Abstract
Background:
Few studies have investigated the dry needling (DN) approach on knee osteoarthritis (KO) patients. The study's aim was to evaluate the short-term efficacy of adding DN to a therapeutic exercise protocol in the treatment of KO in older adults.
Methods:
A double-blind, pilot clinical trial with parallel groups [NCT02698072] was carried out for 12 weeks of treatment and follow-up. Twenty patients aged 65 years and older with myofascial trigger points (MTrPs) in the muscles of the thigh were recruited from older-adult care centers and randomly assigned to a DN + Exercise group or a Sham-DN + Exercise group. The Numeric Rating Scale (NRS; primary outcome) and Western Ontario and McMaster Universities Osteoarthritis Index questionnaire (WOMAC) were assessed before and after the intervention.
Results:
The NRS (analysis of variance, ANOVA) showed statistically significant differences in the time factor (F = 53.038; P < .0001; ηp2 = 0.747). However, it did not show a significant change in the group–time interaction (F = 0.082; P = .777; ηp2 = 0.005). The WOMAC scores (ANOVA) showed statistically significant differences in the time factor for total score WOMAC questionnaire (F = 84.826; P < .0001; ηp2 = 0.825), WOMAC pain (F = 90.478; P < .0001; ηp2 = 0.834), WOMAC stiffness (F = 14.556; P < .001; ηp2 = 0.447), and WOMAC function (F = 70.872; P < .0001; ηp2 = 0.797). However, it did not show a statistically significant change in the group–time interaction.
Conclusion:
Despite the pain intensity and disability clinically relevant improvement for both DN and Sham-DN combined with exercise, 6 sessions of DN added to a therapeutic exercise program for older adults with KO did not seem to improve pain intensity and functionality.
Keywords: complementary medicine, geriatric medicine, musculoskeletal, pain management, rehabilitation medicine, rheumatology
1. Introduction
Knee osteoarthritis (KO) is a syndrome clinically characterized by the presence of pain, and it is correlated with radiological and laboratory tests.[1] The prevalence of osteoarthritis is estimated as affecting 7 million people in the United States,[2] and many of the clinical symptoms that lead to disability are caused by KO, more than in any other joint.[3,4] In Spain, there is an estimated prevalence of osteoarthritis in people over 45 years of age of 46% in women and 21% in men[5] with KO representing 10% of this prevalence.[6]
In Spain, this syndrome had an economic cost of 4700 million euros in 2007, equivalent to 0.5% of the gross domestic product (GDP) in that year. However, it has been shown that KO is a major health problem in all countries.[7]
The etiology of KO has not yet been fully clarified, but its incidence certainly increases with age.[8] In addition, obesity is a risk factor for the development and progression of this syndrome and even a relationship with the requirement for total joint replacement.[9,10]
Fundamentally, KO causes musculoskeletal pain and impaired physical function.[11] Physical therapy has been shown to be highly effective in the treatment of KO.[12] It achieves functional and overall classification improvements by therapeutic exercise,[13] emphasizing an exercise program that focuses on training the quadriceps muscle strength and improving the aerobic capacity and performance of the lower limbs.[14]
A recent set of clinical practice guidelines that focused on the nonpharmacological treatment of pain and dysfunction caused by KO syndrome recommends the use of therapeutic exercise based on strength training and aerobic exercise to give this treatment a high level of efficacy.[15] A similar conclusion and recommendations can be drawn from a recent Cochrane review that focused on therapeutic exercise based on strength training, aerobic exercise, and range of motion as a treatment of this syndrome.[16] Likewise, it has become clear that physical therapy through therapeutic exercise is effective in delaying or avoiding the need for total knee joint replacement surgery.[17]
However, only a few studies have investigated the “dry needling” (DN) approach to the treatment of this syndrome in such a population, although the results of these studies are very positive about the improvement in pain and function.[18–20]
DN uses a needle similar to those used in acupuncture, but the technique of application is different. Regarding DN, the needle is moved up and down within the muscle, exactly at the myofascial trigger points (MTrPs) areas. Indeed, an MTrP may be considered as a hyperirritable spot in a taut band of skeletal muscle that may produce sensitive, motor, or autonomic symptoms and signs, whose prevalence may reach the 100% level of patients with KO.[19]
Mayoral et al[18] explored the muscles of the lower limb related to knee pain in 40 older adults, finding latent and active MTrPs, and divided them into 2 groups: a first group was treated with DN in muscles related to the KO pain of each subject, and a second group was given a placebo treatment with sham-DN in those same points. All patients were sedated and underwent total knee arthroplasty surgery when given both treatments. The authors found a significant reduction in pain in the group that had undergone treatment by DN at 1-month follow-up compared with the sham-DN group; the intensity of pain was reduced for both groups in equal proportions after 6 months from the surgery. In addition, the DN group required fewer analgesics in the first days after surgery compared with the sham-DN group.
Henry et al[19] achieved significant knee pain relief from the first session with injection by local anesthetic infiltration (bupivacaine) in 92% of patients in a sample of 25 older adults recruited from a waiting list for surgery for total knee arthroplasty. They concluded that the knee pain in almost all patients of their sample had a myofascial origin.
Itoh et al[20] compared 3 types of needling techniques in 30 older adults diagnosed with KO using the American College of Rheumatology criteria. The patients were divided into 3 groups: a first group of 10 patients was treated by acupuncture, a second group was treated by sham acupuncture, and a third group was treated by DN at MTrPs in the musculature involved in the clinical features. The authors found lower pain intensity measured by the visual analogue scale (VAS) and less disability by the Western Ontario and McMaster Universities Osteoarthritis Index questionnaire (WOMAC) questionnaire (highest score) in the group of patients who had undergone treatment by DN technique in MTrPs.
