Abstract
Aims
To compare recruitment, refusal and randomisation rates of older adults into a general practice-based clinical trial with two versions (varied format, content and language) of the Participant Information and Consent Form (PICF).
Methods
This prospective PICF study was conducted within the STAREE (STAtins in Reducing Events in the Elderly) clinical trial. Participants phone screened between October 2015 to February 2016 formed Group 1 and were mailed the extended PICF version and participants phone screened between October 2016 to February 2017 formed Group 2 and were mailed the shortened PICF version. Participants who attended a subsequent baseline screening visit were guided through a comprehensive informed consent process.
Results
During the screening phase of the trial, the likelihood of refusing trial participation was lower in Group 2 compared to Group 1 equating to an overall 23% reduction in risk (RR 0.77, P = 0.005, 95% CI 0.62–0.95). Group 2 had a 6.4% higher randomisation rate compared with Group 1 (65.3% versus 58.9% respectively) but this difference was not statistically significant. Factors associated with trial participation were male gender, age between 70 and 75 years and living alone (all p < .0.05).
Conclusions
Whilst avoiding lengthy and complex PICF documents may assist with initial trial engagement, it needs to be supplemented with other strategies to support ongoing trial interest to randomisation and beyond. Participants refused trial participation throughout the screening phase indicating that the PICF was only one factor among several affecting an individual's decision to participate in this clinical trial.
Keywords: Informed consent, Clinical trial, Participant recruitment, Public health, Preventive medicine, Elderly
Highlights
-
•
A short and concise clinical trial document is recommended.
-
•
Avoiding lengthy and complex clinical trial documents may contribute to increasing older adult trial participation rates.
-
•
Strategies are sought to increase participation of older adults in research to garner more age-specific public health data.
1. Background
To date, there has been an underrepresentation of older adults in clinical trials. Offering older adults the opportunity to participate in clinical research is paramount given that life expectancy and the proportion of older people living beyond their seventh decade is dramatically increasing. In Australia there are close to 4 million people aged 65 years and over, representing 15% of the population [1]. This is consistent with the global population total of 13%, which is expected to increase to more than 2 billion people by 2050 [2]. Thus there is a compelling argument for including older adults in clinical trials and establishing age-specific evidence to support best practice in the clinical and medical care of this population. Furthermore garnering data around the clinical efficacy and safety (or risks and benefits) of medical treatments in this age group is crucial as they represent a group that are among the highest medication users [3].
Despite the growing need for more clinical trial evidence in older populations, there are known barriers to recruiting older adults into clinical research studies. Recruitment barriers include difficulty locating willing older adults, access issues, ethnicity barriers, heightened personal fears or concerns about clinical research and other existing comorbidities that may exclude older adults from participating [4,5]. The informed consent process itself may also pose a potential barrier to trial participation. Specifically, how to best tailor the ‘how much, when to and what sort’ of information to provide to older research trial participants, allowing for variations in health literacy, education and age-related sensory (visual and aural) and cognitive changes is unclear [6,7].
The Participant Information and Consent Form (PICF) is one aspect of the informed consent process that can be a critical factor influencing willingness to participate in a clinical trial [7,8]. With respect to the PICF content and language, balancing the requirements of any local human research ethics committee with the needs of the potential research participants can be challenging. Ethically, potential participants must be given sufficient information to make an informed decision about trial participation and not simply opt into a research trial [9]. It has been suggested that if the PICF document is too long, features complex language and provides too much detailed information on the risks and benefits of participating, it may likely be perceived as confusing by the older adult population [6,10]. Some of the issues around misunderstanding of information may be mitigated if the PICF document is supported by an interactive opportunity to discuss its content [7,11]. This is positively viewed if, when interacting with older adults, there is an extended amount of time to read and review the document provided [5].
There is no gold standard for how the PICF should be presented and in most instances it is provided before a face to face discussion with the research team. This is the approach taken in the STAtins in Reducing Events in the Elderly (STAREE) trial, a general practice based, pragmatic, public health randomised control trial (RCT) exploring the potential impact of statin therapy on healthy ageing in adults 70 years of age and older (ClinicalTrials.gov Identifier: NCT0299123). As part of our process evaluation during the vanguard phase, we explored the factors influencing willingness to participate in the clinical trial. It was noted that 1 in 4 invited participants opt out of the study following a successful eligibility phone screen. After receiving the PICF in the mail (with their screening appointment confirmation letter), a significant number of people reported they were concerned about the large number of reported potential side effects of statin treatment.
