Supplemental Digital Content is available in the text
Keywords: activity, biomarker, e-healthcare system, lupus nephritis
Abstract
The current methods of monitoring the activity of lupus nephritis (LN) may cause unnecessary hospital visits or delayed immunosuppressive therapy. We aimed to find a urinary biomarker that could be developed as a home-based test for monitoring the activity of LN.
Urine samples were collected immediately before a renal biopsy from patients of suspected active LN, and also from patients with inactive LN, systemic lupus erythematous without LN or healthy controls. Biomarker search was conducted on a cytokine antibody array and confirmation was done by quantitative evaluation with enzyme-linked immunosorbent assay. The Mann–Whiney test or Student t test was used to compare the levels of 9 cytokines between different groups. The sensitivity and specificity of each cytokine for diagnosis of LN was evaluated by receiver operating characteristic curve. A rapid test based on colloidal gold immunochromatography was then developed for bedside or home use. Furthermore, an experimental e-healthcare system was constructed for recording and sharing the results of the rapid test a cloud-assisted internet of things (IoT) consisting of a sensing device, an IoT device and a cloud server.
Adiponectin (Acrp30), soluble intercellular cell adhesion molecule-1 (sICAM-1), neural cell adhesion molecule 1 (NCAM-1), and CD26 were significantly higher in urine samples of active LN patients. sICAM-1 appeared more sensitive and specific among these candidates. When the cut-off value of sICAM-1 was set at 1.44 ng/mL, the sensitivity reached 98.33% with a specificity at 85.71%. The sICAM-1 strip test showed comparable sensitivity of 95% and a specificity of 83.3% for assessing the LN activity. Meanwhile, the e-healthcare system was able to conveniently digitize and share the sICAM-1 rapid test results.
sICAM-1 appeared to be an excellent biomarker for monitoring LN activity. The e-healthcare system with cloud-assisted IoT could assist the digitalization and sharing of the bedside or home-based sICAM-1 test results.
1. Introduction
Systemic lupus erythematous (SLE) is a systemic autoimmune disease that may be induced by multiple risk factors such as genetics, environment, sex hormones, and drugs.[1,2] Lupus nephritis (LN) is one of the most common complications of SLE. LN-associated end-stage kidney disease (ESKD) is a major cause of mortality in SLE patients. The poor outcomes from LN leading to ESKD are associated with delayed treatment of active LN,[3] as active LN would initiate fibrosis which further results in glomerulosclerosis, and interstitial and vascular fibrosis.[4]
To avoid or delay ESKD, a timely suppression of active LN with immunosuppressants is essential for preservation of the glomeruli and renal tubules. Nonetheless, excessive immunosuppression may cause fatal infections or tumors in the long term.[5] Therefore, a well-adjusted immunosuppression is the key to keep the balance. However, as active LN is usually asymptomatic and determination of LN activity is based on a complicated system of multiple parameters, it is impossible for patients to judge whether LN is active or inactive based on the current criteria. This could consequently lead to a prolonged over-immunosuppression or a delayed treatment of the active LN.
Clinical symptoms, blood examinations, urinary tests, and occasional kidney biopsies are used for monitoring the LN activities.[6] These tests need to be conducted at a hospital and the results interpreted by medical professionals. As a result, the majority of LN patients are monitored by regular or clinical symptom-initiated hospital visits. These traditional methods cause unnecessary hospital visits or delayed treatment of active LN. A home-based accurate urinary test conducted and interpreted by patients may improve the monitoring of LN activity, reduce unwanted hospital visits, help with a timely management of active LN, and decrease the occurrence of over-immunosuppression. These home-based urinary tests must be adequately sensitive and specific, easy to conduct and interpret, and affordable. Furthermore, the results should be recordable for tracing the changes of the LN activities.
We initiated an investigation to search for urinary biomarkers that could be developed into a home based simple test. We collected urine samples from SLE patients just before kidney biopsy. Consequently, all active LN patients were biopsy-proven. After a screening test and several rounds of verification, we found that soluble intercellular adhesion molecule-1 (sICAM-1) in urine was an excellent indicator for monitoring LN activity. We then developed a colloid gold-based strip test that could be conducted by patients at home for self-examination. Furthermore, taking advantage of the development of Internet of things (IoT) and cloud computing,[7] we successfully established an e-healthcare system for the rapid test with a cloud-assisted IoT in the hope to provide a digital and recordable home-based monitoring system to LN patients.
