Abstract
This study aimed to investigate the time-related association between cumulative fluid balance (FB) and mortality. Data were extracted from the Medical Information Mart for Intensive Care (MIMIC) III. FB data on 8584 patients at the first (FB-fir24hr) and second (FB-sec24hr) 24 hours after intensive care unit admission were analysed. Compared to the combination of FB-fir24hr ≤ 0 and FB-sec24 hr ≤ 0, the combination of FB-fir24hr > 0 and FB-sec24hr ≤ 0 had significantly higher FB, with an insignificant odds ratio (OR) for mortality. However, the mortality ORs of two other combinations (FB-fir24hr ≤ 0 and FB-sec24hr > 0; FB-fir24hr > 0 and FB-sec24hr > 0) were significantly high. Furthermore, multivariable logistic analysis showed a significant stepwise increase ORs for mortality with increasing FB-sec24hr quartiles, with no significant increase in FB-fir24hr quartiles aside from quartile 4. In patients with negative FB, a stepwise decrease in mortality ORs with increasing FB-sec24hr quartiles was found with no significant difference in FB-fir24hr quartiles. In conclusion, the positive FB during the second but not the first 24 hours was associated with increased mortality in sepsis. Achieving more negative FB was associated with decreased mortality only in the second 24 hours.
Introduction
Fluid management is critically important in the initial stages of sepsis resuscitation. Since the 2001 landmark study by Rivers et al. reporting early goal-directed therapy1, several recommendations2,3 concerning fluid management have been suggested; these mainly consist of the use of an adequate and large volume of fluid resuscitation, especially during the first six hours after sepsis onset4.
As relative hypovolemia induced by vasodilatation disorder and capillary leak is common in patients with sepsis, appropriate fluid resuscitation is necessary to maintain adequate tissue perfusion. However, excess fluid administration can also lead to adverse outcomes, such as worsening respiratory function5, increased intra-abdominal pressure6, and coagulation disorder7. A post-hoc analysis in the Vasopressin and Septic Shock Trial (VASST) found that cumulative positive fluid balance (FB) over four days after admission was reported to be associated with higher mortality in septic shock8. Micek et al. also reported that the highest quartile of positive FB at eight days was an independent predictor of hospital mortality9. Consistent with these findings, another observational study10 found that the accumulated positive FB in the first 48, 72, and 96 hours was associated with higher mortality in sepsis. However, conclusions regarding the association between early positive FB and mortality are inconsistent. Several studies11,12 reported that positive FB within 24 hours after admission was not associated with increased death in sepsis, while other studies8,13 reported the opposite conclusion. There are several possible reasons for this. First, in patients with unstable haemodynamics, the detrimental effect of positive FB may be overwhelmed by the benefit of adequate fluid resuscitation, thus leading to an insignificant association. On the other hand, the true benefit of negative FB in this association may be overestimated, as the lowest FB quartile was commonly used as the reference quartile8,11. Whether there is a volume-related association between negative FB and mortality in sepsis remain unclear.
Therefore, this study had two main objectives: (1) To explore whether haemodynamic status played a role in the association between FB and mortality in sepsis, and (2) To explore whether achieving more negative FB would be beneficial in patients with sepsis. We speculated that a possible interaction between FB and haemodynamic status could lead to the disputed conclusions regarding the association between early positive FB and sepsis-related mortality.
Results
Comparisons of combinations
Data of 8584 patients with sepsis were included in this analysis, with an overall mortality rate of 21.8%. Comparisons of baseline characteristics between survivors and non-survivors are listed in Table 1. The mean age upon admission was 65.9 years, and 45.1% were male. Compared to Combination I (FB in the first 24 hours after ICU admission [FB-fir24hr] ≤ 0 and FB in the second 24 hours [FB-sec24hr] ≤ 0) (Table 2), Combination III (FB-fir24hr > 0 and FB-sec24hr ≤ 0) had significantly higher FB at 48 hours (FB-48hr) (−31.9 ± 19.9 vs. 17.7 ± 32.1, p < 0.001). However, the mortality rate between these two groups was quite similar (140/1040 (13.5%) vs. 226/1836 (12.3%), p = 0.373), and the adjusted odds ratio (OR) (Table 3) was insignificant in multivariable logistic regression analysis (OR, 0.86; 95% confidence interval [CI], 0.68–1.09, p = 0.206). The other two combinations (II: FB-fir24hr ≤ 0 and FB-sec24hr > 0; IV: FB-fir24hr > 0 and FB-sec24hr > 0) had significantly higher mortality (192/851 (22.5%) and 1320/4857 (27.1%), respectively) and the ORs were statistically significant (OR, 1.84; 95% CI, 1.44–2.35 and OR, 1.99; 95% CI, 1.64–2.42, respectively).