As a result, the PICF was updated to present a more balanced view of the potential risks and benefits of statins. In addition to this, the format was altered and the content shortened in specific parts. By comparing two versions of the PICF, with varied format, content and language, we aimed to assess if a shorter and more concise version led to a difference in the number of participants who remain interested in taking part in our clinical trial and proceeding to randomisation.
2. Methods
This prospective cohort study was conducted through Monash University, School of Public Health and Preventive Medicine (Melbourne, Australia). Ethical approval was obtained from the local institutional research board (Monash University Human Research Ethics Committee, MUHREC) and the Royal College of General Practitioners (RACGP), and the study adhered to the tenets of the Declaration of Helsinki. The study formed part of process evaluation during the early period of participant recruitment of a larger RCT (ClinicalTrials.gov Identifier: NCT0299123).
2.1. Participants
Participants who had been invited to take part in the STAREE trial, a phase IV pragmatic and general practice-based RCT, were eligible to take part in the PICF-study. Given the increase in recruitment numbers as the study progressed and the potential impact of season on recruitment numbers [12], the same enrolment months were selected for group comparison. In addition, the PICF-study was conducted across two time periods reflecting the stages of the informed consent process evaluation. Potentially eligible participants for the STAREE trial were identified from their general practice clinical database, wherein their usual general practitioner (GP) then sent them an invitation letter with a trial summary information brochure, and a request to call the trial office if they were interested in participating. At the time of the call, potential participants were informed more about the key aspects of the trial and guided through a phone eligibility screening questionnaire comprising 12 questions including past/current history of cardiovascular disease, current/previous statin treatment, and current diagnosis of diabetes. Following a successful phone screening, an appointment was made to attend two baseline screening visits four weeks apart with a trained research nurse. These were designated Baseline Visit 1 (BV1) and Baseline Visit 2 (BV2).
Participants for this PICF-study were assigned to the intervention based on the date they were invited into the STAREE trial (see below). Purposive sampling was used and groups were unmatched.
2.2. Intervention
2.2.1. Group 1 = standard PICF (extended version)
Potential participants phone screened during October 2015 to February 2016 were allocated to Group 1 and were sent the extended version of the PICF (version 1.4) with their appointment confirmation letter in the mail 2–3 weeks prior to BV1. PICF v 1.4 had 9 pages presented in a single column of text and featured a complete list (476 words) of the potential risks, or side effects, of the trial therapy based on the Therapeutic Goods Association (TGA) Product Information sheet. Therefore, the list included the most common, uncommon and rare potential side effects. The most common potential side effects, affecting ≥1% of consumers, were listed under seven symptom categories and included gastrointestinal disorders such as diarrhoea. The uncommon potential side effects, affecting ≥0.1–1% of consumers, were listed under twelve symptom categories and included musculoskeletal disorders such as tenderness or pain (myopathy), and finally the rare potential side effects affecting ≥ 0.01% to < 0.1% of consumers were listed under three symptom categories and included effects such as severe hypersensitivity/anaphylaxis. The potential benefits of statin therapy were not listed but rather a standard statement reporting that “participants may experience health benefits from being placed on study medication” and that if shown, this “may enable this treatment to be available to more people in the future”. This version of the PICF was approved for distribution to trial participants by MUHREC and the RACCP ethics committee in September 2015.
2.2.2. Group 2 = condensed PICF (version 1.5)
Potential participants phone screened during October 2016 to February 2017 were allocated to Group 2 and were sent the shortened version of the PICF (version 1.5) with their appointment confirmation letter in the mail 2–3 weeks prior to BV1. PICF v 1.5 had 6 pages presented in two columns of text and featured additional information about the potential benefits of trial medication including a list of the known potential benefits (i.e. reduction in levels of cholesterol in the blood) and also those less certain supporting the need for more research (i.e. reduced risk of dementia) (54 words). In addition, PICF v1.5 had a simplified list of potential side effects with the prevalence percentages and 2–3 symptom examples for the most common, uncommon and rare divisions listed above (248 words). Both the potential benefits and risks of participating in the trial were listed in point form along with a note that the TGA Consumer Medicine sheet would be provided for more information with study medication. This version of the PICF was approved by MUHREC and the RACGP ethics committee in February 2016.
