Abstract:
Gemcabene, a late-stage clinical candidate, has shown efficacy for LDL-C, non-HDL cholesterol, apoB, triglycerides, and hsCRP reduction, all risk factors for cardiovascular disease. In rodents, gemcabene showed changes in targets, including apoC-III, apoA-I, peroxisomal enzymes, considered regulated through peroxisome proliferator–activated receptor (PPAR) gene activation, suggesting a PPAR-mediated mechanism of action for the observed hypolipidemic effects observed in rodents and humans. In the current study, the gemcabene agonist activity against PPAR subtypes of human, rat, and mouse were compared with known lipid lowering PPAR activators. Surprisingly, gemcabene showed no or little PPAR-α transactivation compared with reference agonists, which showed concentration-dependent transactivation against human PPAR-α of 2.4- to 30-fold (fenofibric acid), 17-fold (GW590735), and 2.3- to 25-fold (WY-14643). These agents also showed robust transactivation of mouse and rat PPAR-α in a concentration-dependent manner. The known PPAR-δ agonists, GW1516, L165041, and GW0742, showed potent agonist activity against human, mouse, and rat receptors (ranging from 165- to 396-fold). By contrast, gemcabene at the highest concentration tested (300 μM) showed no response in mouse and rat and a marginal response against human PPAR-δ receptors (3.2-fold). For PPAR-γ, gemcabene showed no agonist activity against all 3 species at 100 μM and marginal activity (3.6- to 5-fold) at 300 μM. By contrast, the known agonists, rosiglitazone, indomethacin, and muraglitazar showed strong activation against the mouse, rat, and human PPAR-γ receptors. No clear antagonist activity was observed with gemcabene against any PPAR subtypes for all 3 species over a wide range of concentrations. In summary, the transactivation studies rule out gemcabene as a direct agonist or antagonist of PPAR-α, PPAR-γ, and PPAR-δ receptors of these 3 species. These data suggest that the peroxisomal effects observed in rodents and the lipid regulating effects observed in rodents and humans are not related to a direct activation of PPAR receptors by gemcabene.
Key Words: gemcabene, PPAR-α, PPAR-δ, PPAR-γ, transactivation, nuclear hormone receptors
INTRODUCTION
Cardiovascular diseases (CVDs) constitute a broad number of conditions including heart and vascular disease, atherosclerosis, stroke, and hypertension and are the leading cause of global morbidity and mortality.1,2 Both genetic and environmental factors contribute to dyslipidemia and type 2 diabetes and can increase the risk for CVD.1,3–5 The discovery that peroxisome proliferator–activated receptors (PPARs) are key regulators of metabolic pathways that have led to significant research, drug discovery, and understanding of the mechanisms of action (MOA) of PPAR receptors and their implications for the prevention and treatment of metabolic disorders and CVD.6–9 Ligand-activated nuclear receptors have been exploited as treatments for the regulation of lipid and glucose metabolism to treat and reduce the risk of diabetes and CVD.4,8–10
PPARs are ligand-activated transcription factors belonging to the nuclear receptor super family.11 PPARs are type II nuclear receptors containing a cysteine-rich Zn finger–motif DNA-binding domain.12,13 The subtypes PPARα, PPARγ, and PPARδ (also known as PPARβ)14 differ with respect to their tissue distribution and distinct roles in glucose and lipid homeostasis15–19 and energy homeostasis,19–22 as well as in other cellular functions.23–30 PPAR-α receptors are involved in ensuring energy availability during fasting with a key role in starvation.31 Their state of activation in the liver is regulated by dietary fatty acids and during fasting by fatty acids generated through de novo lipogenesis.32–34 In humans, these receptors play a broader role in lipid metabolism,32,35 regulating apolipoprotein (apo) genes such as apoA-I, apoA-II, apoA-V, and apoC-III, fatty acid ß-oxidation genes such as acyl CoA oxidase, CPT-I, and CPT-II, and fatty acyl CoA desaturase genes (eg, delta-6-desaturase) involved in fatty acid interconversion,36,37 phospholipid transfer protein involved in HDL metabolism, and HMGCoA synthase 2 (HMGCS2) involved in ketone body synthesis.36,37 Many physiological effects of PPAR-δ activators overlap with those of PPAR-α activators.
Once the PPAR nuclear receptors were molecularly characterized and it was realized that fibrates and thiazolidinediones are PPAR ligands, an enormous effort was made within the pharmaceutical industry to create a large number of synthetic, specific, and potent direct PPAR activating molecules. However, there are also natural physiological ligands that can trigger PPAR activation. For instance, there is a natural importance for providing fatty acid ligands to activate PPAR-α,31,32,34 thereby converting energy balance from a lipogenic to a ketogenic state in extended fasting periods.