Table 1.
Comparisons of baseline characteristics between survivors and non-survivors.
| Variables | Overall (n = 8584) | Survivors (n = 6706) | Non-survivors (n = 1878) | p |
|---|---|---|---|---|
| Age (years) | 65.9 ± 16.0 | 64.9 ± 16.3 | 69.4 ± 14.3 | <0.001 |
| Male [n (%)] | 3878 (45.1) | 3064 (45.6) | 814 (43.3) | 0.071 |
| Weight (kg) | 82.1 ± 26.0 | 82.7 ± 26.3 | 80.0 ± 24.8 | <0.001 |
| Emergency [n (%)] | 7811 (90.9) | 5998 (89.4) | 1813 (96.5) | <0.001 |
| Ethnicity | ||||
| White [n (%)] | 6150 (71.6) | 4843 (72.0) | 1307 (69.5) | 0.026 |
| Black [n (%)] | 662 (7.7) | 546 (8.1) | 116 (6.1) | 0.005 |
| Asian [n (%)] | 201 (2.3) | 159 (2.4) | 42 (2.2) | 0.773 |
| ICU types | ||||
| MICU [n (%)] | 4075 (48.6) | 3030 (45.0) | 1045 (55.6) | <0.001 |
| SICU [n (%)] | 998 (11.6) | 883 (13.1) | 115 (6.1) | <0.001 |
| CCU [n (%)] | 1147 (13.3) | 865(12.8) | 282 (15.0) | 0.017 |
| Infection sites | ||||
| Respiratory [n (%)] | 5117 (59.6) | 3858 (57.5) | 1259 (67.0) | <0.001 |
| Urinary [n (%)] | 2780 (32.3) | 2326 (34.7) | 454 (24.1) | <0.001 |
| Bloodstream [n (%)] | 3038 (35.4) | 2076 (30.8) | 962 (51.2) | <0.001 |
| Abdominal [n (%)] | 1113 (12.9) | 877 (13.0) | 236 (12.5) | 0.560 |
| Intracranial [n (%)] | 607 (7.1) | 501 (7.6) | 106 (5.6) | 0.006 |
| Laboratory indexes | ||||
| Maximum serum creatinine | 2.19 ± 2.19 | 2.03 ± 2.21 | 2.76 ± 2.03 | <0.001 |
| Minimum serum haemoglobin | 8.56 ± 1.72 | 8.63 ± 1.70 | 8.31 ± 1.76 | <0.001 |
| Maximum serum lactate | 3.40 ± 3.17 | 2.90 ± 2.35 | 4.95 ± 4.58 | <0.001 |
| Maximum serum sodium | 144.4 ± 5.2 | 144.2 ± 4.9 | 145.1 ± 6.1 | 0.001 |
| Maximum white blood cell count | 20.9 ± 12.5 | 20.3 ± 14.1 | 23.3 ± 15.3 | <0.001 |
| Clinical characteristics | ||||
| SOFA at ICU admission [median (IQR)] | 5 (4–8) | 5 (3–7) | 6 (4–9) | <0.001 |
| Fluid balance (ml/kg/48hr) | 40.5 ± 56.7 | 34.9 ± 53.4 | 60.2 ± 63.2 | <0.001 |
| Fluid balance (ml/kg/first-24hr) | 28.3 ± 40.6 | 26.1 ± 39.3 | 36.3 ± 44.1 | <0.001 |
| Fluid balance (ml/kg/second-24hr) | 12.1 ± 28.3 | 8.8 ± 26.3 | 23.9 ± 31.8 | <0.001 |
| Fluid intake (ml/kg/48 hr) | 93.91 ± 58.2 | 91.3 ± 55.6 | 103.5 ± 64.7 | <0.001 |
| Intravenous fluid intake (ml/kg/48 hr) | 84.8 ± 58.6 | 81.8 ± 56.3 | 95.6 ± 64.7 | <0.001 |
| Output (ml/kg/48 hr) | 53.5 ± 35.0 | 56.3 ± 34.9 | 43.3 ± 33.7 | <0.001 |
| Urine output (ml/kg/48 hr) | 43.1 ± 30.2 | 45.9 ± 30.2 | 32.9 ± 28.1 | <0.001 |
| Haemodialysis | 969 (11.2) | 638 (9.5) | 331 (17.6) | <0.001 |
| Vasopressor-use | 2513 (29.2) | 1846 (27.5) | 667 (35.5) | <0.001 |
Abbreviations: MICU, medical intensive care unit; SICU, surgical intensive care unit; CCU, coronary care unit; SOFA sequential organ failure assessment; ICU, intensive care unit; IQR, interquartile range.
