Abstract
The 8th edition of TNM staging system has been released and it incorporates many changes to the T and N classifications for pancreatic cancer. Comparative study between the 7th and 8th edition of TNM staging system from Asian population has not been reported yet. This study aimed to compare the 7th and 8th edition of staging system for pancreatic cancer by using a cohort of pancreatic cancer patients from China after R0 pancreaticoduodenectomy and adjuvant chemotherapy. The results showed according to the pT classification of 7th edition, pT3 was predominant (87.25%), however, the new edition led to a more equal distribution of pT classification. pT1, pT2 and pT3 was 27.45%, 56.86% and 15.69%, respectively. According to the new pN classification, 18.63% of the patients were pN2. The pT classification in the 8th edition was significantly superior to that in the 7th edition at stratifying patients by overall survival. The pN classification in the 8th edition failed to show an advantage over the 7th edition in stratifying patients by overall survival. Therefore, the new pT classification, but not the new pN classification, showed a significant advantage over the previous edition at predicting the overall survival of pancreatic cancer patients.
Introduction
Despite tremendous efforts to elucidate the mechanisms underlying the initiation, progression and metastasis of pancreatic ductal adenocarcinoma (PDAC), its 5-year overall survival remains approximately 8.2% in America1–3. During the last several decades, the incidence of PDAC has increased in both Western countries and China4,5.
Accurate evaluation of tumor stage is a prerequisite for further treatment and prognostic prediction. The AJCC/UICC TNM staging system has been widely applied worldwide as the most authorized tool for tumor staging assessment. The first edition was released in 1977, and it has been updated several times every 5–7 years. The 5th edition for pancreatic cancer was released in 1997, and no changes have been made in the 6th and 7th editions in the last 20 years6.
In October of 2016, AJCC/UICC released the 8th edition, which incorporated significant changes in the T and N classification of PDAC. In the 8th edition, stages T1-T3 are redefined according to tumor size (T1 ≤ 2 cm; 2 cm ≥ T2 ≤ 4 cm; T3 > 4 cm). When the tumor invades the celiac axis, common hepatic artery and/or superior mesenteric artery, it is defined as T4, and the classification as “unresectable” was removed. In the 8th edition, the N classification was further subdivided according to the number of positive lymph nodes as N0, N1 (≥1 and ≤3) and N2 (>3). In the 8th edition, T1–3N2M0 was defined as stage III, and the other stages remained unchanged (Table 1).
Table 1.
The definitions of the 7th and 8th edition of TNM staging system of pancreatic cancer by AJCC/UICC.
| 7th | 8th | 7th | 8th | ||||||
|---|---|---|---|---|---|---|---|---|---|
| T1 | Tumor limited to the pancreas, ≤2 cm in greatest dimension | Maximum tumor diameter ≤2 cm | T | N | M | T | N | M | |
| T2 | Tumor limited to the pancreas, >2 cm in greatest dimension | Maximum tumor diameter >2, ≤4 cm | IA | T1 | N0 | MO | T1 | N0 | M0 |
| T3 | Tumor extends beyond the pancreas but without involvement of the celiac axis or the superior mesenteric artery | Maximum tumor diameter >4 cm | IB | T2 | N0 | M0 | T2 | N0 | M0 |
| T4 | Tumor involves the celiac axis or the superior mesenteric artery (unresectable primary tumor) | Tumor involves the celiac axis, common hepatic artery or the superior mesenteric artery | IIA | T3 | N0 | M0 | T3 | N0 | M0 |
| N0 | No regional lymph node metastasis | No regional lymph node metastasis | IIB | T1-T3 | N1 | M0 | T1-T3 | N1 | M0 |
| N1 | Regional lymph node metastasis | Metastasis in 1–3 regional lymph nodes | III | T4 | any N | M0 | T4 (any T) | any N (N2) | M0 |
| N2 | — | Metastasis in ≥ 4 regional lymph nodes | IV | any T | any N | M1 | any T | Any N | M1 |
| M0 | No distant metastasis | No distant metastasis | |||||||
| M1 | Distant metastasis | Distant metastasis | |||||||
Recently, the superiority of the 8th edition at stratifying patients by survival was evaluated in two validation studies from America and two studies from Germany; however, the results were inconsistent. At present, the superiority of the 8th edition to the 7th edition at predicting the prognosis of PDAC has not been evaluated in an Asia population7–10. Since many confounding factors, such as tumor location, tumor margin11–13, and adjuvant chemotherapy14, could affect the clinical value of the TNM staging system in predicting patients survival, we rigorously enrolled 102 PDAC patients who underwent R0 pancreaticoduodenectomy and at least three cycles of gemcitabine-based chemotherapy with a long follow-up period to validate the potential superiority of the new TNM staging system in stratifying patients based on survival.
