioxidants such as vitamin C, leads to cellular injury, widespread endothelial dysfunction, and progressive organ dysfunction.
FIGURE 1.
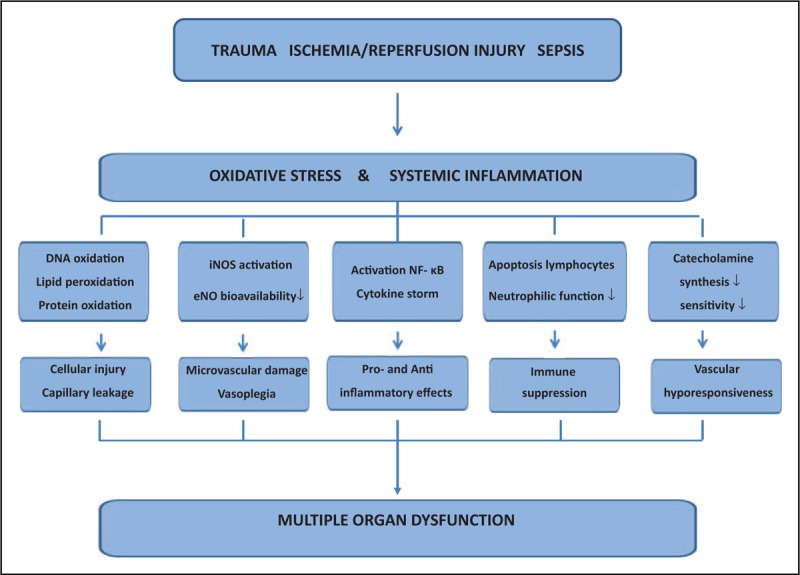
Pathophysiological pathways after a primary insult (trauma, ischemia/reperfusion injury, sepsis) which induces oxidative stress and systemic inflammation, and can lead to remote organ dysfunction. IL-1β, interleukin-1β; IL-6, interleukin-6; NF-κB, nuclear factor-κB; NO, nitric oxide; TNF-α, tumor necrosis factor-α.
WHY VITAMIN C COULD WORK
During critical illness, vitamin C has pleiotropic effects as key circulating antioxidant with anti-inflammatory and immune-supporting effects, and as cofactor for mono and dioxygenase enzymes (Table 1). All of these crucial functions are based on electron donation. The pleiotropic action could decrease cellular and organ injury.
Table 1.
Pleiotropic effects of vitamin C
| Pleiotropic effects | |
| Antioxidative | |
| Direct radical scavenger [10▪▪] | Superoxide Peroxynitrite |
| Reduction of ROS-production [11] | Inhibition of activation of NADPHoxidase |
| Inhibition of activation of xanthine oxidase | |
| Reduction of leakage of electrons from the dysfunctional electron transport chain in the mitochondria | |
| Inhibition of iNOS expression, preventing abundant NO production and peroxynitrite generation | |
| Regeneration of antioxidants [12] | α-tocopherol, protecting against lipid peroxidation |
| Glutathione | |
| Urate | |
| Tetrahydrobiopterin | |
| Anti-inflammatory | |
| Inhibition of NF-κB, reducing proinflammatory mediators | |
| Immune-supporting [13▪▪] | |
| Improvement of chemotaxis | |
| Stimulation interferon production | |
| Enhancement of neutrophilic bacterial killing | |
| Support of lymphocyte proliferation | |
| Modulating regulatory T-cells | |
| Inhibiting bacterial replication | |
| Production of host defence peptides [14] | |
| Cofactor/cosubstrate biosynthesis [15] | |
| Dopamine | Recycling BH4, cofactor of tyrosine hydroxylase |
| Norepinephrine | Cofactor dopamine β-hydroxylase |
| Vasopressin | Cofactor peptidylgylcine α-amidating monooxygenase |
| Serotonin | |
| Cortisol | |
| Collagen | Type IV collagen hydroxylation Oxidative protein folding |
| Increase of catecholamine sensitivity | |
| Binding to adrenergic receptors | |
| Protection microcirculation [11] | |
| Tightening endothelial barrier | Inhibition PP2A activation, increasing phosphorylated occludin crucial for maintenance of tight junctions Inhibition of apoptosis of endothelial progenitor cells |
| Improving microcirculatory patency | Inhibition of TNF-α -induced ICAM expression, reducing leucocyte stickiness and sludging |
| Decrease of endothelial permeability and protection against pathological vasoconstriction | Prevention eNOS uncoupling and eNO depletion |
| Improvement of wound healing [16] | |
eNO, endothelial nitric oxide; eNOS, endothelial nitric oxide synthase; NADPH, nicotinamide adenine dinucleotide phosphate oxidase.