Table 2.
Characteristics and outcomes by four combinations of FB-fir24hr and FB-sec24hr.
| Variables | FB-fir24hr ≤ 0 and FB-sec24hr ≤ 0 (n = 1040) |
FB-fir24hr ≤ 0 and FB-sec24hr > 0 (n = 851) |
FB-fir24hr > 0 and FB-sec24hr ≤ 0 (n = 1836) |
FB-fir24hr > 0 and FB-sec24hr > 0 (n = 4857) |
p |
|---|---|---|---|---|---|
| Hospital mortality [n (%)] | 140 (13.5) | 192 (22.5)# | 226 (12.3) | 1320 (27.1)# | <0.001 |
| ICU LOS [median (IQR)] | 4.1 (2.9–7.3) | 5.7 (3.2–11.3)# | 4.0 (2.8–7.6) | 6.0 (3.3–11.5)# | <0.001 |
| Hospital LOS [median (IQR)] | 10.7 (4.8–17.0) | 12.4 (7.7–20.8)# | 10.8 (7.1–17.7) | 13.2 (7.9–21.5)# | <0.001 |
| SOFA on admission [median (IQR)] | 4 (3–6) | 4 (3–6) | 5 (4–7)# | 6 (4–8)# | <0.001 |
| Fluid balance (ml/kg/48 hr) | −31.9 ± 19.9 | 1.9 ± 25.3# | 17.7 ± 32.1# | 71.3 ± 56.0# | <0.001 |
| Fluid intake (ml/kg/48 hr) | 42.1 ± 26.7 | 67.3 ± 40.6# | 84.9 ± 47.8# | 113.1 ± 59.9# | <0.001 |
| Output (ml/kg/48 hr) | 74.0 ± 34.7 | 65.4 ± 40.0# | 67.1 ± 35.1# | 49.8 ± 29.0# | <0.001 |
| Urine output (ml/kg/48 hr) | 64.2 ± 32.0 | 49.9 ± 31.8# | 54.2 ± 30.9# | 33.1 ± 24.7# | <0.001 |
| Haemodialysis [n (%)] | 84 (8.1) | 77 (9.0) | 156 (8.5) | 652 (13.4)# | <0.001 |
Abbreviations: FB-fir24hr, fluid balance within the first 24 hours after ICU admission; FB-sec24 hr, fluid balance within the second 24 hours (25 to 48 hours) after ICU admission; ICU intensive care unit; LOS, length of stay; SOFA, sequential organ failure assessment; IQR, interquartile range.
Note: Comparisons between combination I and other combinations were made and # indicated p value < 0.001.
Table 3.
Odds ratio of death according to four combinations of FB-fir24hr and FB-sec24hr in logistic regression.
| Variables | Crude Odds ratio (95% CI) | p | Adjusted Odds ratio (95% CI) | p |
|---|---|---|---|---|
| FB-fir24hr ≤ 0 and FB-sec24hr ≤ 0 | Ref. | — | Ref. | — |
| FB-fir24hr ≤ 0 and FB-sec24hr > 0 | 1.87 (1.47–2.38) | <0.001 | 1.84 (1.44–2.35) | <0.001 |
| FB-fir24hr > 0 and FB-sec24hr ≤ 0 | 0.90 (0.71–1.13) | 0.373 | 0.86 (0.68–1.09) | 0.206 |
| FB-fir24hr > 0 and FB-sec24hr > 0 | 2.39 (1.98–2.89) | <0.001 | 1.99 (1.64–2.42) | <0.001 |
| SOFA on ICU admission | 1.12 (1.10–1.14) | <0.001 | ||
| Urinary infection | 0.69 (0.61–0.78) | <0.001 | ||
| Respiratory infection | 1.46 (1.29–1.63) | <0.001 | ||
| Haemodialysis | 1.07 (0.87–1.31) | 0.491 | ||
| Maximum serum creatinine | 1.06 (1.03–1.09) | <0.001 | ||
| Serum sodium on ICU admission (<135 mmol/L) | 1.35 (1.19–1.54) | <0.001 | ||
| Platelet count on ICU admission (<150 *10^9/L) | 1.20 (1.06–1.35) | 0.003 | ||
| Serum calcium on ICU admission (<8 mg/dl) | 0.87 (0.78–0.90) | 0.020 |
Abbreviations: FB-fir24hr, fluid balance within the first 24 hours after ICU admission; FB-sec24hr, fluid balance within the second 24 hours (25 to 48 hours) after ICU admission; ICU intensive care unit; SOFA, sequential organ failure assessment.