A recent critical review suggested a myofascial component of pain in KO and the presence of MTrPs in the surrounding muscles of the knee may play a key role in the pain and disability of osteoarthritis.[21]
Therefore, the aim of this pilot study was to assess the short-term efficacy of adding DN to a therapeutic exercise protocol (12 weeks) in the treatment of KO in older adults.
2. Material and methods
2.1. Study design
A double-blind, pilot clinical trial was performed with parallel groups, between March 2016 and June 2017.
2.2. Participants
Twenty-seven patients with KO were recruited from older-adult care centers and these were screened for possible eligibility criteria. The inclusion and exclusion criteria were based on previous studies.[17,20] The inclusion criteria were as follows: participants aged 65 years or older with knee pain and uni- or bilateral dysfunction, primary KO fulfilling the American College of Rheumatology criteria for clinical and radiographic diagnostic,[1] and at least 1 active or 1 latent myofascial trigger point (MTrP) elicited by palpation ipsilateral to the painful knee(s) situated in a taut band of a skeletal muscle of the lower limb(s), which usually has referred pain.
The diagnosis of active and/or latent MTrPs followed the essential and confirmatory criteria described by Travell and Simons.[22] Active MTrPs produce spontaneous and recognizable pain under stimulation, whereas latent MTrPs generate localized pain or unrecognizable referred pain upon stimulation.[23,24]
Patients were excluded from the study if they suffered from any other condition that could cause myofascial or neuropathic pain in the lower limb; previous total replacement of the same knee; any other surgical procedure of the lower limbs in the previous 6 months; prior diagnoses or prescriptions in the medical record for myopathy or lumbosacral neuropathy; rheumatoid arthritis; initiation of opioid analgesia or corticosteroid or analgesic injection intervention for hip or knee pain within the previous 30 days; alcohol or drug consumption; uncontrolled hypertension or moderate to high risk for cardiac complications during exercise; conservative or invasive physical therapy (previous 6 months or during follow-up); or physical impairments unrelated to the hip or knee preventing safe participation in exercise and walking, such as vision problems that affect mobility, body weight greater than 155 kg, neurogenic disorder, primary or significantly limiting back pain, advanced osteoporosis, or inability to walk 10 m without an assistive device, inability to comprehend and complete study assessments or comply with study instructions, stated inability to attend or complete the proposed course of intervention and follow-up schedule, fibromyalgia syndrome, or other altered affective/cognitive modulation processes of pain perception.
After signing informed consent forms, the 20 patients (8 males, 12 females) who matched the inclusion criteria were randomly allocated into 2 groups using Graph Pad Software and were associated with a letter (A or B). Therefore, 9 patients completed the study in the Sham-DN + exercise group (4 males, 5 females) and 11 patients in the DN + exercise group (4 males, 7 females).
The study was approved by the Clinical Intervention Ethics Committee of Rey Juan Carlos University (Approval Number: 13/2015) and registered in ClinicalTrials.gov Identifier: NCT02698072. All participants gave written informed consent before data collection began.
2.3. Outcome measurement
Physiotherapist 2 (MVOS) and the occupational therapist (VBC), blinded to the patient group assignment, carried out all the assessments at baseline (A0) and after intervention (A1). Sociodemographic data such as age, sex, and body mass index were collected at baseline (A0), before the intervention. The primary outcomes measured were pain intensity, symptomatology, and function in KO and were measured at baseline (A0) and after the intervention (A1).
Pain intensity was measured with the numeric pain rating scale (NRS) of 11 points (interval from 0 to 10), where 0 corresponds to no pain, and 10 corresponds to the worst pain imaginable. A graphical representation of 11 spaces was used to indicate the patient's own evaluation of his or her pain. The patients were asked to assess the subjective pain intensity of the painful knee and lower limb by pointing with one of their fingers to mark the level of pain on the scale. The NRS is a valid and reliable tool for use in older adults,[25,26] and its correlation with the VAS shows a high convergent validity (0.79–0.95).[27]
Regarding the NRS, the minimal detectable change (MDC) was established at 1.5 points[28] and the minimal clinically important difference (MCID) was set at 2 points in KO pain intensity.[29–30]
Function was measured with the WOMAC.[31] The WOMAC is the most widely used instrument to evaluate symptomatology and function in KO. It contains 24 questions — 5 about pain (range: from 0 to 20 points), 2 about stiffness (range: from 0 to 8 points), and 17 about difficulty with physical functions (range: from 0 to 68 points) — and can be completed in less than 5 minutes.[32]
An increase in the WOMAC scores (WOMAC pain, WOMAC stiffness, and WOMAC physical function) indicates a greater degree of deterioration. It has been widely tested in surgical and hospital-based populations and is extensively used in clinical trials because of its sensitivity to change and construct validity.[32] The MDC for the WOMAC was established in 14.1 points and an MCID of 8.5 points in patients with KO.[33]
2.4. Procedures and intervention
This study was carried out by 2 physical therapists (EASR and MVOS; each with more than 4 years clinical experience) experienced in MPS and an occupational therapist (VBC) with more than 10 years of clinical experience. Physical therapist 2 (MVOS) carried out the assessments at baseline (A0) and after intervention (A1) to collect sociodemographic and primary outcomes measurements. Physical therapist 1 (EASR) performed the physical examination for the presence of active or latent MTrPs in the muscles of the involved lower limb(s) using the criteria described by Travell and Simons[22] as well as the DN and Sham-DN therapy interventions in each group. Half of the participants received a therapeutic exercise program and TrP-DN (trigger point DN) in combination (DN + Exercise), while the other half received the same therapeutic exercise program and sham DN (Sham-DN + Exercise). The therapeutic exercise program was performed by 2 physical therapists (EASR and MVOS).