Results
Patient demographics
One hundred two patients were enrolled, and survival information was available for all patients. The follow-up time ranged from 36 to 90 months. The median survival was 32.00±4.91 months (95% CI 22.37±41.62), and the 1-, 2- and 3-year survival rates were 88.2%, 70.5%, and 40.5%, respectively. The detailed clinicopathological information was provided in Table 2.
Table 2.
Patient demographics.
| Characteristics | N0. of patients |
|---|---|
| Age | |
| ≤60 y | 51 |
| >60 y | 51 |
| Gender | |
| Male | 66 |
| Female | 36 |
| pT classification (8th) | |
| pT1 (≤2 cm) | 27 |
| pT2 (>2 cm, ≤4 cm) | 57 |
| pT3 (>4 cm) | 18 |
| pT classification (7th) | |
| pT1 | 7 |
| pT2 | 6 |
| pT3 | 89 |
| pN classification (8th) | |
| pN0 (0) | 40 |
| pN1 (1~3) | 43 |
| pN2 (≥4) | 19 |
| TNM stage (7th) | |
| IA | 5 |
| IB | 2 |
| IIA | 34 |
| IIB | 61 |
| TNM stage (8th) | |
| IA | 12 |
| IB | 23 |
| IIA | 6 |
| IIB | 44 |
| III | 17 |
| Differentiation | |
| Well/moderate | 75 |
| Poor | 27 |
| Perineural invasion (PNI) | |
| Yes | 21 |
| No | 81 |
| Micro-cancerous embolus | |
| Yes | 12 |
| No | 90 |
| CA19-9 | |
| ≤34U/ml | 27 |
| >34U/ml | 74 |
| NA | 1 |
| CA242 | |
| ≤20U/ml | 46 |
| >20Uml | 41 |
| NA | 15 |
| CEA | |
| ≤5 μg/ml | 79 |
| >5 μg/ml | 21 |
| NA | 2 |
| Perioperative bile drainage | |
| Yes | 45 |
| NO | 57 |
| Diabetes mellitus | |
| Yes | 20 |
| No | 82 |
NA: Not Available.
Comparison of the 7th and 8th editions of the TNM staging system for patients
Stages pT1 and pT2 in the 7th edition were well matched with those in the 8th edition, but only 18/89 (20.2%) stage pT3 cases in the 7th edition were matched with those in the 8th edition (Table 3) 60.78% of patients had lymph node metastasis, and according to the new pN classification, 18.63% of these patients had metastasis in more than 3 lymph nodes (pN2) (Table 4). Stages IA and IB in these two editions were well matched. According to the 7th edition, 33.3% and 59.8% of patients were stage IIA (T3N0M0) and IIB (T1–3N1M0), respectively. In the new edition, only 5.9% of the patients were stage IIA (T3N0M0); 22.5%, 43.1%, and 16.7% of the patients were stage IB (T2N0M0), IIB (T1–3N1M0) and III (T1–3N2M0), respectively. Moreover, 6/34 (17.7%) stage IIA cases in the 7th edition were matched with those in the 8th edition, and the others were characterized as stage IA and IB by the 8th edition. A total of 17/61 (27.9%) stage IIB cases in the 7th edition were considered stage III by the 8th edition (Table 5).
Table 3.
The comparison of pT classifications of 7th and 8th edition.
| 8th | pT1 | pT2 | pT3 | Total |
|---|---|---|---|---|
| 7th | ||||
| pT1 | 7 | 7 | ||
| pT2 | 6 | 6 | ||
| pT3 | 21 | 50 | 18 | 89 |
| Total | 28 | 56 | 18 | 102 |
Table 4.
