Abstract
Late referral in chronic kidney disease (CKD) is associated with irregular care and poor prognosis. How the specialty of healthcare provider affect late referral and irregular CKD care remain unclear.
We conducted a population-based cross-sectional study to include incident dialysis patients from 2002 to 2007 in Taiwan and observed for 1, 2, and 3 years before dialysis. The medical visits-related information was evaluated every 3 months, retrospectively. Irregular follow-up was defined as missing a follow-up during more than one interval every year.
A total of 46,626 patients were included. At 1, 2, and 3 years prior to maintenance dialysis, 87%, 66%, and 50% of patients had regular medical visits; however, only 49%, 23%, and 12% had estimated glomerular filtration rate (eGFR) regularly monitored, respectively. Independent factors of less regular eGFR follow-up included age (adjusted odds ratio (OR) 0.995, 95% confidence interval 0.993–0.998), cardiac disorder (0.90, 0.82–0.99), and stroke (0.76, 0.69–0.84), as well as regular visits at some other specialties (adjusted OR range: from 0.77 to 0.88); whereas, independent factors of less regular visits at nephrology included diabetes mellitus (0.48, 0.46–0.51), cardiac disorder (0.61, 0.56–0.66), stroke (0.53, 0.48–0.58), and regular visits at any other specialty (adjusted OR range: from 0.22 to 0.78).
Regular medical visits were quite common in late CKD patients, but they received regular eGFR measurement and visit at nephrology much less frequently. Physicians play a major role in the late referrals in CKD and its irregular care.
Keywords: chronic kidney disease, co-care with nephrology, healthcare behavior, physician's practice patterns, quality of predialysis care
1. Introduction
Low awareness of chronic kidney disease (CKD) is quite common in the general population. Even in CKD stage 5, it has been reported that as high as 30% to 50% of patients were never informed of their renal status.[1–4] Low awareness of CKD and late nephrology referral are associated with poor prognosis, such as rapid progress to end stage renal disease (ESRD), higher mortality, and hospitalization.[1,3,5–8] This not only exposes CKD patients to potential renal injuries which otherwise could have been avoided, but also delays the timely treatment necessary to retard the disease progression.[4,9,10] Thus, to address the increasing dialysis burden worldwide, it is critically important to elucidate the mechanisms of the low awareness level and late referral of CKD.
The low awareness and late referral of CKD is multifactorial, and the causative factors may interact with each other.[11,12] Low awareness of chronic disease could come from both patients’ not seeking medical help and physicians’ under-diagnosis. That CKD patients often have other chronic diseases earlier makes this population tend to seek medical care initially from other specialists rather than nephrologists.[8,13–17] The “silent” characteristics associated with CKD progression probably also contribute to keeping these patients from seeking medical care. Furthermore, medical access and insurance coverage have been reported as 2 major barriers of nephrology referral in late CKD patients.[18–22] Given the high prevalence of co-morbidities in CKD, many of this population had non-nephrology medical visit initially before transferring to nephrologists. Little is known about how these initial medical visits affect late CKD care. Thus, we hypothesized that nephrology referral and regular CKD care would be improved in late CKD patients under regular medical visits. Taiwan, a small island, has almost all of its citizens covered by National Health Insurance (NHI), which makes access to medical care relatively comprehensive; thus, it constitutes an appropriate area for testing the abovementioned hypothesis. We conducted this population-based study in order to elucidate how physicians’ specialty affect care in late CKD.
2. Methods
2.1. National health insurance in Taiwan
In 1995, NHI, a government-run insurer with a single-payer insurance system was established for the entire population of Taiwan. The characteristics of NHI include: payroll-related premiums shared among employers, employees, and the government; fee-for-service under the global budget; and the requirement of co-payment for medication, ambulatory care, and inpatient care. As the enrolment of NHI was mandatory, by December 2008, there were 22.918 million individuals enrolled in the program nationwide, with a coverage rate of 99.5%. The registration of Catastrophic Illness Database cases, such as chronic renal disease and cancer, were required by the bureau of NHI before certification for Catastrophic Illness could be granted.
