Abstract
Background
Goal-directed hemostatic resuscitation based on thrombelastography has a survival benefit compared to conventional coagulation assays. While thrombelastography transfusion thresholds for patients at risk for massive transfusion (MT) have been defined, similar cutoffs do not exist for the other commonly used viscoelastic assay, rotational thromboelastometry (ROTEM). The purpose of this study was to develop ROTEM blood product thresholds in patients at risk for MT.
Methods
ROTEM was assessed in trauma activation patients admitted from 2010 to 2016 (n = 222). Receiver operating characteristic curve analyses were performed to test the predictive performance of ROTEM measurements in patients requiring MT. The Youden Index defined optimal thresholds for ROTEM-based resuscitation.
Results
Patients who required MT (n = 37, 17%) were more severely injured. EXTEM clotting time (CT) was longer in patients with MT compared to non-MT (87 versus 64 s, P < 0.0001). EXTEM angle was shallower in MT patients compared to non-MT (54° versus 69°, P < 0.0001). Clot amplitude after 10 min (CA10) was less in MT compared to non-MT patients (30.5 versus 50 mm, P < 0.0001). Clot lysis index 60 min (CLI60) was lower in patients who had MT than non-MT (47 versus 94%, P = 0.0006). EXTEM CT yielded an area under the receiver operating characteristic curve (AUROC) = 0.7116 and a cut point of >78.5 s. EXTEM angle had an AUROC = 0.865 and a cut point of <64.5°. EXTEM CA10 had an AUROC = 0.858, with a cut point of <40.5 mm. CLI60 had an AUROC = 0.6788 with a cut point at <74%.
Conclusions
We have identified ROTEM thresholds for transfusion of blood components in severely injured patients requiring an MT. Based on our analysis, we propose plasma transfusion for EXTEM CT > 78.5 s, fibrinogen for angle <64.5°, platelet transfusion for CA10 < 40.5 mm, and antifibrinolytics for CLI60 < 74%.
Keywords: Massive transfusion, TEG, ROTEM, Coagulopathy, Resuscitation
Introduction
Uncontrolled hemorrhage is the leading cause of preventable death following trauma, accounting for up to 40% of deaths.1 An endogenous trauma-induced coagulopathy accounts for most of these hemorrhagic deaths and is a multifocal process attributed to reduced thrombin generation, fibrinogen depletion, platelet dysfunction, and systemic hyperfibrinolysis.2–5
Half of deaths from acute blood loss occur within the first 2 h after injury, and hemorrhage accounts for the vast majority of deaths within the first 24 h.6 Reliable objective means of early recognition and targeted transfusion interventions are the key to successful management of life-threatening trauma-induced coagulopathy.
Massive transfusion protocols (MTPs) offer a proven benefit in resuscitation of patients in hemorrhagic shock. Much effort has been directed to identifying the ideal ratio of blood products in resuscitation strategies.6,7 Traditional interventions have been guided by conventional coagulation assays (CCAs), such as international normalized ratio (INR), activated partial thromboplastin time (aPTT), fibrinogen level, and platelet count. Our group has shown that a goal-directed, thrombelastography (TEG)-guided MTP improves survival as compared with MTP guided by CCAs. Furthermore, these results were achieved with the transfusion of less plasma and platelets during the early phases of resuscitation.8
We have defined optimal transfusion thresholds using TEG for patients at risk for massive transfusion (MT) as an activated clotting time (ACT) < 128 s, angle <65°, a maximum amplitude (MA) < 55 mm, and lysis 30 min after achieving MA (LY30) >5%.7 However, these thresholds are not directly translatable for use in rotational thromboelastometry (ROTEM), another commonly used viscoelastic assay because of differences in instrumentation and reagents.9 Therefore, we hypothesize that we can identify ROTEM measurements that indicate the need for MT in injured patients using ROTEM and thresholds for specific blood component therapy in these high-risk patients.