The comparison of pN classifications of 7th and 8th edition.
| 8th | pN0 | pN1 | pN2 | Total |
|---|---|---|---|---|
| 7th | ||||
| pN0 | 40 | 40 | ||
| pN1 | 43 | 19 | 62 | |
| Total | 40 | 43 | 19 | 102 |
Table 5.
The comparison of TNM staging system of 7th and 8th edition.
| 8th | IA | IB | IIA | IIB | III | Total |
|---|---|---|---|---|---|---|
| 7th | ||||||
| IA | 5 | 5 | ||||
| IB | 2 | 2 | ||||
| IIA | 7 | 21 | 6 | 34 | ||
| IIB | 44 | 17 | 61 | |||
| Total | 12 | 23 | 6 | 44 | 17 | 102 |
The ability of the 7th and 8th editions of the TNM staging system to stratify patients by overall survival
After multivariate analysis, CA19-9, tumor size larger than 4 cm (pT3 of the 8th edition), poor differentiation and positive lymph node metastasis were independent risk factors for poor survival, but pT classification in the 7th edition and the number of positive lymph nodes (pN1 and pN2 classification in the 8th edition) failed to stratify patients by survival; this finding indicated that pT classification in the 8th edition was superior to that in the 7th edition. However, pN classification in the 8th edition did not show significant superiority to that in the 7th edition at stratifying patients by overall survival (Table 6, Fig. 1). In addition, the 8th edition of the TNM staging system successfully stratified stage I patients from those at other stages based on overall survival, which the 7th edition could not do (Fig. 2).
Table 6.
Univariate analysis and multivariate analysis of the overall survival of the patients.
| Variable | NO. of patients | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|---|
| Median ± SE | 95% CI | P value | HR | 95% CI | P value | ||
| Age | 0.092 | ||||||
| ≤60 y | 51 | 25.00 ± 4.59 | 16.00–34.00 | ||||
| >60 y | 51 | 43.00 ± 7.75 | 27.81–58.20 | ||||
| Gender | 0.677 | ||||||
| Male | 66 | 31.00 ± 6.14 | 18.97–43.03 | ||||
| Female | 36 | 34.00 ± 8.74 | 16.86–51.14 | ||||
| T stage (8th) | 0.033* | 1.69 | 0.91–3.13 | 0.049* | |||
| ≤4 cm (pT1~T2) | 84 | 36.00 ± 6.20 | 23.84–48.16 | ||||
| >4 cm (pT3) | 18 | 18.00 ± 4.24 | 9.68–26.32 | ||||
| T stage (7th) | 0.056 | ||||||
| pT1~T2 | 13 | 38.00 ± 8.40 | 21.70–49.20 | ||||
| pT3 | 89 | 27.65 ± 6.40 | 16.00–42.60 | ||||
| Lymph node metastasis | <0.0001** | 3.29 | 1.75–6.20 | <0.0001** | |||
| pN0 | 40 | 57.00 ± 5.89 | 45.47–68.53 | ||||
| pN1 | 62 | 20.00 ± 2.61 | 14.86–25.14 | ||||
| Differentiation | <0.0001** | 0.47 | 0.26–0.82 | 0.008** | |||
| Well/moderate | 75 | 41.00 ± 8.32 | 24.69–57.31 | ||||
| Poor | 27 | 15.00 ± 2.60 | 9.91–20.09 | ||||
| Micro-cancerous embolus | 0.571 | ||||||
| Yes | 12 | 26.00 ± 7.75 | 10.81–41.19 | ||||
| No | 90 | 34.00 ± 6.37 | 21.51–46.49 | ||||
| Perineural invasion (PNI) | 0.540 | ||||||
| Yes | 21 | 24.00 ± 6.87 | 10.54–37.46 | ||||
| No | 89 | 36.00 ± 5.47 | 25.27–46.73 | ||||
| CA199 | 0.001** | 2.74 | 1.12–6.67 | 0.027* | |||
| ≤34U/ml | 27 | NA | NA | ||||
| >34U/ml | 74 | 24.00 ± 3.31 | 17.51–30.49 | ||||
| CA242 | 0.044 | 1.09 | 0.61–1.97 | 0.744 | |||
| ≤20U/ml | 46 | 43.00 ± 5.28 | 32.65–53.35 | ||||
| >20Uml | 41 | 23.00 ± 3.20 | 16.73–29.27 | ||||
| CEA | 0.861 | ||||||
| ≤5 μg/ml | 79 | 35.00 ± 5.04 | 25.12–44.88 | ||||
| >5 μg/ml | 21 | 24.00 ± 16.96 | 0–47.25 | ||||
| Perioperative bile drainage | 0.151 | ||||||
| Yes | 45 | 26.00 ± 6.02 | 14.19–37.81 | ||||
| No | 57 | 40.00 ± 8.33 | 23.68–56.33 | ||||
| Diabetes mellitus | 0.133 | ||||||
| Yes | 20 | 55.00 ± 13.62 | 28.30 ± 81.7 | ||||
| NO | 82 | 28.00 ± 4.03 | 20.11–35.89 | ||||
*P < 0.05; **P < 0.01.