2.2. Definition of study population
We conducted a retrospective, population-based cross-sectional study, which was approved by the Institutional Review Board, Kaohsiung Medical University Hospital (KMUH-IRB-EXEMPT-20130012). All research procedures follow the guidelines of the Declaration of Helsinki. Because the patient identifiers were scrambled in the data to the public for research purpose, the Institutional Review Board agreed the study exempted from the requirement for written or verbal inform consents from participants. The research data were obtained from the NHI Research Database provided by the National Health Research Institute and included outpatient, ambulatory care, hospital inpatient care, as well as dental service. It covered all claims submitted from 1997 to 2008, but did not contain laboratory results. Any insured person who needs maintenance dialysis should be issued the Catastrophic Illness certificate for dialysis to waive the deduction. Thus, all patients in the NHI Research Database were screened, and the study cohort included those who met both of the following criteria: having a Catastrophic Illness certificate for maintenance dialysis [International Classification of Disease, Ninth Revision, Clinical Modification, (ICD-9-CM) code 585]; and having the prescription of dialysis for 90 d after the issuance of the Catastrophic Illness certificate for maintenance dialysis. Any person who was younger than 18 year old when dialysis started or received treatment for renal graft (ICD-9-CM code V42.0) during the observation period was excluded. Patients’ demographic information (i.e., age, gender, living area, and socioeconomic status), information regarding medical visits, and comorbidity were obtained after exclusion criteria were applied. The relevant comorbidities in this study included diabetes mellitus (ICD-9-CM code 250), hypertension (ICD-9-CM codes 401–405), cardiac disorders [ICD-9-CM code 410 (myocardial infarction), 428 (congestive heart failure)], stroke (ICD-9-CM code 433, 434, 436), and gout (ICD-9-CM code 274). The presence of comorbidity was defined as a disease diagnosis code shown at least 2 times from outpatient claim data or one time from inpatient claim data at the second year before maintenance dialysis. All these comorbidities are the possible confounding factors in our model to explain the association between medical visit and eGFR measurement base on our clinical experience. Furthermore, we have included Charlson score to reduce the confounding effect by selecting these comorbidities. The Charlson comorbidity index scores were calculated according to the method listed in a previous study.[23]
2.3. Information regarding medical visits
The observation period in this study comprised the 3 years before the first maintenance dialysis. All parameters of interest were further investigated at 1 year, 2 years, and 3 years of the observation period, respectively. In these observation periods, each participant was screened for the timing and frequency of the outpatient visits and the tests prescribed to monitor CKD progression and complications. Any visits or tests occurring in the emergency department were excluded. In addition to frequency, hospital level and specialty of each medical visit were collected for each outpatient visit. Hospitals in Taiwan are classified into 4 levels by Bureau of NHI, that is, primary clinic, local hospital, general hospital, and medical center hospital, according to the numbers of beds, specialties, and kinds of services available. The tests of interest included hemoglobin, serum creatinine, serum potassium, serum calcium, serum phosphorus, intact parathyroid hormone (iPTH), gas analysis, urine albumin-creatinine ratio (ACR), and protein-creatinine ratio (PCR). Tests for ACR and PCR were included only when both urinary creatinine and urinary albumin/protein survey were arranged on the same prescription. Given that the prescription could be valid for medication refill for no longer than 90 d, all of the identified medical visits and tests were further grouped every 3 months, retrospectively since the maintenance dialysis for the evaluation of regularity.
2.4. Definition of regular medical visit
The regularity of medical visits for each participant was evaluated by the frequency of clinic visits at the same hospital or clinic every 3 months, retrospectively since the maintenance dialysis. No medical visit in any 3-month interval was defined as one missing. Regular medical visit was defined as having no more than one missing visit every year in the observation period.
2.5. Definition of regular follow-up of CKD progression
The follow-up of CKD progression was evaluated by the frequency of the estimated glomerular filtration rate (eGFR) and proteinuria measurement every 3 months at the same hospital or clinic in the observation period. Similar to medical visit, one missing measurement for any test above was defined as no prescription in any 3-month interval. Regular eGFR follow-up was defined as no more than one missing measurement every year in the observation period, and the same applied to regular proteinuria follow-up.
2.6. Definition of regular follow-up of CKD complications
The CKD complications follow-up was evaluated by the frequency of tests for hemoglobin, serum potassium, serum calcium, serum phosphorus, iPTH, and gas analysis at least once every 3 months at the same hospital or clinic in the observation period. Regular CKD complications follow-up were tested for all participants at the 1-, 2-, and 3-year observation periods. Again, one missing measurement for any test above was defined as no prescription in any 3-month interval. Regular CKD complications follow-up for each item in the observation period was defined as no more than one missing measurement for the item every year.
2.7. Statistical analysis
A descriptive analysis was carried out to describe the social-demographics, clinical characteristics, proportions of regular measurement of eGFR, and proportions of regular medical visits at the 1-, 2-, and 3-year observation periods. Because no prior study ever tested our main hypothesis, we used G∗Power to estimate sample size by assuming clinical reasonable conditions as α=0.05, β=0.8, 2-tailed test, prevalence of irregularity of eGFR measurement or medial visit at nephrology=70%, prevalence of regular medical visit=90%, and the odds ratio of regular eGFR measurement is 1.5 times in regular medical visit group compared to irregular group. The expected sample size for this study is 2684 by G∗Power estimation.[24] Descriptive statistics, such as mean, standard deviation, median, interquartile range, and proportions were utilized when appropriate. The normal distribution was evaluated through the Kolmogorov–Smirnov test. Chi-square test and Mann–Whitney U test were used to assess the different distributions of patient characteristics between regular and irregular medical visit groups. Univariable logistic analysis was performed to select the variables with P value < .25 to be candidates for inclusion in multivalable analysis. Multiple logistic regression analysis with stepwise variable selection then was construct to identify significant independent factors related to the irregularity of eGFR measurement and irregular medical visits at nephrology. Variance inflation factor was used to detect multicollinearity in the regression model. We used Akaike information criterion to determine the best set of variables and c-statistic to evaluate goodness-of-fit of the final models. Odds ratio (OR) was calculated as exponential of the estimated regression coefficients and the 95% confident interval (CI) for each OR was also computed. Furthermore, linear trend test was used to evaluate the effect of the cohort. All statistical tests were carried out by using SAS 9.2 software. A 2-sided P <.05 was considered as significant.
