Abstract
This study investigated the effect of supplemental iron intake (SII) in early singleton pregnancy women with the risk of developing gestational diabetes mellitus (GDM) among Chinese population.
This study included 259 singleton pregnancy participants. Of those, 135 women underwent SII and were assigned to an intervention group, while 124 participants received no SII and were assigned to a control group. The outcome measurements consisted of the number of patients with GDM development, the levels of hemoglobin (Hb) and ferritin, and the outcomes of infant at delivery.
No significant difference in the number of patients with GDM development was found between 2 groups at delivery. However, when compared with control group, subjects in the intervention group showed greater efficacy in delivery mode choice of vaginal delivery (P = .04), and cesarean section (P = .01), as well as the birthweight of infants (P < .01). Moreover, Hb and ferritin levels were also significantly higher in the intervention group than those in the control group (P < .01).
The results of this retrospective study showed that SII may not increase risk of developing GDM in singleton pregnancy women; and also may benefit both pregnancy women and infants among Chinese population.
Keywords: effect, gestational diabetes mellitus, pregnancy, Supplemental iron
1. Introduction
Gestational diabetes mellitus (GDM) is one of the most common complications during the period of pregnancy.[1–5] It is reported that the prevalence of GDM ranges from 1.7% to 11.7% around the world.[6–7] Many factors can result in such condition. These factors included age, race, and family history of diabetes mellitus (DM).[8–11] If it cannot be controlled very well, it may result in several complications which can affect both mother and the baby, such as high blood pressure and preeclampsia, and future DM for the mother; and excessive birth weight, preterm birth, respiratory distress syndrome, hypoglycemia, and type 2 DM later in life for the baby.[8–11]
Several previous studies have reported that supplementation iron intake (SII) is associated with the risk of type 2 DM in healthy population.[12–15] However, some other studies found that there is no association between SII and GDM among the pregnancy population.[16–19] Although many studies have explored the effect of SII among both normal and GDM population, this issue is still controversial.
Presently, no specific study focuses on the investigation of the effect of SII in Chinese pregnancy women. Thus, in this retrospective study, we investigated the effect of SII in singleton pregnancy women at less than 16-week gestation with risk of GDM among Chinese population.
2. Methods
2.1. Ethics
This study was approved by the ethics committee of The People's Hospital of Yan’an, and Affiliated Hospital of Yan’an University. It was also conducted at these 2 hospitals from January 2011 to December 2015. All patients provided the informed consent.
2.2. Patients
In this retrospective study, 259 singleton pregnancy women at gestational age less than 16 weeks, and Hb levels between 8 and 14 g/dL were included in this study. All participants started to receive SII at less than 16 weeks of gestation with normal level of hemoglobin (Hb). However, subjects were excluded if they had existing DM, abnormal Hb (Hb < 8 g/dL, or Hb > 14 g/dL), and received SII after 16 weeks gestation.
2.3. Interventions
Around 135 pregnancy women received SII 300 mg daily, 7 days weekly until the baby delivery, and were assigned to an intervention group. A total of 124 women did not receive SII, and were assigned to a control group.
2.4. Outcome measurements
The outcomes were measured by the number of subjects with development of GDM, the levels of Hb, and ferritin, and the outcomes of infant at delivery.
2.5. Statistical analysis
All data were analyzed by using the Statistical Package for the Social Sciences software v.17.0 (SPSS Inc., Chicago, IL). The minimum sample size of 135 patients in the intervention, and 124 patients in the control group with α = 0.05, 1−β = 0.8. Student's t-test or Wilcoxon and Mann–Whitney tests were used to analyze the continuous outcome data. Pearson's chi-square or Fisher's exact tests were utilized to analyze the categorical outcome data. P value of P < .05 was regarded as the statistically significant.