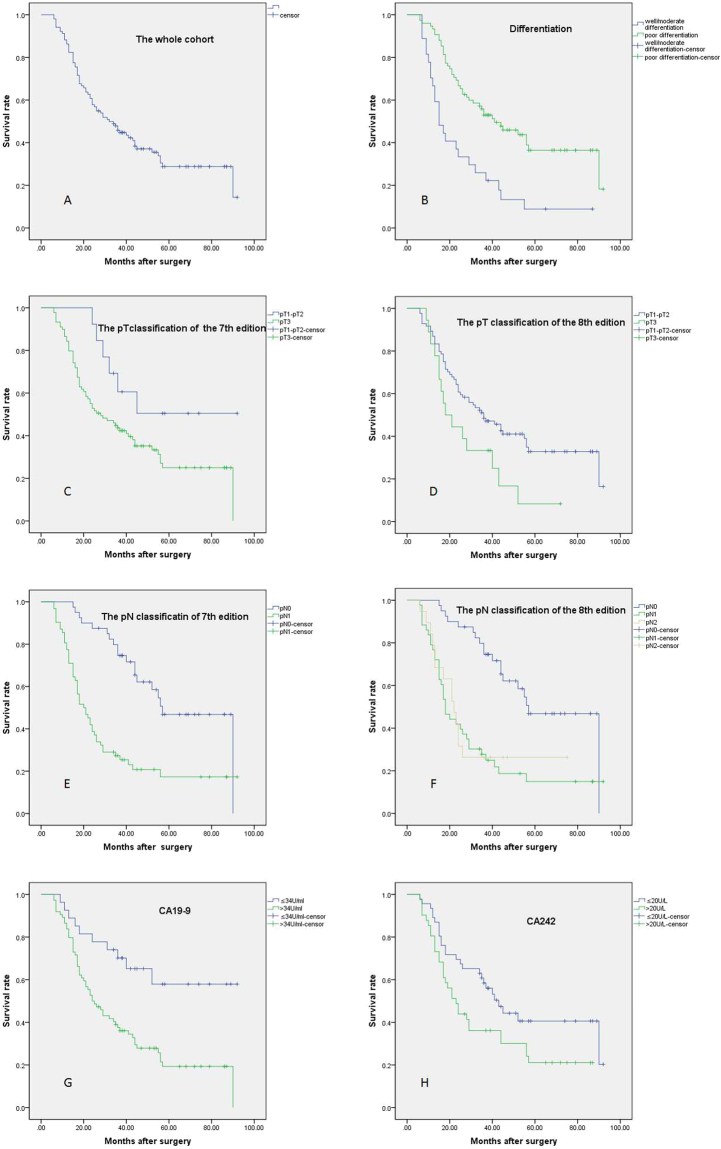
Figure 1.
Survival curves of patients with different clinicopathological characteristics. (A) Overall survival of all patients. (B) Poor differentiation predicted worse prognosis (P < 0.0001). (C) The pT classification in the 7th edition failed to stratify by prognosis (P = 0.054). (D) The pT classification in the 8th edition successfully stratified by prognosis (P = 0.033). (E) The pN classification in the 7th edition stratified by prognosis (P < 0.0001). (F) The survival of patients classified as pN1 and pN2 in the 8th edition was not significantly different (P > 0.05). (G) The OS was worse for patients with elevated CA19-9 than for those with decreased or normal CA19-9 (P = 0.001). (H) The OS of patients with elevated CA242 was worse than that of the other patients (P = 0.044).