3. Results
3.1. Demographic characteristics
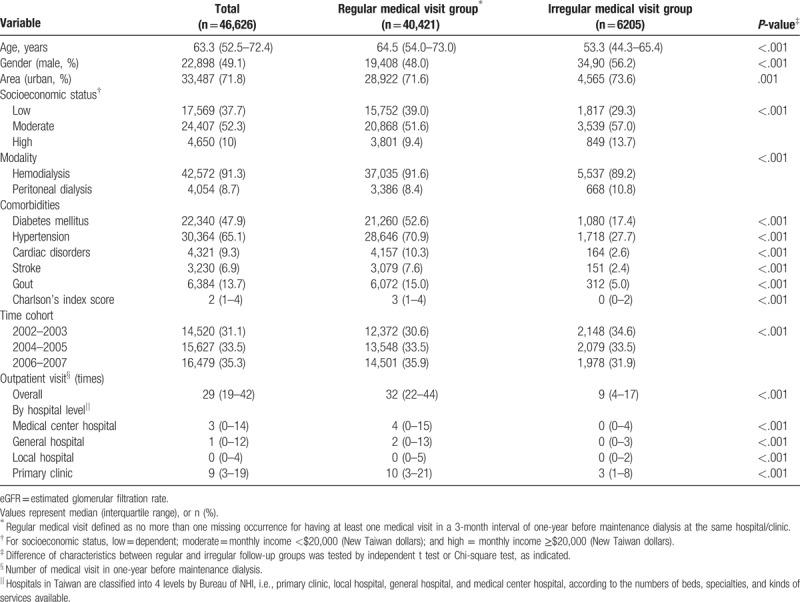
In the Taiwan National Health Research Dataset from 2002 to 2007, we identified 57,339 patients who had been issued the Catastrophic Illness certificate for maintenance dialysis. Later, 48,283 patients (84.2%) had survived for more than 3 months after their first dialysis. Patients who were younger than 18 year old (n = 190) or had received treatment for renal graft (n = 1467) in the observation period were excluded. Thus, 46,626 subjects formed the study cohort. Their clinical characteristics, obtained by regular medical follow-up at the same hospital/clinic in the year before the first maintenance dialysis, are shown in Table 1.
Table 1.
Clinical characteristics of the subjects by regular medical visit in 1-year before maintenance dialysis.
3.2. First eGFR measurement and nephrologist visit before maintenance dialysis in the observation period
The first eGFR measurements and nephrologist visit for all subjects in the 3-year observation period (the 3 years before maintenance dialysis) are shown in Figure 1A and B. Two-thirds of them had the first eGFR measurement in the third year before maintenance dialysis, one-sixth in the second year, and the remaining one-sixth in the first year. More than 10% even had the first eGFR measurement within half-a-year before the first maintenance dialysis, and nearly 5% never had eGFR measurement at an outpatient clinic (parachute dialysis). For those who had one-year regular medical visits before maintenance dialysis at any specialty, the first eGFR measurement in the observation period did not appear to be much earlier than those without regular medical visits (Fig. 1A); however, there seemed to be more first nephrologist visits in the beginning of the observation period when patients were under regular medical visits (Fig. 1B). In addition, 62.8%, 76.1%, and 68.9% of the first eGFR measurement occurring at the first-, second-, and third-year before maintenance dialysis, respectively, were prescribed at specialties other than nephrology.
Figure 1.
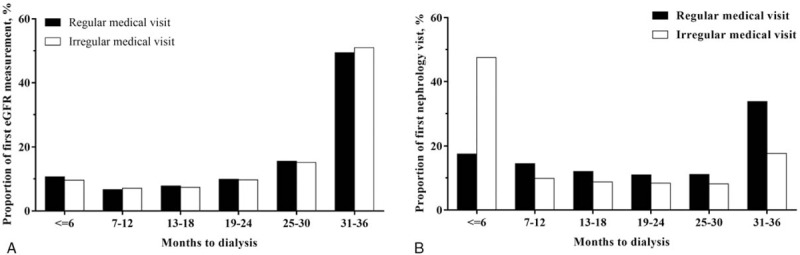
Regular medical visit is not associated with first estimated glomerular filtration rate (eGFR) measurement in predialysis care, but has more first nephrologist visits in the beginning of observation before dialysis. Time distribution between (A) first eGFR measurement; or (B) first nephrology visit to the maintenance dialysis in the observation period by one-year regular medical visit. eGFR = estimated glomerular filtration rate.
3.3. Regularity of medical visit and eGFR measurement before maintenance dialysis
Table 1 presents the clinical characteristics of the study subjects by one-year regular medical visit (at least 3 out of the 4 quarters in which the patient had visited the same hospital or clinic at least one time). Near 90% of incident dialysis patients had their regular medical visit in one year before maintenance dialysis. This group was a little older, more likely to be female, and had a slightly lower income. The prevalence of diabetes mellitus, hypertension, cardiac disease, stroke, gout, and Charlson Index score were much higher in the regular group. In different time cohorts, a continuous improvement of regularity can be seen.