PHARMACOKINETICS
Dietary vitamin C is absorbed via the saturable mucosal sodium-dependent vitamin C transporter 1 (SCVC1) of with maximum plasma vitamin C levels after enteral intake of 220 μmol/l in volunteers. Supraphysiological levels can only be attained by intravenous vitamin C supplementation [17]. Vitamin C is filtered by the glomerulus and actively reabsorbed in the proximal tubule (SVCT1). Urine excretion of vitamin C starts from plasma levels around 55 μmol/l. Tubular reabsorption is saturable. Vitamin C is actively transported into the tissue cells (SCVC2), leading to higher intracellular concentrations than in plasma, especially in activated leucocytes and in neurons protecting them against ROS. Vitamin C enters the brain both via SCVT2 and as DHA via glucose transporter 1.
In critically ill patients enteral uptake is insufficient to normalize plasma levels, due to saturable enteral transport, diminished mucosal function, and increased needs. Deficiency persists, despite receiving recommended intakes by (par) enteral nutrition [1▪,2▪▪,6]. To restore plasma levels in critically ill patients, a minimum of 2–3 g intravenous (i.v.) vitamin C is necessary [18▪▪,19]. With 10 g, and respectively, approximately 16 g i.v. vitamin C/day [18▪▪,20] plasma levels of above 1000 μmol/l can be achieved with a linear dose–response relationship [18▪▪].
PRECLINICAL STUDIES
Many studies investigating the use of pharmacological doses of vitamin C in preclinical models of sepsis and ischemia/reperfusion showed attenuation of oxidative stress and inflammation restoring endothelial and organ function, as we reviewed in 2014 [11]. Since then, several new relevant studies in preclinical models of trauma, ischemia/reperfusion injury, and sepsis were performed.
In a swine polytrauma model, i.v. vitamin C (50 and 200 mg/kg) significantly reduced proinflammatory mediators [interleukin (IL)-1, IL-8, and plasminogen activator inhibitor-1) and greatly reduced injury of the lung (hemorrhage, septal edema, and inflammation), liver, and kidney (microangiopathy and cellular infiltration) [21].
In a cardiac arrest rat model, vitamin C (50 and 100 mg/kg i.v.) decreased myocardial damage and improved neurological outcome and survival [22]. However, in another study 250 mg/kg vitamin C compromised resuscibility, possibly due to the much higher dose used [23]. In an isolated perfused rat heart and isolated cardiomyocytes vitamin C inhibited ROS generation, reduced infarct area, and improved cardiac function by reducing calcium overload and impeding the opening of the mitochondrial permeability transition pore [24]. In a renal ischemia/reperfusion model in rats, i.v. 50 mg/kg vitamin C reduced renal injury and preserved renal tissue, especially when combined with hydrocortisone [25].
In a cecal ligation/perforation sepsis model, multiple organ dysfunction and mortality were lower in wild-type mice compared to Gulo−/− knockout mice, which are incapable to synthesize vitamin C. Subcutaneous administration of 200 mg/kg vitamin C improved cellular immunosuppression and reduced organ dysfunction and mortality in both Gulo−/− and Gulo+/+ mice, supporting additional benefit of supraphysiological plasma vitamin C levels [26▪]. In human lung endothelial cells with lipopolysaccharide-induced hyperpermeability vitamin C enhanced endothelial barrier function only in combination with hydrocortisone [27▪].
CLINICAL STUDIES
As previously reviewed by us [1▪,11], two older studies in trauma patients [one randomized controlled trial (RCT) of 595 patients [28] and one before/after study in 4294 patients [29] using repletion doses of 3 gram i.v. vitamin C/day combined with vitamin E] showed reduced organ dysfunction, length of stay [28,29], and mortality [29]. In this population, no new clinical trials have been performed since. Similarly, in burn patients, no new clinical studies have been performed since 2014. That leaves us with two old small studies performed with huge doses of vitamin C (66 mg/kg/h for 24 h) which reduced fluid requirements and increased urine output [30,31].