Figure 2.
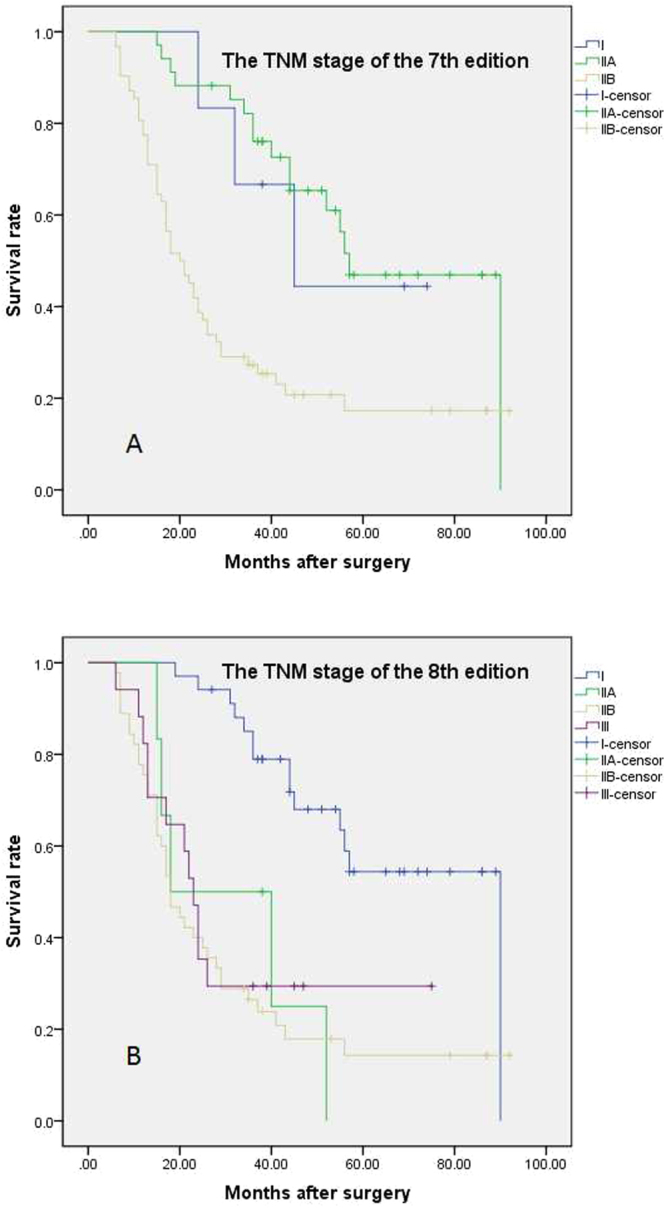
Survival curves based on TNM staging systems. (A) The 7th edition TNM staging system failed to discriminate patients with stage I disease from those at other stages by overall survival. (B) The 8th edition TNM staging system could differentiate patients with stage I disease from those at other stages by overall survival.
Discussion
Since the 1st edition of the TNM staging system was published in 1977, the AJCC/UICC has released a total of eight editions. During the last 40 years, considerable improvements have been achieved in many malignancies, such as melanoma, gastric cancer, colorectal cancer, breast cancer and lung cancer. However, despite tremendous efforts, a diagnosis of PDAC remains devastating15–18. There has not been any change in the TNM staging system for PDAC in the last three editions. Finally, in the new 8th edition, many changes in both T and N classification for PDAC have been incorporated, but whether these changes make the 8th edition superior to the 7th edition at stratifying patients by prognosis remains unclear.
