Supplemental Digital Content is available in the text.
Keywords: atherosclerosis, galactosyl-(1-3)galactose, immunoglobulin E, red meat, risk factors
Abstract
Objective—
Emerging evidence suggests a link between coronary artery disease and type 2 immunity. We sought to test the hypothesis that IgE sensitization to the mammalian oligosaccharide galactose-α-1,3-galactose (α-Gal)—the target allergen of delayed anaphylaxis to red meat—is associated with coronary artery disease.
Approach and Results—
Total IgE and specific IgE to α-Gal were assayed on sera from 118 subjects who presented for cardiac catheterization and underwent intravascular ultrasound. IgE to α-Gal was detected in 26%, and atheroma burden was higher in sensitized subjects (P=0.02). Because α-Gal sensitization relates to an environmental exposure that could be a risk factor for early-onset coronary artery disease (ie, tick bites), we age stratified the cohort. In subjects ≤65 years of age, the strength of the association with atheroma burden was stronger (P<0.001), and plaques in the sensitized group had less stable features based on intravascular ultrasound. To address the specificity of the association with IgE to α-Gal, IgE to inhalants and peanut were assayed and were not associated with coronary artery disease. Total IgE and α-Gal–specific IgE were strongly associated with each other, but the strength of the relationship with atheroma burden was stronger for α-Gal–specific IgE. This association was significant when adjusted for sex, diabetes mellitus, hypertension, statin use, and total IgE (regression coefficient, 12.2; SE, 5.2; P=0.02).
Conclusions—
Increased atheroma burden and plaques with more unstable features were associated with IgE to α-Gal—an effect most pronounced in subjects ≤65 years of age. IgE sensitization to α-Gal may represent a novel, and potentially modifiable, risk factor for coronary atherosclerosis.
Inflammation is integral to the pathogenesis of coronary artery disease (CAD), and a role for food-specific antibodies and mast cells has long been a subject of inquiry.1–3 Recent reports have confirmed a role for mast cells and demonstrated an association between levels of total serum IgE and cardiovascular disease, though no specific allergens have been identified.4–6 Galactose-α-1,3-galactose (α-Gal) is a blood group-like oligosaccharide of nonprimate mammals and is recognized in humans as a target of natural IgM, IgA, and IgG antibodies.7,8 In 2008, IgE specific to α-Gal was identified as the cause of anaphylactic reactions to cetuximab.9 Subsequently, it became clear that this IgE antibody is also the primary cause of delayed anaphylaxis to red meat (α-Gal syndrome).10 In the United States, sensitization to α-Gal is recognized as a consequence of bites of the tick Amblyomma americanum, which is in keeping with a prevalence of sensitization to α-Gal as high as 20% in areas of the southeast.11 Although α-Gal is present in mammalian meat as both glycolipids and glycoproteins, the delay in reactions to red meat is best explained by the time taken for processing of lipids into chylomicrons and smaller lipoprotein particles, such as LDL (low-density lipoprotein) or HDL (high-density lipoprotein).12,13 Taken together, α-Gal represents a foreign epitope, which is regularly consumed as glycolipids and to which a subset of the population has a strikingly different type of immune response. Thus, it is possible that specific IgE (sIgE) on mast cells, including those in coronary arteries, could increase the inflammatory response to dietary glycolipids. Here, we sought to use intravascular ultrasound imaging in a population where sensitization is prevalent to investigate whether the presence of IgE sensitization to α-Gal is related to the burden of CAD.14
Methods
Data are available on request from the authors.
Study Cohort
The studied population consisted of subjects between the ages of 30 to 80 years who were undergoing medically warranted cardiac catheterization and were enrolled into ongoing studies at the University of Virginia as described previously.15 Written consent was obtained from all subjects, and the studies were approved by the Institutional Review Board at the University of Virginia.
