ABSTRACT
OBJECTIVE:
Wound dressings that use biosynthetic cellulose may be a good alternative to dressings currently used to treat chronic and acute ulcers because their nanostructure is similar to collagen. The objective of this study was to evaluate a wound dressing created with a new material that is composed of a fibrillary network of biosynthetic cellulose.
METHODS:
A case series of 8 patients in primary healthcare centers in Östergötland county council, Sweden, with chronic and acute lower limb wounds were treated with a wound dressing based on eiratex (S2Medical AB, Linköping, Sweden). The dressing was applied to traumatic (n = 5) and venous ulcers (n = 3). All ulcers were considered healed at the end of the treatment.
MAIN OUTCOME MEASURE:
The wounds were examined at regular intervals by a physician to determine healing time, number of dressing changes, and number of visits.
MAIN RESULTS:
Mean healing time was 43 ± 6 days after the first application of the dressing. The mean number of visits was 5.7 ± 0.6, and the mean number of dressings used per patient was 1.7 ± 0.2.
CONCLUSIONS:
These results demonstrate the efficacy of a wound dressing made of eiratex to heal chronic and acute ulcers. The data show that the number of dressings used and dressing changes needed to heal the ulcers are lower than what have been reported in the literature for other dressing materials.
KEYWORDS: acute ulcers, biosynthetic cellulose, chronic ulcers, collagen, nanomaterials, wound care, wound dressing
INTRODUCTION
Chronic ulcers, defined as ulcers that do not heal in 6 to 9 weeks,1 can be colonized by bacteria, viruses, parasites, and fungi.2 This type of ulcer has a big impact on patient quality of life and the healthcare system. There are different types of chronic ulcers, such as venous, arterial, neurotrophic, lymphatic, and drug induced.3 Venous ulcerations account for 70% to 90% of all chronic wounds and are most commonly found in the lower extremities. Reoccurrence is high, and the estimated treatment cost can exceed US $40,000 during the lifetime of each patient. Further, more than $1 billion is spent annually in the United States alone on treatment of chronic venous disease.4 The prevalence is 0.18% to 2% in the European population, and in patients older than 65 years, the prevalence is reaching 5%.5,6
The standard of care when treating venous ulcers is compression treatment to improve the venous return from the limb. However, this can lead to drying of the wound and increase the risk of infection.7 Different types of dressings are used, including hydrofibers, hydrocolloids, hydrogels, silicones, alginates, or polyurethanes to optimize the level of moisture in the wound depending on the healing phase.8
New, alternative wound dressings are based on a dense fibrillar network of the natural carbohydrate cellulose. Eiratex, one such dressing, was developed at Linköping University in Sweden. The membrane has a nanostructure that is similar to collagen in the skin, is highly hydrated, and shows excellent mechanical properties with high tensile strength and conformability.
The material is based on biosynthetic cellulose (BC) that is highly biocompatible and has been examined in various biomedical applications, including drug delivery, wound healing, and tissue engineering.9–13 Traditional BC has been problematic for use on infected wounds because blood and exudate can become trapped under the membrane, causing escalation of an infection. It can, in some cases, integrate with the wound tissue. However, because of its nanostructure, a wound dressing based on eiratex is permeable to water vapor, oxygen, and carbon dioxide but impermeable to bacteria and water (data not shown). Moreover, the material is highly conformable, which facilitates covering of exposed nerve endings in the wound, as well as rendering it transparent and flexible.
The hypothesis of this study was that a wound dressing based on eiratex could facilitate the healing of the wounds and overcome the problems related to the use of the traditional BC. The aim of this case series was to evaluate the efficacy of an eiratex-based wound dressing in treatment of chronic and acute lower limb venous ulcers.
METHODS
A case series/observational study was done in primary healthcare centers in Östergötland county council, Sweden. Inclusion criteria were: all patients with lower-extremity ulcers who were treated with eiratex for the whole study period; both uninfected and infected ulcers were included. Venous ulcers were diagnosed with duplex scan. In these patients, standard compression therapy was used. Exclusion criteria were: patients with exposure of bone, muscle, ligaments, or tendons. Patients with mental disorders were excluded to guarantee a conscious agreement to participate. Patients visiting local health centers in southeast Sweden who met the inclusion and exclusion criteria were asked to participate in the study. The procedure was conducted in accordance with the Declaration of Helsinki. Written consent for publication was collected from all of the patients involved. Data were blinded in a way that cannot be traced back to the research subjects.