Gemcabene lipid–regulating activities were discovered after screening of small molecules with similar chemical functionalities for triglyceride lowering and other lipid-regulating activities (ie, HDL-C elevation) in chow-fed Sprague–Dawley rats.38 Structurally, gemcabene is a fraudulent fatty acid with 2 terminal gem-dimethyl carboxylate moieties (Fig. 1). Although gemcabene dose dependently increases liver weight and expression of hepatic peroxisomal enzymes in rats,38 there are insufficient data to conclude direct activation of the PPAR-α receptor by gemcabene. In chow-fed Sprague–Dawley rats, gemcabene dose dependently reduced hepatic apoC-III mRNA levels, plasma triglycerides, LDL-C, VLDL-C, apoC-II, apoC-III, and apoB and elevated HDL-C, apoA-I, and apoE.38
FIGURE 1.
Structures of gemcabene and reference compounds evaluated in the PPAR transactivation assays.
In hypertriglyceridemic patients (TG ≥200 mg/dL) with low HDL-C (HDL-C <35 mg/dL) and normal LDL-C levels, gemcabene lowered plasma apoC-III, triglycerides, LDL-C, non–HDL-C, apoB, and apoE and elevated apoA-I, apoA-II, and HDL-C.39 In a placebo-controlled double-blind clinical study in hypercholesterolemic patients on background statin therapy, gemcabene further reduced LDL-C, apoB, non–HDL-C, and C-reactive protein.40 Because gemcabene showed increased peroxisomal activity in rats and modulated some of the known hepatic PPAR-α responsive genes,38 we evaluated the potential involvement of PPAR subtype activities by transactivation assays in PPAR subtype constructs. We present data demonstrating that gemcabene lacks significant activities against any of the PPAR subtypes for either mouse, rat, or human receptors.
METHODS
Plasmid and Transactivation Assay
This study was commissioned to and conducted by Indigo Biosciences, State College, PA, 16801, USA. The nuclear receptor reporter cells used were available from Indigo Biosciences, a proprietary cell line expressing a hybrid receptor comprising the N-terminal Gal4 DNA-binding domain fused to the ligand-binding domain of the specific nuclear receptor. The reporter gene, firefly luciferase, is functionally linked to the Gal4 upstream activation sequence. Descriptive information on reference compounds (known drugs and clinical candidates) used for assay validation or comparative purposes is displayed in Figure 1 and Table 1. The nuclear receptor assays were performed in 3 steps as described below.
TABLE 1.
Descriptive Information on Gemcabene and Reference Agents: Type, Chemical Abstract Service (CAS) Number, Molecular Weights (MWs), and Molecular Formulas (MFs)
In step 1, as shown in Figure 2, a suspension of reporter cells was prepared in cell recovery medium containing 10% charcoal-stripped fetal bovine serum. For antagonist assays, reporter cells were first supplemented with 2x-EC80 concentration of the appropriate reference agonist (Table 1). Then, 100 μL of the reporter cell suspension treated with or without 2x-EC80 was dispensed into wells in a white 96-well assay plate. In step 2, before assay setup, test compounds were diluted using compound screening medium containing 10% charcoal-stripped fetal bovine serum to generate “2x-concentration” treatment media. Treatment medium (100 μL of each) was dispensed into wells predispensed with the reporter cells. The assay was conducted in triplicate. Assay plates were incubated at 37°C for 24 hours. Finally, in step 3, after the 24-hour incubation period, treatment media were discarded and the wells were rinsed once with Live Cell Multiplex Assay (LCMA) buffer and subsequently treated with LCMA substrate. After incubation at 37°C for 45 minutes, fluorescence was measured to determine the relative number of live cells expressed as relative fluorescence unit per assay well. The percent live cells were taken into consideration in the calculation of EC50. Some of the reference agents were toxic to the cells, and they have been mentioned in the comments section of Table 2. LCMA substrate was then discarded, and 100 μL/well of luciferase detection reagent was added. Relative luciferase units were quantified from each assay well to determine PPAR agonist and antagonist activities.
FIGURE 2.