With regard to ischemia/reperfusion injury no clinical studies have been performed in critically ill patients after cardiac arrest or stroke. Patients with ST-elevation myocardial infarction receiving i.v. vitamin C prior to percutaneous coronary intervention and achieving plasma levels above 1 mmol/l had significantly better left ventricular function and microvascular function than patients with levels below 1 mmol/l [32]. In a RCT of 532 patients, infusion of 3 g vitamin C before elective percutaneous coronary intervention showed a significant decrease of myocardial injury and blunted the increase of 8-hydroxy-2-deoxyguanosine – an oxidative stress marker [33].
Several recent clinical studies applying pharmacological doses of vitamin C focussed on sepsis. In a small RCT in 24 patients with severe sepsis and septic shock, 50 and 200 mg/kg/day reduced the inflammatory response, decreased circulating levels of cell-free and mitochondrial DNA, increased levels of antimicrobial proteins [14], and reduced the SOFA score, all to a larger extent in the 200 mg/kg/day group [20]. In second small RCT in 28 surgical ICU patients with septic shock, 100 mg i.v. vitamin C/kg/day reduced vasopressor requirements and mortality [34▪]. A very recent before and after study investigating the ‘cocktail’ of vitamin C, hydrocortisone, and thiamine amazed not only the critical care community but also the lay press due to the considerable effect on mortality (40 in the standard care group vs. 8% in the vitamin C/hydrocortisone/thiamine group). In addition, the duration of vasopressor therapy was significantly reduced (54.9 vs. 18.3 h) and SOFA score decreased faster. Importantly, kidney function was better in the vitamin C group, which is reassuring with regard to the potential risk of oxalate nephropathy. However, acknowledged limitations of the study are the low number of patients, the historical control group (although well matched), and the single-center design [35▪▪].
ADVERSE EFFECTS
Potential side effects of pharmacological doses of i.v. vitamin C are oxalate nephropathy, pro-oxidative activity, and factitious hyperglycemia in point- of-care glucose measurements [36].
Vitamin C is mainly metabolized (reversibly) to DHA and (when not recycled) subsequently to 2,3-diketo-1-gulonic acid and oxalate (Fig. 2). Oxalate is excreted in the urine. In primary hyperoxaluria, oxalate nephropathy takes months to years to develop. Prolonged supplementation of high-dose vitamin C can increase the risk of oxalate nephropathy. In the recent controlled studies in critically ill patients with high doses for a short period, no oxalate nephropathy was reported [1▪], and kidney function even improved [35▪▪]. Few case reports describe oxalate nephropathy in burn patients after vitamin C administration (101 and 224 g <24 h) much higher than used in most clinical trials [36].
FIGURE 2.
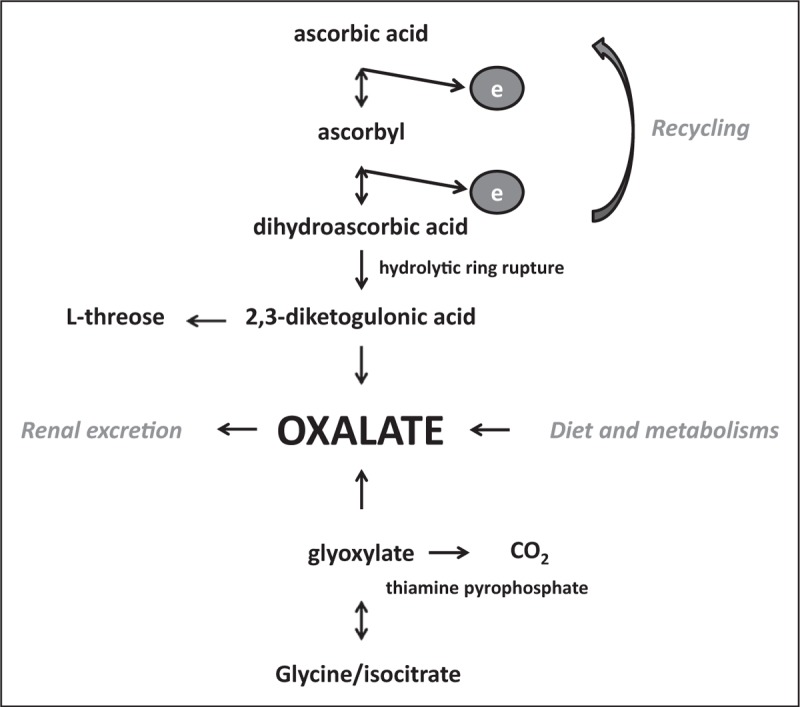
Pathways of oxalate biosynthesis.