Methods
Study design
This is an analysis of prospectively collected data from our Trauma Activation Protocol registry, which includes consecutive adult (age ≥ 18 y old) patients who met criteria for the highest level of trauma team activation at the Denver Health Medical Center, an American College of Surgeons-verified and state-certified level 1 trauma center affiliated with the University of Colorado Denver and were at risk for MT. Exclusion criteria were unsalvageable injuries (defined by patients in asystole at emergency department arrival), isolated gunshot wounds to the head, pregnancy, documented chronic liver disease, or a known coagulation disorder.
The studies contributing to this database were approved by the Colorado Multiple Institution Review Board and performed under a waiver of consent. Trained research professional assistants performed all viscoelastic assays within 1-h post-injury. Clinicians were blinded to these research data. TEGs were ordered at the discretion of the care team and processed in the hospital clinical laboratory to guide resuscitation in injured patients.
The transfusion of products other than red blood cells during this period was guided by rapid thrombelastography (rTEG) criteria, as previously described.8,10 The primary endpoint of this study was MT, defined as >10 units of red blood cells or death in first 6 h from injury based on findings previously published by our group.11
TEG is used at our institution and more commonly in the United States, while ROTEM is used widely in European centers.9 Although the technologies and reagents are somewhat similar, the measurements reported are not the same and cannot be generalized from one instrument to the other.12
Rotational thromboelastometry
ROTEM was performed on whole blood collected in vacuum tubes with citrate added to prevent clotting before the analysis. The specific ROTEM assays used were EXTEM (activated with tissue factor), FIBTEM (activated similar to EXTEM but with cytochalasin D to inhibit the contribution of platelets to the clot),13 and APTEM, which is a modified EXTEM, in which aprotinin inhibits plasmin in vitro if systemic fibrinolysis is present.14 ROTEM tests yield the following variables that were used to assess the dynamic process of clot formation and breakdown in this study: time to clot initiation (clotting time [CT, s]), dynamics of clot formation (angle [degrees] and clot formation time [CFT, s]), clot strength (maximum clot formation [MCF, mm] and clot amplitude after 10 min [CA10, mm]), and fibrinolysis (clot lysis index at 30 min [CLI30, %] and 60 min after CT [CLI60, %]).14 Prolonged clot initiation is an indication for plasma and reflected by EXTEM CT. Abnormal dynamics of clot formation is an indication for fibrinogen products and reflected by EXTEM CFT, EXTEM angle, FIBTEM angle, FIBTEM MCF, and FIBTEM CA10. Low clot strength is an indication for platelets and reflected by EXTEM CA10 and EXTEM MCF. Increased fibrinolysis is an indication for antifibrinolytics and reflected by EXTEM CLI30 and CLI60. Clinicians based blood product transfusions on clinical rapid TEG and were blinded to ROTEM values.
Rapid thrombelastography
Thrombelastography (TEG-5000 analyzer; Haemonetics Corp, Stoughton, MA) was performed on whole blood collected in vacuum tubes with citrate added to prevent clotting before analysis. This assay incorporates tissue factor to the whole blood sample immediately before test initiation to expedite results, also known as rTEG. rTEG yields the following variables: ACT (the time to beginning of clot formation, s), angle (α; rate of clot strength increase, degrees), MA (maximal clot strength achieved, millimeters), and percent clot LY30. Thresholds for determination of predictive capacity have previously been defined as an ACT < 128 s, angle <65°, a MA < 55 mm, and LY30 >5%.7
Conventional coagulation assays
Samples were collected during trauma activations upon arrival to the emergency department in tubes containing 3.2% citrate and 4 mL of heparin (19 units/mL). Values for CCAs (INR and aPTT) were determined by the clinical laboratory at Denver Health Medical Center by a standard protocol. Abnormal values for CCAs were set as INR>1.3 and aPTT>34 as previously published.15–17
Statistical analysis
GraphPad Prism version 7.0a (GraphPad Software, Inc; La Jolla, CA) and Excel version 12.2.5 (Microsoft Corporation; Redmond, WA) were used for statistical analysis. For nonnormally distributed variables, data were expressed as median and interquartile range using a two-tailed Mann–Whitney test. Area under the receiver operating characteristic (AUROC) curve analysis was performed for each ROTEM measurement to assess its predictive performance for MT. For each of the ROTEM measurements, we selected the thresholds with the strongest differentiation of the outcome (MT) as identified by the maximum Youden Index (J = sensitivity + specificity − 1). The maximum Youden’s Index is the value closest to one.18 The sensitivity, specificity, positive predictive values (PPVs), and negative predictive values (NPVs) correspondent to the chosen cutoff were determined for the ROTEM indices for clot initiation, dynamics of clot formation, clot strength, and fibrinolysis. The sensitivity, specificity, PPVs, and NPVs were also determined for rapid TEG variables, INR, and aPTT.