Table 2 shows the regularity of medical visits, co-care with nephrologist, and eGFR measurement at various specialties in one-year before the maintenance dialysis. As expected, the proportion of regular eGFR measurement decreased as the observation period increased. The percentages of regular eGFR measurement for 1-, 2-, and 3-years were 48.6%, 22.8%, and 11.7%, respectively (Fig. 2). The follow-up of urine protein seemed not be a routine practice in daily CKD care, as <1% of the patients in this study met our definition of regularity in any observation period (Fig. 2)
Table 2.
Proportions of regular medical visits, co-care with nephrologist, and regular eGFR measurement in one-year before maintenance dialysis by specialty and hospital level.
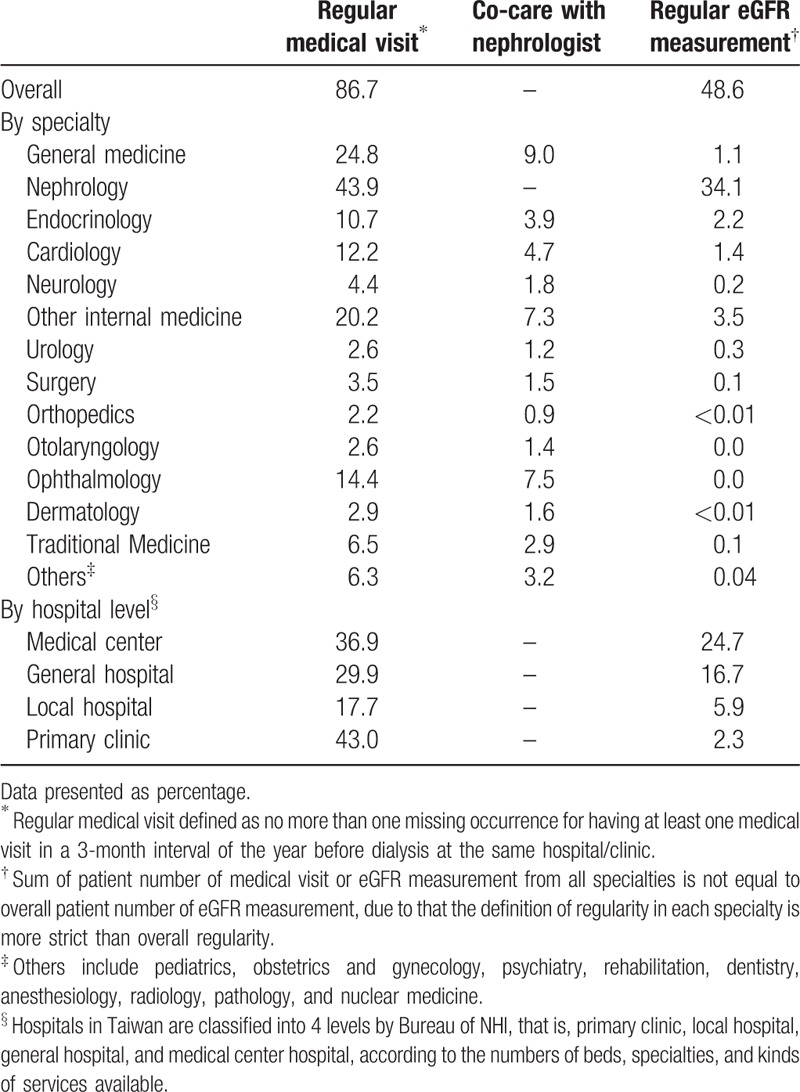
Figure 2.
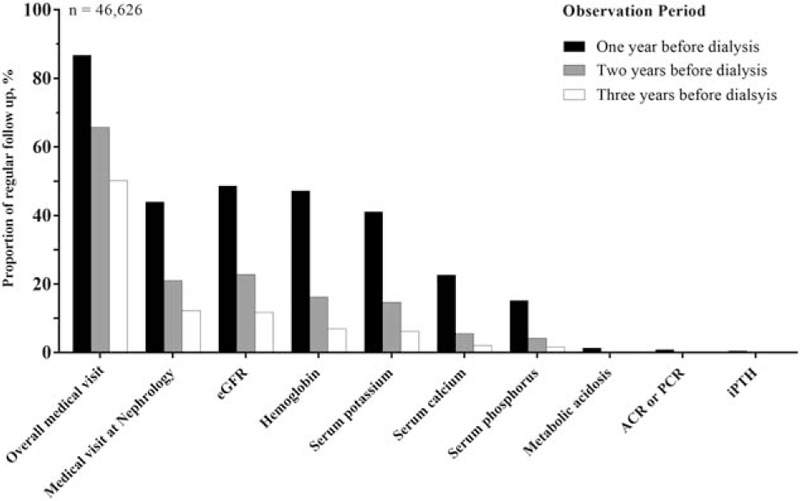
Proportions of regular medical visit and regular follow-up of renal function and CKD complications in various observation periods. Regular follow-up in a certain observation period defined as no more than one missing occurrence for having at least one visit/survey in a 3-month interval of every year at the same hospital/clinic. ACR = albumin creatinine ratio, CKD = chronic kidney disease, eGFR = estimated glomerular filtration rate, iPTH = intact parathyroid hormone, PCR = protein creatinine ratio.