2.3. Consent process
Regardless of consent version, each participant presenting to BV1 was guided through a comprehensive informed consent process, adhering to The International Council for Harmonisation - Good Clinical Practice (ICH-GCP) guidelines, including a full and open discussion around all aspects of the clinical trial, answering any questions raised by the participant and getting the participant to repeat the key aspects of the trial. The informed consent process was undertaken in a private consulting room at the participant's primary general practice. Research staff were trained in GCP and effective communication including active listening skills, maintaining eye contact and speaking in a soft and welcoming manner.
2.4. Sample size calculation
Based on a binary primary outcome (recruited/consented; yes or no), and estimating a 10% proportional difference in event rate between the two groups of unequal size, a sample size of 300 individuals for Group 1 and 400 individuals for Group 2 yielded 80% power to detect a 10% difference in recruitment rate with a two sided alpha level of 0.05 [13]. Unequal sample sizes were expected given Group 1 participants were enrolled at the beginning of the recruitment phase in year 1 and Group 2 participants enrolled in year 2 when an increase in recruitment numbers was anticipated.
2.5. Statistical analysis
Pearson's chi-square tests were used to compare groups on demographic categories including age group, gender and living arrangements. Modified Poisson regression (using robust error variances), was used to compute relative risks for binary (yes/no) outcomes: exclusion rate (participants excluded based on study inclusion/exclusion criteria not being met), recruitment rate (participants who attended BV1 and offered written informed consent), refusal rate (participants refusing trial participation during the screening baseline visits) and randomisation rate (participants who were allocated to trial intervention) [14]. Randomisation rates were analysed following removal of participants who were excluded during the baseline screening visits. In secondary adjusted analyses, identification of factors that may influence outcomes in each group were explored using the same model with 3 covariates: age group (reference 70–75 years vs ≥ 76 years), gender (reference female vs male) and living arrangement (reference living alone vs with others). Other potential covariates were considered, such as season and state, but they were not found to be significant in preliminary stepped multivariate analyses. The reasons provided by participants who refused trial participation were examined using Pearson's chi square tests. All analyses were performed using STATA statistical software (version 11, StataCorp LLC, USA).