3. Results
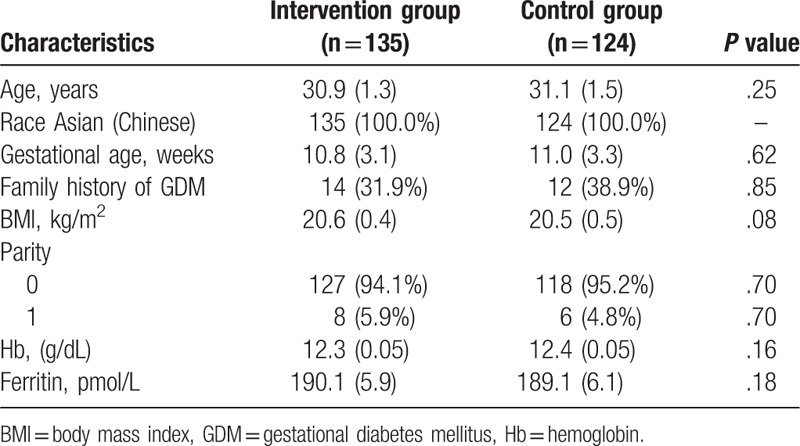
The patient characteristics at baseline are listed in Table 1. No significant differences of characteristic values were found between 2 groups (Table 1). These characteristic values consisted of age, race, and gestational age, family history of GDM, body mass index, parity, hemoglobin, and ferritin.
Table 1.
Patients characteristic at baseline.
The outcome measurements of pregnancy women at delivery were showed in Table 2.
Table 2.
Outcome measurements of pregnancy women at delivery.
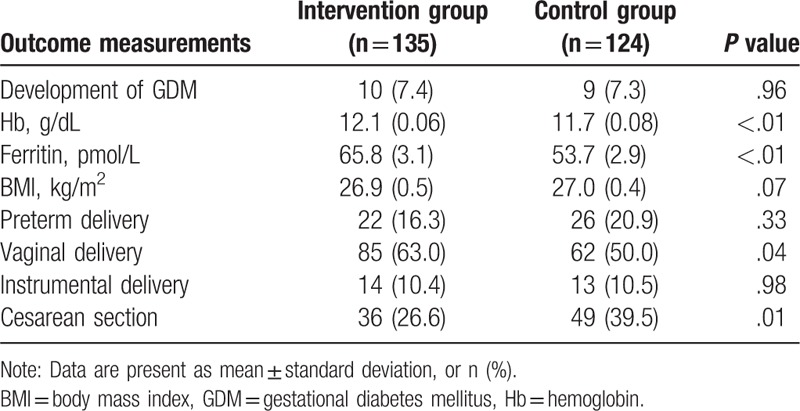
At delivery, there were no significant differences in the development of GDM (P = .96), BMI (P = .07), preterm delivery (P = .33), and instrumental delivery (P = .98) between 2 groups (Table 2). However, significant differences were found in vaginal delivery (P = .04), cesarean section (P = .01), and the levels of Hb groups (P < .01), and ferritin (P < .01) between the 2 groups (Table 2).
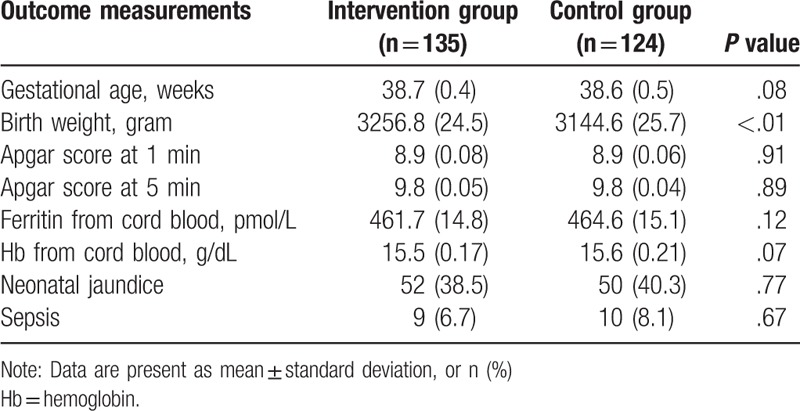
The outcome measurements of infant at delivery were summarized in Table 3. There was no significant in the gestational age (P = .08), Apgar score at 1 and 5 minute (P = .91, P = .89), respectively, ferritin (P = .12) and Hb (P = .07) from cord blood, neonatal jaundice (P = .77), and sepsis (P = .67) between the 2 groups (Table 3). However, the birth weight of infants was significantly higher in the intervention group than that in the control group (P < .01, Table 3).