Association between FB and mortality
Quartiles of all patients
Further multivariable logistic analysis (Table 4) showed a significant stepwise increase in mortality rate with increasing FB-sec24hr quartiles (from Q2 [OR, 1.56; 95% CI, 1.31–1.86] to Q4 [OR, 3.33; 95% CI, 2.82–3.92]); however, in FB-fir24hr quartiles (using Q1 as the reference quartile), no other significant difference occurred aside from Q4 (OR, 1.41; 95% CI, 1.20–1.65). This increasing trend of ORs became less significant in FB-48hr quartiles (from Q2 [OR, 1.20; 95% CI, 1.01–1.42] to Q4 [OR, 2.31; 95% CI, 1.88–2.59]).
Table 4.
Adjusted odds ratio of mortality using fluid balance as design variables in logistic models.
| Variables | Odds ratio (95% CI) | p | Odds ratio (95% CI) | p | Odds ratio (95% CI) | p |
|---|---|---|---|---|---|---|
| All patients | ||||||
| Model 1 | All FB-fir24hr (n = 8584) | All FB-sec24hr (n = 8584) | All FB-48hr (n = 8584) | |||
| FB Q1 | 1 | — | 1 | — | 1 | — |
| FB Q2 | 1.05 (0.89–1.23) | 0.540 | 1.56 (1.31–1.86) | <0.001 | 1.20 (1.01–1.42) | 0.035 |
| FB Q3 | 1.11 (0.95–1.31) | 0.164 | 1.94 (1.64–2.31) | <0.001 | 1.57 (1.33–1.85) | <0.001 |
| FB Q4 | 1.41 (1.20–1.65) | <0.001 | 3.33 (2.82–3.92) | <0.001 | 2.31 (1.88–2.59) | <0.001 |
| Patients with negative FB | ||||||
| Model 1 | Negative FB-fir24hr (n = 1882) | Negative FB-sec24hr (n = 2866) | Negative FB-48hr (n = 1950) | |||
| FB Q1 | 1 | — | 1 | — | 1 | — |
| FB Q2 | 0.86 (0.61–1.21) | 0.391 | 0.97 (0.72–1.32) | 0.891 | 0.87 (0.61–1.24) | 0.459 |
| FB Q3 | 0.88 (0.62–1.24) | 0.480 | 0.62 (0.44–0.86) | 0.005 | 0.74 (0.52–1.07) | 0.115 |
| FB Q4 | 0.77 (0.54–1.09) | 0.145 | 0.59 (0.43–0.82) | 0.002 | 0.53 (0.36–0.78) | <0.001 |
Abbreviation: FB-fir24hr, FB-sec24hr, and FB-48hr represented the fluid balance within the first 24 hours, the second 24 hours and the 48 hours after ICU admission, respectively.
Note: All three models were adjusted for infection site, disease severity score, haemodialysis, and laboratory factors (including serum creatinine, sodium, calcium and platelet count).
Quartiles of patients with negative FB
Patients who achieved negative FB during the three periods (the first and second 24 hours, and the entire 48 hours) were further divided into four quartiles (Table 4). Logistic regression analysis was used to explore the volume-related association. ORs of mortality decreased stepwise with increasing FB-sec24hr (high quartile indicating a more negative FB) (from Q2 [OR, 0.97; 95% CI, 0.72–1.32] to Q4 [OR, 0.59; 95% CI, 0.43–0.82]) and FB-48hr quartiles (from Q2 [OR, 0.87; 95% CI, 0.61–1.24] to Q4 [OR, 0.53; 95% CI, 0.36–0.78]), with no significant association in FB-fir24hr quartiles.