2.4.1. Detection of active or latent MTrPs
The tensor fasciae latae, hip adductors, hamstrings, quadriceps, gastrocnemius, and popliteus muscles were examined in each subject following a protocol regarding patient and limb positions exactly reproduced from the study by Mayoral et al,[18] as these muscles are frequently involved in myofascial knee pain. Patients were considered according to this syndrome if they had at least 1 active (pain-generating) MTrP.[22]
2.4.2. Therapeutic exercise
A therapeutic exercise program previously shown to be feasible and effective for reducing pain and improving physical function in patients with KO was administered in 1-hour, group-based, supervised sessions twice weekly over 12 weeks.[13–17,34] On average, about 10 patients attended each training session. Each patient was monitored individually for exercise quality. The subjective pain level was used to guide progression. To support adherence to the exercise, a secretary contacted the patients twice a month by telephone for the 12-week treatment period. Training took place in groups under the supervision of an experienced physical therapist specializing in therapeutic exercise to treat musculoskeletal disorders. A total of 24 therapeutic exercise sessions were conducted in a land-based therapeutic exercise program consisting of aerobic exercise (20–25 minutes warm-up), lower-limb muscle strengthening (20–25 minutes), and lower-limb muscle stretching (10–15 minutes).
2.4.3. Dry needling and sham dry needling
Half of the participants (DN + Exercise group; n = 10) were randomly assigned to a treatment with 6 DN sessions (once a week, for the first 6 weeks) at all MTrPs of the involved symptomatic lower limb(s) using the fast-in and fast-out technique with multiple rapid needle insertions[32] (the needle was moved up and down within the muscle). Needle insertion was repeated 15 times.[18,35] The procedure of TrP-DN was similar to that used by Hong.[36,37]
If patients had symptoms in both knees, both lower limbs were treated. Patients with symptoms in both knees but who had previous knee surgery were only treated on the nonoperated lower limb. To identify the lower limb(s) MTrPs that were homolateral to the painful knee, a grid with 4 perpendicular lines was drawn using a permanent marker to determine the active MTrPs (evoked subject knee pain), and a grid of 2 perpendicular lines was drawn to determine the most mechanosensitive latent MTrPs of each muscle. A headless 0.30 x 40 mm needle, 0.30 x 60 mm needle, or 0.30 x 0.75 mm needle (AGU-PUNT) was inserted perpendicularly directly to the selected muscle of the lower limb toward the MTrP located between the fingers of the subdominant hand, and the guide tube was removed. By means of metacarpophalangeal flexion/extension of the first and second fingers of the dominant hand, the area was probed in different directions until a minimum of 1 local twitch response (LTR), a local pain response, and usually the referred pain pattern of the MTrP were obtained.[22] The penetration depth varied according to the selected muscle and to the subject.
The observation of sensitivity to 1 LTR (if possible, not in deeper muscles) is considered an indispensable confirmatory criterion when performing DN in both active and latent MTrPs. After extracting the needle from the dominant hand, ischemic compression was applied with the fingers for 1 minute.[22,38]
The other half of the participants (Sham-DN + Exercise; n = 10) were randomly assigned to a treatment with 6 sham DN sessions (once a week, for the first 6 weeks) at all MTrPs of the involved symptomatic lower limb(s) with the park sham device, DONGBANG AcuPrime.[39–41] The sham DN looked exactly like a real DN, except it penetrated only a few millimeters of the skin without inducing any LTR.
2.5. Statistical analysis
Data were analyzed using the Statistical Package for Social Sciences (SPSS) software version 20.0. Mean, standard deviation (SD), and 95% confidence interval (95% CIs) were calculated for each variable. The Shapiro–Wilk test did not detect significant departures from normality (P > .05). A 1-way analysis of variance (ANOVA) was used to compare baseline continuous data, and Chi-square tests were used to test for the independence of baseline categorical data. Repeated-measures ANOVA with 2 factors, 2 (group) X 2 [time: pre-intervention (A0) and after the treatment (A1)], were performed for NRS and WOMAC scores. The proportion of subjects who recorded an improvement superior to the MCID of the NRS (1.3 points) or to MCID of the WOMAC (8.5 points) was calculated for each group and compared between groups by using Fisher exact test. Bonferroni correction was applied to group and time comparisons for all variables. The effect size was calculated for NRS and WOMAC variables. For all analyses, the statistical significance was set at P < .05.
3. Results
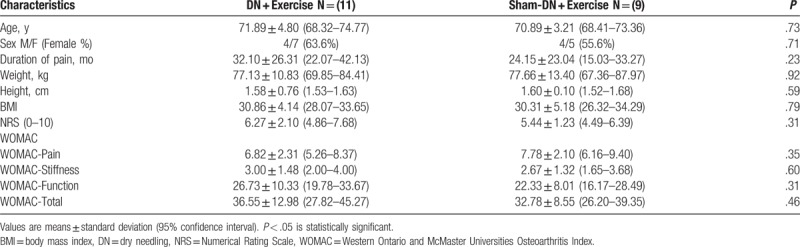
Twenty-seven patients with KO were screened for possible eligibility criteria, and 20 patients finally were randomized and successfully completed the study protocol (8 males, 12 females). In all, 9 patients completed the study in the Sham-DN + exercise group (4 males, 5 females; mean age ± SD, 70.89 ± 3.21 years) and 11 patients in the DN + exercise group (4 males, 7 females; mean age ± SD, 71.55 ± 4.80 years). Figure 1 shows the process of recruitment and dropouts. There were no significant differences between groups in terms of demographic or clinical characteristics at the time of the baseline screening (P > .05). Demographic and pre-intervention data are summarized in Table 1.