Wound dressings were acquired free of charge from S2Medical AB (Linköping, Sweden). The eiratex dressing was applied on the wound after surgical debridement, where applicable. The wounds were examined every second day by the attending physician during the first week and then once per week until final healing, unless otherwise indicated according to the attending physician. Healing was defined as full epithelial coverage as assessed by the attending physician.
When the wound started to heal, the dressing was found to adhere to the wound, typically within 2 days from initiation of treatment. In case of lack of improvement of the wound healing, a new debridement was carried out, and a new eiratex membrane was applied to the wound. In 3 patients, clinical signs of infection were seen. Those patients were treated with systemic administration of clindamycin (300 mg 3 times per day for 10 days). Once the dressing had adhered to the wound, it was not removed until it spontaneously fell off. An examination by the attending physician was then performed to assess wound healing.
At each treatment visit, information regarding adverse events, medications, and other aspects of care since the last visit was recorded. All wounds were photographed before treatment and at each follow-up visit after treatment. Primary objectives were to assess wound healing time and number of dressing changes.
RESULTS
Eight patients were included in the study; 1 was male (12.5%) and 7 were females (87.5%). The mean age was 76 ± 3 years (minimum, 62 years; maximum, 85 years); 5 ulcers were traumatic (62.5%) and 3 were venous (37.5%). The time from first occurrence of the ulcer ranged from 18 to 180 days, with a mean of 88 ± 21 days (Table 1). Mean healing time was 43 ± 6 days after the first application of the dressing, with a minimum of 18 days and a maximum of 73 days. The mean number of visits was 5.7 ± 0.6, whereas the mean number of dressings used per patient was 1.7 ± 0.2 (Table 2). In 3 patients, ulcers were infected before the application of the dressing. The Figure shows the progress of wound healing in this group.
Table 1.
CLINICAL DATA
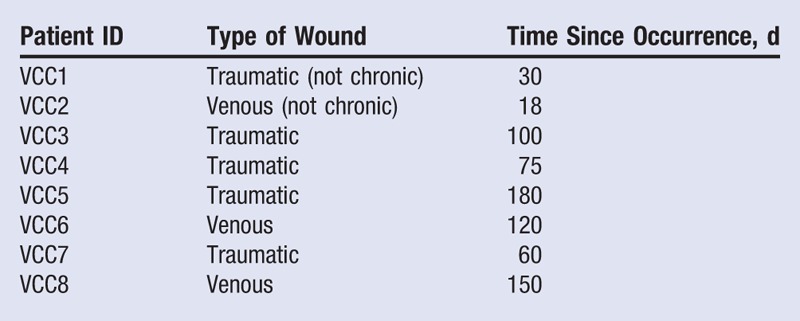
Table 2.
DATA ACQUIRED DURING THE STUDY
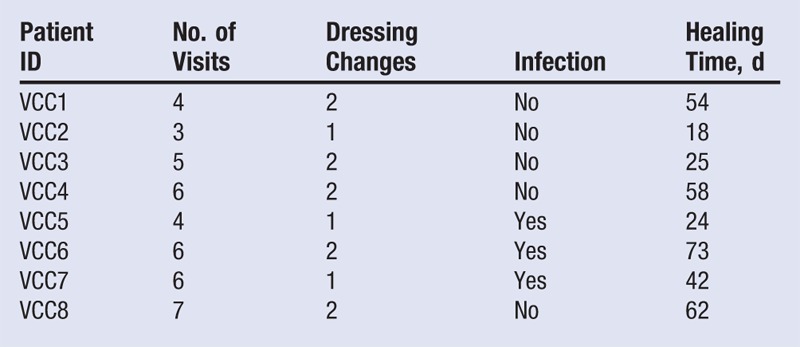
Figure.