Table 3.
Outcome measurements of infant.
4. Discussion
Iron deficiency anemia is a very common issue during the period of pregnant women. Such condition often results in many adverse events on both mother and the baby. It often includes pre-eclampsia, preterm delivery, and even can increase the mortality in some severe cases for mother and the baby. Previous study has been reported that pregnant women require a greater amount of iron to meet the needs of pregnancy and delivery,[20] and at least 50% anemia results from the iron deficiency.[21] Thus, at such situation, SII is recommended to correct this condition.[21]
Previous related studies have also reported the association of SII in pregnant participants. However, this issue remains a controversial topic. One study conducted in Iran included normal and GDM population to investigate the risk factors of GDM.[14] Its results found that the levels of serum ferritin were significantly higher among the GDM population than that among the healthy population.[14] The other study conducted a randomized controlled study to explore the effect of SII from early pregnancy population.[17] The results of this study demonstrated that SII cannot increase the risk of GDM for the pregnancy women. Moreover, it can also benefit their pregnancy outcomes.[17]
The result of our study is partly consistent with the previous study.[17] In our study, we found that SII can reduce the numbers of patients to receive cesarean, and can help to increase vaginal delivery for the pregnancy women. Moreover, the Hb and ferritin levels are also significantly higher in the patients who received SII than those who did not receive it. Additionally, in the intervention group, the infants also had significant higher in birth weight than those in the control group.
This retrospective study had its strength of cutting out the variability outcome assessments among different centers and different population, because this study only included Chinese Han population. On the other hand, its results may affect the generalizability of this finding to the other ethnicities. Moreover, more clinical trials, especially the randomized controlled trials are still needed to warrant our results, and to explore the effect of SII to the other population.
5. Conclusion
The results of this study found that SII may not increase the risk of GDM development among Chinese population. Moreover, it may benefit the outcomes of both pregnancy women and infants.
Author contributions
Conceptualization: Jing Pang, Xiao-ni Liu.
Data curation: Jing Pang, Xiao-ni Liu.
Formal analysis: Xiao-ni Liu.
Investigation: Xiao-ni Liu.
Methodology: Xiao-ni Liu.
Project administration: Jing Pang.
Resources: Jing Pang, Xiao-ni Liu.
Software: Xiao-ni Liu.
Supervision: Xiao-ni Liu.
Validation: Jing Pang.
Visualization: Jing Pang.
Writing – original draft: Jing Pang, Xiao-ni Liu.
Writing – review & editing: Jing Pang, Xiao-ni Liu.
Footnotes
Abbreviations: DM = diabetes mellitus, GDM = gestational diabetes mellitus, Hb = hemoglobin, SII = supplemental iron intake.
The authors have no conflicts of interest to disclose.