The role of the previous T classification in predicting the prognosis of PDAC patients is controversial19. It is difficult to accurately ascertain the T classification according to the previous editions; in the 8th edition, only tumor size was considered, significantly increased the accuracy of the pathological assessment7,10. The number of positive lymph nodes was adopted to define the N classification in the new edition, but the role of this factor remained unclear20–22. Allen et al.7 analyzed 2318 cases of PDAC after resection from 3 large volume centers in America and found that the 8th edition increased the reproducibility of the T3 classification among different centers and that the N classification in the 8th edition was able to discriminate the prognosis of patient subgroups. Kamarajah et al.10 collected data from the Surveillance, Epidemiology and End Results (SEER) database from 2004 to 2013 and analyzed 8960 pancreatic cancer patients without metastasis who underwent surgical resection. The results showed that the 8th edition allowed for finer stratification of patients according to the extent of nodal involvement. Although these two studies had a large number of patients, there were some limitations: (1) all the data were from America; (2) the time interval during which patients were enrolled was more than 10 years; and (3) information on adjuvant treatment was missing. More recently, the results of two validation studies from Germany were inconsistent with the significance of the N classification in the 8th edition. Welsch et al.9 reported a cohort of 256 PDAC patients who underwent curative resection from 2005 to 2015, and the results showed that the new N and T classifications both better discriminated PDAC patients by survival. Schlitter et al.8 reported two cohorts of 523 PDAC patients from Germany who underwent surgery in two hospitals over two decades (1991–2006; 2007–2014). They found that the T classification in the 7th edition could not discriminate patient prognosis, whereas the 8th edition showed substantial success in stratifying patients by prognosis. The new N classification failed to show high clinical relevance in either cohort.
Herein, we report the first validation of the 8th edition in an Asia population. To test the reliability of stratification based on the new TNM staging system, we enrolled patients according to rigorous criteria to avoid confounding factors to the maximal extent possible: (1) only patients treated after 2010 were enrolled; (2) there was a long follow-up period; (3) all cases achieved R0 resection; (4) only patients with tumors located in the head of the pancreas were enrolled; and (5) all the enrolled patient underwent at least 3 cycles of adjuvant chemotherapy based on gemcitabine. The median age of the patients was 60 years, which was a little younger than that of the above reports from Germany and America. There were slightly more male patients than female patients (1.8:1.0), which was in accordance with the ratio reported in the above studies from Germany. CA19-9, CA242 and CEA were elevated in 72.5%, 40.5% and 20.6% of the patients, respectively. Some previous studies have reported that elevated CA19-9 and CA242 are risk factors for poor prognosis23,24; in this study, we also found that elevated CA19-9 and CA242 were associated with poor prognosis.
Overall, 60.7% of the patients had lymph node metastasis, and 42.2% and 18.5% of the patients were pN1 and pN2, respectively. The pN classification in the 7th edition successfully stratified patient based on survival, but the new pN classification did not show an advantage over the 7th edition in discriminating patients by prognosis. In the 7th edition, 87.3% of patients were pT3, whereas 54.9% of patients were pT2 in the 8th edition, which was similar to the percentage reported in the above studies from America and Germany. pT3 classification in the 7th edition was redefined as pT1, pT2 and pT3 classification in the 8th edition, with a predominance of pT2 (51.2%); only 20.2% of pT3 classifications in the 8th edition corresponded with those in the 7th edition. pT classification in the 7th edition failed to stratify patients by survival. However, the prognosis of patients classified by the 8th edition as pT1-pT2 or pT3 was significantly different, suggesting that the new T classification was superior to the previous one at stratifying patients by survival. Although the 8th edition of the TNM staging system did not stratify patients by survival for all stages, it could discriminate stage I patients from those at other stages by survival, which the 7th edition of the staging system failed to do, further showing the superiority of the new edition.
In conclusion, this study is the first to validate the 8th edition of the TNM staging system for PDAC in an Asian population after its release. Compared to the validation studies from America and Germany, this study was performed at only a single center with a relatively small number of patients, which may weaken the reliability of the results. As previously stated, to validate the value of the TNM staging system, we enrolled patients according to rigorous criteria to minimize possible confounding factors that may affect the efficacy of the TNM staging system. The results supported that the new pT classification had a substantial advantage over the previous edition in predicting the overall survival of PDAC patients undergoing R0 pancreaticoduodenectomy and gemcitabine-based adjuvant chemotherapy. In this study, the new pN classification failed to show superiority over the previous edition in stratifying patients by overall survival. To further validate the superiority of the new edition of the TNM staging system at stratifying PDAC patients in Asia (China) by survival, a multicenter study with a large number of patients will be needed.