Intravascular Ultrasound
Intravascular ultrasound was conducted as previously described and in accordance with the standards of the American College of Cardiology for image acquisition on a noninfarct-related artery.14,15 Analysis was conducted by an investigator (A.T.N. or A.M.T.) who was blind to IgE assay results. The fraction of atherosclerotic plaque that was fibrous, fibrofatty, necrotic, or calcified (as determined by intravascular ultrasound) was calculated as a percentage.
Assays
Total IgE and sIgE to α-Gal, dust mite (Dermatophagoides pteronyssinus), oak, timothy grass, ragweed, and peanut were assayed using ImmunoCAP 250 (Thermo-Fisher/Phadia, Kalamazoo, MI) as described previously.10 The solid phase for the α-Gal assay utilized biotinylated cetuximab conjugated to streptavidin ImmunoCAP.9
Statistics
Prism 7.0 (GraphPad, San Diego, CA) was used for graphs and statistics. Categorical variables were compared with Fisher exact test. Continuous data were compared by the Mann–Whitney U test. Spearman rank correlation was used to compare the relationship between α-Gal IgE and total IgE. Regression modeling was performed with IBM SPSS statistics, version 24.0 (IBM Corp, Armonk, NY).
Results
Using a cutoff of ≥0.1 kU/L, 26.3% of the subjects had detectable levels of IgE to α-Gal, and, not unexpectedly, there was higher total IgE in the sensitized group. There was no difference in established risk factors or markers of CAD, such as hypertension, lipid levels, or diabetes mellitus, though the sensitized group had a higher percentage of males and black subjects (Table I in the online-only Data Supplement). Intravascular ultrasound revealed greater burden and volume of atheroma in α-Gal–sensitized subjects (Figure [A and B]). Because induction of IgE to α-Gal results from an environmental exposure that could disproportionately impact younger adults, we stratified the cohort into 3 age groups with roughly equal numbers of subjects. This stratification revealed a striking difference between subjects of relatively younger versus older age. The significant association of atheroma burden and volume in α-Gal–sensitized compared with nonsensitized subjects was predominantly driven by results in subjects ≤65 years of age (Figure [B]), so additional analysis focused on this group (n=79). The demographic characteristics and risk factors in these subjects (≤65 years) were similar to the cohort as a whole (Table I in the online-only Data Supplement), and atherosclerotic plaques had significantly more fibrofatty, necrotic, and calcified content but were less fibrous in α-Gal–sensitized than nonsensitized subjects (Figure [C]). IgE to a panel of inhalant allergens (including dust mite, oak, timothy grass, and ragweed) and IgE to peanut, in contrast to IgE to α-Gal, did not have a significant relationship with atheroma burden, atheroma volume, or maximal stenosis (Table 1). To model the association of CAD with α-Gal sIgE status, we conducted linear regression of atheroma burden as a continuous dependent variable with the independent variable of presence or absence of α-Gal sIgE. Although there was a significant association between α-Gal sIgE and total IgE (Figure I in the online-only Data Supplement), in regression modeling, the strength of the relationship with atheroma burden was stronger for IgE to α-Gal than for high levels of total IgE (using 100 kU/L as a cutoff; Table 2). Moreover, total IgE >100 kU/L did not have a significant association with atheroma volume or maximal stenosis (Table 1). Collectively, these results support the specificity of the relationship between α-Gal sIgE and CAD. Finally, the relationship between α-Gal IgE status and atheroma burden was preserved in regression analysis adjusted for sex, diabetes mellitus, hypertension, statin use, and total IgE (regression coefficient, 12.2; SE, 5.2; P=0.02; Table 2).
Table 1.
Comparison of Atheroma Measurements Based on sIgE Status to α-Gal, a Panel of Common Inhalants,* Peanut, or Total IgE >100 kU/L

Table 2.