3. Results
3.1. Characteristics of the study population
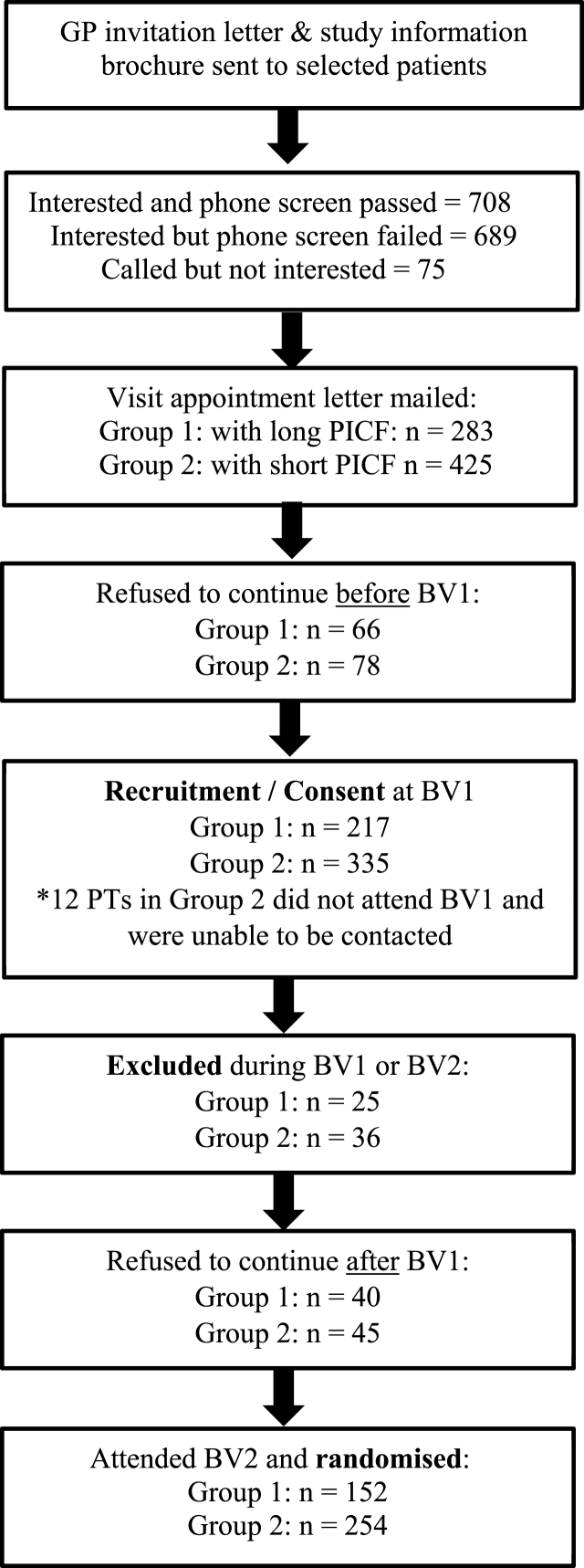
1472 individuals contacted the trial office following receipt of a GP invitation letter during the PICF study period. Of these, 1397 were interested in STAREE trial participation and proceeded to the first eligibility step via a phone screening; 689 were excluded at this stage and 708 had baseline screening visits (BV1 and BV2) booked and were eligible for the PICF process evaluation study (Fig. 1). The mean age of the PICF study participants was 76.9 ± 5.5 years with 28% of the sample aged 80 years and above. Gender disposition was similar within the PICF study population with 56% being female.
Fig. 1.
Flowchart of participants from study invitation to randomisation by group.
Notes: BV1 = Baseline Visit 1, BV2 = Baseline Visit 2, PICF = Participant Information and Consent Form, PT = Participant.
Within the PICF study population, 283 participants formed Group 1 and were allocated to receive the PICF version 1.4. An additional 30 people were eligible to participate in this arm of the study however for a variety of reasons (i.e. were not available on the select appointments days) a baseline screening visit appointment could not be made and thus they were excluded from the PICF study. Group 2 compromised 425 participants who were eligible following phone screening and allocated to receive the PICF version 1.5. Of these, 12 participants received the PICF in the mail but did not attend baseline screening appointments and could not be contacted for a follow up outcome. The two groups had similar proportions of participants who were male, aged 76 and above, spoke English as their first language and lived alone (P < 0.05; Table 1) Groups varied slightly based on the numbers of participants from each state, reflecting the on boarding of additional sites as study recruitment expanded from the primary site (Melbourne, Australia) (P = <0.0001; Table 1).
Table 1.
Characteristics of the PICF study groups.
| Factor | Group 1 PICF v 1.4 n = 283 Count (%) |
Group 2 PICF v 1.5 n = 425 Count (%) |
|---|---|---|
| Age Group | ||
| 70–75 years | 147 (51.9%) | 231 (54.4%) |
| 76–98 years | 136 (48.1%) | 194 (45.6%) |
| Gender | ||
| Male | 118 (41.7%) | 197 (46.3%) |
| Female | 165 (58.3%) | 228 (53.7%) |
| Primary Language | ||
| English | 186 (65.7%) | 278 (65.4%) |
| Non-English | 97 (34.3%) | 147 (34.6%) |
| Living Arrangement | ||
| Alone | 70 (24.7%) | 96 (22.6%) |
| With others | 213 (75.3%) | 329 (77.4%) |
| State | ||
| Victoria | 194 (68.5%) | 231 (54.3%) |
| Tasmania | 67 (23.7%) | 52 (12.2%) |
| Western Australia | 22 (7.8%) | 142 (33.4%) |
Note: PICF = Participant Information and Consent Form.