2. Methods
2.1. Patients
One hundred twenty-five active LN patients, 31 inactive LN patients, 36 SLE patients without LN, and 55 healthy controls were enrolled in this study. The clinical characteristics of all subjects are presented in table, Supplemental Content 1, which illustrates clinical characteristic of the subjects.
All patients were from West China Hospital of Sichuan University, Chengdu, China. SLE was diagnosed according to the 1997-updated American College of Rheumatology criteria for SLE.[8] LN was diagnosed and classified by SLE history plus renal biopsy results which were assessed according to International Society of Nephrology/Renal Pathology Society (ISN/RPS) guidelines. All active LN patients were diagnosed based on SLE history plus clinical evidence of active LN (persistent proteinuria >0.5 g per day or greater than 3+ by dipstick, and/or cellular casts including red blood cells, hemoglobin, granular, tubular, or mixed[6]) and confirmed by biopsy histology with renal activity index (AI) ≥ 1. Inactive LN patients were defined by LN history plus absence of clinical evidence of active LN.
The SLE disease activity index (SLEDAI) score were evaluated by 2 independent specialists[9] (interobserver agreement: kappa = 0.98, 95% confidence internal = 0.95–1.00). AI was described and scored by 2 independent pathologists according to the criterion published by Austin et al.[10] The appearance of cellular proliferation, fibrinoid necrosis, karyorrhexis, cellular crescents, hyaline thrombi, wire loops, leukocyte infiltration, or mononuclear-cell infiltration was considered as an active change. Each feature mentioned above was scored as 0, 1, 2, or 3 (absent, mild, moderate, and severe, respectively) (interobserver agreement: kappa = 0.84, 95% confidence internal = 0.80–0.90).
Controls were recruited from health check volunteers at West China Second University Hospital of Sichuan University, Chengdu, China as described in a previous study.[11] This study was approved by the ethics committees of West China Hospital and West China Second Hospital of Sichuan University. All subjects signed the informed consent forms.
2.2. Urine sample collection
Urine samples were collected immediately before the performance of renal biopsies in LN patients to keep a good comparability. A clean midstream urine was collected in a sterile 15 mL tube and centrifuged at 400 g for 5 minutes. The supernatant was aliquoted and then stored at −80°C until further use. Each urine sample was performed spot urine protein quantitative test (sulfosalicylic acid-sodium sulfate method) and urine creatinine test (enzymatic method) by Roche Cobas 6000 Analyzer (Roche Diagnostics, Mannheim, Germany). Ratio of spot urine protein to creatinine (SUP/Cr) was then calculated automatically.
2.3. Cytokine antibody array detection
Four urine samples (from 2 SLE patients with active LN and 2 SLE patients with inactive LN) were used to perform the semi-quantitative detection of 274 human proteins (C-Series human cytokine antibody array C4000, RayBiotech, Inc., Norcross, GA). The assay was carried out according to the manufacturer's instruction. Briefly, a set of 5 membranes was incubated with blocking buffer for 30 minutes, followed by incubation with 1.5 mL of a urine sample at 4°C overnight. After washing, membranes were incubated with a biotinylated antibody cocktail for 2 hours at room temperature (RT) with gentle shaking. After another round of washing, membranes were incubated with horseradish peroxidase (HRP)-streptavidin for 2 hours at RT with gentle shaking. Finally, signals on the membranes were detected by chemiluminescence using BIO-RAD ChemiDoc XRS system (Bio-Rad Laboratories, Inc., Ontario, CA).
2.4. Enzyme-linked immunosorbent assay (ELISA)
The urinary concentrations of adiponectin (Acrp30), human growth-regulated oncogene β (GRO β), human growth-regulated oncogene γ (GRO γ), soluble intercellular cell adhesion molecule-1 (sICAM-1), matrix metalloproteinase-8 (MMP-8), neural cell adhesion molecule 1 (NCAM-1), Trappin-2, and CD26 were detected with commercial ELISA kits (RayBiotech, Inc., and Cloud-Clone Corp, TX). The assay procedure was performed according to the manufacturer's instructions. Samples were diluted and added into wells, then incubated at RT. After washing, plates were incubated with biotinylated antibody and streptavidin-HRP before the color development. After stopping, reactions were read immediately at 450 nm using a full wavelength microplate reader (Tecan Trading AG, Männedorf, Switzerland).