References
- [1].Buchanan TA, Xiang AH. Gestational diabetes mellitus. J Clin Invest 2005;115:485–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Wei J, Heng W, Gao J. Effects of low glycemic index diets on gestational diabetes mellitus: a meta-analysis of randomized controlled clinical trials. Medicine (Baltimore) 2016;95:e3792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Cho NH, Ahn CH, Moon JH, et al. Metabolic syndrome independently predicts future diabetes in women with a history of gestational diabetes mellitus. Medicine (Baltimore) 2016;95:e4582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Cho HY, Jung I, Kim SJ. The association between maternal hyperglycemia and perinatal outcomes in gestational diabetes mellitus patients: A retrospective cohort study. Medicine (Baltimore) 2016;95:e4712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Wang L, Xu W, Wang X. Peroxisome proliferator-activated receptor Pro12Ala polymorphism and the risks of gestational diabetes mellitus: an updated meta-analysis of 12 studies. Medicine (Baltimore) 2016;95:e5090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Coustan DR. Gestational diabetes mellitus. Clin Chem 2013;59:1310–21. [DOI] [PubMed] [Google Scholar]
- [7].Liang HL, Ma SJ, Xiao YN, et al. Comparative efficacy and safety of oral antidiabetic drugs and insulin in treating gestational diabetes mellitus: an updated PRISMA-compliant network meta-analysis. Medicine (Baltimore) 2017;96:e7939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Minooee S, Ramezani Tehrani F, Rahmati M, et al. Diabetes incidence and influencing factors in women with and without gestational diabetes mellitus: a 15 year population-based follow-up cohort study. Diabetes Res Clin Pract 2017;128:24–31. [DOI] [PubMed] [Google Scholar]
- [9].Lee CH, Kim J, Jang EJ, et al. Inhaled corticosteroids use is not associated with an increased risk of pregnancy-induced hypertension and gestational diabetes mellitus: two nested case-control studies. Medicine (Baltimore) 2016;95:e3627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Lee J, Ouh YT, Ahn KH, et al. Preeclampsia: a risk factor for gestational diabetes mellitus in subsequent pregnancy. PLoS One 2017;12:e0178150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Adam S, Rheeder P. Screening for gestational diabetes mellitus in a South African population: Prevalence, comparison of diagnostic criteria and the role of risk factors. S Afr Med J 2017;107:523–7. [DOI] [PubMed] [Google Scholar]
- [12].Park JS, Kim DW, Kwon JY, et al. Development of a screening tool for predicting adverse outcomes of gestational diabetes mellitus: a retrospective cohort study. Medicine (Baltimore) 2016;95:e2204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Khambalia AZ, Aimone A, Nagubandi P, et al. High maternal iron status, dietary iron intake and iron supplement use in pregnancy and risk of gestational diabetes mellitus: a prospective study and systematic review. Diabet Med 2016;33:1211–21. [DOI] [PubMed] [Google Scholar]
- [14].Javadian P, Alimohamadi S, Gharedaghi MH, et al. Gestational diabetes mellitus and iron supplement; effects on pregnancy outcome. Acta Med Iran 2014;52:385–9. [PubMed] [Google Scholar]
- [15].Rajpathak S, Ma J, Manson J, et al. Iron intake and the risk of type 2 diabetes in women: a prospective cohort study. Diabetes Care 2006;29:1370–6. [DOI] [PubMed] [Google Scholar]
- [16].Luan de C, Li H, Li SJ, et al. Body iron stores and dietary iron intake in relation to diabetes in adults in North China. Diabetes Care 2008;31:285–6. [DOI] [PubMed] [Google Scholar]
- [17].Chan KK, Chan BC, Lam KF, et al. Iron supplement in pregnancy and development of gestational diabetes-a randomised placebo-controlled trial. BJOG 2009;116:789–97. [DOI] [PubMed] [Google Scholar]
- [18].Chen X, Scholl TO, Stein TP. Association of elevated serum ferritin levels and the risk of gestational diabetes mellitus in pregnant women: the Camden study. Diabetes Care 2006;29:1077–82. [DOI] [PubMed] [Google Scholar]
- [19].Milman N. Iron and pregnancy-a delicate balance. Ann Hematol 2006;85:559–65. [DOI] [PubMed] [Google Scholar]
- [20].Milman N. Oral iron prophylaxis in pregnancy: not too little and not too much!. J Pregnancy 2012;2012:514345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Ministry of Health and Family Welfare, Government of India: Guidelines for the control of iron deficiency anaemia: A national iron plus initiative. Ministry of Health and Family Welfare: Adolescent division 2013. http://www.pbnrhm.org/docs/iron_plus_guidelines.pdf. Accessed April 1, 2018. [Google Scholar]