3.2. Trial participation outcomes
A smaller proportion of participants refused to continue STAREE trial participation before attending BV1 in Group 2 (18.4%) compared to Group 1 (23.3%) (Table 2). A similar proportion of participants across the two groups were recruited into the STAREE trial, namely they completed BV1 and provided written informed consent (Table 2). A smaller proportion of participants refused to continue after BV1 in Group 2 (10.6%) compared to Group 1 (14.2%). When the refusal rate was considered before and after BV1, the overall likelihood of not continuing in the trial was lower in Group 2 compared to Group 1 equating to a 23% relative risk reduction (RR 0.77, P = 0.017, 95% CI 0.62–0.95). There was no significant difference in the proportion of participants being excluded due to abnormalities of routine pathology testing or having a confirmed history of cardiovascular disease during the screening phase between the two groups (RR 0.99, P = 0.961, 95% CI 0.95–1.04). A larger proportion of participants were randomised into the trial in Group 2 (65.3%) compared with Group 1 (58.9%) but the 6.4% rate difference did not equate to a significant relative risk difference (Table 2).
Table 2.
Unadjusted relative risk levels on trial participation outcomes.
| Outcome | Group 1 PICF v 1.4 Count/283 (%) |
Group 2 PICF v 1.5 Count/425 (%) |
RR 95% CI |
P Value |
|---|---|---|---|---|
| Refusal % before BV1 | 66 (23.3%) | 78 (18.4%) | 0.78 | 0.107 |
| 0.58–1.05 | ||||
| Recruitment % (written informed consent completed at BV1) | 217 (76.7%) | 335* (78.8%) | 1.02 | 0.505 |
| 0.94–1.11 | ||||
| Refusal % after BV1 | 40 (14.2%) | 45 (10.6%) | 0.74 | 0.156 |
| 0.50–1.11 | ||||
| Refusal % total before and after BV1 | 106 (37.5%) | 123 (28.9%) | 0.77 | 0.005 |
| 0.62–0.95 | ||||
| Randomisation %** | 152 (58.9%) | 254 (65.3%) | 1.10 | 0.110 |
| 0.97–1.25 |
Notes: Significant at probability alpha level 0.05; Reference group in statistical models is Group 1; PICF = Participant Information and Consent Form; BV1 = Baseline Visit 1; RR = Relative Risk or Risk Ratio; *12 participants are not included in this total as they did not attend their first baseline visit & were unable to be contacted for follow up, **Total randomised is adjusted by removing participants excluded due to pathology etc. after their first baseline visit (Group 1 n = 258, Group 2 n = 389).
3.3. Factors associated with trial participation
Factors associated with trial consent, refusal and randomisation irrespective of PICF version were gender, age group and living arrangement (Table 3). When compared with females, males were more likely to consent and continue in the trial to randomisation (Table 3). When compared with participants aged 70–75 years, those aged 76 years and above were less likely to consent and continue in the trial to randomisation (Table 3). When compared with participants who live alone, those who live with others were less likely to consent and continue in the trial beyond the first baseline visit (Table 3). When examining the total number of participants who refused to continue in the trial before and after the first baseline visit, males were 27% less likely to refuse trial participation than females, as were participants aged between 70 and 75 years compared with those aged ≥76 years (Table 3).
Table 3.
Adjusted relative risk levels across groups on study outcomes.
| Outcome | RR (95% CI) | P Value |
|---|---|---|
| Recruitment - Consent | ||
| Group (2) | 1.06 (0.98–1.15) | 0.130 |
| Gender (male) | 1.25 (1.15–1.35) | <0.001 |
| Age (≥76 group) | 0.88 (0.81–0.96) | 0.004 |
| Lives with other/s | 0.63 (0.59–0.68) | <0.001 |
| Refusal total before and after first baseline visit | ||
| Group (2) | 0.74 (0.59–0.91) | 0.004 |
| Gender (male) | 0.63 (0.50–0.79) | <0.001 |
| Age (≥76 group) | 1.26 (1.02–1.56) | 0.028 |
| Lives with other/s | 2.80 (1.91–4.10) | <0.001 |
| Refusal before first baseline visit | ||
| Group (2) | 0.79 (0.60–1.04) | 0.087 |
| Gender (male) | 0.46 (0.34–0.63) | <0.001 |
| Age (≥76 group) | 1.31 (1.00–1.72) | 0.046 |
| Lives with other/s | 2.36 (1.47–2.40) | <0.001 |
| Refusal after first baseline visit | ||
| Group (2) | 0.65 (0.51–1.13) | 0.047 |
| Gender (male) | 1.09 (0.71–1.67) | 0.677 |
| Age (≥76 group) | 1.19 (0.75–1.83) | 0.399 |
| Lives with other/s | 0.64 (0.41–1.00) | 0.053 |
| Randomised | ||
| Group (2) | 1.10 (0.97–1.24) | 0.110 |
| Gender (male) | 1.28 (1.14–1.44) | <0.001 |
| Age (≥76 group) | 0.82 (0.73–0.93) | 0.002 |
| Lives with other/s | 0.63 (0.56–0.71) | <0.001 |
Notes: Significant at probability alpha level 0.05; reference variable in brackets; RR = Relative Risk or Risk Ratio.