Figure 1.
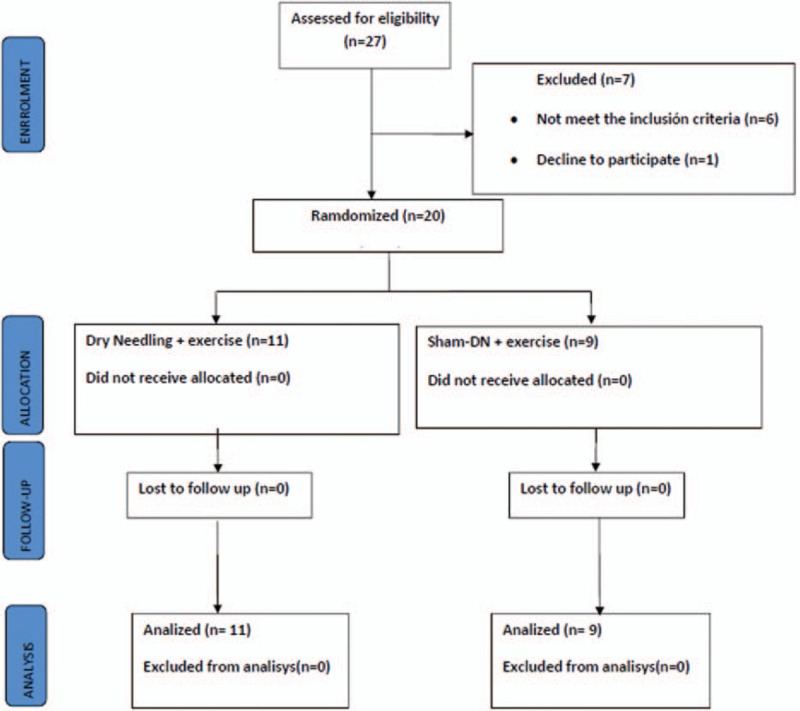
Process of recruitment and dropouts.
Table 1.
Baseline (A0) patient's characteristics.
3.1. Pain intensity
The 2x2 mixed-model ANOVA showed statistically significant differences in the time factor (F = 53.038; P < .0001; ηp2 = 0.747). However, it did not show a significant change in the group–time interaction (F = 0.082; P = .777; ηp2 = 0.005). No other comparison between groups achieved the level of significance (P > .05). The NRS intensities of patients obtained at the end of the intervention are summarized in Table 2 and Fig. 2.
Table 2.
Adjusted means and post-hoc tests with Bonferroni correction for time x group interaction and time factor.
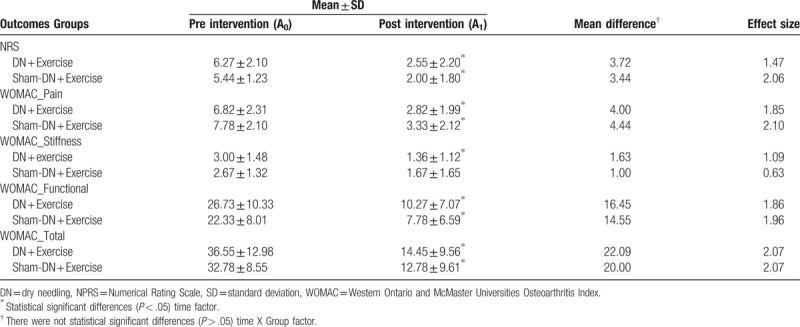
Figure 2.
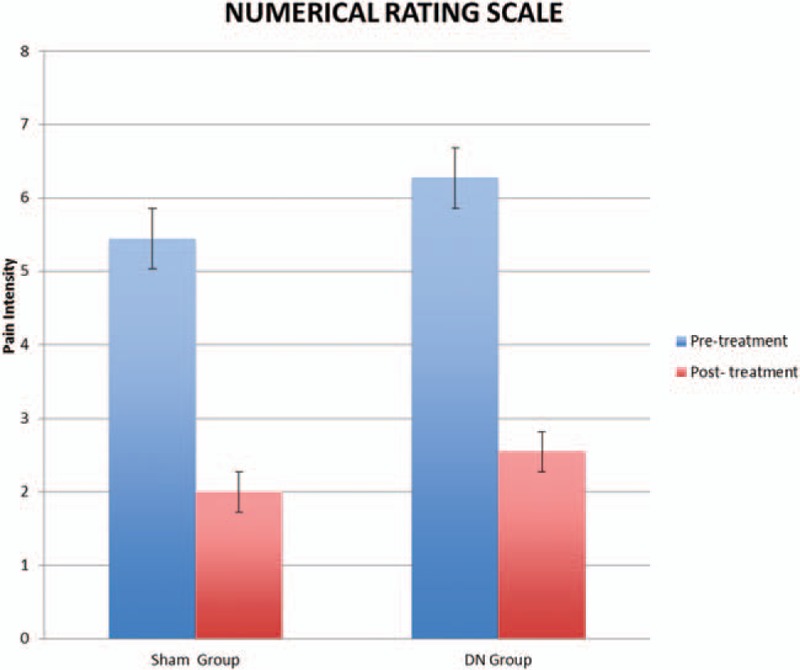
Numerical rating scale of pain intensity differences between both groups. DN = dry needling.