High concentrations of vitamin C can affect blood glucose measurements by some, not all, point-of-care glucometers, leading to falsely higher results [37]. This can lead to hypoglycemia if aggressive insulin therapy is erroneously applied. Therefore, glucose measurements after the administration of pharmacological doses of vitamin C should be performed at the central laboratory or with blood gas analysis [38].
Vitamin C can induce pro-oxidative effects. When donating one electron to ROS, vitamin C converts to the ascorbate radical, which can be pro-oxidant, but frequently has substituted a much stronger and unstable radical. Furthermore, vitamin C can reduce the ion status of metals like copper and iron, and by the Fenton reaction lead to production of superoxide and hydrogen peroxide. A recent review showed that vitamin C in vivo predominantly reduced oxidative damage, despite the described in-vitro pro-oxidative effects [39]. High-dose i.v. vitamin C should be avoided in patients with glucose-6-phosphate deficiency, who can develop hemolysis.
SYNERGISM WITH THIAMINE AND HYDROCORTISONE
Vitamin C can reverse the oxidation of the glucocorticoid receptor and restore the activity of glucocorticoids. Vice versa, glucocorticoids stimulate the expression of SVCT2, which actively transports vitamin C into the tissue cells, but is down-regulated in sepsis.
Thiamine deficiency frequently occurs in septic patients due to increased consumption [40] and can enhance reduction of glyoxylate to oxalate instead of oxidation to CO2 (Fig. 2), increasing hyperoxaluria. In addition, thiamine is an important cofactor in the energy metabolism, and thiamine deficiency causes mitochondrial dysfunction and oxidative stress [41,42]. Supplementation of thiamine could reduce hyperoxaluria and act synergistically with hydrocortisone and vitamin C to reduce organ dysfunction and improve outcome [35▪▪].
DISCUSSION
When considering all the available evidence, i.v. vitamin C – in repletion dose, but maybe even more in pharmacological dose – is a promising potential adjuvant therapy for critical illnesses with increased oxidative stress, such as trauma, ischemia/reperfusion injury, and sepsis. There is a good pathophysiological rationale supported by an increasing number of (pre)clinical studies.
Preclinical studies, however, do not show univocal results, and the clinical studies are mostly small, single-centered, and not all are randomized. Doses differ widely and are often combined with other antioxidants or hydrocortisone. Therefore, requests for immediate wide-scale implementation of vitamin C in pharmacological doses should not be honored at this time. Several large, double-blinded RCTs in the sepsis population are under way, but other ICU populations might benefit, such as patients after cardiac arrest, trauma, or burn. They are necessary to provide more evidence.
If vitamin C does indeed benefit critically ill patients, there still remain many unanswered questions such as the follows:
-
(1)
Is my patient deficient? First of all, we need a reliable screening of vitamin C status for clinical practice. At present, determination of plasma vitamin C levels is complicated and expensive, and not available for daily practice.
-
(2)
Should we provide repletion or pharmacological doses? There are no comparative studies showing that 1.5 g q 6 hourly is optimal as was recently suggested [35▪▪]. Because pharmacological doses are possibly needed to optimize the antioxidant effects of vitamin C [43], the clinical effect of these doses might be stronger [20,32,34▪,35▪▪]. However, large studies on side effects are not available.
-
(3)
What is the optimal timing and duration of supplementation? Probably, vitamin C should be administered as soon as possible after the primary insult, when oxidative stress is maximal. The optimal duration probably differs per patient. We think that high-dose vitamin C should be stopped after the acute phase to allow the beneficial signaling function of ROS necessary for cell survival.
-
(4)
Should we use bolus or continuous administration? The antioxidant effect is dose-related, thus temporarily high peaks might be beneficial.