3.4. Reasons given for refusing trial participation
The main reason/s given for refusing trial participation are provided in Table 4. These included concerns over potential treatment side effects, family influence, unstable health and too many competing demands in life. Of note, around 1 in 5 participants in both groups chose not to report a reason for their decision to refuse trial participation. When comparing reasons across the two PICF groups, concerns over potential treatment side effects were more commonly reported by participants in Group 1 compared with Group 2, 23.6% versus 13.7% respectively (P = 0.034) whereas changes to health affecting their decision to continue in the trial were more commonly reported by participants in Group 2 compared with Group 1, 11.1% versus 5% respectively (P = 0.032).
Table 4.
Reasons participants provided for deciding not to continue in the trial during the screening phase.
| Main reason for refusing to continue trial participation | Group 1 PICFv 1.4 % response |
Group 2 PICFv 1.5 % response |
Pearson's chi square P Value |
|---|---|---|---|
| Concerns over potential treatment side effects | 23.6 | 13.7 | 4.481 |
| 0.034 | |||
| Family influence | 17.0 | 10.2 | 2.704 |
| 0.100 | |||
| Unstable health | 5.0 | 11.1 | 4.606 |
| 0.032 | |||
| Too many competing demands | 7.0 | 9.4 | 0.130 |
| 0.718 | |||
| Unwilling to take study medication | 12.3 | 12.0 | 0.054 |
| 0.816 | |||
| Other reasons (combined categories with smaller %) | 35.1 | 43.6 | – |
Notes: Significant at probability alpha level 0.05; PICF = Participant Information and Consent Form.
4. Discussion
Our study showed that a shortened more concise version of a PICF compared with an extended more detailed version led to fewer participants refusing to continue in the trial during the screening phase. This equated to a cumulative incidence difference of 8.6% (between PICF groups) or relative risk reduction of 23% in participants who received the shortened version of the PICF. Whilst this indicated an initial impact of the shorter PICF on willingness to participate, it did not support sustained trial interest across the cohort to the time of randomisation. Factors associated with trial consent, refusal and randomisation irrespective of PICF version were male gender, being aged between 70 and 75 years, and living alone. Reasons provided by participants who refused trial participation were varied and included concerns over potential side effects. These reasons, along with the finding that participants refused trial participation throughout the screening phase, indicate that the PICF was only one factor among several affecting an individual's decision to participate in this clinical trial.
Critically these findings support previous literature suggesting older adults need more time to make an informed decision regarding clinical trial participation (compared to younger adults) and that more time should be spent discussing all the perceived benefits and risks to potential participants and family members [5,15]. Providing an optimal level of information around side effects of study treatment to older trial participants so that they are not overwhelmed by the details yet have understood the most important potential risks is reportedly key in enhancing understanding and reducing anxiety [15]. From an ethical perspective, the provision of less information still needs to be weighed against providing enough information to make an informed decision.
Ethical principles recommend that research participants must be provided a comprehensive and balanced overview of the available evidence that is relevant to garner an evaluation of the individual risks and benefits of participating, and it is the responsibility of the researchers and research committees to determine how much information is sufficient to uphold these principles [16]. Following careful consultation between the STAREE trial researchers, ethics committees and community advisory group, the shortened PICF was created with the aim of providing a more realistic reflection of the risks and benefits of participating as well as adding important information about how the risks may be minimised (i.e. emphasising that participants should notify and discuss any new symptom or potential side effect with their treating GP) in this phase IV trial. Regardless of study group, research staff were trained to focus discussion at screening visits on what individual risks and benefits were likely and how any potential risks could be managed. In this PICF study, whilst the reasons for refusing continued research participation after phone screening were varied, the number of participants citing concerns over potential side effects of treatment were significantly greater in those receiving the more detailed PICF compared with the shortened condensed version. Irrespective of version, coupling the PICF delivery with a face to face, individually-focused and interactive discussion of the trial components is a known facilitator to boosting acceptance and comprehension of clinical trial information [6,17]. Indeed, having the same group of participants compare both versions of the PICF (direct comparison) in accompaniment with a face to face discussion, would likely provide more information about the acceptability of our modified PICF.