Subgroup analysis of FB-fir24hr
As we speculated that haemodynamic status might have an interaction with FB, we performed a subgroup analysis according to the use of vasopressors within 24 hours after ICU admission. In patients without vasopressor-use, the mortality rate significantly increased stepwise with the increased quartile of FB-fir24hr (from Q2: OR, 1.05; 95%CI, 0.87–1.27 to Q4: OR, 1.32; 95%CI, 1.09–1.59) withinsignificant in patients with vasopressor-use aside from Q4 (see Supplementary Table S1).
Fluid intake/output distribution
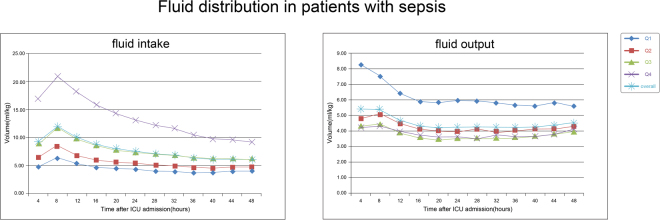
To explicitly show the fluid distribution in patients within each quartile, the amount of fluid intake/output is shown in four-hour intervals in Fig. 1. The fluid intake curve fluctuated during the first 24 hours and peaked within 4–8 hours, whereas the curve during the second 24 hours after admission was relatively smooth.
Figure 1.
Volume distribution of fluid intake and output over the first 48 hours after ICU admission in patients with sepsis.
Discussion
Our analysis, including 8584 patients with sepsis from a large online database (Medical Information Mart for Intensive Care III [MIMIC III]), revealed two key findings. First, daily FB in the second—but not the first—24 hours after ICU admission was positively associated with hospital mortality. Second, achieving more negative FB during this same time period (second but not first 24 hours) was associated with decreased mortality.
Associations between cumulative positive FB and adverse outcomes have been well established in patients with sepsis. We hypothesized that there was an interaction between early FB and haemodynamic status that contributed to the insignificant association between positive FB-fir24hr and mortality. Results from one large, multicentre, observational study indicated that the volume of positive FB was an independent risk factor for mortality14. The post-hoc analysis in VASST showed that cumulative positive FB over four days was associated with higher mortality in septic shock8, while Micek et al. also found the highest positive FB quartile at eight days was an independent predictor of hospital mortality9. Consistent with previous findings, we also found that high fluid accumulation at 48 hours was significantly associated with increased mortality. However, conclusions regarding the association between early FB and mortality are conflicting in reported studies. Boyd et al.8 and Sadaka et al.13 found that a more positive FB early in the resuscitation was associated with increased mortality in septic shock. However, in a multi-centre observational study, Sakr et al.11 reported otherwise, that early fluid resuscitation (within 24 hours after admission) was not associated with increased mortality in sepsis, and that it might even be associated with reduced mortality in patients that have septic shock for more than three days12. In the current study, we also found that early fluid accumulation (FB-fir24hr) was not associated with mortality rate. However, in the subgroup analysis, FB-fir24hr was still significantly associated with increased mortality in patients without vasopressor-use, while insignificant in the patients with vasopressor-use. Interestingly, a similar but insignificant pattern was also shown in Table 3 of the study by Sakr et al.11, as insignificant OR for mortality increased stepwise in the subgroup with no septic shock. As both the necessity of adequate fluid resuscitation and the detrimental effect of fluid accumulation have been well established, we speculated that the detrimental effect of positive FB accumulated during fluid resuscitation may be reduced or overwhelmed by the benefits of adequate fluid resuscitation. This speculation could also explain the inconsistent findings of significant8,13 or insignificant12,15,16 associations of early positive FB with increased sepsis-related mortality, as haemodynamic status was largely different between these cohorts. If this is the case, fluid accumulation during the resuscitation stage needs to be re-evaluated. Additionally, we noticed that, despite a significantly higher cumulative FB-48hr in Combination III than in Combination II (Table 2), the mortality in Combination III was significantly lower.
Furthermore, as the available data renders it impossible to identify patients who underwent fluid resuscitation, we presented the fluid intake/output distribution at the first and the second 24 hours (Fig. 1). Compared to the smooth curve at the second 24 hours, the fluid intake curve at the first 24 hours fluctuated and peaked within 4–8 hours after ICU admission, which, to a certain degree, implies that fluids during this interval may be administered therapeutically instead of by a protocol-driven programme. However, without additional information, we cannot definitively assume that fluctuations in the fluid intake curve indicate fluid resuscitation.