3.4. Regularity of CKD complications follow-up before maintenance dialysis
Figure 2 presents the poor follow-up for CKD complications in predialysis. Less than half of all incidental dialysis patients had their hemoglobin and potassium levels under regular follow-up in the year before maintenance dialysis. Only approximately 20% had regular follow-up for their mineral disorders and <1% for iPTH and metabolic acidosis. Similar to eGFR measurement, the complications survey decreased dramatically as the observation period increased (Fig. 2).
3.5. Nephrology visit and regular eGFR follow-up before maintenance dialysis
In contrast to low regular follow-up for CKD progress and complications, incident dialysis patients had very regular medical visits in predialysis. As shown in Figure 2, nearly 90% of incident dialysis patients at the 1-year, 70% at 2-year, and 50% at 3-year observation period had regular medical visits at the same hospital or clinic. Further classifying these regular visits by specialty, we found that nephrology was first, but only accounted for 44%, 21%, and 12% in the 1-, 2-, and 3-year observation period, respectively. Among those under regular nephrology clinic, 78%, 67%, and 58% in the 1-, 2-, and 3-year observation period, respectively, had regular eGFR measurement. In addition, regular co-care with nephrologists for predialysis patients was not common. There was a trend of increasing rate of regular co-care when approaching the beginning of maintenance dialysis; however, almost none of the other specialties had a co-care percentage higher than 50% at the last year before maintenance dialysis (Table 2).
Regular medical visits did not produce regular eGFR measurement in predialysis. Regardless of whether or not co-care occurred with nephrologists, physicians in other specialties did not usually prescribe regular eGFR measurement to this population (Table 2). In addition, those under regular co-care were prescribed less regular eGFR measurement when compared with those only under regular follow-up in nephrology clinics (42% vs 84%, P < .001).
3.6. Independent factors of regular eGFR measurement and regular medical visit at nephrology before maintenance dialysis
Table 3 presents the independent factors of regular eGFR measurement at the year before maintenance dialysis. Patients of a younger age (adjusted OR 0.995, 95% CI: 0.993–0.998, P < .001) tended to receive regular eGFR measurement. There was significant continuous improvement of regular eGFR measurement in different time cohorts (linear trend P < .001). The presence of hypertension (adjusted OR 1.21, 95% CI 1.14-1.28, P < .001), and gout (adjusted OR 1.12, 95% CI 1.04-1.20, P = .004) were independently positive predictors; whereas, cardiac disorders (adjusted OR 0.90, 95% CI 0.82-0.99, P = .03) and stroke (adjusted OR 0.76, 95% CI 0.69–0.84, P < .001) were negative ones. As expected, regular visits at nephrology (adjusted OR 19.16, 95% CI 18.06–20.33, P < .001) had the strongest predictive power of regular eGFR measurement. For the rest of the specialties, the association varied from positively to negatively significant. Regular medical visits at higher hospital levels were associated with more regular eGFR measurement. However, this association was totally the opposite at local hospital or primary clinics, which means that predialysis patients with regular OPD visits at local hospital or primary clinics had a lower chance of their eGFR being regularly monitored.
Table 3.
Factors associated with regular medical visit at nephrology and eGFR measurement before maintenance dialysis.
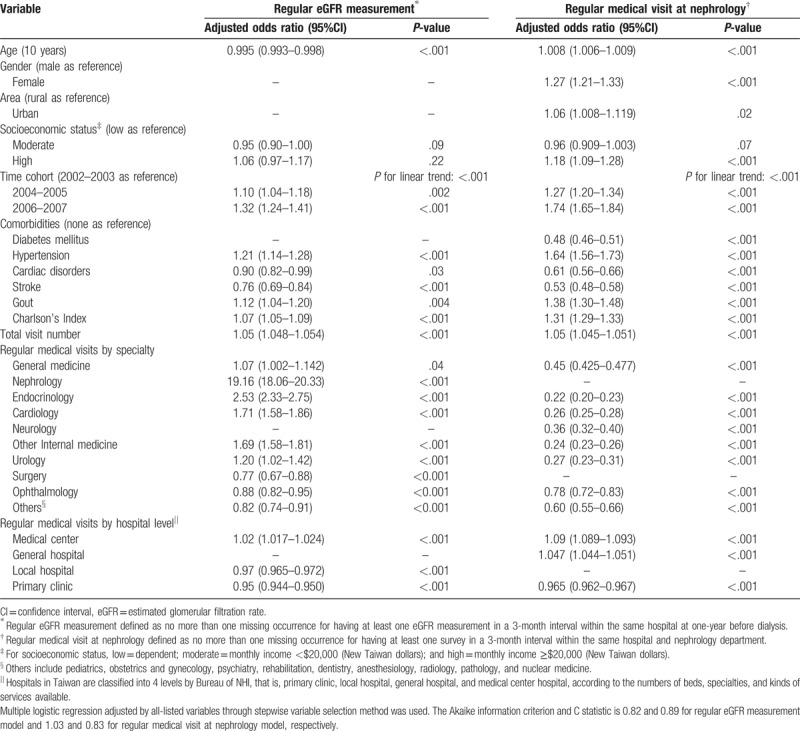
The independent factors of one-year regular medical visit at nephrology are also shown in Table 3. Patients of an older age (adjusted OR 1.008, 95% CI 1.006–1.008, P < .001), female gender (adjusted OR 1.27, 95% CI 1.21–1.33, P < .001), living in an urban area (adjusted OR 1.06, 95% CI 1.008–1.119, P = .02), and having a slightly higher income (adjusted OR 1.18, 95% CI 1.09–1.28, P < .001) tended to have regular nephrology follow-up. The presence of diabetes mellitus (adjusted OR 0.48, 95% CI 0.46–0.51, P < .001), cardiac disorders (adjusted OR 0.61, 95% CI 0.56–0.66, P < .001), and stroke (adjusted OR 0.53, 95% CI 0.48–0.58, P < .001) were independently negative predictors; whereas, hypertension (adjusted OR 1.64, 95% CI 1.56–1.73, P < .001) and gout (adjusted OR 1.38, 95% CI 1.30–1.48, P < .001) were positive ones. Higher Charlson's Index (adjusted OR 1.31, 95% CI 1.29–1.33, P < .001) was associated with regular visits at nephrology. There was still significantly continuous improvement of regular visits at nephrology in different time cohorts (linear trend P < .001). Surprisingly, regular visits at other specialists all decreased the regular visits at nephrology at very low odds ratios (adjusted OR range: from 0.22 to 0.78). Regular medical visits at higher hospital levels were associated with more regular nephrology visits. Patients who had regular medical visits at medical center hospitals were approximately 1.09 times more likely to have regular nephrology care, compared to only 2 times while having regular medical visits at primary clinics.