Few studies have directly examined the effect of content and language of the PICF on clinical trial participation rates in older adults. Furthermore, it is not clearly reported what the average refusal rate might be for older adults considering trial participation [18]. Two early studies examined the impact of the PICF on recruitment rates and both studies concluded that a short and concise PICF, with only key potential side effects listed, compared with a long and more detailed PICF, led to a statistically significant improvement in recruitment rates [19,20]. The study by Del-ra and colleagues, also showed that anxiety levels were much lower in participants, whilst the understanding of the key points much higher, with the short PICF version [19]. These studies were conducted on young adults with small sample sizes yet suggest that the PICF is an influential part of the informed consent procedure. Indeed a systematic review conducted in 1998 examining the informed consent process across a broad range of clinical trials highlighted that few studies explored the impact of the quantity of information given to potential middle-aged participants on consent but in those that did (4 trials) more information was generally associated with either a lower or unchanged consent rate [15].
Older participant involvement in clinical research is low to modest, varying between 10 and 50% across the small number of trials that focus on this population [21]. Although our recruitment rate reflects the upper range of reported recruitment rates, a large number of older adults are needed to be invited into the trial to reach this level. Nonetheless, our study highlights the importance of exploring ways to reduce the known barriers to inviting and enrolling older adults into clinical trials.
It would have been of interest to quantify each participant's level of comprehension of their PICF version, for example level of understanding of the possible individual risks and benefits of trial involvement. Whilst this was beyond the scope of this study it would be an important area for future research especially given the need to establish a validated method to measure level of participant understanding of a consent form contents [16]. In addition, future research could also collect more detailed information about the specific concerns participants reported either during the informed consent process or when participants decided to discontinue from the trial aside from collecting themes. Collecting information about which specific side effects were of most concern, for example, would assist focused discussion around these areas during the consent process. Methods to capture this specific information are currently being explored in the trial.
Our study may have been limited by comparing two PICF versions a year apart. It is possible that there could be differences in external influences on participant involvement (i.e. media exposure). In addition, we did not have the opportunity to enquire as to the reasons why non-responders of the GP invitation letter did not consider clinical trial participation. Despite this, we were able to identify potential risk factors for refusing continued trial participation in the recruitment phase, namely female gender, increasing age and living with others. Coupled with a focused discussion on crucial potential treatment side effects, it may be that the informed consent process could be further tailored to suit the needs of these high risk ‘refusers’, a study for future work in this area.
Tailoring the Participant Information and Consent Form to suit the needs of older adults is critical in promoting greater awareness and understanding of public health research. This study suggested that a short and concise PICF document with an easy to read and follow format may improve the willingness of older adults to consider participation in clinical research trials. Whilst this strategy had an initial beneficial impact on early trial engagement, it did not lead to any sustained improvement on randomisation rates into the trial. Further research would be valuable in exploring additional strategies that facilitate recruitment and retention of older adults into clinical trials given the increasing need to establish age-appropriate health care therapies that support independence and a positive quality of life in later years.
Financial acknowledgement
This work (base trial) was supported by the National Health and Medical Research Council (NH&MRC) of Australia (APP1068146). The NH&MRC had no role in the writing of this paper.
Declaration
The Authors declare that there are no conflict of interest.
Acknowledgements
STAREE Study Group, Professor John McNeil, Professor Christopher Reid, Dr Alissia Kost and Professor Andrew Tonkin.