2.5. Strip test for rapid detection
The urinary sICAM-1 rapid detection qualitative strip test was developed using a colloidal gold immunochromatography assay as described previously.[12] Briefly, 0.8 milligrams per milliliter (mg/mL) of goat antihuman ICAM-1 polyclonal antibody (R&D Systems, Minneapolis, MN) was jetted as a test (T) line and 1 mg/mL of goat antimouse IgG (ZSGB-BIO, Beijing, China) was jetted as a control (C) line. Glass fiber pads (Merck, Kenilworth, NJ) were dipped in gold-conjugated mouse antihuman ICAM-1 monoclonal antibody solution (R&D Systems). Eighty microliters of urine sample was added to the sample well of the test card. One or 2 lines could be observed in the control and test window within 10 to 15 minutes. Presence of both the red test and control lines was determined as positive for sICAM-1. Absence of test line and a presence of control line was determined as negative for sICAM-1.
2.6. Cloud-assisted IoT construction for strip test results processing
The system model for processing the strip test results with cloud-assisted IoT involves 5 entities: a patient, a sensing device, an IoT device, a cloud server, and healthcare service providers (HSPs). Sensing device for processing the rapid detection test results consists of 4 modules: sensor module senses the colors of control line and test line of the strip; microprogrammed control unit (MCU) is responsible for processing the raw sensing data and scheduling the tasks of the device; random access memory (RAM) temporarily stores the sensing data that is waiting to upload to the cloud server; IoT communication module transmits the sensing data to the cloud server through a 2G-IoT network supported by China Mobile Group Co., Ltd, ,Beijing,China.
The workflow of e-healthcare system is as follow: IoT device, which acts as the data collector, gathers the results from the sensor module. The sensing data are aggregated into a single file called personal health information (PHI). Then, the PHI is transmitted to a cloud server for storage via IoT. The HSPs act as the data user and can monitor PHI of the patient and provide healthcare services (e.g., timely diagnosis and reactions) by querying and retrieving sensing data of the patient from cloud.
2.7. Statistical analysis
The normality of distribution of continuous variables was evaluated by one-sample Kolmogorov–Smirnov test in SPSS software version 13.0 (SPSS, Inc., Chicago, IL).
The continuous variables were described as the mean value ± standard deviation (SD) (data with a normal distribution) or as the median (interquartile range) (data with a nonnormal distribution). Kruskal–Walis test was used to detect the difference among the four groups. If P-value of Kruskal–Walis test was lower than .05, Dunn post hoc test was used to determine the difference between 2 groups. The sensitivity and specificity of each cytokine for diagnosis of LN was evaluated by receiver operating characteristic (ROC) curve. Correlation coefficient between sICAM-1/Cr and SUP/Cr, sICAM-1/Cr and AI, and SUP/Cr and AI was calculated by Spearman correlation. P < .05 was considered as statistically significant. The interobserver agreements for SLEDAI score between 2 independent specialists, and for AI score between 2 independent pathologists were described by kappa statistic. All statistical analysis was performed using GraphPad Prism 5 software (San Diego, CA).
3. Results
3.1. Renal biopsy histopathology
According to the histologic classification, 7.2%, 4.0%, 18.4%, 60.0%, and 32.0% of the 125 active LN patients were diagnosed with class I, II, III, IV, and V, respectively. Among them, class V in combination with class III was noted in 8.0% of the LN patients, with class IV in 9.6% of the LN patients, and with class II in 1.6% of the LN patients.
The AI score was in the range of 1 to 12 with a median score at 5 in the 125-enrolled active LNs. The proliferation of mesangial cells was observed in almost all the patients and approximately 68.0% of the biopsies was scored 3. Fibrinoid necrosis or karyorrhexis was present in 11.0% of the patients with a maximum score of 2. Cellular crescents were seen in 45.0%, hyaline thrombi or wire loops in 8.8%, and mild leukocyte infiltration in 8.8%, and focal tubulointerstitial inflammation in 80.0% of the active LN patients.
3.2. Biomarker screening
Potential biomarkers in the urine samples for evaluating LN activity were screened using a cytokine array. As shown in figure, Supplemental Content 2, which illustrates result of human cytokine antibody array, multiple positive spots were observed in each of the 4 samples assessed. Intensity for MCP-1, Acrp30, GRO, sICAM-1, MMP-8, NCAM-1, Trappin-2, and CD26 was much stronger in the active LN samples than in inactive LN samples. This result indicates that increased concentrations of these cytokines in urine were associated with the active LN, and would likely be useful as biomarkers for monitoring LN activity in patients. Therefore, these cytokines were further examined by a quantitative detection.