3.2. Disability
The 2x2 mixed-model ANOVA showed statistically significant differences in the time factor for total score WOMAC questionnaire (F = 84.826; P < .0001; ηp2 = 0.825), WOMAC pain (F = 90.478; P < .0001; ηp2 = 0.834), WOMAC stiffness (F = 14.556; P < .001; ηp2 = 0.447), and WOMAC function (F = 70.872; P < .0001; ηp2 = 0.797). However, it did not show a significant change in the group–time interaction for total score WOMAC questionnaire (F = 0.209; P = .653; ηp2 = 0.011), WOMAC pain (F = 0.251; P = .623; ηp2 = 0.014), WOMAC stiffness (F = 0.848; P = .369; ηp2 = 0.045), and WOMAC function (F = 0.266; P = .612; ηp2 = 0.015). WOMAC scores of patients obtained at the end of the intervention are summarized in Table 2 and Fig. 3.
Figure 3.
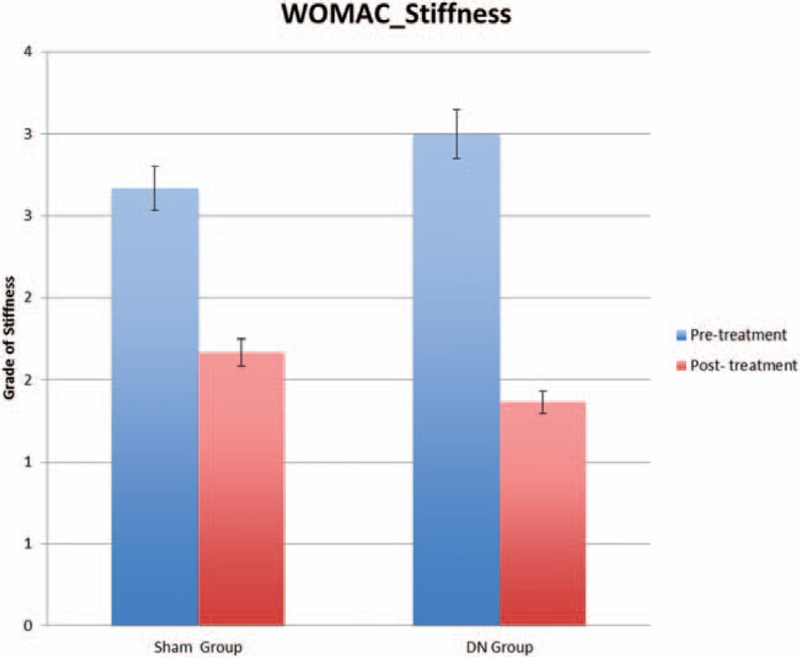
WOMAC scores differences between both groups. DN = dry needling; WOMAC = Western Ontario & McMaster Universities Osteoarthritis Index.
4. Discussion
This pilot clinical trial showed that adding a DN intervention to an exercise program is no more efficacious in improving pain and disability in patients with KO than adding sham DN to an exercise program. Concerning knee pain and function, the DN + Exercise group experienced similar reduction in pain intensity and WOMAC scores as the Sham-DN + Exercise group; therefore, no significant differences were found between the groups.
The clinical relevance of this pain reduction was estimated on the basis of the mean difference between before and immediately after the treatment. On the basis of a between-group comparison, the difference in NRS immediately after the treatment in the DN + Exercise group was 3.72 points and in the Sham-DN + exercise group was 3.44 points. These differences represent a 40.6% and 36.7% reduction in pain, respectively. Therefore, both groups reported a reduction in the NRS greater than 3 points, demonstrating a clinically important change.[28]
The NRS reflects that the patients’ perception of pain improved in both groups postintervention, which may be related to patient expectations in the Sham-DN + Exercise. This is in line with a recent study that found that the acupuncture group was similar to the sham acupuncture group. However, the acupuncturist's style of communication had a significant effect on pain reduction, suggesting the influence of patients’ expectations of invasive treatment.[42] We did not measure this aspect in our study.
Regarding the WOMAC, the clinical relevance of disability reduction was estimated on the basis of the mean difference between before and immediately after the treatment. On the basis of a between-group comparison, the difference in the WOMAC immediately after the treatment in the DN + Exercise group was 22.09 points and in the Sham-DN + Exercise group was 20.00 points. Therefore, both groups reported a reduction of the WOMAC total score greater than 8.5 points, demonstrating a clinically important change (MCID).[29,30]
Skou et al[17] examined 100 patients with moderate-to-severe KO who were eligible for unilateral total knee replacement. In their study, half of the participants received nonsurgical treatment (therapeutic exercise, education, dietary advice, use of insoles, and pain medication), while the other half were treated by surgery. The researchers found that nonsurgical treatment followed by total knee replacement resulted in greater pain relief and functional improvement after 12 months than nonsurgical treatment alone. However, most patients who were assigned to receive nonsurgical treatment alone did not undergo total knee replacement before the 12 months follow-up. In our study, 3 surgeries were cancelled during the intervention because of pain reduction and improvement of function, but it would be necessary to perform a similar study with examination of medium and long-term effects.
In our study, 6 sessions of DN were used for the first 6 weeks of treatment. Previous studies[19,20] only evaluated post-treatment after DN intervention, except in the study by Mayoral et al,[18] that 6 months of follow-up were used. Nevertheless, patients only achieved less pain after intervention and 1-month follow-up, and improved the need for immediate postsurgery analgesics. We desired to evaluate if the analgesic effect of DN was maintained during the following 6 weeks of treatment, while the beneficial mid-term effect of therapeutic exercise appeared.
Regarding previous studies of therapeutic exercise[13–17] and DN,[18–20] and according to the results of the present study, we can establish a relationship between the treatment by therapeutic exercise and the improvement in pain and function of patients with KO. It seems that the improvements obtained by DN in previous studies could be due to analgesic effects in the short-term.