Methods
Patients and follow-up
In total, 102 consecutive cases of PDAC were enrolled from January 2010 to October 2014 according to the following inclusion criteria: (1) the final pathological examination confirmed PDAC; (2) none of the patients underwent neoadjuvant treatment; (3) radical R0 pancreaticoduodenectomy was achieved (microscopic margin > 1 mm); (4) all the patients underwent at least three cycles of gemcitabine-based adjuvant chemotherapy; (5) information on postoperative survival time was available, and patients who died within 3 months after surgery were excluded; and (6) all the patients signed the informed consent form. The clinicopathological information, including age, gender, tumor size, differentiation, perineural invasion (PNI), micro-cancerous embolus, CA19-9, CA242, CEA, pT classification, pN classification and TNM stage of the 7th and 8th editions, was extracted from the PACS system. After operation, the patients were followed up every 3∼6 months by outpatient clinic visits or telephone calls until patient death. Overall survival (OS) was defined as the survival time after surgery. All the patients agreed to donate bio-specimens for scientific research and publication and signed the informed consent form before surgery. The study was approved by the ethics committee of Peking Union Medical College Hospital.
Statistics
IBM SPSS Statistics software version 22.0 was applied for statistical analysis. Overall survival was analyzed using the Kaplan-Meier method, and the values were compared using the log-rank test. Multivariate analysis was performed using the Cox proportional hazard model. A P value less than 0.05 indicated statistical significance.
Data Availability
The primary data is available if reasonable requested.
Ethical approval
All procedures involving human participants were in accordance with the ethical standards of the ethical committee of Peking Union Medical College Hospital and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. All of the patients signed the informed consent for the scientific use of their information or bio-specimen before surgery.
Acknowledgements
This work was supported by National Natural Science Foundation of China (81502068, 81673023 and 81272573) and Beijing Natural Science Foundation of China (7172177) and CAMS innovation Fun for Medical Sciences (CIFMS) 2016-12M-3-005.
Author Contributions
L.Q. and Z.Y.P. designed the study. C.L., L.Q.F., Z.R.H., G.X., C.M., Z.X. collected and analyzed the data. C.L. and L.Q.F. wrote the manuscript. G.J.C., D.M.H., Z.T.P., L.Q. and Z.Y.P. performed the operation and revised the manuscript.
Competing Interests
The authors declare no competing interests.
Footnotes
Lin Cong and Qiaofei Liu contributed equally to this work.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Quan Liao, Email: lqpumc@126.com.
Yupei Zhao, Email: zhao8028@263.net.
References
- 1.The Lancet Gastroenterology, H. Pancreatic cancer: how can we tackle the lack of progress? Lancet Gastroenterol Hepatol2, 73 (2017). [DOI] [PubMed]
- 2.Ryan DP, Hong TS, Bardeesy N. Pancreatic adenocarcinoma. N Engl J Med. 2014;371:1039–1049. doi: 10.1056/NEJMra1404198. [DOI] [PubMed] [Google Scholar]
- 3.Aung, K. L. et al. Genomics-Driven Precision Medicine for Advanced Pancreatic Cancer - Early Results from the COMPASS Trial. Clin Cancer Res (2017). [DOI] [PMC free article] [PubMed]
- 4.Chen W, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115–132. doi: 10.3322/caac.21338. [DOI] [PubMed] [Google Scholar]
- 5.Liu Q, et al. Immunoglobulin G4 (IgG4)-positive plasma cell infiltration is associated with the clinicopathologic traits and prognosis of pancreatic cancer after curative resection. Cancer Immunol Immunother. 2016;65:931–940. doi: 10.1007/s00262-016-1853-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Crippa, S., Partelli, S. & Falconi, M. Pancreatic Ductal Adenocarcinoma: A New TNM Staging System is Needed! Ann Surg (2016). [DOI] [PubMed]