Results of Univariate and Multivariate Regression Analysis of Relationship With Atheroma Burden in Subjects ≤65 y of Age (n=79)
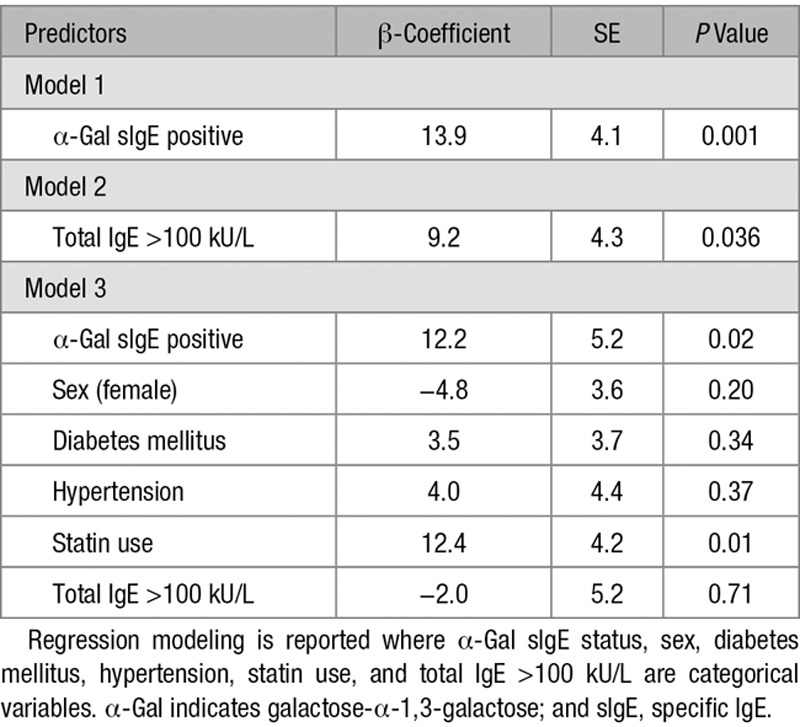
Figure.
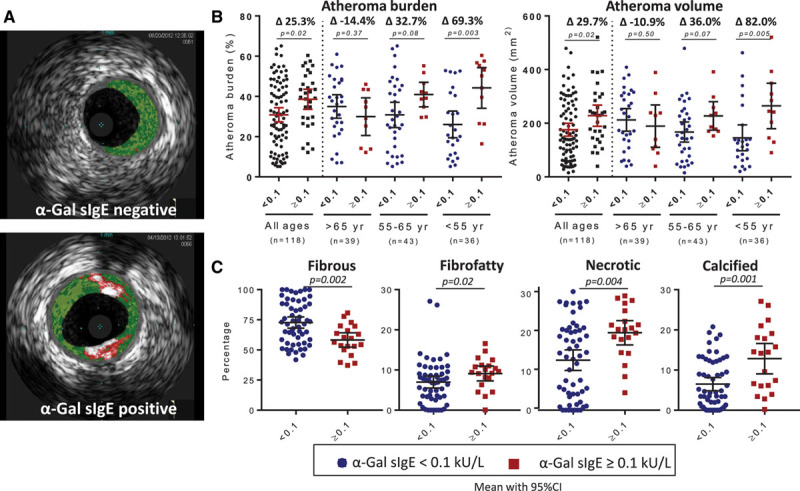
A, Representative examples of intravascular ultrasound (IVUS) with fibrous (dark green), fibrofatty (light green), necrotic (red), and calcified plaques (white) evident. B, Results of IVUS in total cohort and when stratified by age. C, Results of IVUS virtual histology in subjects ≤65 y of age. Data in B and C show mean with 95% confidence interval (CI) where each data point represents 1 subject and comparison is with the Mann–Whitney U test. Percentage differences in the mean value between galactose-α-1,3-galactose (α-Gal)–positive and α-Gal–negative subjects within an age group are represented in B by Δ. sIgE indicates specific IgE.