Transactivation methodology, for the agonist (A) and antagonist (B) assays. Assays were performed in 96-well plates using a proprietary cell line from Indigo Biosciences, State College, PA. This proprietary cell line expresses a hybrid receptor comprising the N-terminal Gal4 DNA-binding domain fused to the ligand-binding domain of the specific nuclear receptor. The reporter gene used in this assay is the firefly luciferase, which is functionally linked to the Gal4 upstream activation sequence. The fluorescence-based LCMA and luminescence-based nuclear receptor assay were performed sequentially using the same assay wells. For the agonist assay, nuclear receptor reporter cells were incubated with the test compound for 24 hours followed by the LCMA as shown, and relative fluorescence was read. Media were removed by aspiration, the detection reagent was added, and relative luciferase activity was measured in a luminometer. The antagonist assay was performed in the same manner except in the presence of agonist at a concentration at 2× than that of the EC80. NR, nuclear receptor; RFU, relative fluorescence unit; RLU, relative luciferase unit.
TABLE 2.
PPAR Transcriptional Activation by Gemcabene and Reference Compounds (or Commercial Drugs) in Cell-Based Transactivation Assays Using Gal-4 PPAR Chimeras Receptors
Assay Validation
Reference compounds (Table 1 and Fig. 1) were used to confirm the performance of the specific lot of PPAR reporter cells. Reference compounds and gemcabene were assayed simultaneously to insure comparability (see individual data sets for identity and treatment concentration ranges of reference compounds). A vehicle control allowed for determination of background activity in the assay and to calculate values of fold activation and percent inhibition of receptor activity.
Graphical Data Methods
Dose–response curve analyses of the tested agents were performed through nonlinear curve fitting of fold activation versus log[compound concentration] for PPAR agonist assays. Percent inhibition versus log[gemcabene concentration] for PPAR antagonist assays was calculated using GraphPad Prism software.
RESULTS
To determine whether gemcabene is a direct activator of PPAR subtypes, transactivation assays were performed in Chinese hamster ovary cells in both agonist and antagonist mode using mouse, rat, and human PPAR subtype constructs. In this assay, activation of Gal4 expression induces luciferase activity, a measure of extent of PPAR gene activation. PPAR transcriptional activation values are presented in Table 2.
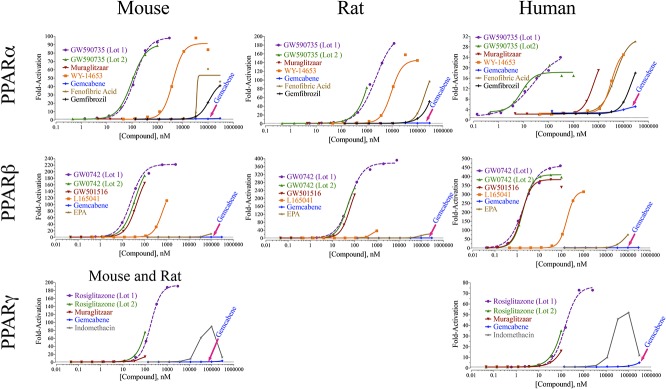
The results of PPAR-α activation in human, rat, and mouse are shown in Figure 3. Two lots of a potent PPAR-α agonist, GW590735, were tested for their PPAR-α agonist activities and, as expected, both showed potent agonist activity with an EC50 of 23 nM against human, 98 nM against mouse, and 2.2 μM against rat PPAR-α. WY-14643, a widely used PPAR-α activator reference agent,41,42 showed PPAR-α activation (EC50) against human (32.6 μM), mouse (4.1 μM), and rat (9.5 μM) PPAR-α. The PPAR-α agonist GW59073543 showed robust maximal activation of 17-, 89-, and 83-fold against human, mouse, and rat PPAR-α, respectively (Fig. 3), whereas gemfibrozil showed similar values to the originally reported EC50 of 193 μM.44 Muraglitazar, with dual PPAR-α/PPAR-γ agonist activity,45 was used to evaluate the robustness of this assay and to ensure that the assay can detect simultaneous activation of multiple PPAR subtypes for dual or pan agonists. In this assay, the dual PPAR-α/-γ agonist muraglitazar showed agonist activity against human PPAR-α and PPAR-γ, but not against mouse and rat PPAR-α, consistent with the literature.45
FIGURE 3.