Based on the current evidence, conservative fluid administration has been widely adopted after achieving relatively stable haemodynamic status, to avoid further positive FB. However, the true benefit of negative FB in sepsis may be overestimated, as the lowest quartile of FB is commonly used as the reference quartile8,11,13. Balakumar et al.17 reported that among critically ill patients, negative FB was more associated with long-term mortality than euvolaemia. However, this conclusion became unstable18 in sensitivity analysis and the great heterogeneity within included patients may lead to potential bias. There are no known reports evaluating whether there is a volume-related association between negative FB and mortality in sepsis. In this study, we found that the volume in negative FB was associated with decreased hospital mortality only in the second, but not the first, 24 hours after ICU admission. Based on our hypothesis in this study, it is reasonable to speculate that negative FB is only “beneficial” after haemodynamic status is stable. However, the underlying mechanism of this association, and whether the amount of negative FB relied on previous cumulative FB, could not be inferred from our study and requires further investigation.
Based on our findings, we suggest that the interactive effect of fluid accumulation and fluid resuscitation should be re-considered when making the fluid management protocol decision in sepsis. In patients with poor haemodynamics, adequate fluid resuscitation is necessary, and fluid accumulation during this stage is unlikely to be associated with poor outcomes. However, subsequent fluid management is critical after the patient’s haemodynamic status becomes relatively stable. Additionally, it is beneficial in this stage to avoid further fluid accumulation or achieve more negative FB.
One advantage of the present study is the large sample size from the MIMIC III database. Additionally, the detailed data enabled us to present fluid distribution by hour, adjust for confounding factors, and perform subgroup analysis. However, information bias is still possible, and other unmeasured factors (such as haemodynamic targets and fluid administered before ICU admission) were unrecorded in the present study. A second limitation is that the database used in this study comprised data from patients admitted to an ICU between 2001 and 2012, but sepsis 3.04 was used as inclusion criteria in the current study. Thirdly, septic shock is an important subgroup of sepsis; however, due to a lack of related information in this database, patients with septic shock could not be separated from patients with sepsis. Thus, we selected patients with a record of vasopressor-use as potential cases of septic shock. However, the related selection bias cannot be avoided; as an example, epinephrine may have been used for bradycardia rather than for septic shock. Finally, due to the retrospective nature of the study, an association between FB and mortality can only be inferred, and cannot be established as a causal relationship. Further studies focusing on the interaction between FB and fluid resuscitation are needed to verify this speculation.
In conclusion, we found in this large cohort study that positive FB-sec24hr was significantly associated with increased hospital mortality, while FB-fir24hr was not. However, in the subgroup without vasopressor-use, the association between FB-fir24hr and mortality became significant. The amount of negative FB during the second—but not the first—24 hours after ICU admission showed a stepwise association with decreased mortality in patients with sepsis.
Methods
Data sources
All of the data in this study were extracted from a large online international database, Medical Information Mart for Intensive Care III (MIMIC III), which is a single-centre database comprising information of patients admitted to the Beth Israel Deaconess Medical Center in Boston, Massachusetts19,20. This database comprises over 58 000 hospital admissions for 38 645 adults and 7875 neonates from 2001 to 2012, and the use of the database was approved by the institutional review boards of the Massachusetts Institute of Technology. The included patients’ information was anonymised, and thus the need for patients’ informed consent was waived for this study. All data were extracted by the corresponding author (certification number: 1564657), who passed the online training on protecting human subjects research participants.
Inclusion and exclusion criteria
Adult patients meeting the criteria for sepsis were initially screened. Sepsis definition was adapted from the recommendations in Surviving Sepsis Campaign 20164, defined as sequential organ failure assessment (SOFA) score ≥2 within 24 hours after ICU admission, accompanied by at least one infection site. To accurately identify patients with a diagnosis that suggested sepsis, we manually reviewed all International Classification of Diseases, Ninth Revision codes indicating the existence of infection. The following criteria were used to exclude patients from this analysis: (1) younger than 18 years, (2) spent less than 48 hours in the ICU, and/or (3) without data on fluid management. For patients who stayed in the ICU more than once, only the first ICU stay was used in this study.