3.3. Biomarker verification
We initially analyzed the levels of 8 cytokines in a small panel of urinary samples. GRO β/Cr, GRO γ/Cr, and Trappin-2/Cr levels displayed no significant differences between the active and inactive LN groups or the group of SLE without LN (data not shown). Therefore, these cytokines were excluded from subsequent evaluation.
As shown in table (Supplemental Content 3, which illustrates levels of urinary Acrp30/Cr, sICAM-1/Cr, NCAM-1/Cr, CD26/Cr, and MMP-8/Cr in 4 groups) and Fig. 1, urinary Acrp30/Cr, sICAM-1/Cr, NCAM-1/Cr, and CD26/Cr levels in the active LN group were significantly higher than in other 3 groups (P < .05). MMP-8/Cr levels were significantly higher in the active LN group compared to inactive LN group and the healthy controls (P < .05), but not in the SLE without LN group (P > .05).
Figure 1.
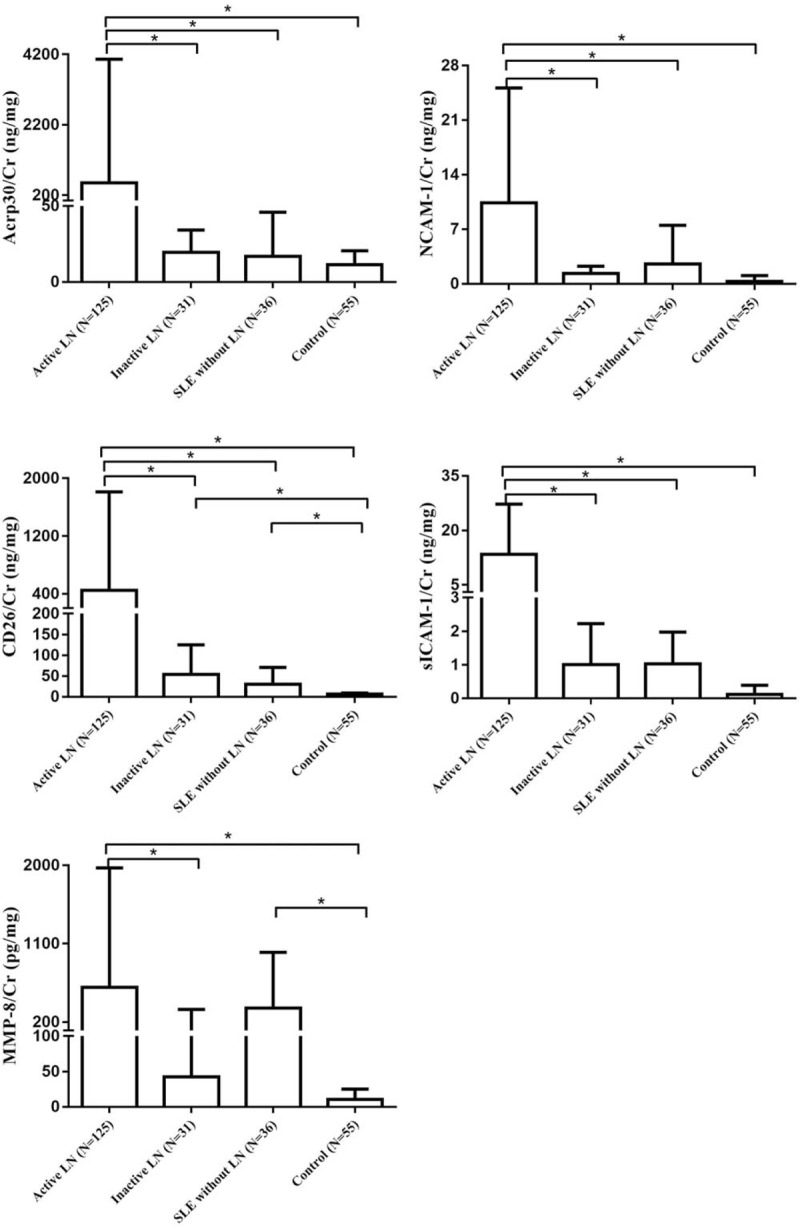
Urinary concentration/Cr value of the 5 cytokines. ∗P < .05.