-
(5)
What is the relevance of co-administration of thiamine and hydrocortisone? Pathophysiologically, this is plausible in septic patients, but no data are available for patients after trauma or cardiac arrest. At this moment, at least eight RCTs are being performed with either vitamin C alone, or combined with hydrocortisone or thiamine (ClinicalTrials.gov), which will answer some of these questions.
CONCLUSION
Many critically ill patients, especially those exhibiting oxidative stress, are vitamin C-deficient, because needs are increased and intake diminished. An intravenous dose of 2–3 g/day is needed to restore normal plasma concentrations, as enteral uptake is insufficient due to maximum enteral absorption capacity. Several older studies suggest less organ failure when vitamin C is administered in repletion dose. Some recent small controlled studies suggest that vitamin C used in pharmacological doses (6–16 g/day) reduces vasopressor support, fastens recovery from organ failure, and may even reduce mortality. In addition, it may, despite increased oxalate excretion, even improve kidney function. Whether the combination with thiamine and hydrocortisone is even more beneficial needs to be confirmed. Large RCTs are necessary to provide more evidence and safety data before wide-scale implementation can be recommended. Up to now, a short course of vitamin C seems well tolerated, making this cheap and widely available substance extremely promising to modulate the overwhelming oxidative stress in severe sepsis, trauma, and reperfusion after ischemia.
Acknowledgements
None.
Financial support and sponsorship
None.
Conflicts of interest
The authors have received a research grant of the Netherlands Organisation of Health Research and Development to perform a randomized controlled trial on the effect of high-dose vitamin C in postcardiac arrest syndrome.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
▪ of special interest
▪▪ of outstanding interest
REFERENCES
- 1▪.Amrein K, Oudemans-van Straaten HM, Berger MM. Vitamin therapy in critically ill patients: focus on thiamine, vitamin C, and vitamin D. Intensive Care Med 2018; doi: 10.1007/s00134-018-5107-y [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]; A recent high-quality review of the studies in intensive care patients reporting vitamin C plasma levels and the controlled studies on the effect of vitamin C in critically ill patients.
- 2▪▪.Carr AC, Rosengrave PC, Bayer S, et al. Hypovitaminosis C and vitamin C deficiency in critically ill patients despite recommended enteral and parenteral intakes. Crit Care 2017; 21:300. [DOI] [PMC free article] [PubMed] [Google Scholar]; An important study describing hypovitaminosis C and vitamin C deficiency in critically ill patients.
- 3.Rice ME, Lee EJ, Choy Y. High levels of ascorbic acid, not glutathione, in the CNS of anoxia-tolerant reptiles contrasted with levels in anoxia-intolerant species. J Neurochem 1995; 64:1790–1799. [DOI] [PubMed] [Google Scholar]
- 4.Spoelstra-de Man AME, Elbers PWG, Oudemans-van Straaten HM. Making sense of early high-dose intravenous vitamin C in ischemia/reperfusion injury. Crit Care 2018; 22:70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nakano K, Suzuki S. Stress-induced change in tissue levels of ascorbic acid and histamine in rats. J Nutr 1984; 114:1602–1608. [DOI] [PubMed] [Google Scholar]
- 6.Borrelli E, Roux-Lombard P, Grau GE, et al. Plasma concentrations of cytokines, their soluble receptors, and antioxidant vitamins can predict the development of multiple organ failure in patients at risk. Crit Care Med 1996; 24:392–397. [DOI] [PubMed] [Google Scholar]
- 7.Grooth HJ, Spoelstra-de Man AME, Oudemans-van Straaten HM. Early plasma vitamin C concentration, organ dysfunction and ICU mortality. Intensive Care Med 2014; 40 10 Suppl 1:S199. [Google Scholar]
- 8.Lenz A, Franklin GA, Cheadle WG. Systemic inflammation after trauma. Injury 2007; 38:1336–1345. [DOI] [PubMed] [Google Scholar]
- 9.Berger MM, Oudemans-van Straaten HM. Vitamin C supplementation in the critically ill patient. Curr Opin Clin Nutr Metab Care 2015; 18:193–201. [DOI] [PubMed] [Google Scholar]
- 10▪▪.Padayatty SJ, Levine M. Vitamin C: the known and the unknown and Goldilocks. Oral Dis 2016; 22:463–493. [DOI] [PMC free article] [PubMed] [Google Scholar]; An excellent and extensive review describing the general aspects of vitamin C biology.