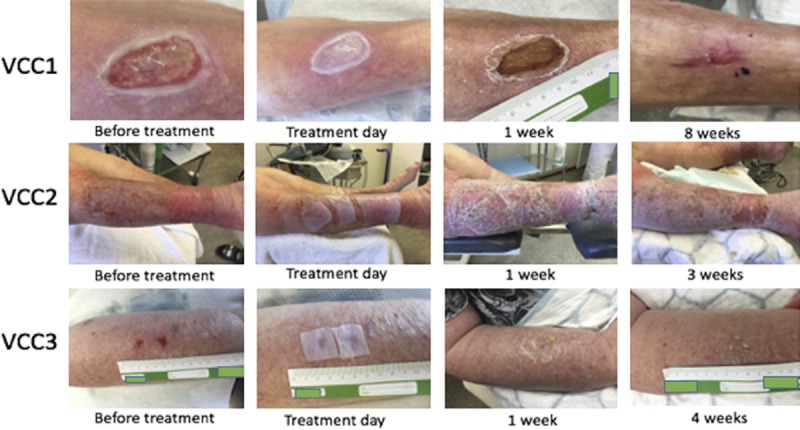
PHOTOGRAPHS OF ULCERS IN PATIENTS VCC1-VCC3 BEFORE TREATMENT, DIRECTLY AFTER APPLICATION OF THE WOUND DRESSING, AND 1 AND 3 TO 8 WEEKS AFTER TREATMENT WAS INITIATED, RESPECTIVELY
DISCUSSION
Dressing Changes
After dividing the number of dressings used to heal the wounds by the healing time, the frequency of dressing changes in this study was 0.24 dressing per week. This low number is because dressings made from eiratex, once applied, do not have to be removed from the wound until healing is complete. The dressing will adhere to the wound if there is no infection; if the wound is infected, it will not attach. Once adhered, the wound will form a scab under the dressing. Because of the lack of cellulase enzyme in the human organism, the dressing cannot be absorbed by the body and will therefore peel off from the wound once the wound is healed. Moreover, because eiratex helps to promote scab formation, the amount of exudate will be lower. Interestingly, in studies on advanced wound dressings such as the ones created from cells found in healthy human skin to treat venous ulcers,14 the dressing was changed up to 5 times during the first 3 weeks of treatment.
O’Donnell and Lau15 analyzed the results of 20 randomized controlled trials of wound dressings for chronic venous ulcers. In trial subjects, wound dressings were divided into different groups: nonocclusive, semiocclusive/occlusive, dressings containing growth factors, and the human skin equivalent group. The results of this review reveal that 8 wound dressing types were changed once per week, and in 5 studies, the dressings were changed twice per week. In another study from Andersen et al,16 a polyurethane dressing was tested on patients with lower leg ulcers. The dressing was changed once per week during the treatment.
Wound Healing Time
In a study from 2002 on diabetic foot ulcers, Zimny et al17 evaluated wound healing in 31 patients with type 1 or 2 diabetes with plantar foot ulcers receiving wound care treatment including use of proper footwear, non–weight-bearing limb support, appropriate antibiotics, and debridement. The authors report that the mean healing time for these patients was 77.7 (95% confidence interval, 62–93) days. In contrast, the eiratex dressings used in this study resulted in a healing time of 42 ± 8 days.
Wound Dressing Cost Comparison
In a study from 2009, Payne et al18 calculated the average treatment cost per week in the United States to treat patients with pressure injuries. In this study, the costs for the use of a polyurethane foam (Allevyn; Smith and Nephew, Fort Worth, Texas) and a saline-soaked gauze were compared. By taking into account the frequency of dressing change reported by the authors and considering a dressing area of 10 cm2, the cost of using Allevyn is US $71.54 per week, whereas the saline-soaked gauze is US $179.31 per week, assuming 30 minutes of work for a nurse at US $27.54 per hour. The results of the current study show that an average of 0.24 dressing per week was needed to heal participant wounds. Therefore, the cost for material and labor is US $7.60 per week with eiratex. This result shows that, more than the price of the dressing itself, the frequency of dressing changes affects the final cost of the treatment.
Limitations
Because of the low number of patients included in this case series, a larger prospective randomized controlled trial is needed to verify these results. Such a study is currently being planned by the authors.
CONCLUSIONS
The results of this case series, compared with the data reported in the studies mentioned above, suggest that the use of a wound dressing made of eiratex could decrease the healing time and frequency of dressing changes when used on venous ulcers. The use of advanced materials such as eiratex for wound healing can increase the quality of life for these patients by reducing the number of dressing changes during the treatment. In addition, a decrease in the number of dressings used for the whole treatment will result in a reduced cost for the healthcare system. Most importantly, eiratex represents a new and efficient method to increase the healing rate of chronic venous ulcers and can reduce the associated costs for the healthcare system.
Footnotes
Acknowledgments: The authors thank all of the patients involved in the study, which was performed exclusively in Sweden (trial registration no. ISRCTN14756102). Petter Sivlér, Mårten Skog, and Tobias Sivlér are founders of S2Medical AB but are not direct shareholders of the company. Wound dressings were provided free of charge by S2Medical AB for the purposes of this study. The authors have disclosed no other financial relationships related to this article.
