Abstract
This study aims to investigate relationship between the level of uric acid (UA) and UA/creatinine ratios (UA/Cr) to the stage of Parkinson disease (PD).
A total of 120 cases of PD patients who were admitted in our hospital between 2013 and 2015 were enrolled into this study; these 120 cases of PD patients were divided into 3 groups, according to Hoehn–Yahr (H-Y) classification: early stage (1–2 classification), medium stage (2.5–3 classification), and advanced stage (4–5 classification); UA and UA/Cr level in each group was compared. Then, factors including age, gender, dopamine dosage, UA, and UA/Cr levels were analyzed to find the independent predictive factors of PD by logistic regression.
UA and UA/Cr levels in the early and medium stage PD patients were significantly higher than in the advanced stage ones. UA and UA/Cr levels in patients with good prognosis were significantly higher than in the poor ones.
UA and UA/Cr levels are negatively correlated with the stages of PD and are independent negatively predicting biological indexes of PD incidence and progression.
Keywords: H-Y classification, Parkinson disease, prognostic factor, uric acid, uric acid/creatinine
1. Introduction
Parkinson disease (PD) is a common neurodegenerative disease. Its etiology and pathogenesis are very complex, and remains unclear at present. These may be related to the interactions of age, environment, heredity and oxidative stress, and other factors.[1,2] Uric acid (UA) is a natural antioxidant that has effects of scavenging free radicals, and antioxidation.[1,2] It has been reported that serum UA concentrations are closely related to the development of some neurodegenerative diseases such as PD, multiple system atrophy (MSA), amyotrophic lateral sclerosis (ALS), and Alzheimer disease (AD).[3–6] Therefore, we explored the correlation between this disease classification versus UA and UA/Cr levels by investigating differences in UA and UA/Cr levels in PD patients at the early, medium, and advanced stages.
2. Materials and methods
2.1. Research objects
Hospitalized PD patients admitted in our hospital from 2013 to 2015 were included into this retrospective study. These patients were diagnosed by 2 experienced neurologists, strictly in accordance with the UK Parkinson's Disease Society Brain Bank Clinical Diagnostic Criteria. Exclusion criteria were as follows: PD induced by chemical drugs, trauma, cerebrovascular disease, and other reasons; PD patients complicated with nephropathy, tumor, gout, vegetarians; PD patients who received thiazide diuretics and patients of nervous system disease were excluded. All subjects provided a signed informed consent. Among these subjects, 63 subjects were male and 57 subjects were female. The age of these subjects ranged from 27 to 86 years, with an average age of 69.10 ± 0.78 years, and the course of the disease was 0.5 to 20 years. According to the Hoehn–Yahr (H-Y) classification, the 120 PD patients were divided into 3 groups: early stage (1–2 classification, 37 cases), medium stage (2.5–3 classification, 49 cases), and advanced stage (4–5 classification, 34 cases). Then, UA and UA/Cr levels in each group were compared.[7] Exclusion criteria included chemical drugs, trauma, cerebrovascular disease, and other causes of PD, and further exclusion of nephropathy, tumor, gout, use of seperazine diuretics, and vegetarians.
This study was conducted in accordance with the declaration of Helsinki.
This study was conducted with approval from the Ethics Committee of Huai’an First People's Hospital. Written informed consent was obtained from all participants.
2.2. Methods
Four milliliters of fasted elbow venous blood were collected from all the subjects in the morning, and UA was determined using an RL 7600 automatic biochemical analyzer (Hitachi, Tokyo, Japan).
2.3. Statistical analysis
Graphpad 6.0 and SPSS 17.0 software were used for statistical analysis. Quantitative data were expressed as 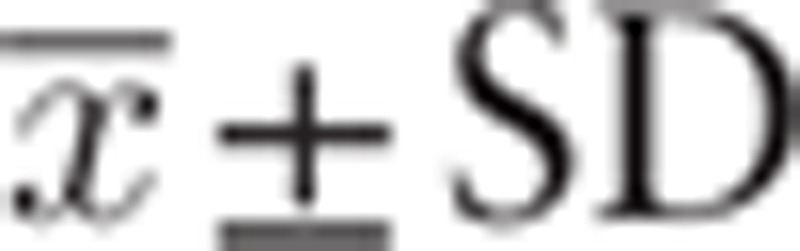 and performed with the variance test and Turkey HSD test. In addition, hierarchical multiple factors and univariate and multivariate logistic regression were performed. P < .05 was considered statistically significant. The Graphpad 6.0 software was used for mapping figures.
and performed with the variance test and Turkey HSD test. In addition, hierarchical multiple factors and univariate and multivariate logistic regression were performed. P < .05 was considered statistically significant. The Graphpad 6.0 software was used for mapping figures.