- 11.Oudemans-van Straaten HM, Spoelstra-de Man AM, de Waard MC. Vitamin C revisited. Crit Care 2014; 18:460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Buettner GR, Jurkiewicz BA. Catalytic metals, ascorbate and free radicals: combinations to avoid. Radiat Res 1996; 145:532–541. [PubMed] [Google Scholar]
- 13▪▪.Carr AC, Maggini S. Vitamin C and immune function. Nutrients 2017; 9: [DOI] [PMC free article] [PubMed] [Google Scholar]; An excellent review that depicts the various roles of vitamin C in the immune system, including barrier integrity and leukocyte function, and discusses the relevance of the immune-modulating effects of vitamin C in the context of infections and conditions leading to vitamin C insufficiency.
- 14.Natarajan RFBJ, Syed AA, Fowler AA. Impact of intravenous ascorbic acid infusion on novel biomarkers in patients with severe sepsis. J Pulm Respir Med 2014; 4:214. [Google Scholar]
- 15.Carr AC, Shaw GM, Fowler AA, Natarajan R. Ascorbate-dependent vasopressor synthesis: a rationale for vitamin C administration in severe sepsis and septic shock? Crit Care 2015; 19:418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mohammed BM, Fisher BJ, Kraskauskas D, et al. Vitamin C promotes wound healing through novel pleiotropic mechanisms. Int Wound J 2016; 13:572–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Padayatty SJ, Sun H, Wang Y, et al. Vitamin C pharmacokinetics: implications for oral and intravenous use. Ann Intern Med 2004; 140:533–537. [DOI] [PubMed] [Google Scholar]
- 18▪▪.de Grooth HJ, Manubulu-Choo WP, Zandvliet AS, et al. Vitamin-C pharmacokinetics in critically ill patients: a randomized trial of four intravenous regimens. Chest 2018; doi: 10.1016/j.chest.2018.02.025 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]; A clinically very relevant study investigating the pharmacokinetics of two different doses of intravenously administered vitamin C.
- 19.Long CL, Maull KI, Krishnan RS, et al. Ascorbic acid dynamics in the seriously ill and injured. J Surg Res 2003; 109:144–148. [DOI] [PubMed] [Google Scholar]
- 20.Fowler AA, III, Syed AA, Knowlson S, et al. Phase I safety trial of intravenous ascorbic acid in patients with severe sepsis. J Transl Med 2014; 12:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reynolds PS, Fisher BJ, McCarter J, et al. Interventional vitamin C: a strategy for attenuation of coagulopathy and inflammation in a swine polytrauma model. J Trauma Acute Care Surg 2018; doi: 10.1097/TA.0000000000001844 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 22.Tsai MS, Huang CH, Tsai CY, et al. Combination of intravenous ascorbic acid administration and hypothermia after resuscitation improves myocardial function and survival in a ventricular fibrillation cardiac arrest model in the rat. Acad Emerg Med 2014; 21:257–265. [DOI] [PubMed] [Google Scholar]
- 23.Motl J, Radhakrishnan J, Ayoub IM, et al. Vitamin C compromises cardiac resuscitability in a rat model of ventricular fibrillation. Am J Ther 2014; 21:352–357. [DOI] [PubMed] [Google Scholar]
- 24.Hao J, Li WW, Du H, et al. Role of vitamin C in cardioprotection of ischemia/reperfusion injury by activation of mitochondrial KATP channel. Chem Pharm Bull (Tokyo) 2016; 64:548–557. [DOI] [PubMed] [Google Scholar]
- 25.Azari O, Kheirandish R, Azizi S, et al. Protective effects of hydrocortisone, vitamin C and E alone or in combination against renal ischemia-reperfusion injury in rat. Iran J Pathol 2015; 10:272–280. [PMC free article] [PubMed] [Google Scholar]
- 26▪.Gao YL, Lu B, Zhai JH, et al. The parenteral vitamin c improves sepsis and sepsis-induced multiple organ dysfunction syndrome via preventing cellular immunosuppression. Mediators Inflamm 2017; 2017:4024672. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]; A very interesting preclinical study using GULO−/− knockout mice supporting additional benefit of pharmacological doses of vitamin C.