Results
There were 222 nonconsecutive patients with ROTEM completed. All patients had EXTEM performed, while due to technical difficulties, only 212 patients also had FIBTEM performed and 128 APTEM. Table 1 illustrates the demographic characteristics of patients who required MT (17%) and those who did not. Patients who had an MT were more severely injured, had signs of more pronounced shock, and more abnormal ROTEM indices compared to those who did not require an MT (Table 2).
Table 1.
Demographic characteristics of patients.
| Variable | Massive transfusion | No massive transfusion | P value |
|---|---|---|---|
| Age (y) | 36 (27–48) | 36 (26–49) | 0.9749 |
| NISS | 46.5 (38–57) | 17 (4–28) | <0.0001 |
| ED SBP (mmHg) | 60 (0–100) | 112 (92–138) | <0.0001 |
| ED heart rate (BPM) | 95 (0–128) | 100.5 (85–116) | 0.2381 |
| ED GCS | 3 (3–13) | 15 (9–15) | <0.0001 |
| ED temp (celsius) | 36.3 (35.65–36.95) | 36.6 (36.3–36.8) | 0.3059 |
| Calcium (mg/dL) | 7.65 (6.8–8.65) | 8 (7.5–8.5) | 0.201 |
| Lactate (mg/dL) | 8.1 (4.7–14.55) | 3.35 (2.1–5.0) | <0.0001 |
| Base deficit | 14 (8.5–20.5) | 6.5 (3–9.25) | <0.0001 |
| Hgb (g/dL) | 11.6 (9.7–13.95) | 13.9 (12.6–15.3) | 0.0001 |
| Platelet count (1000/mL) | 176.5 (112–244) | 258 (213–311) | <0.0001 |
| INR | 1.72 (1.33–2.07) | 1.1 (1.04–1.23) | <0.0001 |
| PTT (s) | 43.4 (33.7–80.5) | 27.4 (24.1–31.2) | <0.0001 |
| Fibrinogen (mg/dL) | 122 (68.75–237.5) | 208 (170–265) | 0.0243 |
| D-Dimer (ng/mL) | 19.86 (8.82–20.01) | 2.145 (0.405–7.615) | 0.0018 |
Data are presented as median and interquartile range.
NISS = new injury severity score; ED = emergency department; SBP = systolic blood pressure; Hgb = hemoglobin; PTT = partial thromboplastin time; GCS = glasgow coma scale; BPM = beats per minute.
Table 2.
ROTEM indices by massive transfusion status.
| Massive transfusion | No massive transfusion | P value | |
|---|---|---|---|
| EXTEM | |||
| CT (s) | 87 (67.25–118.3) | 64 (56–75) | <0.0001 |
| CFT (s) | 160 (107.8–281.5) | 106 (87–131.8) | <0.0001 |
| Angle (degrees) | 54 (40.5–64) | 69 (65–72) | <0.0001 |
| CA10 (mm) | 30.5 (21.5–42) | 50 (44–56) | <0.0001 |
| MCF (mm) | 42 (24.75–52.75) | 59 (54–64) | <0.0001 |
| CLI30 (%) | 100 (22.75–100) | 100 (100–100) | 0.0003 |
| CLI60 (%) | 47 (1.25–96.75) | 94 (91.5–97) | 0.0006 |
| FIBTEM | |||
| Angle (degrees) | 55 (0–68) | 66 (60–71.25) | 0.0211 |
| CA10 (mm) | 5 (3–9) | 12 (9–15) | <0.0001 |
| MCF (mm) | 5 (3–9) | 13 (10–16) | <0.0001 |
| APTEM | |||
| CT (s) | 86.5 (63.75–126) | 61.5 (51.75–149) | <0.0001 |
| MCF (mm) | 47 (39–55.5) | 60 (54–63.25) | <0.0001 |
Data are presented as median and interquartile range.