References
- 1.(ABS) vol. 2015. 2015. (A.B.o.S., 3235.0-Population by Age and Sex, Regions of Australia). [Google Scholar]
- 2.United Nations, D.o.E.a.S.A., Population Division . 2017. World Population Ageing 2017-Highlights (ST/ESA/SER.A/397) [Google Scholar]
- 3.Koronkowski M., Eisenhower C., Marcum Z. An update on geriatric medication safety and challenges specific to the care of older adults. Ann. Long Term Care. 2016;24(3):37–40. [PMC free article] [PubMed] [Google Scholar]
- 4.Crome P., Cherubini A., Oristrell J. The PREDICT (increasing the participation of the elderly in clinical trials) study: the charter and beyond. Expet Rev. Clin. Pharmacol. 2014;7(4):457–468. doi: 10.1586/17512433.2014.922864. [DOI] [PubMed] [Google Scholar]
- 5.Ridda I. Difficulties in recruiting older people in clinical trials: an examination of barriers and solutions. Vaccine. 2010;28(4):901–906. doi: 10.1016/j.vaccine.2009.10.081. [DOI] [PubMed] [Google Scholar]
- 6.Hallinan Z.P. Barriers to change in the informed consent process: a systematic literature review. IRB. 2016;38(3):1–10. [PubMed] [Google Scholar]
- 7.Lentz J. Paving the way to a more effective informed consent process: recommendations from the Clinical Trials Transformation Initiative. Contemp. Clin. Trials. 2016;49:65–69. doi: 10.1016/j.cct.2016.06.005. [DOI] [PubMed] [Google Scholar]
- 8.Harris R., Dyson E. Recruitment of frail older people to research: lessons learnt through experience. J. Adv. Nurs. 2001;36(5):643–651. doi: 10.1046/j.1365-2648.2001.02029.x. [DOI] [PubMed] [Google Scholar]
- 9.I.H.T.g.f.g.c.p.r.E.R. 1996. Guideline for Good Clinical Practice. Step 4 Version Dated 10 June 1996. [Google Scholar]
- 10.Lorell B.H. Informed consent in clinical research: consensus recommendations for reform identified by an expert interview panel. Clin. Trials. 2015;12(6):692–695. doi: 10.1177/1740774515594362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pick A., Gilbert K., McCaul J. The role of effective communication in achieving informed consent for clinical trials. Nurs. Stand. 2014;29(10):45–48. doi: 10.7748/ns.29.10.45.e9443. [DOI] [PubMed] [Google Scholar]
- 12.Haidich A.B., Ioannidis J.P. Determinants of patient recruitment in a multicenter clinical trials group: trends, seasonality and the effect of large studies. BMC Med. Res. Meth. 2001;1:4. doi: 10.1186/1471-2288-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wittes J. Sample size calculations for randomized controlled trials. Epidemiol. Rev. 2002;24(1):39–53. doi: 10.1093/epirev/24.1.39. [DOI] [PubMed] [Google Scholar]
- 14.Zou G. A modified Poisson regression approach to prospective studies with binary data. Am. J. Epidemiol. 2004;159(7):702–706. doi: 10.1093/aje/kwh090. [DOI] [PubMed] [Google Scholar]
- 15.Edwards S.J. Informed consent for clinical trials: in search of the "best" method. Soc. Sci. Med. 1998;47(11):1825–1840. doi: 10.1016/s0277-9536(98)00235-4. [DOI] [PubMed] [Google Scholar]
- 16.Nijhawan L.P. Informed consent: issues and challenges. "J. Adv. Pharm. Technol. Research"" (JAPTR)". 2013;4(3):134–140. doi: 10.4103/2231-4040.116779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Giampieri M. Communication and informed consent in elderly people. Minerva Anestesiol. 2012;78(2):236–242. [PubMed] [Google Scholar]
- 18.Leppik I.E. Outcomes research: clinical trials in the elderly. Epilepsy Res. 2006;68(Suppl 1):S71–S76. doi: 10.1016/j.eplepsyres.2005.07.019. [DOI] [PubMed] [Google Scholar]
- 19.Dal-Re R. Clinical trial of drugs: a study of the influence of information on adverse reactions on obtaining of informed consent. Med. Clin. 1991;96(15):566–569. [PubMed] [Google Scholar]
- 20.Epstein L.C., Lasagna L. Obtaining informed consent. Form or substance. Arch. Intern. Med. 1969;123(6):682–688. [PubMed] [Google Scholar]
- 21.Hawranik P., Pangman V. Recruitment of community-dwelling older adults for nursing research: a challenging process. Can. J. Nurs. Res. 2002;33(4):171–184. [PubMed] [Google Scholar]