- 7.Allen PJ, et al. Multi-institutional Validation Study of the American Joint Commission on Cancer (8th Edition) Changes for T and N Staging in Patients With Pancreatic Adenocarcinoma. Ann Surg. 2017;265:185–191. doi: 10.1097/SLA.0000000000001763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schlitter AM, et al. pT but not pN stage of the 8th TNM classification significantly improves prognostication in pancreatic ductal adenocarcinoma. Eur J Cancer. 2017;84:121–129. doi: 10.1016/j.ejca.2017.06.034. [DOI] [PubMed] [Google Scholar]
- 9.Welsch, T. et al. The “T” now Matters: The Eighth Edition of the Union for International Cancer Control Classification of Pancreatic Adenocarcinoma. Ann Surg (2017). [DOI] [PubMed]
- 10.Kamarajah SK, Burns WR, Frankel TL, Cho CS, Nathan H. Validation of the American Joint Commission on Cancer (AJCC) 8th Edition Staging System for Patients with Pancreatic Adenocarcinoma: A Surveillance, Epidemiology and End Results (SEER) Analysis. Ann Surg Oncol. 2017;24:2023–2030. doi: 10.1245/s10434-017-5810-x. [DOI] [PubMed] [Google Scholar]
- 11.Vaccaro, V., Sperduti, I. & Milella, M. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med365, 768–769, author reply 769 (2011). [DOI] [PubMed]
- 12.Liu Q, Liao Q, Zhao Y. Chemotherapy and tumor microenvironment of pancreatic cancer. Cancer Cell Int. 2017;17:68. doi: 10.1186/s12935-017-0437-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Conroy T, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364:1817–1825. doi: 10.1056/NEJMoa1011923. [DOI] [PubMed] [Google Scholar]
- 14.Bender O, et al. Clinical Significance of Intraoperative Frozen Section Analysis of Pancreatic Cancer Surgical Margin at the Time of Pancreaticoduodenectomy. Chirurgia (Bucur) 2015;110:446–450. [PubMed] [Google Scholar]
- 15.Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin. 2017;67:7–30. doi: 10.3322/caac.21387. [DOI] [PubMed] [Google Scholar]
- 16.Siegel RL, et al. Colorectal cancer statistics, 2017. CA Cancer J Clin. 2017;67:177–193. doi: 10.3322/caac.21395. [DOI] [PubMed] [Google Scholar]
- 17.Miller KD, et al. Cancer treatment and survivorship statistics, 2016. CA Cancer J Clin. 2016;66:271–289. doi: 10.3322/caac.21349. [DOI] [PubMed] [Google Scholar]
- 18.Bowyer S, Lorigan P. The place of PD-1 inhibitors in melanoma management. Lancet Oncol. 2015;16:873–874. doi: 10.1016/S1470-2045(15)00094-7. [DOI] [PubMed] [Google Scholar]
- 19.Marchegiani G, et al. Does Size Matter in Pancreatic Cancer?: Reappraisal of Tumour Dimension as a Predictor of Outcome Beyond the TNM. Ann Surg. 2017;266:142–148. doi: 10.1097/SLA.0000000000001837. [DOI] [PubMed] [Google Scholar]
- 20.Tarantino I, et al. Staging of pancreatic cancer based on the number of positive lymph nodes. Br J Surg. 2017;104:608–618. doi: 10.1002/bjs.10472. [DOI] [PubMed] [Google Scholar]
- 21.Kim SH, Hwang HK, Lee WJ, Kang CM. Identification of an N staging system that predicts oncologic outcome in resected left-sided pancreatic cancer. Medicine (Baltimore) 2016;95:e4035. doi: 10.1097/MD.0000000000004035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Malleo G, et al. Reappraisal of Nodal Staging and Study of Lymph Node Station Involvement in Pancreaticoduodenectomy with the Standard International Study Group of Pancreatic Surgery Definition of Lymphadenectomy for Cancer. J Am Coll Surg. 2015;221:367–379 e364. doi: 10.1016/j.jamcollsurg.2015.02.019. [DOI] [PubMed] [Google Scholar]
- 23.Ni XG, et al. The clinical value of serum CEA, CA19-9, and CA242 in the diagnosis and prognosis of pancreatic cancer. Eur J Surg Oncol. 2005;31:164–169. doi: 10.1016/j.ejso.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 24.Dong Q, et al. Elevated serum CA19-9 level is a promising predictor for poor prognosis in patients with resectable pancreatic ductal adenocarcinoma: a pilot study. World J Surg Oncol. 2014;12:171. doi: 10.1186/1477-7819-12-171. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The primary data is available if reasonable requested.