3. Results
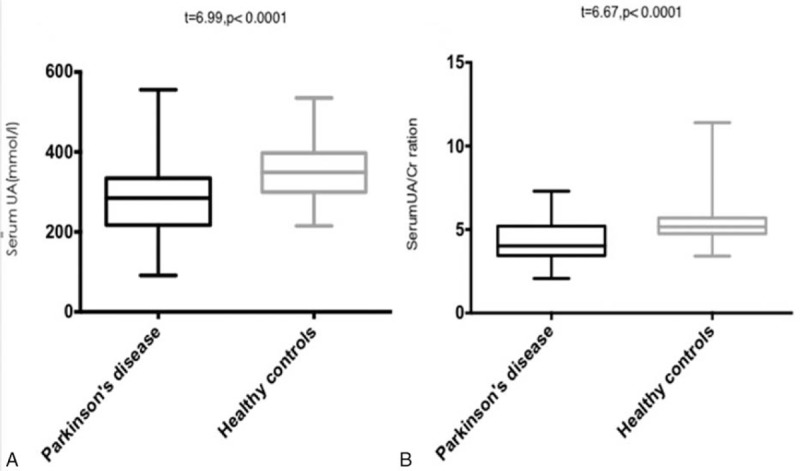
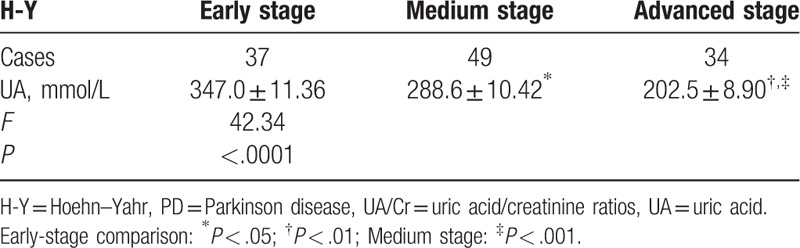
Healthy group UA and UA/Cr were higher than pd group. There was no significant difference in age and disease duration between the 3 groups. UA and UA/Cr values in the different classifications of disease conditions in the PD group: When variance analysis was used among groups with different H-Y classifications, statistically significant differences were found in comparison with the 3 groups (F = 42.34, P < .0001), and there were also statistically significant differences in UA/Cr among these 3 groups (F = 63.15, P < .0001). Results of the Turkey HSD test in each group revealed that UA value (3.47 ± 11.36 μmol/L) in the early stage was higher than in the medium (288.60 ± 10.42 μmol/L) and advanced (202.50 ± 8.9 μmol/L) stages in the PD group, and the differences were statistically significant (t = 3.76, P = .0003; and t = 9.90, P < .0001, respectively). UA value in the medium stage (288.60 ± 10.42 μmol/L) was higher than that in the advanced stage (202.50 ± 8.90 μmol/L), and the difference was statistically significant (t = 5.92, P < .0001). UA/Cr value in early stage (5.44 ± 0.13) was higher than in the medium (4.31 ± 0.15) and advanced (3.16 ± 0.10) stages in the PD group, and the difference was statistically significant (t = 5.47, P < .0001; t = 14.3, P < .0001, respectively). UA/Cr value in the medium stage (4.31 ± 0.15) was higher than in the advanced stage (3.16 ± 0.10), and the difference was statistically significant (t = 5.84, P < .0001; Table 1; Fig. 1).
Table 1.
The value of blood uric acid/creatinine in the early middle and late stages of different H-Y grades  .
.
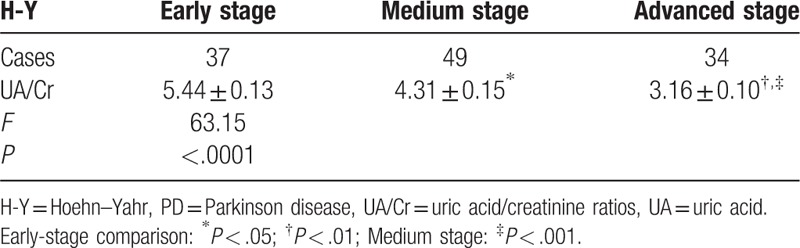
Figure 1.