REFERENCES
- 1.Kahle B, Hermanns HJ, Gallenkemper G. Evidence-based treatment of chronic leg ulcers. Dtsch Arztebl Int 2011;108(14):231-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adam DJ, Naik J, Hartshorne T, Bello M, London NJ. The diagnosis and management of 689 chronic leg ulcers in a single-visit assessment clinic. Eur J Vasc Endovasc Surg 2003;25:462-8. [DOI] [PubMed] [Google Scholar]
- 3.Moffat CJ, Franks PJ, Doherty DC, et al. Prevalence of leg ulceration in a London population. QJM 2004;97(7):431-7. [DOI] [PubMed] [Google Scholar]
- 4.Lazarides MK, Giannoukas AD. The role of hemodynamic measurements in the management of venous and ischemic ulcers. Int J Low Extrem Wounds 2007;6(4):254-61. [DOI] [PubMed] [Google Scholar]
- 5.Lautenschlager S, Eichmann A. Differential diagnosis of leg ulcers. Curr Probl Dermatol 1999;27:259-70. [DOI] [PubMed] [Google Scholar]
- 6.Mekkes JR, Loots MAM, Van der Wal AC, Bos JD. Causes, investigation and treatment of leg ulceration. Br J Dermatol 2003;148(3):388-401. [DOI] [PubMed] [Google Scholar]
- 7.Atiyeh BS, Costagliola M, Hayek SN, Dibo SA. Effect of silver on burn wound infection control and healing: review of the literature. Burns 2007;33(2):139-48. [DOI] [PubMed] [Google Scholar]
- 8.Queen D, Orsted H, Sanada H, Sussman G. A dressing history. Int Wound J 2004;1(1):59-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Czaja W, Krystynowicz A, Bielecki S, Brown RM., Jr Microbial cellulose-the natural power to heal wounds. Biomaterials 2006;27:145-51. [DOI] [PubMed] [Google Scholar]
- 10.Grande CJ, Torres FG, Gomez CM, Martinez-Pastor J. Morphological characterisation of bacterial cellulose-starch nanocomposites. Polymers Polymer Composites 2008;16:181-5. [Google Scholar]
- 11.Li J, Wan Y, Li L, Liang H, Wang J. Preparation and characterization of 2,3-dialdehyde bacterial cellulose for potential biodegradable tissue engineering scaffolds. Mater Sci Eng 2009;29(5):1635-42. [Google Scholar]
- 12.Grande CJ, Torres FG, Gomez CM, Bañó MC. Nanocomposites of bacterial cellulose/hydroxyapatite for biomedical applications. Acta Biomater 2009;5:1605-15. [DOI] [PubMed] [Google Scholar]
- 13.Helenius G, Bäckdahl H, Bodin A, Nannmark U, Gatenholm P, Risberg B. In vivo biocompatibility of bacterial cellulose. J Biomed Mater Res A 2006;76:431-8. [DOI] [PubMed] [Google Scholar]
- 14.Falanga V, Margolis D, Alvarz O, et al. Rapid healing of venous ulcers and lack of clinical rejection with an allogeneic cultured human skin equivalent. Arch Dermatol 1998;134(3):293-300. [DOI] [PubMed] [Google Scholar]
- 15.O’Donnell TF, Jr, Lau J. A systematic review of randomized controlled trials of wound dressings for chronic venous ulcer. J Vasc Surg 2006;44(5):1118-25. [DOI] [PubMed] [Google Scholar]
- 16.Andersen KE, Franken CPM, Gad P, et al. A randomized, controlled study to compare the effectiveness of two foam dressings in the management of lower leg ulcers. Ostomy Wound Manage 2002;48(8):34-41. [Google Scholar]
- 17.Zimny S, Schatz H, Pfohl M. Determinants and estimation of healing times in diabetic foot ulcers. J Diabetes Complications 2002;16(5):327-32. [DOI] [PubMed] [Google Scholar]
- 18.Payne WG, Posnett J, Alvarez O, et al. A prospective, randomized clinical trial to assess the cost-effectiveness of a modern foam dressing versus a traditional saline gauze dressing in the treatment of stage II pressure ulcers. Ostomy Wound Manage 2009;55(2):50-5. [PubMed] [Google Scholar]