The AUROC for ROTEM measurements are shown in Table 3. Of all ROTEM variables that measured dynamics of clot formation, EXTEM angle was the best predictor of MT (AUROC = 0.865 95% confidence interval [CI] 0.7961–0.934, P < 0.0001). CA10 was the best predictor of MT of the ROTEM indices measuring clot strength (AUROC = 0.8580, 95% CI 0.7884–0.9275, P < 0.0001). CLI60 was the best predictor of MT for ROTEM parameters assessing fibrinolysis (AUROC = 0.6788, 95% CI 0.5565–0.8010, P = 0.0007).
Table 3.
Area under the receiver operating characteristic curve for all ROTEM measurements.
| Clot initiation | AUROC | 95% confidence interval | P value |
|---|---|---|---|
| EXTEM CT | 0.7116 | 0.6003–0.8229 | <0.0001 |
| Dynamics of clot formation | |||
| EXTEM CFT | 0.7315 | 0.6173–0.8458 | <0.0001 |
| EXTEM angle | 0.8650 | 0.7961–0.9340 | <0.0001 |
| FIBTEM angle | 0.7084 | 0.4987–0.9181 | 0.0225 |
| FIBTEM CA10 | 0.8492 | 0.7773–0.9212 | <0.0001 |
| FIBTEM MCF | 0.8515 | 0.7803–0.9226 | <0.0001 |
| Clot strength | |||
| EXTEM CA10 | 0.8580 | 0.7884–0.9275 | <0.0001 |
| EXTEM MCF | 0.8499 | 0.7775–0.9224 | <0.0001 |
| Fibrinolysis | |||
| EXTEM CLI30 | 0.6116 | 0.4987–0.7245 | 0.0343 |
| EXTEM CLI60 | 0.6788 | 0.5565–0.8010 | 0.0007 |
Table illustrates the AUROC with 95% confidence intervals of multiple ROTEM parameters for each aspect of clot formation and breakdown (clot initiation, dynamics of clot formation, clot strength, and fibrinolysis or clot breakdown).
The ROTEM predicted thresholds for the ROTEM-guided MT based on the Youden Index are shown in Table 4. CLI60 of <74% had a much greater specificity and PPV while sensitivity and NPV were better with CT and MCF EXTEM/APTEM. The predictive capacity of rapid TEG and CCA (INR and aPTT) are shown in Table 5. The predictive capacity is similar between both ROTEM and TEG for clot initiation, dynamics of clot formation, and clot strength. However, the PPV of LY30 is better than CLI60 but still remains fairly low at 58%.
Table 4.
Predictive performance of ROTEM variables for MT.
| ROTEM variables | Sensitivity | Specificity | PPV | NPV |
|---|---|---|---|---|
| EXTEM CT > 78.5 s | 58.33% | 81.62% | 42.98% | 57.74% |
| EXTEM angle <64.5° | 80.56% | 77.72% | 52.02% | 48.62% |
| EXTEM CA10 < 40.5 mm | 77.22% | 89.19% | 46.24% | 54.38% |
| EXTEM CLI60 < 74% | 52.78% | 96.76% | 37.68% | 63.01% |
| EXTEM CT > APTEM CT | 65.00% | 33.33% | 20.00% | 80.95% |
| EXTEM MCF < APTEM MCF | 76.90% | 58.82% | 32.25% | 90.90% |
Table 5.