Graphical presentation of the transactivation assays for PPAR-α, PPAR-δ, and PPAR-γ agonists using GAL4-LBD. Luciferase activity was measured after treatments with the indicated reference agents or test agent, gemcabene. Strong (GW590735, muraglitazar), mild (WY-14643, fenofibric acid), and weak (gemfibrozil) PPAR-α agonists were used as reference agents in this assay against human, rat, and mouse PPAR-α as indicated. For PPAR-δ, strong (GW0742, GW501516, L165041) PPAR-δ agonists were used as reference agents in this assay against human, rat, and mouse PPAR-δ as shown. Similarly, strong PPAR-γ agonists were used as reference agents in this assay against human, rat, and mouse PPAR-γ as indicated in the figure. Note that there is a single graph for mouse and rat PPAR-γ assay, as only the mouse transactivation assays was performed because the DNA-binding domain between the mouse and rat receptors are homologous.
Under the assay conditions and construct used, gemcabene showed essentially little or no PPAR-α activity against rat and mouse, but low marginal PPAR-α activity against human at the highest concentration (300 μM) tested (Fig. 3).
Furthermore, 2 lots of the potent PPAR-δ agonist GW074221,46 were tested, and both gave identical activation values (Fig. 3) with an EC50 of 0.0019 μM against human, and 0.036 μM and 0.059 μM against mouse and rat PPAR-δ, respectively (Table 2). PPAR-δ agonist L16504144,47,48 showed potent PPAR-δ agonist activity against human PPAR-δ, with an EC50 of 0.176 μM, but less activation against the mouse PPAR-δ (EC50 of 0.640 μM). Similarly, the reference compound GW501516, widely studied for its hypolipidemic activities,49,50 showed potent PPAR-δ agonist activity with an EC50 of 0.0016 μM against human, and EC50 of 0.044 μM and 0.063 μM against mouse and rat PPAR-δ, respectively (Fig. 3 and Table 2). In conclusion, PPAR-δ reference compounds GW1516, L165041, and GW0742 confirm their robust agonist activity against human, mouse, and rat PPAR-δ with maximal activation in the range of 37- to 409-fold. By contrast, gemcabene showed no activation up to 300-μM concentration against rat or mouse PPAR-δ and marginal activation against human PPAR-δ only at the highest concentration tested. This clearly shows that gemcabene does not possess any direct PPAR-δ agonist activity under the assay conditions, whereas known PPAR-δ agonists showed expected level of agonist activities in a concentration-dependent manner.
As displayed in Table 2 and Figure 3, known PPAR-γ activators showed expected activity in this assay. Gemcabene did not show concentration-dependent activity up to 100-μM concentration, in the 3 species tested. However, rodent and human PPAR-γ receptors showed relatively marginal (3.6- to 5-fold) activation at (300 μM).
Because PPARs may influence lipid and carbohydrate metabolism through antagonist activity,51 we tested whether gemcabene has antagonist activity against PPAR subtypes in human, rat, and mouse. As shown in Figure 4, gemcabene did not display antagonist activity against any PPAR subtypes, whereas known PPAR subtype antagonists51,52 showed expected activities.
FIGURE 4.
Graphical presentations of the transactivation assay for PPAR antagonist activity. Luciferase activity was measured after treatments with the indicated test agent, gemcabene, against human, rat, and mouse PPAR-α, PPAR-δ, and PPAR-γ in the presence of appropriate agonists (WY-1453 for PPAR-α, GW501516 for PPAR-δ, and rosiglitazone for PPAR-γ). Although we could not find a reference agent for PPAR-α antagonist, we used GSK378758 and T007090751 as PPAR-δ and PPAR-γ antagonists, respectively. Note that there is a single graph for mouse and rat PPAR-γ assay, as only the mouse transactivation assays was performed because the DNA-binding domain between the mouse and rat receptors are homologous.
DISCUSSION
This study evaluated gemcabene as a direct agonist/antagonist for the PPAR receptor subtypes in 3 species: rat, mouse, and human, to corroborate the earlier physiological findings of gemcabene-induced modulation of a subset of PPAR-α responsive genes in rodents.38 Specifically, we sought to determine whether the observed biological effects were a direct or indirect result of gemcabene activation of PPAR-α–related genes.