Discussion
To our knowledge, this is the first report that has described an association between the IgE response to α-Gal, or any specific allergen, and the burden of CAD. A link between a food allergen and CAD may not seem a priori obvious, but there are several elements of the immune response to α-Gal that argue for biological plausibility. Because many, or perhaps most, subjects with IgE to α-Gal do not have allergic symptoms, an implication is that many people, despite mounting a unique and potentially detrimental immune response to α-Gal, continue to consume foods that contain the oligosaccharide allergen.16 In regard to the cause-effect relationship, we are not aware of any evidence that dietary exposure to mammalian products can promote IgE to α-Gal, and it is similarly unlikely that atherosclerosis itself could contribute to this response.11
The study design limits mechanistic insight, but we hypothesize that chronic ingestion of α-Gal–glycosylated mammalian products, particularly the glycolipid form of the allergen, can lead to recurrent release of inflammatory products from mast cells bearing sIgE.4 A role for other cells, which express members of the Fcε or Fcγ family of receptors, such as macrophages or basophils, cannot be discounted, especially given that IgG1 often increases in parallel with IgE.17 The induction of sIgE (and IgG1) to the same antigen that is the target of abundant natural antibodies, as occurs with α-Gal, is also interesting because it is in keeping with prior reports that natural antibodies to oxidation-specific epitopes are protective in atherosclerosis, whereas class-switched antibodies can be proatherogenic.18 Relatedly, an inverse association between levels of α-Gal–specific IgM and IgG2 antibodies and CAD has been reported previously.19
There are several limitations to consider. We do not know the allergic histories or dietary habits of the participating subjects. Our assumption, based on clinical experience and other cohorts, is that most subjects in this study did not have a history of symptomatic α-Gal syndrome (ie, urticaria or anaphylaxis) and consequently are regularly consuming mammalian products.16 The known association of total IgE with atherosclerosis is a potential confounder; however, in this cohort, the association with CAD is stronger for α-Gal sIgE than total IgE. Moreover, in areas where delayed anaphylaxis to red meat is common, it is likely that elevated total IgE levels are often directly attributable to the same environmental trigger that generates IgE to α-Gal (ie, tick bites).11,20 The specificity of the relationship with α-Gal sIgE is also supported by the finding that sIgE antibodies to a panel of inhalants and peanut did not have a significant relationship with CAD. Finally, our current cohort is relatively small, and the effect was predominantly in subjects ≤65 years of age.
In summary, we report here that a specific antibody response to the dietary antigen α-Gal is associated with an increased burden of atherosclerosis and plaques with less stable characteristics. As the causal agent of a delayed allergic reaction to mammalian meat, which is IgE mediated, the glycolipid form of α-Gal may be particularly relevant to understanding its role in atherosclerosis. Mechanistic studies in animal models are suggested, but ultimately, efforts to translate this finding into clinical relevance will benefit from analysis of larger cohorts, including disparate geographic areas and prospective studies of adult subjects, which include information on diet, allergic history, and detailed investigation into sIgE antibodies.
Acknowledgments
We are grateful to the participants in the cardiovascular cohort. We thank Lisa Workman for assistance with IgE assays and Jim Garmey for sample acquisition.
Sources of Funding
This study was supported by the National Institutes of Health funding KO8-AI085190 (Dr Commins), K23-HL093118 (Dr Taylor), RO1-AI 20565 (Dr Platts-Mills), PO1-HL55798 (Dr McNamara), RO1-HL136098-01 (Dr McNamara), and RO1-HL107490 (Dr McNamara).
Disclosures
Dr Commins has been on the speaker’s bureau for Genentech. Dr Platts-Mills has a patent on ImmunoCAP IgE assays to galactose-α-1,3-galactose (α-Gal) and has received assay support from Thermo-Fisher/Phadia. Drs McNamara and Taylor have had research grants with Astra-Zeneca.