The value of blood uric acid/creatinine and blood uric acid in the early middle and late stages of different H-Y grades. Low = Early stage; mild = medium stage; high = advanced stage.
Taking the PD classification as the dependent variable, and taking UA level, UA/Cr ratio, gender, age, and dopamine dose as independent variables, univariate and multivariate ordered logistic regression were carried out. Results revealed that gender, age, and dopamine dose had no correlation with disease conditions, while UA level [hazards ratio (HR): 0.986, 95% confidence interval (95% CI): 0.976–0.995] and UA/Cr value (HR: 0.292, 95% CI: 0.165–0.517) were independent prognostic factors of PD (P < .01). Furthermore, UA/Cr value had a more significant predictability for PD disease (P < .001, Tables 2 and 3).
Table 2.
Logistic regression of all factors of PD patients in different stages.
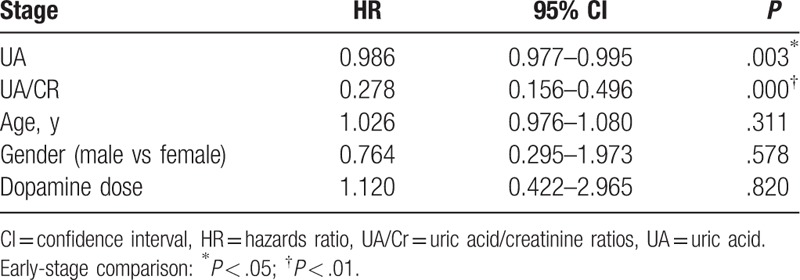
Table 3.
The value of blood uric acid in the early middle and late stages of different H-Y grades 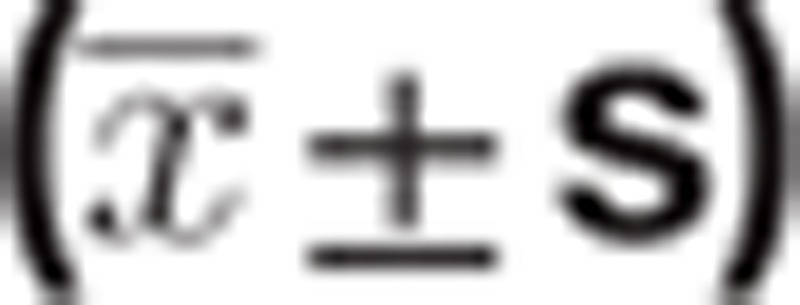 .
.
4. Discussion
PD is a common neurodegenerative disease with main clinical features of resting tremor, bradykinesia, myotonia, and gait posture abnormities, and is associated with depression, constipation, sleep disorders, and other nonmotor symptoms.[8] Although the pathogenesis of PD remains unclear, oxidative stress has been the hot topic in the research of the pathogenesis of PD. In pathological conditions, there is an overproduction of reactive oxygen, and reactive nitrogen may damage cells and almost all DNAs in the mitochondria. More evidences have continued to indicate that oxidative stress could lead to injury of the nigral neurons.[3,9] UA is a natural antioxidant in blood and the brain. It can reduce brain damage caused by oxidative stress.[10] Studies have shown that more than half of the antioxidant capacities of humans are derived from UA. UA is an important endogenous antioxidant that can scavenge reactive oxygen species (ROS), as well as nitro oxide and chelate ferric ions, proving that UA plays an important role in the pathogenesis of PD.[4,10–12] Urate is present in bodily fluids as the anionic form of UA (2,6,8-trioxy-purine). It possesses antioxidant properties comparable to those of ascorbate, and accounts for most of the antioxidant capacity in human plasma.[13] In vitro, in PD models, urate prevents spontaneous degeneration of cultured nigral neurons, as well as dopaminergic cell death induced by oxidative and mitochondrial toxins.[14] In vivo, genetic manipulation of urate oxidase and resulting increased concentrations of urate in the central nervous system (CNS) led to improved phenotype and histopathologic findings in PD mouse models.[15] These data substantiate earlier find-ings in PC12 cells, in which urate blocked cell death and oxidative damage induced by either dopamine or 6-hydroxydopamine.[16] Radhika and other researchers have found that serum UA levels in PD patients may decrease to levels lower than that in the normal population, through the internal control mechanism. Thus, we can speculate that lower UA may be a potential risk factor for PD.