4. Discussion
This is the first study investigating CKD care in predialysis on a national basis after the launch of the CKD concept by NKF-KDOQI in 2002. Even with the nationwide health insurance coverage making medical care more accessible, patients in Taiwan did not have satisfactory follow-up for CKD progression and complications in predialysis. It was found that under-diagnosis of late CKD was still common, and up to one-third of incident dialysis patients might not have their eGFR measured until 2 years before the maintenance dialysis. At the same time, regular medical visits were common, but did not bring earlier eGFR screen in this population. Furthermore, these regular medical visits were diverse among many specialties and accompanied by low nephrologist co-care and follow-up of CKD progression and complications. In multivariate analysis, numerous comorbidities and regular medical visits at non-nephrology subspecialists were proven to be risk factors of irregular eGFR measurement and follow-up at nephrology. Our findings above highlight some obstacles in contemporary CKD care in Taiwan, and physicians might play a determinant role in them.
Screening for high-risk populations of developing ESRD is one of the main components of the CKD concept.[25,26] Prior to this study, it was known that under-diagnosis of CKD was common, but why the screen rate and awareness level were so low was unclear. Since health insurance in Taiwan is obligatory, people in Taiwan have less limited medical access when compared with those in other areas. This can be seen from the high number and diverse specialties of medical visits in predialysis. Probably due to the same reason and the high comorbidities, around two-thirds of these late CKD patients had their first eGFR measurement prescribed by specialties other than nephrologists in the observation period. However, that these late CKD patients under regular medical visits did not have their first eGFR measurement earlier when compared with those without, suggested that these frequent outpatient visits seemed not increase the CKD screen rate (Fig. 1A). This implied that CKD screen was not considered as a routine daily practice in other specialties, and this can be reflected in the current guideline recommendations. Diabetes is the only common comorbidity of CKD that has its care guidelines recommending renal function survey in routine regular practice.[27] High comorbidities and silent clinical characteristics of CKD easily lead these patients to seek medical care in specialties other than nephrology in the beginning of disease course; however, CKD screen is not executed routinely in the practice of many physicians. This constitutes the first obstacle of contemporary CKD care.
Although there is no strong evidence of benefit to suggest how frequent eGFR should be measured in a late CKD patient, regular eGFR follow-up is clearly fundamental to CKD care.[28,29] It provides information about not only the CKD progression rate and treatment response, but also some subclinical renal injuries which, if detected earlier, may be reversed. As previously mentioned, a very large number of late CKD patients were under regular medical visits in other specialties, but had very low regular eGFR measurement. This result, although not unexpected,[17] raised the concern that physicians in charge could probably make the referral based on the uremic symptoms which usually appear at a very late CKD stage. Furthermore, we found that some comorbidity and associated regular medical visits conversely deteriorate regular CKD care (eGFR measurement as representative in this study). In other words, although many late CKD patients were under regular medical visits at non-nephrology clinics, these physicians neither offered basic CKD care nor did they encourage co-care with nephrologists (this was especially the case at primary clinics, as shown in the results). Further investigation is needed to clarify whether the way that health insurance reimburses the practice affects the referral and co-care in these late CKD patients. The lack of policy and target for co-care between non-nephrologists and nephrologists for CKD patients constitute the second obstacle of contemporary CKD care.