3.4. Biomarker confirmation
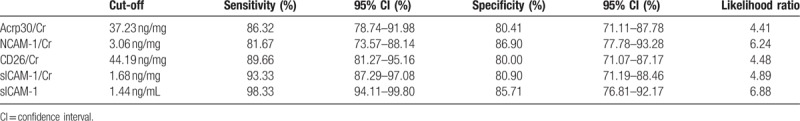
According to the quantitative results, we found that Acrp30/Cr, sICAM-1/Cr, NCAM-1/Cr, and CD26/Cr might be used for monitoring LN activity in patients. Consequently, the diagnostic values for these markers were evaluated by ROC curve analysis (see Fig. 2). The cut-off values were chosen by considering the maximum sensitivity and acceptable specificity for diagnosing LN activity in patients (see Table 1). The sensitivity and specificity of the 4 analytes reached above 80%, especially for sICAM-1/Cr. When the cut-off value was 1.68 ng/mg, the sensitivity of sICAM-1/Cr reached 93.33%. Thus, among the cytokines tested in our study, sICAM-1/Cr may serve as a potential urine biomarker for detecting LN activity.
Figure 2.
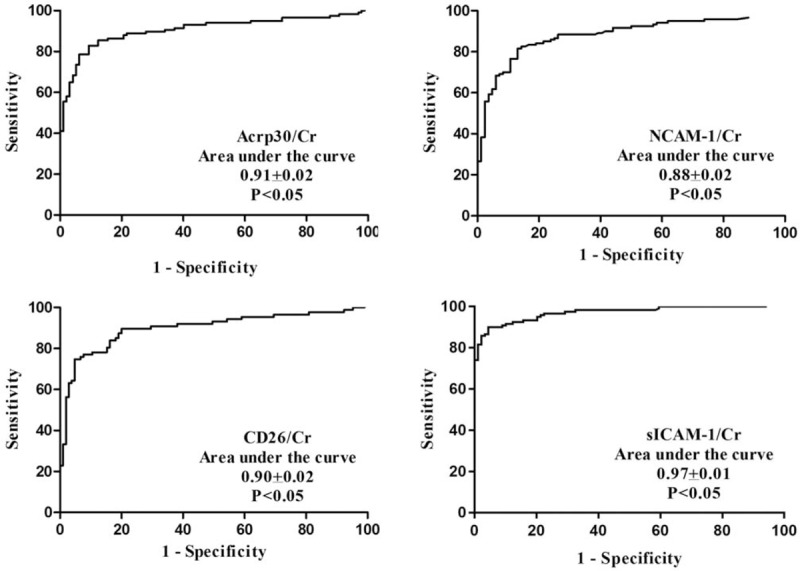
Diagnostic value of concentration/Cr of the 4 cytokines.
Table 1.
Diagnostic value of Acrp30/Cr, NCAM-1/Cr, CD26/Cr, sICAM-1/Cr, and sICAM-1 for active LN.
3.5. Correlation analysis
Correlation between the sICAM-1/Cr and SUP/Cr values or biopsy pathology AI in active LN patients was determined. A linear correlation was observed between sICAM-1/Cr and SUP/Cr (r2 = 0.27, P < .01). However, a very weak correlation was noted between sICAM-1/Cr and AI (r2 = 0.06, P < .05) and between SUP/Cr and AI (r2 = 0.08, P < .05).
3.6. Diagnostic value of sICAM-1 for monitoring LN activity
As the levels of sICAM-1 and Cr in urine could not be quantified at home by the patients themselves, we therefore examined if the urine sICAM-1 concentration by itself would be enough for monitoring LN activity. As shown in Fig. 3, the urine sICAM-1 concentration was 15.75 ± 20.78 ng/mL, 0.48 (0.05–1.18) ng/mL, 0.88 ± 0.92 ng/mL, and 0.14 (0.05–0.66) ng/mL in active LN samples, inactive LN samples, SLE without LN samples, and healthy controls, respectively. The levels in the active LN group were significantly higher than in the patients with inactive LN, SLE without LN and in healthy controls (P < .05). The area under the curve for sICAM-1 was 0.96 ± 0.01. When the cut-off value was set at 1.44 ng/mL, the sensitivity reached 98.33% and the specificity was as high as 85.71% (see Table 1) for revealing active LN.
Figure 3.