Nonstandard Abbreviations and Acronyms
- α-Gal
- galactose-α-1,3-galactose
- CAD
- coronary artery disease
- HDL
- high-density lipoprotein
- LDL
- low-density lipoprotein
- sIgE
- specific IgE
The online-only Data Supplement is available with this article at http://atvb.ahajournals.org/lookup/suppl/doi:10.1161/ATVBAHA.118.311222/-/DC1.
Highlights
IgE to galactose-α-1,3-galactose (α-Gal) is common in adults in Central Virginia who are clinically judged to be at cardiovascular risk.
In subjects ≤65 years of age, sensitization to α-Gal is associated with greater burden of atheroma and with plaques that have less stable characteristics.
As an oligosaccharide that is present on glycolipids in mammalian meat, α-Gal may represent a novel risk factor for coronary artery disease in IgE-sensitized subjects.
References
- 1.Hansson GK, Hermansson A. The immune system in atherosclerosis. Nat Immunol. 2011;12:204–212. doi: 10.1038/ni.2001. doi: 10.1038/ni.2001. [DOI] [PubMed] [Google Scholar]
- 2.Davies DF, Davies JR, Richards MA. Antibodies to reconstituted dried cow’s milk protein in coronary heart disease. J Atheroscler Res. 1969;9:103–107. doi: 10.1016/s0368-1319(69)80071-2. [DOI] [PubMed] [Google Scholar]
- 3.Constantinides P. Mast cells and susceptibility to experimental atherosclerosis. Science. 1953;117:505–506. doi: 10.1126/science.117.3045.505. [DOI] [PubMed] [Google Scholar]
- 4.Sun J, Sukhova GK, Wolters PJ, Yang M, Kitamoto S, Libby P, MacFarlane LA, Mallen-St Clair J, Shi GP. Mast cells promote atherosclerosis by releasing proinflammatory cytokines. Nat Med. 2007;13:719–724. doi: 10.1038/nm1601. doi: 10.1038/nm1601. [DOI] [PubMed] [Google Scholar]
- 5.Wang J, Cheng X, Xiang MX, et al. IgE stimulates human and mouse arterial cell apoptosis and cytokine expression and promotes atherogenesis in Apoe-/- mice. J Clin Invest. 2011;121:3564–3577. doi: 10.1172/JCI46028. doi: 10.1172/JCI46028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kounis NG, Hahalis G. Serum IgE levels in coronary artery disease. Atherosclerosis. 2016;251:498–500. doi: 10.1016/j.atherosclerosis.2016.05.045. doi: 10.1016/j.atherosclerosis.2016.05.045. [DOI] [PubMed] [Google Scholar]
- 7.Galili U, Rachmilewitz EA, Peleg A, Flechner I. A unique natural human IgG antibody with anti-alpha-galactosyl specificity. J Exp Med. 1984;160:1519–1531. doi: 10.1084/jem.160.5.1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Galili U. The Natural Anti-Gal Antibody as Foe Turned Friend in Medicine. 1st ed. Cambridge, MA: Elsevier; 2017. [Google Scholar]
- 9.Chung CH, Mirakhur B, Chan E, et al. Cetuximab-induced anaphylaxis and IgE specific for galactose-alpha-1,3-galactose. N Engl J Med. 2008;358:1109–1117. doi: 10.1056/NEJMoa074943. doi: 10.1056/NEJMoa074943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Commins SP, Satinover SM, Hosen J, Mozena J, Borish L, Lewis BD, Woodfolk JA, Platts-Mills TA. Delayed anaphylaxis, angioedema, or urticaria after consumption of red meat in patients with IgE antibodies specific for galactose-alpha-1,3-galactose. J Allergy Clin Immunol. 2009;123:426–433. doi: 10.1016/j.jaci.2008.10.052. doi: 10.1016/j.jaci.2008.10.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Commins SP, James HR, Kelly LA, Pochan SL, Workman LJ, Perzanowski MS, Kocan KM, Fahy JV, Nganga LW, Ronmark E, Cooper PJ, Platts-Mills TA. The relevance of tick bites to the production of IgE antibodies to the mammalian oligosaccharide galactose-α-1,3-galactose. J Allergy Clin Immunol. 