Further randomized clinical trials with larger sample sizes and follow-up at medium or long-term are necessary to recommend the use of DN in patients with MTrPs and KO. Others authors[43–46] have found positive effects of treatment with manual therapy and therapeutic exercise in mid-term, providing individually or combined improvements in pain, stiffness, and functional limitation in patients suffering from KO.
To effectively compare results from different treatments for patients with KO, the sample should be as homogeneous as possible. Indeed, objective information about the type of feeding of the subjects of the study was not collected, although it should be mentioned that all of them belonged to a middle class, as documented by a social worker[47,48] that was fed on a Mediterranean diet,[49] and DN was never done under fasting conditions to any of them.
Fan et al[50] affirmed that DN is a form of simplified acupuncture using biomedical language in treating myofascial pain. Also, they affirm that the “ashi” points are the MTrPs, but this does not seem to be true, especially when applying DN according to the fast-in and fast-out technique with multiple rapid needle insertions[32] within the muscle using the diagnosis by the essential and confirmatory criteria described by Travell and Simons.[22]
In our study, BMI data were collected, which resulted in the overweight in both groups at baseline, being distributed homogeneously. We know that this condition favors knee pain, and that there is a directly proportional relationship between weight loss and pain decrease.[9,10] However, these data were not collected post intervention. Although the present study was not based on weight loss, the benefit of physical exercise is presupposed in the form of caloric expenditure, among others.
4.1. Study limitations
The present study has several limitations. First, there were no control groups, so we cannot exclude the effect of DN alone or the influence of the exercise alone on KO pain and disability. Second, there was only a short-term (immediately and after the treatment) follow-up (12 weeks) of the variables. It would be useful to perform studies with a longer follow-up period to demonstrate whether any clinical effects could be modified over time. Third, 6 DN sessions were performed, but different outcomes could appear with more sessions. Fourth, 15 needle insertions were applied in all subjects in the DN + Exercise group, following the insertions average calculated from 2 prior studies.[18,35] More data from different muscles were not collected at the lower limb treated with DN, but these data should be considered for futures studies. Finally, the small sample size could be considered a limitation, and a larger sample size could show a significant difference between groups.
Despite its limitations, we believe that this novel study helps to clarify the utility of DN in patients suffering KO. However, pending a study with a larger sample size and monitoring medium- and long-term follow-up that takes into account more variables, such as functional independence or speed walking in older adults, we cannot recommend the use of DN as a treatment of MTrPs associates to KO patients. Therefore, the results of this study should be viewed with caution.
5. Conclusion
Despite the pain intensity and disability clinically relevant improvement for both DN and Sham-DN combined with exercise, 6 sessions of DN added to a therapeutic exercise program for older adults with KO did not seem to improve pain intensity and functionality. However, these results should be interpreted with caution because of the small sample size and short follow-up.
Acknowledgments
The authors would like to thank Carmen Romero for help and advice in setting up a telephone survey tool and preparing the materials for the study.
Author contributions
ESR, DPM, and JFC contributed to the study concept and design. JFC and CCL did the main statistical analysis and interpretation of data. ESR, VOS, and VBC contributed to draft the report and collect the data. DPM, CCL, and JFC supervised the study. All authors revised the text for intellectual content and have read and approved the final version of the manuscript.
Conceptualization: Eleuterio Sánchez Romero, Daniel Pecos Martín, Josué Fernández Carnero.
Data curation: César Calvo Lobo, Josué Fernández Carnero.
Formal analysis: César Calvo Lobo, Josué Fernández Carnero.
Investigation: Eleuterio Sánchez Romero, Verónica Burgos Caballero, Josué Fernández Carnero.
Methodology: Eleuterio Sánchez Romero, Daniel Pecos Martín, César Calvo Lobo, Josué Fernández Carnero.
Project administration: Victoria Ochoa Sáez, Verónica Burgos Caballero.
Resources: Eleuterio Sánchez Romero, Daniel Pecos Martín, Victoria Ochoa Sáez, Verónica Burgos Caballero.
Supervision: Daniel Pecos Martín, César Calvo Lobo, Victoria Ochoa Sáez, Verónica Burgos Caballero, Josué Fernández Carnero.
Validation: Daniel Pecos Martín, César Calvo Lobo, Verónica Burgos Caballero, Josué Fernández Carnero.
Visualization: Daniel Pecos Martín, César Calvo Lobo, Verónica Burgos Caballero, Josué Fernández Carnero.
Writing – original draft: Eleuterio Sánchez Romero, Daniel Pecos Martín, César Calvo Lobo, Victoria Ochoa Sáez, Verónica Burgos Caballero, Josué Fernández Carnero.
Writing – review & editing: Eleuterio Sánchez Romero, Daniel Pecos Martín, César Calvo Lobo, Victoria Ochoa Sáez, Verónica Burgos Caballero, Josué Fernández Carnero.
Footnotes
Abbreviations: BMI = body mass index, DN = dry needling, KO = knee osteoarthritis, MTrPs = myofascial trigger points, NRS = Numeric Pain Rating Scale, ROM = range of motion, WOMAC = Western Ontario and McMaster Universities Osteoarthritis Index questionnaire.
XI Award for Best Research Project awarded by the Ilustre Colegio Profesional de Fisioterapeutas de la Comunidad de Madrid (Spain), December 2015.
The authors have no conflicts of interest.