Grouping method
Cumulative FB was calculated as follows: (fluid intake - fluid output) in millilitre/body weight (ml/kg). Fluid intake indicated all fluids (including colloid, crystalloid, or blood products) administered to the patient within 48 hours after ICU admission. As the main purpose of this study was to investigate the time-related association, daily FB instead of cumulative FB was determined. Thus, patients were stratified into four categories according to the four combinations of daily FB-fir24hr and FB-sec24hr after ICU admission. The categories were: Combination I (FB-fir24hr ≤ 0 and FB-sec24hr ≤ 0), II (FB-fir24hr ≤ 0 and FB-sec24hr > 0), III (FB-fir24hr > 0 and FB-sec24hr ≤ 0), and IV (FB-fir24hr > 0 and FB-sec24hr > 0). Furthermore, all patients were further stratified into four quartiles. Logistic regression analysis was performed to explore the time-related association between FB and mortality. In all logistic models, quartile 1 represented the lowest FB and quartile 4 represented the highest FB. For negative FB, quartile 1 represented the least negative and quartile 4 represented the most negative FB. The primary endpoint was hospital mortality, defined as observed death during hospitalisation.
Subgroup analysis
Subgroup analysis was performed to explore possible interactions between FB and haemodynamic status. All patients were divided into two subgroups according to the use of any vasopressor within 24 hours after ICU admission, including norepinephrine, dopamine, dobutamine, and epinephrine.
Missing data management
Variables with missing data were common in the MIMIC III database. In this analysis, the percentages of most missing variables were less than 5%. Thus, we replaced missing variables with their mean/median value21.
Statistical analysis
Continuous variables were expressed as mean ± standard deviation (SD) or median (interquartile range) as appropriate. Categorical data were expressed as proportions22. Variables, including demographic characteristics, infection site, disease severity score, and laboratory measures potentially associated with mortality or which had a p value < 0.20 in univariate analyses were included in the multivariate logistic regression analyses18,23. Multi-collinearity was tested using variance inflation factor (VIF) method, with VIF ≥ 5 indicating the existence of multi-collinearity. The goodness of fit was checked for all logistic regression models. The two-tailed test was used, and p < 0.05 was considered statistically significant. All statistical analyses were performed using the software STATA 11.2 (StataCorp LLC, College Station, TX, USA).
Data availability
The data that support the findings of this study were extracted from the MIMIC III database, https://physionet.org/works/MIMICIIIClinicalDatabase/files/, by the corresponding author, who passed the online training. Thus, the data are only available from the corresponding author upon reasonable request and with permission of MIMIC III.
Electronic supplementary material
Author Contributions
Y.S. designed the study, extracted the data, and wrote the draft of the manuscript. W.R. and X.H. performed all statistical analyses and revised the manuscript for important intellectual content. W.Z. revised the manuscript for the final version. All the authors gave final approval of the version to be published and agreed to be accountable for all aspects of the work, including accuracy or integrity of any part of the work.
Competing Interests
The authors declare no competing interests.
Footnotes
Yanfei Shen and Weizhe Ru contributed equally to this work.
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-28781-4.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Rivers E, et al. Early goal-directed therapy in the treatment of severe sepsis and septic shock. The New England journal of medicine. 2001;345:1368–1377. doi: 10.1056/NEJMoa010307. [DOI] [PubMed] [Google Scholar]
- 2.Yealy DM, et al. A randomized trial of protocol-based care for early septic shock. The New England journal of medicine. 2014;370:1683–1693. doi: 10.1056/NEJMoa1401602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Peake SL, et al. Goal-directed resuscitation for patients with early septic shock. The New England journal of medicine. 2014;371:1496–1506. doi: 10.1056/NEJMoa1404380. [DOI] [PubMed] [Google Scholar]
- 4.Rhodes A, et al. Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock: 2016. Intensive care medicine. 2017;43:304–377. doi: 10.1007/s00134-017-4683-6. [DOI] [PubMed] [Google Scholar]
- 5.Wiedemann HP, et al. Comparison of two fluid-management strategies in acute lung injury. The New England journal of medicine. 2006;354:2564–2575. doi: 10.1056/NEJMoa062200. [DOI] [PubMed] [Google Scholar]