Predictive performance of rapid TEG variables for MT.
| rTEG variables | Sensitivity | Specificity | PPV | NPV |
|---|---|---|---|---|
| ACT >128 s | 58.33% | 74.32% | 30.88% | 90.07% |
| Angle <65° | 75.00% | 81.97% | 49.09% | 94.34% |
| MA <55 mm | 72.22% | 83.61% | 46.43% | 93.87% |
| LY30 > 5% | 41.67% | 93.99% | 58% | 89.12% |
| INR >1.3 | 66.67% | 88.20% | 46.15% | 94.58% |
| aPTT >34 s | 69.23% | 86.36% | 42.86% | 93.83% |
Discussion
In this study, we determined the degree of discrimination offered by ROTEM output values with respect to the need for MT. Based on the ROTEM parameters evaluated, thresholds were identified that could be used to guide hemostatic resuscitation for injured patients who require MT. We would therefore propose plasma transfusion for EXTEM CT > 78.5 s, fibrinogen products for EXTEM angle <64.5°, platelet transfusion for EXTEM CA10 < 40.5 mm, and antifibrinolytics for EXTEM CLI60 < 74% (Figure).
Fig 1.
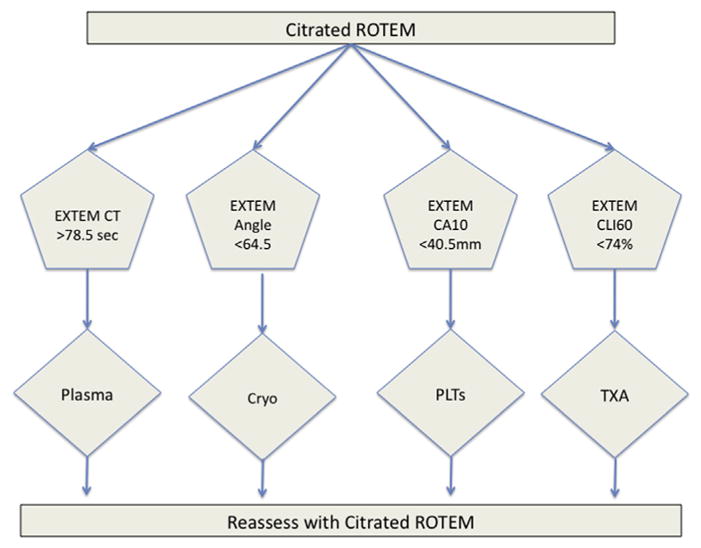
Proposed resuscitation thresholds based on ROTEM parameters implicating need for MT. A schematic representation of the proposed thresholds in a ROTEM-guided MTP for injured patients. Cryo = cryoprecipitate; PLTs = platelets; TXA = tranexamic acid. (Color version of figure is available online.)
The purpose of goal-directed therapy is to optimize a normal hemostatic competence until surgical hemostasis is obtained, which has been shown to improve mortality.19 Our primary aim was to determine optimal thresholds for blood component therapy in patients at risk for MT. Viscoelastic assays illustrate multiple, different hemostatic indices but not all are used clinically. Prolonged clot initiation is an indication for plasma, abnormal dynamics of clot formation is a signifier for fibrinogen products, low clot strength is an indication for platelets, and increased fibrinolysis is an implication for antifibrinolytics.8 Therefore, we used ROTEM measurements that focus on these aspects of clot formation and breakdown to develop a proposal for ROTEM-guided resuscitation. Studies have correlated EXTEM CT with coagulation factor activity; EXTEM Angle, EXTEM CFT, FIBTEM CA10, FIBTEM Angle, and FIBTEM MCF with fibrinogen level and function; EXTEM CA10 and EXTEM MCF with platelet-fibrinogen interactions; EXTEM CLI30, EXTEM CLI60, and EXTEM CT > APTEM CT or EXTEM MCF < APTEM MCF as evidence for fibrinolysis.14,20–24
In this study, we found that the ROTEM measurements that yielded the greatest AUROC curves were EXTEM angle (AUROC = 0.865) and EXTEM CA10 (AUROC = 0.858). This would correspond to deficiencies in dynamics of clot formation and clot strength, respectively. EXTEM CT yielded a weaker but fair AUROC curve of 0.7116. EXTEM CLI60 yielded a suboptimal AUROC curve of 0.6788.25 Extent of fibrinolysis, measured by TEG, and early mortality exhibit a quadratic relationship.26 This observation may be responsible for the diminished ability of viscoelastic measurement of fibrinolysis to predict MT. Furthermore, because of the suboptimal AUROC for CLI60, the predictive performance was compared to EXTEM CT > APTEM CT and EXTEM MCF < APTEM MCF. The addition of APTEM permits the quantitative assessment of fibrinolysis and thus an estimate of therapeutic benefit from response to an anti-fibrinolytic agent, specifically, tranexamic acid. An improvement in CT or MCF on APTEM compared to EXTEM unmasks a hyperfibrinolytic state.27,28 CLI60 of <74% had a much greater specificity and PPV while sensitivity and NPV were better with CT and MCF EXTEM/APTEM comparison (Table 4). This indicates fibrinolysis is driven by mechanisms beyond tissue plasminogen activator; for example, poor clot structure that may not be inhibited by tranexamic acid. EXTEM CT > APTEM CT and EXTEM MCF < APTEM MCF are determined more rapidly than EXTEM CLI60 based on the time for results. However, if the amount of blood obtained is limited, EXTEM CLI60 should be used to determine fibrinolysis as all aspects of proposed ROTEM thresholds can be determined from the EXTEM test alone.