References
- [1].Altman R, Asch E, Bloch D, et al. Development of criteria for the classification and reporting of osteoarthritis: classification of osteoarthritis of the knee. Arthr Rheum 1986;29:1039–49. [DOI] [PubMed] [Google Scholar]
- [2].D’Ambrosia RD. Epidemiology of osteoarthritis. Orthopedics 2005;28(2 suppl):s201–5. [DOI] [PubMed] [Google Scholar]
- [3].Felson DT, Zhang Y, Hannan MT, et al. The incidence and natural history of knee osteoarthritis in the elderly: the Framingham Osteoarthritis Study. Arthritis Rheum 1995;38:1500–5. [DOI] [PubMed] [Google Scholar]
- [4].Felson DT, Naimark A, Anderson J, et al. The prevalence of knee osteoarthritis in the elderly: the Framingham Osteoarthritis Study. Arthr Rheum 1987;30:914–8. [DOI] [PubMed] [Google Scholar]
- [5].Enquesta de Salut de Catalunya (Catalonia Health Survey). Generalitat de Catalunya. 2011. [Google Scholar]
- [6].Carmona L, Ballina J, Gabriel R, et al. The burden of musculoskeletal diseases in the general population of Spain: results from a national survey. Ann Rheum Dis 2001;60:1040–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Deyle GD, Allison SC, Matekel RL, et al. Physical therapy treatment effectiveness for osteoarthritis of the knee: a randomized comparison of supervised clinical exercise and manual therapy procedures versus a home exercise program. Phys Ther 2005;85:1301–17. [PubMed] [Google Scholar]
- [8].Felson DT, Zhang Y, Hannan MT, et al. Risk factors for incident radiographic knee osteoarthritis in the elderly: the Framingham Study. Arthr Rheum 1997;40:728–33. [DOI] [PubMed] [Google Scholar]
- [9].Messier SP, Loeser RF, Mitchell MN, et al. Exercise and weight loss in obese older adults with KO: a preliminary study. J Am Geriatr Soc 2000;48:1062–72. [DOI] [PubMed] [Google Scholar]
- [10].Christensen R, Astrup A, Bliddal H. Weight loss: the treatment of choice for knee osteoarthritis? A randomized trial. Osteoarthr Cartil 2005;13:20–7. [DOI] [PubMed] [Google Scholar]
- [11].Jansen MJ, Viechtbauer W, Lenssen AF, et al. Strength training alone, exercise therapy alone, and exercise therapy with passive manual mobilization each reduce pain and disability in people with knee osteoarthritis: a systematic review. J Physiother 2011;57:11–20. [DOI] [PubMed] [Google Scholar]
- [12].Muraja S, Markulincic B. (FRI0597-HPR). The effect of physical therapy on functional status and synovial perfusion in patients with knee osteoarthritis. Ann Rheum Dis 2011;72:578. [Google Scholar]
- [13].Fitzgerald GK, Piva SR, Gil AB, et al. Agility and perturbation training techniques in exercise therapy for reducing pain and improving function in people with knee osteoarthritis: a randomized clinical trial. Phys Ther 2011;91:452–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Juhl C, Christensen R, Roos EM, et al. Impact of exercise type and dose on pain and disability in knee osteoarthritis: a systematic review and meta-regression analysis of randomized controlled trials. Arthritis Rheumatol 2014;66:622–36. [DOI] [PubMed] [Google Scholar]
- [15].McAlindon TE, Bannuru RR, Sullivan MC, et al. OARSI guidelines for the non-surgical management of knee osteoarthritis. Osteoarthr Cartil 2014;22:363–88. [DOI] [PubMed] [Google Scholar]
- [16].Fransen M, McConnell S, Harmer AR, et al. Exercise for osteoarthritis of the knee: a Cochrane systematic review. Br J Sports Med 2015;49:1554–7. [DOI] [PubMed] [Google Scholar]
- [17].Skou ST, Roos EM, Laursen MB, et al. A randomized, controlled trial of total knee replacement. N Engl J Med 2015;373:1597–606. [DOI] [PubMed] [Google Scholar]
- [18].Mayoral O, Salvat I, Martín MT, et al. Efficacy of myofascial trigger points dry needling in the prevention of pain after total knee arthroplasty: a randomized, double-blinded, placebo-controlled trial. Evid Based Complement Alternat Med 2013;2013:694941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Henry R, Cahill CM, Wood G, et al. Myofascial pain in patients waitlisted for total knee arthroplasty. Pain Res Manage 2012;17:321–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Itoh K, Hirota S, Katsumi Y, et al. Trigger point acupuncture for treatment of knee osteoarthritis: a preliminary RCT for a pragmatic trial. Acupunct Med 2008;26:17–26. [DOI] [PubMed] [Google Scholar]
- [21].Dor A, Kalichman L. A myofascial component of pain in knee osteoarthritis. J Bodyw Mov Ther 2017;21:642–7. [DOI] [PubMed] [Google Scholar]
- [22].Travell JG, Simons DG. Myofascial Pain and Dysfunction. The Trigger Point Manual. Volume I. The Lower Extremities. Baltimore, MA: Williams & Wilkins; 1999. [Google Scholar]
- [23].Srbely JZ, Dickey JP, Lee D, et al. Dry needle stimulation of myofascial trigger points evokes segmental anti-nociceptive effects. J Rehabil Med 2010;42:463–8. [DOI] [PubMed] [Google Scholar]
- [24].Hsieh YL, Kao MJ, Kuan TS, et al. Dry needling to a key myofascial trigger point may reduce the irritability of satellite MTrPs. Am J Phys Med Rehabil 2007; 86:397–403. [DOI] [PubMed] [Google Scholar]
- [25].Williamson A, Hoggart B. Pain: A review of three commonly used pain rating scales. J Clin Nurs 2005;14:798–804. [DOI] [PubMed] [Google Scholar]
- [26].Taylor LJ, Harris J, Epps CD, et al. Psychometric evaluation of selected pain intensity scales for use with cognitively impaired and cognitively intact older adults. Rehabil Nurs 2005;30:55–61. [DOI] [PubMed] [Google Scholar]