- 6.Balogh Z, Moore FA, Moore EE, Biffl WL. Secondary abdominal compartment syndrome: a potential threat for all trauma clinicians. Injury. 2007;38:272–279. doi: 10.1016/j.injury.2006.02.026. [DOI] [PubMed] [Google Scholar]
- 7.Coats TJ, Brazil E, Heron M, MacCallum PK. Impairment of coagulation by commonly used resuscitation fluids in human volunteers. Emergency medicine journal: EMJ. 2006;23:846–849. doi: 10.1136/emj.2006.036574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boyd JH, Forbes J, Nakada TA, Walley KR, Russell JA. Fluid resuscitation in septic shock: a positive fluid balance and elevated central venous pressure are associated with increased mortality. Critical care medicine. 2011;39:259–265. doi: 10.1097/CCM.0b013e3181feeb15. [DOI] [PubMed] [Google Scholar]
- 9.Micek ST, et al. Fluid balance and cardiac function in septic shock as predictors of hospital mortality. Crit Care. 2013;17:R246. doi: 10.1186/cc13072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sirvent JM, Ferri C, Baro A, Murcia C, Lorencio C. Fluid balance in sepsis and septic shock as a determining factor of mortality. The American journal of emergency medicine. 2015;33:186–189. doi: 10.1016/j.ajem.2014.11.016. [DOI] [PubMed] [Google Scholar]
- 11.Sakr Y, et al. Higher Fluid Balance Increases the Risk of Death From Sepsis: Results From a Large International Audit. Critical care medicine. 2017;45:386–394. doi: 10.1097/CCM.0000000000002189. [DOI] [PubMed] [Google Scholar]
- 12.Smith SH, Perner A. Higher vs. lower fluid volume for septic shock: clinical characteristics and outcome in unselected patients in a prospective, multicenter cohort. Crit Care. 2012;16:R76. doi: 10.1186/cc11333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sadaka F, Juarez M, Naydenov S, O’Brien J. Fluid resuscitation in septic shock: the effect of increasing fluid balance on mortality. Journal of intensive care medicine. 2014;29:213–217. doi: 10.1177/0885066613478899. [DOI] [PubMed] [Google Scholar]
- 14.Vincent JL, et al. Sepsis in European intensive care units: results of the SOAP study. Critical care medicine. 2006;34:344–353. doi: 10.1097/01.CCM.0000194725.48928.3A. [DOI] [PubMed] [Google Scholar]
- 15.Carlsen S, Perner A. Initial fluid resuscitation of patients with septic shock in the intensive care unit. Acta anaesthesiologica Scandinavica. 2011;55:394–400. doi: 10.1111/j.1399-6576.2011.02399.x. [DOI] [PubMed] [Google Scholar]
- 16.McIntyre LA, et al. Resuscitating patients with early severe sepsis: a Canadian multicentre observational study. Canadian journal of anaesthesia = Journal canadien d’anesthesie. 2007;54:790–798. doi: 10.1007/BF03021706. [DOI] [PubMed] [Google Scholar]
- 17.Balakumar V, et al. Both Positive and Negative Fluid Balance May Be Associated With Reduced Long-Term Survival in the Critically Ill. Critical care medicine. 2017;45:e749–e757. doi: 10.1097/CCM.0000000000002372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shen Y, Huang X, Zhang W. Association between fluid intake and mortality in critically ill patients with negative fluid balance: a retrospective cohort study. Crit Care. 2017;21:104. doi: 10.1186/s13054-017-1692-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Johnson AE, et al. MIMIC-III, a freely accessible critical care database. Scientific data. 2016;3:160035. doi: 10.1038/sdata.2016.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goldberger AL, et al. PhysioBank, PhysioToolkit, and PhysioNet: components of a new research resource for complex physiologic signals. Circulation. 2000;101:E215–220. doi: 10.1161/01.CIR.101.23.e215. [DOI] [PubMed] [Google Scholar]
- 21.Zhang Z. Missing data imputation: focusing on single imputation. Annals of translational medicine. 2016;4:9. doi: 10.21037/atm.2016.09.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang Z. Univariate description and bivariate statistical inference: the first step delving into data. Annals of translational medicine. 2016;4:91. doi: 10.21037/atm.2016.02.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang Z. Model building strategy for logistic regression: purposeful selection. Annals of translational medicine. 2016;4:111. doi: 10.21037/atm.2016.02.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study were extracted from the MIMIC III database, https://physionet.org/works/MIMICIIIClinicalDatabase/files/, by the corresponding author, who passed the online training. Thus, the data are only available from the corresponding author upon reasonable request and with permission of MIMIC III.