In the current study, although the reference compounds showed significant agonist activity in human, mouse, and rat toward PPAR subtypes in transactivation assays, gemcabene essentially lacked agonist activity against rat and mouse PPAR-α up to the highest concentration tested (300 μM). Unlike gemcabene, the reference PPAR-α activators showed robust concentration-dependent transactivation against human PPAR-α ranging from 2.4-fold to 30-fold for fenofibric acid20 and 2.3- to 25-fold for WY-1464329 (Table 2). These reference agents also showed marked concentration-dependent transactivation for mouse and rat PPAR-α. Muraglitazar, a dual PPAR-α and -γ agonist,45 showed 19- and 16-fold activation of human PPAR-α and PPAR-γ, respectively (Table 2). The PPAR-α agonist GW59073543 also showed robust maximal activation of 21-, 89-, and 83-fold against human, mouse, and rat PPAR-α, respectively (Table 2). These findings suggest that gemcabene lacks direct rat and mouse PPAR-α activation and is an extremely weak direct agonist for the human PPAR-α receptor. Given the central role of PPAR-α in lipid metabolism,7,11,13 it is possible and likely that gemcabene treatment in rodents causes an indirect PPAR-α activation by endogenous cellular metabolites,27,33 as observed during fasting and starvation.31
Recognized PPAR-δ agonists L165041,44,47,48 GW0742,21,46 and GW151649,50 showed potent agonist activity against human, mouse, and rat PPARs with maximal activation in the range of 37- to 409-fold (Table 2). In this experiment, gemcabene showed little or no activity and lacked a dose–response for PPAR-δ agonist activity against all species tested.
Finally, we investigated PPAR-γ agonist activity of gemcabene, as PPAR-γ activation is associated with anti-inflammatory activities25,26 and insulin sensitization.53,54 As expected, the known PPAR-γ activator rosiglitazone showed potent agonist activity (Table 2), whereas gemcabene did not show any activity up to 100-μM concentration and modest activation at 300 μM, suggesting that gemcabene possesses little or no direct PPAR-γ activation properties depending on the species.
In terms of antagonist activities, the data do not indicate a clear antagonist activity of gemcabene against any PPAR subtype (human, mouse, and rat), with a clear lack of concentration response.
CONCLUSION
In conclusion, these results rule out efficacious direct agonist or antagonist activities of gemcabene against all 3 PPAR subtypes: -α, -γ, and -δ, in human, rat, and mouse and infer that gemcabene does not bind to, nor directly activate, the PPAR nuclear hormone receptors to any appreciable extent. These findings are in part in agreement with the significant hypolipidemic efficacy of gemcabene when administered to PPAR-α knockout mice,55,56 suggesting that at least some of gemcabene-mediated lipid regulation is independent of the PPAR-α receptor. The MOA of currently available therapies for hypertriglyceridemia rely on their PPAR activation,56 which incur an inherent safety concern, because PPAR-α activation in rodents is associated with specific cancer pathologies.56,57 Gemcabene seems to work through a number of pleiotropic MOAs that we are in the process of elucidating, but the current study shows that gemcabene does not involve a direct PPAR agonist or antagonist component, and that, most likely, in rodents, gemcabene activates PPAR-α–responsive genes indirectly.
Footnotes
All authors are employed by and own equity in Gemphire Therapeutics inc.
REFERENCES
- 1.Kleinman JC, Donahue RP, Harris MI, et al. Mortality among diabetics in a national sample. Am J Epidemiol. 1988;128:389–401. [DOI] [PubMed] [Google Scholar]
- 2.American Heart Association HDass-u. A report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2007;115:e69–e71. [DOI] [PubMed] [Google Scholar]
- 3.Genest JJ, McNamara JR, Salem DN, et al. Prevalence of risk factors in men with premature coronary artery disease. Am J Cardiol. 1991;67:1185–1189. [DOI] [PubMed] [Google Scholar]
- 4.Williams KJ, Wu X. Imbalanced insulin action in chronic over nutrition: clinical harm, molecular mechanisms, and a way forward. Atherosclerosis. 2016;247:225–282. [DOI] [PubMed] [Google Scholar]