CKD progression and complications follow-up for late predialysis patients, although not satisfactory, has exhibited continuous improvement. This was partially due to the launch of the CKD Preventive Project in Taiwan in 2001 and reimbursement for that integrated care beginning in 2006.[30] However, regular eGFR measurement at 2- and 3-years before maintenance dialysis was unexpectedly low. The poor regular follow-up of eGFR was certainly related to the abovementioned 2 obstacles. However, the eGFR prescribed by nephrologists for regular visit patients were not so frequent. Without a long enough period of regular eGFR follow-up, it is difficult to evaluate the efficacy of CKD progression retardation. Both CKD progression retardation and preparation for dialysis initiation constitute the therapeutic goal at late CKD stage.[26] The above result seemed to indicate that preparing for dialysis initiation, rather than retarding CKD progression, should be the target of the physician in the observation period. This might also be one of the reasons why many late CKD patients visited nephrologists in the beginning (the first 6 months of the observation period in this study, Fig. 1B), but finally only approximately one-half of them had regular nephrology visits and eGFR measurements in the last year prior to dialysis. In addition, the poorer performance on regular eGFR monitoring by nephrologists in the co-care group was probably due to the same reason, but confirmation of this requires further investigation. Regular eGFR measurement is fundamental to retard CKD progression, and our study results revealed that even nephrologists exhibited unsatisfactory performance. That there is no clear goal and principle for retarding CKD progression constitutes the third obstacle of contemporary CKD care.
There are several limitations in this study. First, the lack of laboratory results in claim data makes it impossible to include all of the late CKD patients, such as those who either died before dialysis or had not yet received dialysis. Second, for the same above reason, we could not exclude incident dialysis patients with acute kidney injury course. This subgroup, although very small, is not the target of interest and may further interfere with interpretation of the results since they did not have the chance to receive regular follow-up of CKD progression and complications. Third, we only made a retrospective observation of 3 years before maintenance dialysis, which might miss some earlier eGFR measurement or nephrology visits. Given the 3-year regular medical visit at nephrology already being as low as 12%, extending the observation length is less likely to change the trend observed in this study. Finally, the unique insurance system and physician practice culture in Taiwan may limit the generalizability of our results to other countries.
In this study, the hypothesis that regular medical visits in late CKD patients improve nephrology referral and regular CKD care cannot be proven. In contrast, having certain chronic disease or regular follow-up at some clinics lowered the chance of having regular CKD care. Moreover, even near the start of dialysis, those late CKD patients under regular follow-up at other clinics tended to receive treatment at the same specialists, which means that the regular medical visits did little to facilitate nephrology referrals. Even though it has been years since the CKD concept was initially launched, pre-ESRD care in Taiwan is still not adequate, and physicians here, both nephrologists and other specialists, should take responsibility for this unsatisfactory result in terms of screens, referrals, and co-care. New health policies are urgently needed to solve the abovementioned obstacles in late CKD care.
Acknowledgments
This study is based in part on data from the National Health Insurance Research Database provided by the Bureau of National Health Insurance and the Department of Health, and managed by the National Health Research Institute (no. 99324). The interpretation and conclusions contained herein do not represent the views of the Bureau of National Health Insurance, the Department of Health, or the National Health Research Institute. The authors are thankful for assistance from the Statistical Analysis Laboratory, Department of Internal Medicine, Kaohsiung Medical University Hospital, Kaohsiung Medical University.
Author contributions
Conceptualization: Ming-Yen Lin, Mei-Chuan Kuo, Shang-Jyh Hwang, Hung-Chun Chen, Yi-Wen Chiu.
Data curation: Ming-Yen Lin, Charles Tzu-Chi Lee.
Formal analysis: Ming-Yen Lin, Charles Tzu-Chi Lee.
Methodology: Charles Tzu-Chi Lee, Mei-Chuan Kuo, Yi-Wen Chiu.
Supervision: Shang-Jyh Hwang, Hung-Chun Chen.
Writing – original draft: Ming-Yen Lin, Yi-Wen Chiu.
Writing – review & editing: Ming-Yen Lin, Charles Tzu-Chi Lee, Mei-Chuan Kuo, Shang-Jyh Hwang, Hung-Chun Chen, Yi-Wen Chiu.
Footnotes
Abbreviations: ACR = albumin-creatinine ratio, CI = confidence interval, CKD = chronic kidney disease, eGFR = estimated glomerular filtration rate, ICD-9-CM = International Classification of Disease, Ninth Revision, Clinical Modification, iPTH = intact parathyroid hormone, NHI = National Health Insurance, OR = odds ratio, PCR = protein-creatinine ratio.
The authors have no funding and no conflicts of interest to disclose.