- 27▪.Barabutis N, Khangoora V, Marik PE, Catravas JD. Hydrocortisone and ascorbic acid synergistically prevent and repair lipopolysaccharide-induced pulmonary endothelial barrier dysfunction. Chest 2017; 152:954–962. [DOI] [PMC free article] [PubMed] [Google Scholar]; An interesting preclinical study showing the synergistic effect of hydrocortison and vitamin C in a in-vitro model.
- 28.Nathens AB, Neff MJ, Jurkovich GJ, et al. Randomized, prospective trial of antioxidant supplementation in critically ill surgical patients. Ann Surg 2002; 236:814–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Collier BR, Giladi A, Dossett LA, et al. Impact of high-dose antioxidants on outcomes in acutely injured patients. JPEN J Parenter Enteral Nutr 2008; 32:384–388. [DOI] [PubMed] [Google Scholar]
- 30.Tanaka H, Matsuda T, Miyagantani Y, et al. Reduction of resuscitation fluid volumes in severely burned patients using ascorbic acid administration: a randomized, prospective study. Arch Surg 2000; 135:326–331. [DOI] [PubMed] [Google Scholar]
- 31.Kahn SA, Beers RJ, Lentz CW. Resuscitation after severe burn injury using high-dose ascorbic acid: a retrospective review. J Burn Care Res 2011; 32:110–117. [DOI] [PubMed] [Google Scholar]
- 32.Valls N, Gormaz JG, Aguayo R, et al. Amelioration of persistent left ventricular function impairment through increased plasma ascorbate levels following myocardial infarction. Redox Rep 2016; 21:75–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang ZJ, Hu WK, Liu YY, et al. The effect of intravenous vitamin C infusion on periprocedural myocardial injury for patients undergoing elective percutaneous coronary intervention. Can J Cardiol 2014; 30:96–101. [DOI] [PubMed] [Google Scholar]
- 34▪.Zabet MH, Mohammadi M, Ramezani M, Khalili H. Effect of high-dose Ascorbic acid on vasopressor's requirement in septic shock. J Res Pharm Pract 2016; 5:94–100. [DOI] [PMC free article] [PubMed] [Google Scholar]; A promising pilot RCT of pharmacological dose vitamin C in septic shock.
- 35▪▪.Marik PE, Khangoora V, Rivera R, et al. Hydrocortisone, Vitamin C and thiamine for the treatment of severe sepsis and septic shock: a retrospective before-after study. Chest 2017; 151:1229–1238. [DOI] [PubMed] [Google Scholar]; A very important before and after study in septic patients showing an amazing effect of the cocktail of pharmacological dose vitamin C, hydrocortisone, and thiamine on mortality.
- 36.Buehner M, Pamplin J, Studer L, et al. Oxalate nephropathy after continuous infusion of high-dose vitamin C as an adjunct to burn resuscitation. J Burn Care Res 2016; 37:e374–e379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cho J, Ahn S, Yim J, et al. Influence of vitamin C and maltose on the accuracy of three models of glucose meters. Ann Lab Med 2016; 36:271–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Flannery AH, Bastin MLT, Magee CA, Bensadoun ES. Vitamin C in sepsis: when it seems too sweet, it might (literally) be. Chest 2017; 152:450–451. [DOI] [PubMed] [Google Scholar]
- 39.Carr A, Frei B. Does vitamin C act as a pro-oxidant under physiological conditions? FASEB J 1999; 13:1007–1024. [DOI] [PubMed] [Google Scholar]
- 40.Donnino MW, Andersen LW, Chase M, et al. Randomized, double-blind, placebo-controlled trial of thiamine as a metabolic resuscitator in septic shock: a pilot study. Crit Care Med 2016; 44:360–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu D, Ke Z, Luo J. Thiamine deficiency and neurodegeneration: the interplay among oxidative stress, endoplasmic reticulum stress, and autophagy. Mol Neurobiol 2017; 54:5440–5448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Abdou E, Hazell AS. Thiamine deficiency: an update of pathophysiologic mechanisms and future therapeutic considerations. Neurochem Res 2015; 40:353–361. [DOI] [PubMed] [Google Scholar]
- 43.Jackson TS, Xu A, Vita JA, Keaney JF., Jr Ascorbate prevents the interaction of superoxide and nitric oxide only at very high physiological concentrations. Circ Res 1998; 83:916–922. [DOI] [PubMed] [Google Scholar]