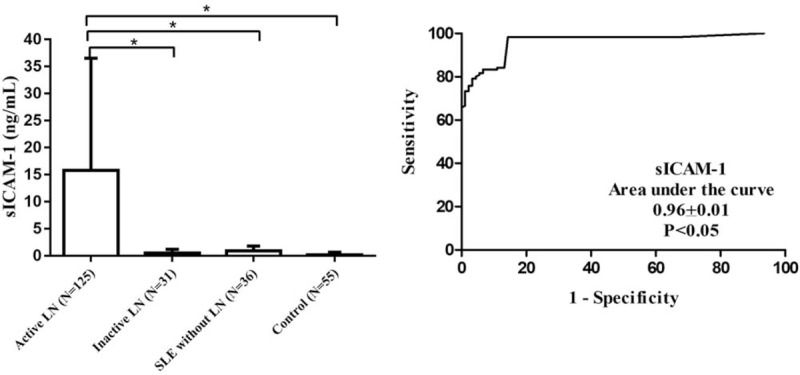
Concentration and diagnostic value of urinary sICAM-1.
3.7. sICAM-1 strip test for monitoring LN activity
A colloid gold-based sICAM-1 strip test was developed which allowed detection within 10 to 15 minutes after dropping the urine samples onto the strip. However, the minimal detection threshold was approximately 2 ng/mL, and therefore, this test may not be sensitive enough for some of the active LNs below this value.
Fifty urine samples, including 20 samples from active LN patients, were assessed with the strip test. The results showed a sensitivity of 95% and a specificity of 83.3% for revealing LN activity in patients. As expected, the signals of the test (T) line increased with increasing concentration of sICAM-1 (see Fig. 4). This indicates that the density of test line roughly reveals the sICAM-1 concentration in urine samples. Furthermore, as a positive T line suggests the sICAM-1 concentration to be above 2 ng/mL, a positive T line therefore indicates an active LN.
Figure 4.
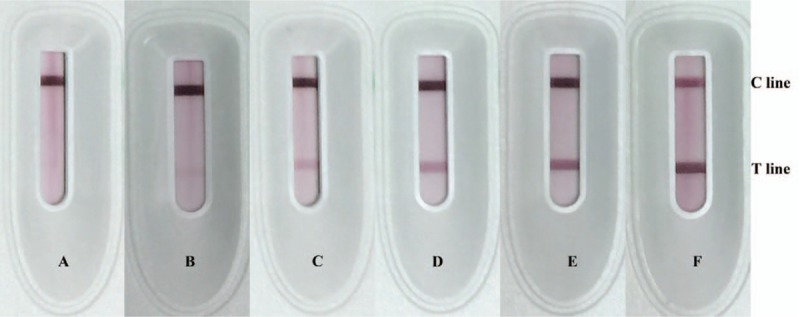
sICAM-1 strip tests for urine samples. Color of test lines became darker with increasing level of urinary sICAM-1. A = 0.98 ng/mL, B = 1.86 ng/mL, C = 4.11 ng/mL, D = 14.71 ng/mL, E = 30.39 ng/mL, F = 82.85 ng/mL.
3.8. e-Healthcare system for reading and storage of test results
An experimental e-healthcare system was prepared for reading and storing the test results (see Video, Supplemental Content 4, which demonstrates the workflow of e-healthcare system). Signal of both test and control lines was presented in numerical grade. The blank reading was Grade 0. The background reading of test line was Grade 1. Grade 2 to Grade 6 indicated the visible line in strip, and a higher numerical grade meant a stronger signal of the lines on the strip. Twenty urine samples with different sICAM-1 levels were examined with the strip test and e-healthcare system. The T line with average optical density (AOD) value of 50 to 80 was shown as Grade 2 or Grade 3, AOD value of 81 to 140 as Grade 3 or Grade 4, and Grade 5 or Grade 6 was shown when the AOD value exceeded 150.
4. Discussion and conclusions
Recurrent renal inflammation results in renal fibrosis and high mortality in patients with LN if the active status is not well controlled in a timely manner.[13,14] Once SLE or LN is diagnosed, regular urine testing is advised by the doctors. Random urinary protein/Cr ratio and/or 24-hour urinary protein quantitation have been frequently used in clinics for monitoring LN activity. However, these tests require special equipment and patients have to be either present in the hospitals or ship samples to hospitals for these tests. Furthermore, the remote tests do not generate results in a real time fashion.