- 5.Srivastava RAK. Dysfunctional HDL in diabetes mellitus and its role in the pathogenesis of cardiovascular disease. Mol Cell Biochem. 2018;440:167–187. [DOI] [PubMed] [Google Scholar]
- 6.Frick MH, Elo O, Haapa K, et al. Helsinki Heart Study: primary-prevention trial with gemfibrozil in middle-aged men with dyslipidemia. Safety of treatment, changes in risk factors, and incidence of coronary heart disease. N Engl J Med. 1987;317:1237–1245. [DOI] [PubMed] [Google Scholar]
- 7.Investigators DAIS. Effect of fenofibrate on progression of coronary-artery disease in type 2 diabetes: the Diabetes Atherosclerosis Intervention Study, a randomised study. Lancet. 2001;357:905–910. [PubMed] [Google Scholar]
- 8.de las Fuentes L, de Simone G, Arnett DK, et al. Molecular determinants of the cardiometabolic phenotype. Endocr Metab Immune Disord Drug Targets. 2010;10:109–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chai JT, Choudhury RP. Cardiometabolic interventions—focus on transcriptional regulators. Eur J Cardiovasc Med. 2013;2:212–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Orekhov AN, Mukhamedova N, Ivanova EA, et al. PPAR in cardiovascular disorders. PPAR Res. 2016;2016:6293629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Forman BM, Chen J, Evans RM. Hypolipidemic drugs, polyunsaturated fatty acids, and eicosanoids are ligands for peroxisome proliferator-activated receptors alpha and delta. Proc Natl Acad Sci U S A. 1997;94:4312–4317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moras D, Gronemeyer H. The nuclear receptor ligand-binding domain: structure and function. Curr Opin Cell Biol. 1998;10:384–391. [DOI] [PubMed] [Google Scholar]
- 13.Carson-Jurica MA, Schrader WT, O'Malley BW. Steroid receptor family: structure and functions. Endocr Rev. 1990;11:201–220. [DOI] [PubMed] [Google Scholar]
- 14.Schoonjans K, Peinado-Onsurbe J, Lefebvre AM, et al. PPARalpha and PPARgamma activators direct a distinct tissue-specific transcriptional response via a PPRE in the lipoprotein lipase gene. EMBO J. 1996;15:5336–5348. [PMC free article] [PubMed] [Google Scholar]
- 15.Dressel U, Allen TL, Pippal JB, et al. The peroxisome proliferator-activated receptor beta/delta agonist, GW501516, regulates the expression of genes involved in lipid catabolism and energy uncoupling in skeletal muscle cells. Mol Endocrinol. 2003;17:2477–2493. [DOI] [PubMed] [Google Scholar]
- 16.Barroso E, Rodriguez-Calvo R, Serrano-Marco L, et al. The PPARbeta/delta activator GW501516 prevents the down-regulation of AMPK caused by a high-fat diet in liver and amplifies the PGC-1alpha-Lipin 1-PPARalpha pathway leading to increased fatty acid oxidation. Endocrinology. 2011;152:1848–1859. [DOI] [PubMed] [Google Scholar]
- 17.Sprecher DL, Massien C, Pearce G, et al. Triglyceride:high-density lipoprotein cholesterol effects in healthy subjects administered a peroxisome proliferator activated receptor delta agonist. Arterioscler Thromb Vasc Biol. 2007;27:359–365. [DOI] [PubMed] [Google Scholar]
- 18.Dimopoulos N, Watson M, Green C, et al. The PPARdelta agonist, GW501516, promotes fatty acid oxidation but has no direct effect on glucose utilisation or insulin sensitivity in rat L6 skeletal muscle cells. FEBS Lett. 2007;581:4743–4748. [DOI] [PubMed] [Google Scholar]
- 19.Barroso E, Rodriguez-Rodriguez R, Chacon MR, et al. PPARbeta/delta ameliorates fructose-induced insulin resistance in adipocytes by preventing Nrf2 activation. Biochim Biophys Acta. 2015;1852:1049–1058. [DOI] [PubMed] [Google Scholar]
- 20.Srivastava RA, Jahagirdar R, Azhar S, et al. Peroxisome proliferator-activated receptor-alpha selective ligand reduces adiposity, improves insulin sensitivity and inhibits atherosclerosis in LDL receptor-deficient mice. Mol Cell Biochem. 2006;285:35–50. [DOI] [PubMed] [Google Scholar]
- 21.Faiola B, Falls JG, Peterson RA, et al. PPAR alpha, more than PPAR delta, mediates the hepatic and skeletal muscle alterations induced by the PPAR agonist GW0742. Toxicol Sci. 2008;105:384–394. [DOI] [PubMed] [Google Scholar]
- 22.Srivastava RA. Fenofibrate ameliorates diabetic and dyslipidemic profiles in KKAy mice partly via down-regulation of 11beta-HSD1, PEPCK and DGAT2. Comparison of PPARalpha, PPARgamma, and liver x receptor agonists. Eur J Pharmacol. 2009;607:258–263. [DOI] [PubMed] [Google Scholar]