[17] At the same time, foreign scholars have found that higher UA could reduce the risk of PD.[18,19] Furthermore, recent studies have found that in addition to antioxidative stress effects, other mechanisms exist between UA and PD. UA may be involved in the brain neurotransmitter transfer process such as the dopaminergic pathway. Moreover, oxidative stress injury of nigral neurons in patients with PD can lead to lower UA levels. Hence, UA levels can be used not only as an effective monitoring index for the progression of PD but also as a potential neuroprotective agent for the treatment of PD.[20–22] It is noteworthy to mention that UA is primarily metabolized through the kidney, and excreted via urine. Men usually have higher levels of UA than women of the same age, which may be promoted by estrogen. The application of UA/Cr values reduce the interference caused by gender and renal function abnormalities.[23]
In this study, differences in UA and UA/Cr values among the different H-Y classifications of PD patients were compared, and the feasibility of applying UA and UA/Cr values as prognostic indexes for PD disease were further explored. Results revealed that there were differences in UA and UA/Cr levels among the different H-Y classifications of PD patients, and these differences were statistically significant. UA in the early stage was higher than in the medium and advanced stages in the PD group; the differences were statistically significant. Furthermore, UA levels in the medium stage were higher than in the advanced stage in the PD group, and the difference was statistically significant. UA/Cr value in the early stage was higher than in the medium and advanced stages in the PD group, and the differences were statistically significant. UA/Cr values in the medium stage were higher than the advanced stage in the PD group, and the difference was statistically significant. Taking UA and UA/Cr values as independent variables for the logistic regression analysis, results revealed that UA and UA/Cr values had correlations with PD. They were independent predictors of PD, and UA/Cr had a more significant correlation with PD.
In conclusion, UA and UA/Cr have an inverse correlation with the H-Y classifications, UA levels gradually decreased with the progression of the disease, and this was consistent with the research conducted by foreign scholars.[24,25] These indicate that lower UA is a risk factor for PD, and UA and UA/Cr can be used as predictive factors for monitoring the progression of the disease, in which UA/Cr values are more effective than UA levels in predicting disease progression.
Author contributions
Conceptualization: Ling-Ling Zhong, Ya-Qi Song.
Data curation: Ling-Ling Zhong, Hua Cao.
Formal analysis: Xiang-Yang Tian.
Funding acquisition: Xiang-Yang Tian.
Methodology: Ya-Qi Song, Hua Cao, Ke-Ju Ju.
Validation: Hua Cao, Ke-Ju Ju.
Writing – original draft: Ling-Ling Zhong.
Writing – review & editing: Xiang-Yang Tian.
Footnotes
Abbreviations: AD = Alzheimer disease, ALS = amyotrophic lateral sclerosis, HR = hazards ratio, H-Y = Hoehn–Yahr, MSA = multiple system atrophy, PD = Parkinson disease, UA = uric acid, UA/Cr = uric acid/creatinine ratios.
The authors have no conflicts of interest.