The test with the greatest PPV was the EXTEM angle (Table 4). None of the parameters evaluated had a PPV that was greater than 53%. However, one could argue that there is greater harm in not identifying the need for MT than overestimating a need for MT. Furthermore, the value of ROTEM and TEG is in identifying which blood component is required.
A comparison of predictive capacity can be made between ROTEM, TEG, and CCAs (Tables 4 and 5). Rapid TEG had a similar PPV as EXTEM with regard to clot initiation (ACT), dynamics of clot formation (angle), and clot strength (MA). The PPV was better with rTEG LY30 compared to EXTEM CLI60 but was still only 58%. From this comparison of viscoelastic assays, it remains difficult to determine which, if either, assay is better. Furthermore, this study was not specifically designed to compare ROTEM versus TEG because it is not the same patient population as the previous study,7 so these comparisons must be taken cautiously. We have previously shown that a TEG-guided MTP improves survival as compared with those guided by CCA. These results were achieved with the use of less plasma and platelet transfusions during the early phases of resuscitation.8 Based on this, we would argue that either viscoelastic assay, ROTEM, or TEG, should be used instead of CCAs to guide resuscitation in injured patients. This is further illustrated by the fact that the PPV for aPTT and INR are no greater than 46.2% for either test.
There are several limitations to this study. These data reflect a single time point of a dynamic process and do not take into account the temporal changes of the coagulation process throughout the acute phase of resuscitation. Transfusion of blood products was based on goal-directed resuscitation based on TEG. These ROTEM values are then predictive of a MT that is already guided by viscoelastic testing. Because the measurements are analyzed for MT and not for individual components, they should be used cautiously. However, at this time, these measurements provide the best guidelines for specific component therapy. Finally, the sample size limits the ability to analyze subgroups.
In conclusion, these thresholds provide an important point in the evolution of viscoelastic-based resuscitation strategies. They may act as a guide to further evaluate ideal thresholds for the transfusion of specific blood products These observations should serve as a building block for a multicenter trial that should validate or refine these recommendations. Furthermore, refinement of the ROTEM-based resuscitation strategies should include optimizing the respective clinical interventions for each given ROTEM output.
Footnotes
Authors’ contributions: G.R.S. implemented the study, interpreted data, and drafted and critically revised the article. G.R.N. interpreted data and drafted and critically revised the article. J.C. collected patient data and compiled database. E.E.M., E.P., C.C.S., A.B., and A.S. are principal investigators and were responsible for study conception, design, and implementation of the study, interpretation of data, article drafting, and critical revision.
Presented at the Academic Surgical Congress; Jacksonville FL, January 30, 2018-February 1, 2018.