- 23.Colville-Nash PR, Qureshi SS, Willis D, et al. Inhibition of inducible nitric oxide synthase by peroxisome proliferator-activated receptor agonists: correlation with induction of heme oxygenase 1. J Immunol. 1998;161:978–984. [PubMed] [Google Scholar]
- 24.Neve BP, Corseaux D, Chinetti G, et al. PPARalpha agonists inhibit tissue factor expression in human monocytes and macrophages. Circulation. 2001;103:207–212. [DOI] [PubMed] [Google Scholar]
- 25.Murphy GJ, Holder JC. PPAR-gamma agonists: therapeutic role in diabetes, inflammation and cancer. Trends Pharmacol Sci. 2000;21:469–474. [DOI] [PubMed] [Google Scholar]
- 26.Moraes LA, Piqueras L, Bishop-Bailey D. Peroxisome proliferator-activated receptors and inflammation. Pharmacol Ther. 2006;110:371–385. [DOI] [PubMed] [Google Scholar]
- 27.Cuzzocrea S, Di Paola R, Mazzon E, et al. Role of endogenous and exogenous ligands for the peroxisome proliferators activated receptors alpha (PPAR-alpha) in the development of inflammatory bowel disease in mice. Lab Invest. 2004;84:1643–1654. [DOI] [PubMed] [Google Scholar]
- 28.Staumont-Salle D, Abboud G, Brenuchon C, et al. Peroxisome proliferator-activated receptor alpha regulates skin inflammation and humoral response in atopic dermatitis. J Allergy Clin Immunol. 2008;121:962–968.e6. [DOI] [PubMed] [Google Scholar]
- 29.Cuzzocrea S, Bruscoli S, Mazzon E, et al. Peroxisome proliferator-activated receptor-alpha contributes to the anti-inflammatory activity of glucocorticoids. Mol Pharmacol. 2008;73:323–337. [DOI] [PubMed] [Google Scholar]
- 30.Hu Y, Chen Y, Ding L, et al. Pathogenic role of diabetes-induced PPAR-alpha down-regulation in microvascular dysfunction. Proc Natl Acad Sci U S A. 2013;110:15401–15406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sun Y, Ng L, Lam W, et al. Identification and characterization of a novel mouse peroxisome proliferator-activated receptor alpha-regulated and starvation-induced gene, Ppsig. Int J Biochem Cell Biol. 2008;40:1775–1791. [DOI] [PubMed] [Google Scholar]
- 32.Chakravarthy MV, Pan Z, Zhu Y, et al. “New” hepatic fat activates PPARalpha to maintain glucose, lipid, and cholesterol homeostasis. Cell Metab. 2005;1:309–322. [DOI] [PubMed] [Google Scholar]
- 33.Chakravarthy MV, Lodhi IJ, Yin L, et al. Identification of a physiologically relevant endogenous ligand for PPARalpha in liver. Cell. 2009;138:476–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sanderson LM, Degenhardt T, Koppen A, et al. Peroxisome proliferator-activated receptor beta/delta (PPARbeta/delta) but not PPARalpha serves as a plasma free fatty acid sensor in liver. Mol Cell Biol. 2009;29:6257–6267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fernandez-Alvarez A, Alvarez MS, Gonzalez R, et al. Human SREBP1c expression in liver is directly regulated by peroxisome proliferator-activated receptor alpha (PPARalpha). J Biol Chem. 2011;286:21466–21477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mandard S, Muller M, Kersten S. Peroxisome proliferator-activated receptor alpha target genes. Cell Mol Life Sci. 2004;61:393–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shah YM, Morimura K, Yang Q, et al. Peroxisome proliferator-activated receptor alpha regulates a microRNA-mediated signaling cascade responsible for hepatocellular proliferation. Mol Cell Biol. 2007;27:4238–4247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bisgaier CL, Essenburg AD, Barnett BC, et al. A novel compound that elevates high density lipoprotein and activates the peroxisome proliferator activated receptor. J Lipid Res. 1998;39:17–30. [PubMed] [Google Scholar]
- 39.Bays HE, McKenney JM, Dujovne CA, et al. Effectiveness and tolerability of a new lipid-altering agent, gemcabene, in patients with low levels of high-density lipoprotein cholesterol. Am J Cardiol. 2003;92:538–543. [DOI] [PubMed] [Google Scholar]
- 40.Stein E, Bays H, Koren M, et al. Efficacy and safety of gemcabene as add-on to stable statin therapy in hypercholesterolemic patients. J Clin Lipidol. 2016;10:1212–1222. [DOI] [PubMed] [Google Scholar]