2011;127:1286–1293.e6. doi: 10.1016/j.jaci.2011.02.019. doi: 10.1016/j.jaci.2011.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Commins SP, Jerath MR, Cox K, Erickson LD, Platts-Mills T. Delayed anaphylaxis to alpha-gal, an oligosaccharide in mammalian meat. Allergol Int. 2016;65:16–20. doi: 10.1016/j.alit.2015.10.001. doi: 10.1016/j.alit.2015.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Commins SP, James HR, Stevens W, Pochan SL, Land MH, King C, Mozzicato S, Platts-Mills TA. Delayed clinical and ex vivo response to mammalian meat in patients with IgE to galactose-alpha-1,3-galactose. J Allergy Clin Immunol. 2014;134:108–115. doi: 10.1016/j.jaci.2014.01.024. doi: 10.1016/j.jaci.2014.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mintz GS, Nissen SE, Anderson WD, Bailey SR, Erbel R, Fitzgerald PJ, Pinto FJ, Rosenfield K, Siegel RJ, Tuzcu EM, Yock PG. American College of Cardiology clinical expert consensus document on standards for acquisition, measurement and reporting of intravascular ultrasound studies (IVUS). A report of the American College of Cardiology task force on clinical expert consensus documents. J Am Coll Cardiol. 2001;37:1478–1492. doi: 10.1016/s0735-1097(01)01175-5. [DOI] [PubMed] [Google Scholar]
- 15.Manichaikul A, Rich SS, Perry H, Yeboah J, Law M, Davis M, Parker M, Ragosta M, Connelly JJ, McNamara CA, Taylor AM. A functionally significant polymorphism in ID3 is associated with human coronary pathology. PLoS One. 2014;9:e90222. doi: 10.1371/journal.pone.0090222. doi: 10.1371/journal.pone.0090222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fischer J, Lupberger E, Hebsaker J, Blumenstock G, Aichinger E, Yazdi AS, Reick D, Oehme R, Biedermann T. Prevalence of type I sensitization to alpha-gal in forest service employees and hunters. Allergy. 2017;72:1540–1547. doi: 10.1111/all.13156. doi: 10.1111/all.13156. [DOI] [PubMed] [Google Scholar]
- 17.Rispens T, Derksen NI, Commins SP, Platts-Mills TA, Aalberse RC. IgE production to α-Gal is accompanied by elevated levels of specific IgG1 antibodies and low amounts of IgE to blood group B. PLoS One. 2013;8:e55566. doi: 10.1371/journal.pone.0055566. doi: 10.1371/journal.pone.0055566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rosenfeld SM, Perry HM, Gonen A, Prohaska TA, Srikakulapu P, Grewal S, Das D, McSkimming C, Taylor AM, Tsimikas S, Bender TP, Witztum JL, McNamara CA. B-1b cells secrete atheroprotective IgM and attenuate atherosclerosis. Circ Res. 2015;117:e28–e39. doi: 10.1161/CIRCRESAHA.117.306044. doi: 10.1161/CIRCRESAHA.117.306044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mosedale DE, Chauhan A, Schofield PM, Grainger DJ. A pattern of anti-carbohydrate antibody responses present in patients with advanced atherosclerosis. J Immunol Methods. 2006;309:182–191. doi: 10.1016/j.jim.2005.12.003. doi: 10.1016/j.jim.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 20.Wilson JM, Schuyler AJ, Schroeder N, Platts-Mills TA. Galactose-α-1,3-galactose: atypical food allergen or model IgE hypersensitivity? Curr Allergy Asthma Rep. 2017;17:8. doi: 10.1007/s11882-017-0672-7. doi: 10.1007/s11882-017-0672-7. [DOI] [PMC free article] [PubMed] [Google Scholar]