References
- [1].Schlesinger I, Schlesinger N. Uric acid in Parkinson's disease. Mov Disord 2008;23:1653–7. [DOI] [PubMed] [Google Scholar]
- [2].Cipriani S, Chen X, Schwarzschild MA. Urate: a novel biomarker of Parkinson's disease risk, diagnosis and prognosis. Biomark Med 2010;4:701–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Shen C, Guo Y, Luo W, et al. Serum urate and the risk of Parkinson's disease: results from a meta- analysis. Can J Neurol Sci 2013;40:73–9. [DOI] [PubMed] [Google Scholar]
- [4].Lee JE, Song SK, Sohn YH, et al. Uric acid as a potential disease modifier in patients with multiple system atrophy. Mov Disord 2011;26:1533–6. [DOI] [PubMed] [Google Scholar]
- [5].Paganoni S, Zhang M, Quiroz Zárate A, et al. Uric acid levels predict survival in men with amyotrophic lateral sclerosis. J Neurol 2012;259:1923–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Ye BS, Lee WW, Ham JH, et al. Alzheimer's Disease Neuroimaging Initiative. Does serum uric acid act as a modulator of cerebrospinal fluid Alzheimer's disease biomarker related cognitive decline? Eur J Neurol 2016;23:948–57. [DOI] [PubMed] [Google Scholar]
- [7].Kocer A, Oktay AB. Nintendo Wii assessment of Hoehn and Yahr score with Parkinson's disease tremor. Technol Health Care 2016;24:185–91. [DOI] [PubMed] [Google Scholar]
- [8].Müller B, Assmus J, Herlofson K, et al. Importance of motor vs. non-motor symptoms for health-related quality of life in early Parkinson's disease. Parkinsonism Relat Disord 2013;19:1027–32. [DOI] [PubMed] [Google Scholar]
- [9].Jomova K, Vondrakova D, Lawson M, et al. Metals, oxidative stress and neurodegenerative disorders. Mol Cell Biochem 2010;345:91–104. [DOI] [PubMed] [Google Scholar]
- [10].Bowman GL, Shannon J, Frei B, et al. Uric acid as a CNS antioxidant. J Alzheimers Dis 2010;19:1331–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Davies KJ, Sevanian A, Muakkassah-Kelly SF, et al. Uric acid-iron ion complexes. A new aspect of the antioxidant functions of uric acid. Biochem J 1986;2359:747–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Kim TH, Lee JH. Serum uric acid and nigral iron deposition in Parkinson's disease: a pilot study. PLoS One 2014;9:e112512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Paganoni S, Schwarzschild MA. Urate as a marker of risk and progression of neurodegenerative disease. Neurotherapeutics 2017;14:148–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Cipriani S, Desjardins CA, Burdett TC, et al. Urate and its transgenic depletion modulate neuronal vulnerability in a cellular model of Parkinson's disease. PLoS One 2012;7:e37331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Chen X, Burdett TC, Desjardins CA, et al. Disrupted and transgenic urate oxidase alter urate and dopaminergic neurodegeneration. Proc Natl Acad Sci U S A 2013;110:300–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Zhu TG, Wang XX, Luo WF, et al. Protective effects of urate against 6-OHDA-induced cell injury in PC12 cells through antioxidant action. Neurosci Lett 2012;506:175–9. [DOI] [PubMed] [Google Scholar]
- [17].Sampat R, Young S, Rosen A, et al. Potential mechanisms for low uric acid in Parkinson disease. J Neural Transm (Vienna) 2016;123:365–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Gao X, O’Reilly ÉJ, Schwarzschild MA, et al. Prospective study of plasma urate and risk of Parkinson disease in men and women. Neurology 2016;86:520–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Fukae J, Ishikawa K, Hatano T, et al. Serum Uric Acid Concentration is linked to wearing-off fluctuation in Japanese Parkinson's disease patients. J Parkinsons Dis 2014;4:499–505. [DOI] [PubMed] [Google Scholar]
- [20].Bucay AH. A new hypothesis about the role of uric acid as a neurotransmitter. Med Hypotheses 2009;73:854–65. [DOI] [PubMed] [Google Scholar]
- [21].Shen L. Evolutionary support for the hypothesis about uric acid as a neurotransmitter. Med Hypotheses 2010;75:131–2. [DOI] [PubMed] [Google Scholar]
- [22].Schwarzschild MA, Ascherio A, Beal MF, et al. Parkinson Study Group SURE-PD Investigators. Inosine to increase serum cerebrospinal fluid urate in Parkinson disease: a randomized clinical trial. JAMA Neurol 2014;71:141–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Lolekha P, Wongwan P, Kulkantrakorn K. Association between serum uric acid and motor subtypes of Parkinson's disease. J Clin Neurosci 2015;22:1264–7. [DOI] [PubMed] [Google Scholar]
- [24].Jesús S, Pérez I, Cáceres-Redondo MT, et al. Low serum uric acid concentration in Parkinson's disease in southern Spain. Eur J Neurol 2013;20:208–10. [DOI] [PubMed] [Google Scholar]
- [25].Vieru E, Köksal A, Mutluay B, et al. The relation of serum uric acid levels with L-Dopa treatment and progression in patients with Parkinson's disease. Neurol Sci 2016;37:743–7. [DOI] [PubMed] [Google Scholar]