Disclosure
Research reported in this publication was supported in part by the National Institute of General Medical Sciences grants: T32-GM008315 and P50-GM49222, the National Heart, Lung, and Blood Institute UM1-HL120877, in addition to the Department of Defense USAMRAA and W81XWH-12-2-0028. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health, the National Heart, Lung, and Blood institute, or the Department of Defense. Additional research support was provided by TEM Systems and Haemonetics with shared intellectual property.
References
- 1.Tisherman SA, Schmicker RH, Brasel KJ, et al. Detailed description of all deaths in both the shock and traumatic brain injury hypertonic saline trials of the resuscitation outcomes consortium. Ann Surg. 2015;261:586–590. doi: 10.1097/SLA.0000000000000837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fries D, Martini WZ. Role of fibrinogen in trauma-induced coagulopathy. Br J Anaesth. 2010;105:116–121. doi: 10.1093/bja/aeq161. [DOI] [PubMed] [Google Scholar]
- 3.Wohlauer MV, Moore EE, Thomas S, et al. Early platelet dysfunction: an unrecognized role in the acute coagulopathy of trauma. J Am Coll Surg. 2012;214:739–746. doi: 10.1016/j.jamcollsurg.2012.01.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moore HB, Moore EE, Chapman MP, et al. Viscoelastic measurements of platelet function, not fibrinogen function, predicts sensitivity to tissue-type plasminogen activator in trauma patients. J Thromb Haemost. 2015;13:1878–1887. doi: 10.1111/jth.13067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rizoli SB, Scarpelini S, Callum J, et al. Clotting factor deficiency in early trauma-associated coagulopathy. J Trauma. 2011;71:S427–S434. doi: 10.1097/TA.0b013e318232e5ab. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Holcomb JB, Tilley BC, Baraniuk S, et al. Transfusion of plasma, platelets, and red blood cells in a 1:1:1 vs a 1:1:2 ratio and mortality in patients with severe trauma: the PROPPR randomized clinical trial. JAMA. 2015;313:471–482. doi: 10.1001/jama.2015.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Einersen PM, Moore EE, Chapman MP, et al. Rapid thrombelastography thresholds for goal-directed resuscitation of patients at risk for massive transfusion. J Trauma Acute Care Surg. 2017;82:114–119. doi: 10.1097/TA.0000000000001270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gonzalez E, Moore EE, Moore HB, et al. Goal-directed hemostatic resuscitation of trauma-induced coagulopathy: a pragmatic randomized clinical trial comparing a viscoelastic assay to conventional coagulation assays. Ann Surg. 2016;263:1051–1059. doi: 10.1097/SLA.0000000000001608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sankarankutty A, Nascimento B, Teodoro da Luz L, et al. TEG® and ROTEM® in trauma: similar test but different results? World J Emerg Surg. 2012;7:S3. doi: 10.1186/1749-7922-7-S1-S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gonzalez E, Pieracci FM, Moore EE, et al. Coagulation abnormalities in the trauma patient: the role of point-of-care thromboelastography. Semin Thromb Hemost. 2010;36:723–737. doi: 10.1055/s-0030-1265289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kashuk JL, Moore EE, Johnson JL, et al. Postinjury life threatening coagulopathy: is 1:1 fresh frozen plasma:packed red blood cells the answer? J Trauma. 2008;65:261–270. doi: 10.1097/TA.0b013e31817de3e1. discussion 70–1. [DOI] [PubMed] [Google Scholar]
- 12.Saracoglu A, Yarat A, Tetik S. The role of viscoelastic tests in trauma: “TEG and ROTEM”. J Pharmacol Med Chem. 2017;1:1–5. [Google Scholar]
- 13.Rahe-Meyer N, Solomon C, Winterhalter M, et al. Thromboelastometry-guided administration of fibrinogen concentrate for the treatment of excessive intraoperative bleeding in thoracoabdominal aortic aneurysm surgery. J Thorac Cardiovasc Surg. 2009;138:694–702. doi: 10.1016/j.jtcvs.2008.11.065. [DOI] [PubMed] [Google Scholar]