- 41.Clavey V, Copin C, Mariotte MC, et al. Cell culture conditions determine apolipoprotein CIII secretion and regulation by fibrates in human hepatoma HepG2 cells. Cell Physiol Biochem. 1999;9:139–149. [DOI] [PubMed] [Google Scholar]
- 42.Liangpunsakul S, Wou SE, Wineinger KD, et al. Effects of WY-14,643 on the phosphorylation and activation of AMP-dependent protein kinase. Arch Biochem Biophys. 2009;485:10–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Riserus U, Sprecher D, Johnson T, et al. Activation of peroxisome proliferator-activated receptor (PPAR)delta promotes reversal of multiple metabolic abnormalities, reduces oxidative stress, and increases fatty acid oxidation in moderately obese men. Diabetes. 2008;57:332–339. [DOI] [PubMed] [Google Scholar]
- 44.Mukherjee R, Sun S, Santomenna L, et al. Ligand and coactivator recruitment preferences of peroxisome proliferator activated receptor alpha. J Steroid Biochem Mol Biol. 2002;81:217–225. [DOI] [PubMed] [Google Scholar]
- 45.Harrity T, Farrelly D, Tieman A, et al. Muraglitazar, a novel dual (alpha/gamma) peroxisome proliferator-activated receptor activator, improves diabetes and other metabolic abnormalities and preserves beta-cell function in db/db mice. Diabetes. 2006;55:240–248. [PubMed] [Google Scholar]
- 46.Sznaidman ML, Haffner CD, Maloney PR, et al. Novel selective small molecule agonists for peroxisome proliferator-activated receptor delta (PPARdelta)–synthesis and biological activity. Bioorg Med Chem Lett. 2003;13:1517–1521. [DOI] [PubMed] [Google Scholar]
- 47.Bonala S, Lokireddy S, Arigela H, et al. Peroxisome proliferator-activated receptor beta/delta induces myogenesis by modulating myostatin activity. J Biol Chem. 2012;287:12935–12951. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 48.Quintela AM, Jimenez R, Piqueras L, et al. PPARbeta activation restores the high glucose-induced impairment of insulin signalling in endothelial cells. Br J Pharmacol. 2014;171:3089–3102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bojic LA, Telford DE, Fullerton MD, et al. PPARdelta activation attenuates hepatic steatosis in Ldlr-/- mice by enhanced fat oxidation, reduced lipogenesis, and improved insulin sensitivity. J Lipid Res. 2014;55:1254–1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Olson EJ, Pearce GL, Jones NP, et al. Lipid effects of peroxisome proliferator-activated receptor-delta agonist GW501516 in subjects with low high-density lipoprotein cholesterol: characteristics of metabolic syndrome. Arterioscler Thromb Vasc Biol. 2012;32:2289–2294. [DOI] [PubMed] [Google Scholar]
- 51.Lee G, Elwood F, McNally J, et al. T0070907, a selective ligand for peroxisome proliferator-activated receptor gamma, functions as an antagonist of biochemical and cellular activities. J Biol Chem. 2002;277:19649–19657. [DOI] [PubMed] [Google Scholar]
- 52.Seimandi M, Lemaire G, Pillon A, et al. Differential responses of PPARalpha, PPARdelta, and PPARgamma reporter cell lines to selective PPAR synthetic ligands. Anal Biochem. 2005;344:8–15. [DOI] [PubMed] [Google Scholar]
- 53.Bisgaier CL, Newton RS. Statin-Carboxyalkylether Combinations. New York, NY: Warner-Lambert Company, LLC; 2013. US Patent No. 8,557,835. [Google Scholar]
- 54.Bakker-Arkema R, Bisgaier C. Effect of gemcabene on insulin sensitivity in nondiabetic, obese subjects. ACC, March 19, 2017, Washington DC, 2017. [Google Scholar]
- 55.Bisgaier C, Srivastava RA. A novel new chemical entity, gemcabene, shows significant lipid regulation in PPRARα knock-out mice supporting a mechanism independent of PPARα. Circulation AHA Abstracts 2015, 2015. [Google Scholar]
- 56.Peters JM, Hennuyer N, Staels B, et al. Alterations in lipoprotein metabolism in peroxisome proliferator-activated receptor alpha-deficient mice. J Biol Chem. 1997;272:27307–27312. [DOI] [PubMed] [Google Scholar]
- 57.Cheung C, Gonzalez FJ. Humanized mouse lines and their application for prediction of human drug metabolism and toxicological risk assessment. J Pharmacol Exp Ther. 2008;327:288–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lehmann JM, Lenhard JM, Oliver BB, et al. Peroxisome proliferator-activated receptors alpha and gamma are activated by indomethacin and other non-steroidal anti-inflammatory drugs. J Biol Chem. 1997;272:3406–3410. [DOI] [PubMed] [Google Scholar]