- [27].Kahl C, Cleland JA. Visual analogue scale, numeric pain rating scale and the Mc Gill pain Questionnaire: an overview of psychometric properties. Phys Ther Rev 2005;10:123–8. [Google Scholar]
- [28].Devji T, Guyatt GH, Lytvyn L, et al. Application of minimal important differences in degenerative knee disease outcomes: a systematic review and case study to inform BMJ Rapid Recommendations. BMJ Open 2017;7:e015587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Katz NP, Paillard FC, Ekman E. Determining the clinical importance of treatment benefits for interventions for painful orthopedic conditions. J Orthop Surg Res 2015;10:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Salaffi F, Stancati A, Silvestri CA, et al. Minimal clinically important changes in chronic musculoskeletal pain intensity measured on a numerical rating scale. Eur J Pain 2004;8:283–91. [DOI] [PubMed] [Google Scholar]
- [31].Cleland JA, Childs JD, Whitman JM. Psychometric properties of the neck disability index and numeric pain rating scale in patients with mechanical neck pain. Arch Phys Med Rehabil 2008;89:69–74. [DOI] [PubMed] [Google Scholar]
- [32].Escobar A, Quintana JM, Bilbao A, et al. Validation of the Spanish version of the WOMAC questionnaire for patients with hip or knee osteoarthritis. Western Ontario and McMaster Universities Osteoarthritis Index. Clin Rheumatol 2002;21:466–71. [DOI] [PubMed] [Google Scholar]
- [33].Jinks C, Jordan K, Croft P. Measuring the population impact of knee pain and disability with the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC). Pain 2002;100:55–64. [DOI] [PubMed] [Google Scholar]
- [34].Williams VJ, Piva SR, Irrgang JJ, et al. Comparison of reliability and responsiveness of patient-reported clinical outcomes measures in knee osteoarthritis rehabilitation. J Orthop Sports Phys Ther 2012;42:716–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Pecos-Martín D, Montáñez-Aguilera J, Gallego-Izquierdo T, et al. Effectiveness of dry needling on the lower trapezius in patients with mechanical neck pain: a randomized controlled trial. Arch Phys Med Rehabil 2015;96:775–81. [DOI] [PubMed] [Google Scholar]
- [36].Hong CZ. Treatment of myofascial pain syndrome. Curr Pain Headache Rep 2006;10:345–9. [DOI] [PubMed] [Google Scholar]
- [37].Hong CZ. Lidocaine injection versus dry needling to myofascial trigger point: the importance of the local twitch response. Am J Phys Med Rehabil 1944;73:256–63. [DOI] [PubMed] [Google Scholar]
- [38].Martín-Pintado-Zugasti A, Pecos-Martín D, Rodríguez-Fernández AL, et al. Ischemic compression after dry needling of a latent myofascial trigger point reduces post-needling soreness intensity and duration. PM R 2015;7:1026–34. [DOI] [PubMed] [Google Scholar]
- [39].Park J, White A, Stevinson C, et al. Validating a new non-penetrating sham acupuncture device: two randomized controlled trials. Acupunct Med 2002;20:168–74. [DOI] [PubMed] [Google Scholar]
- [40].Park J. Developing and validating a sham acupuncture needle. Acupunct Med 2009;27:93. [DOI] [PubMed] [Google Scholar]
- [41].Park J. Sham needle control needs careful approach. Pain 2004;109:195–6. [DOI] [PubMed] [Google Scholar]
- [42].Suarez-Almazor ME, Looney C, Liu Y, et al. A randomized controlled trial of acupuncture for osteoarthritis of the knee: effects of patient-provider communication. Arthritis Care Res (Hoboken) 2010;62:1229–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Abbott JH, Chapple CM, Fitzgerald GK, et al. The incremental effects of manual therapy or booster sessions in addition to exercise therapy for knee osteoarthritis: a randomized clinical trial. J Orthop Sport Phys Ther 2015;45:975–83. [DOI] [PubMed] [Google Scholar]
- [44].Abbott JH, Robertson MC, Chapple C, et al. Manual therapy, exercise therapy, or both, in addition to usual care, for osteoarthritis of the hip or knee: a randomized controlled trial. 1: Clinical effectiveness. Osteoarthr Cartil 2013;21:525–34. [DOI] [PubMed] [Google Scholar]
- [45].Deyle GD, Allison SC, Matekel RL, et al. Physical therapy treatment effectiveness for osteoarthritis of the knee: a randomized comparison of supervised clinical exercise and manual therapy procedures versus a home exercise program. Phys Ther 2005;85: [PubMed] [Google Scholar]
- [46].Deyle GD, Henderson NE, Matekel RL, et al. Effectiveness of manual physical therapy and exercise in osteoarthritis of the knee: a randomized, controlled trial. Ann Intern Med 2000;132:173–81. [DOI] [PubMed] [Google Scholar]
- [47].McMunn A, Nazroo J, Breeze E. Inequalities in health at older ages: a longitudinal investigation of the onset of illness and survival effects in England. Age Ageing 2009;38:181–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Sousa AC, Guerra RO, Thanh Tu M, et al. Lifecourse adversity and physical performance across countries among men and women aged 65-74. PLoS One 2014;9:e102299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Estruch R, Ros E, Salas-Salvadó J, et al. Primary prevention of cardiovascular disease with a Mediterranean diet. N Engl J Med 2013;368:1279–90. [DOI] [PubMed] [Google Scholar]
- [50].Fan AY, Xu J, Li YM. Evidence and expert opinions: dry needling versus acupuncture (I): the American Alliance for Professional Acupuncture Safety (AAPAS) White Paper 2016. Chin J Integr Med 2017;23:3–9. [DOI] [PubMed] [Google Scholar]