- 14.Tanaka KA, Bolliger D, Vadlamudi R, et al. Rotational thromboelastometry (ROTEM)-based coagulation management in cardiac surgery and major trauma. J Cardiothorac Vasc Anesth. 2012;26:1083–1093. doi: 10.1053/j.jvca.2012.06.015. [DOI] [PubMed] [Google Scholar]
- 15.Cohen MJ, Call M, Nelson M, et al. Critical role of activated protein C in early coagulopathy and later organ failure, infection and death in trauma patients. Ann Surg. 2012;255:379–385. doi: 10.1097/SLA.0b013e318235d9e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.MacLeod JB, Lynn M, McKenney MG, et al. Early coagulopathy predicts mortality in trauma. J Trauma. 2003;55:39–44. doi: 10.1097/01.TA.0000075338.21177.EF. [DOI] [PubMed] [Google Scholar]
- 17.Kunitake RC, Howard BM, Kornblith LZ, et al. Individual clotting factor contributions to mortality following trauma. J Trauma Acute Care Surg. 2017;82:302–308. doi: 10.1097/TA.0000000000001313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Perkins NJ, Schisterman EF. The inconsistency of “optimal” cutpoints obtained using two criteria based on the receiver operating characteristic curve. Am J Epidemiol. 2006;163:670–675. doi: 10.1093/aje/kwj063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fahrendorff M, Oliveri RS, Johansson PI. The use of viscoelastic haemostatic assays in goal-directing treatment with allogeneic blood products - a systematic review and meta-analysis. Scand J Trauma Resusc Emerg Med. 2017;25:39. doi: 10.1186/s13049-017-0378-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schochl H, Maegele M, Solomon C, et al. Early and individualized goal-directed therapy for trauma-induced coagulopathy. Scand J Trauma Resusc Emerg Med. 2012;20:15. doi: 10.1186/1757-7241-20-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Haas T, Fries D, Velik-Salchner C, et al. The in vitro effects of fibrinogen concentrate, factor XIII and fresh frozen plasma on impaired clot formation after 60% dilution. Anesth Analg. 2008;106:1360–1365. doi: 10.1213/01.ane.0b013e3181684339. table of contents. [DOI] [PubMed] [Google Scholar]
- 22.Naik BI, Pajewski TN, Bogdonoff DI, et al. Rotational thromboelastometry-guided blood product management in major spine surgery. J Neurosurg Spine. 2015;23:239–249. doi: 10.3171/2014.12.SPINE14620. [DOI] [PubMed] [Google Scholar]
- 23.Girdauskas E, Kempfert J, Kuntze T, et al. Thromboelastometrically guided transfusion protocol during aortic surgery with circulatory arrest: a prospective, randomized trial. J Thorac Cardiovasc Surg. 2010;140:1117–1124.e2. doi: 10.1016/j.jtcvs.2010.04.043. [DOI] [PubMed] [Google Scholar]
- 24.Fayed N, Mourad W, Yassen K, et al. Preoperative thromboelastometry as a predictor of transfusion requirements during adult living donor liver transplantation. Transfus Med Hemothe. 2015;42:99–108. doi: 10.1159/000381733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zaletel-Kragelj L, Bozikov J. Forum for Public Health Collaboration in South Eastern E. Methods and tools in Public Health a Handbook for Teachers, Researchers and Health Professionals. Austin, Texas: Lage: Jacobs; 2010. [Google Scholar]
- 26.Moore HB, Moore EE, Gonzalez E, et al. Hyperfibrinolysis, physiologic fibrinolysis, and fibrinolysis shutdown: the spectrum of postinjury fibrinolysis and relevance to antifibrinolytic therapy. J Trauma Acute Care Surg. 2014;77:811–817. doi: 10.1097/TA.0000000000000341. discussion 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kozek-Langenecker S. Management of massive operative blood loss. Minerva Anestesiol. 2007;73:401–415. [PubMed] [Google Scholar]
- 28.Vucelic D, Miljic P, Antonijevic N, et al. The role of rotational thromboelastometry in real time assessment of haemostasis in surgical settings. Srp Arh Celok Lek. 2010;138:43–49. doi: 10.2298/sarh10s1043v. [DOI] [PubMed] [Google Scholar]