References
- [1].Jha V, Garcia-Garcia G, Iseki K, et al. Chronic kidney disease: global dimension and perspectives. Lancet 2013;382:260–72. [DOI] [PubMed] [Google Scholar]
- [2].Hsu CC, Hwang SJ, Wen CP, et al. High prevalence and low awareness of CKD in Taiwan: a study on the relationship between serum creatinine and awareness from a nationally representative survey. Am J Kidney Dis 2006;48:727–38. [DOI] [PubMed] [Google Scholar]
- [3].Sesso R, Belasco AG. Late diagnosis of chronic renal failure and mortality on maintenance dialysis. Nephrol Dial Transplant 1996;11:2417–20. [DOI] [PubMed] [Google Scholar]
- [4].Jungers P. Late referral: loss of chance for the patient, loss of money for society. Nephrol Dial Transplant 2002;17:371–5. [DOI] [PubMed] [Google Scholar]
- [5].Smart NA, Dieberg G, Ladhani M, et al. Early referral to specialist nephrology services for preventing the progression to end-stage kidney disease. Cochrane Database Syst Rev 2014;6:Cd007333. [DOI] [PubMed] [Google Scholar]
- [6].Smart NA, Titus TT. Outcomes of early versus late nephrology referral in chronic kidney disease: a systematic review. Am J Med 2011;124:1073.e2–80.e2. [DOI] [PubMed] [Google Scholar]
- [7].Minutolo R, Lapi F, Chiodini P, et al. Risk of ESRD and death in patients with CKD not referred to a nephrologist: a 7-year prospective study. Clin J Am Soc Nephrol 2014;9:1586–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Ratcliffe PJ, Phillips RE, Oliver DO. Late referral for maintenance dialysis. Brit Med J (Clin Res Ed) 1984;288:441–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Levin A. The need for optimal and coordinated management of CKD. Kidney Int Suppl 2005;68:S7–10. [DOI] [PubMed] [Google Scholar]
- [10].Wavamunno MD, Harris DC. The need for early nephrology referral. Kidney Int Suppl 2005;67:S128–32. [DOI] [PubMed] [Google Scholar]
- [11].Sprangers B, Evenepoel P, Vanrenterghem Y. Late referral of patients with chronic kidney disease: no time to waste. Mayo Clin Proc 2006;81:1487–94. [DOI] [PubMed] [Google Scholar]
- [12].Wauters JP, Lameire N, Davison A, et al. Why patients with progressing kidney disease are referred late to the nephrologist: on causes and proposals for improvement. Nephrol Dial Transplant 2005;20:490–6. [DOI] [PubMed] [Google Scholar]
- [13].James MT, Hemmelgarn BR, Tonelli M. Early recognition and prevention of chronic kidney disease. Lancet 2010;375:1296–309. [DOI] [PubMed] [Google Scholar]
- [14].El Nahas AM, Bello AK. Chronic kidney disease: the global challenge. Lancet 2005;365:331–40. [DOI] [PubMed] [Google Scholar]
- [15].Levey AS, de Jong PE, Coresh J, et al. The definition, classification, and prognosis of chronic kidney disease: a KDIGO Controversies Conference report. Kidney Int 2011;80:17–28. [DOI] [PubMed] [Google Scholar]
- [16].Hommel K, Madsen M, Kamper AL. The importance of early referral for the treatment of chronic kidney disease: a Danish nationwide cohort study. BMC Nephrol 2012;13:108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Boulware LE, Troll MU, Jaar BG, et al. Identification and referral of patients with progressive CKD: a national study. Am J Kidney Dis 2006;48:192–204. [DOI] [PubMed] [Google Scholar]
- [18].Cass A, Cunningham J, Snelling P, et al. Urban disadvantage and delayed nephrology referral in Australia. Health Place 2003;9:175–82. [DOI] [PubMed] [Google Scholar]
- [19].Jurkovitz CT, Elliott D, Li S, et al. Physician utilization, risk-factor control, and CKD progression among participants in the Kidney Early Evaluation Program (KEEP). Am J Kidney Dis 2012;59(3 suppl 2):S24–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Arora P, Obrador GT, Ruthazer R, et al. Prevalence, predictors, and consequences of late nephrology referral at a tertiary care center. J Am Soc Nephrol 1999;10:1281–6. [DOI] [PubMed] [Google Scholar]
- [21].Kinchen KS, Sadler J, Fink N, et al. The timing of specialist evaluation in chronic kidney disease and mortality. Ann Int Med 2002;137:479–86. [DOI] [PubMed] [Google Scholar]
- [22].Obialo CI, Ofili EO, Quarshie A, et al. Ultralate referral and presentation for renal replacement therapy: socioeconomic implications. Am J Kidney Dis 2005;46:881–6. [DOI] [PubMed] [Google Scholar]
- [23].Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol 1992;45:613–9. [DOI] [PubMed] [Google Scholar]
- [24].Faul F, Erdfelder E, Buchner A, et al. Statistical power analyses using G∗ Power 3.1: tests for correlation and regression analyses. Behavior Res Methods 2009;41:1149–60. [DOI] [PubMed] [Google Scholar]
- [25].Eknoyan G, Levin NW. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis 2002;39(2 suppl 1):S1-266. [PubMed] [Google Scholar]
- [26].Levey AS, Coresh J. Chronic kidney disease. Lancet 2012;379:165–80. [DOI] [PubMed] [Google Scholar]
- [27].American Diabetes Association. Standards of Medical Care in Diabetes 2015. Diabetes Care 2015;38(suppl 1):S5–87.25537709 [Google Scholar]
- [28].Hemmelgarn BR, Manns BJ, Lloyd A, et al. Relation between kidney function, proteinuria, and adverse outcomes. JAMA 2010;303:423–9. [DOI] [PubMed] [Google Scholar]
- [29].Rosansky S. Early dialysis initiation and renal function trajectory. J Int Med 2011;269:275–7. [DOI] [PubMed] [Google Scholar]
- [30].Hwang SJ, Tsai JC, Chen HC. Epidemiology, impact and preventive care of chronic kidney disease in Taiwan. Nephrology (Carlton) 2010;15(suppl 2):3–9. [DOI] [PubMed] [Google Scholar]