There have been many studies on biomarkers for identifying active LN. Elevated anti-C1q autoantibodies, circulating cell-free DNA and renalase, etc. in serum have been reported to correlate with LN activities.[15–17] Elevated urinary cell-free microRNA, neutrophil gelatinase-associated lipocalin, transforming growth factor-β1, and sCD25 and others are present in active LN.[18–20] However, none of these are adequately sensitive and specific to be utilized as clinically useful tests.
We assumed that the expression of the urinary cytokine spectrum would be altered in kidney in an active inflammatory state.[21] Indeed, we observed elevation in multiple inflammatory cytokines in urine of active LN patients. MCP-1 was previously reported as a biomarker for monitoring LN activity.[22–24] However, urinary MCP-1 levels in active LN are at picogram levels, and quantification of this cytokine needs special equipment. This precluded us from using MCP-1 as a bedside or home based rapid test. Further quantitative and diagnostic studies demonstrated that the urinary sICAM-1 levels are in nanogram quantities, allowing the development of a simple and rapid colloid gold-based strip tests, so we developed a strip test, and the diagnostic value of the strip test are indeed acceptable for clinical use. Especially, the test generates results in minutes, and the interpretation of the results is straight forward for both medical professionals and patients. When we examined the intensity of the T lines on the strip test, we found that the T line intensity was in direct proportion to the sICAM-1 concentration with a reader that digitized and recorded the signals. Therefore, it is possible to tell the differences of T line intensities for longitudinal comparison, and thereby to identify the LN activities. Based on these features, the sICAM-1 rapid test has the potential for several clinical usages. First, this test can be used as an aid for diagnosis of active LN at hospitals when a quick decision needs to be made either for the judgment of the LN status or for the adjustment of the immunosuppressive therapy. Second, LN patients can use this affordable test to monitor the LN status periodically for the purpose of early detection of LN disease flare at home, which would offer an advantage over urine dipsticks, as urine dipsticks had a high false positive rate with 59.5% for protein as we described previously.[25] Third, patients can monitor the therapeutic effect of the immunosuppression for an active LN to avoid inadequate or over immunosuppression. However, these potential tests need to be further confirmed in a clinical setting through a formal clinical trial.
In summary, the present study reveals that urinary Acrp30, NCAM-1, CD26, and especially sICAM-1 may be potentially useful biomarkers for the determination of LN activities. sICAM-1 appears to be a clinically useful and could be developed into a rapid test for bedside and home use for the management of LN patients.
Author contributions
Conceptualization: Yanyun Wang, Ye Tao, Yi Liu, Lin Zhang, Huaizhong Hu.
Data curation: Yanyun Wang, Bin Zhou.
Formal analysis: Yanyun Wang, Linbo Gao.
Funding acquisition: Yanyun Wang, Bin Zhou, Lin Zhang.
Investigation: Ye Tao, Yi Liu, Yi Zhao.
Methodology: Yanyun Wang, Yi Zhao, Chao Song, Tao Wang.
Project administration: Lin Zhang, Huaizhong Hu.
Resources: Ye Tao, Yi Liu.
Software: Yanyun Wang, Chao Song.
Supervision: Lin Zhang, Huaizhong Hu.
Writing – original draft: Yanyun Wang.
Writing – review & editing: Ye Tao, Yi Liu, Yi Zhao, Chao Song, Bin Zhou, Tao Wang, Linbo Gao, Lin Zhang, Huaizhong Hu.
Supplementary Material
Footnotes
Abbreviations: Acrp30 = adiponectin, AI = activity index, AOD = average optical density, AUC = areas under the curve, Cr = creatinine, ESKD = end-stage kidney disease, GRO = growth-regulated oncogene, HRP = horseradish peroxidase, HSPs = healthcare service providers, IoT = Internet of things, ISN/RPS = International Society of Nephrology/Renal Pathology Society, LN = lupus nephritis, MCU = microprogrammed control unit, MMP-8 = matrix metalloproteinase-8, NCAM-1 = neural cell adhesion molecule 1, PHI = personal health information, RAM = random access memory, ROC = receiver operating characteristic, RT = room temperature, SD = standard deviation, sICAM-1 = soluble intercellular adhesion molecule-1, SLE = systemic lupus erythematous, SLEDAI = SLE disease activity index, SUP = spot urine protein.
Funding: This work was supported by a grant from the National Natural Science Foundation of China (No. 81501261); the Sichuan Provincial Science & Technology Project (No. 2016SZ0013); and the Office of Science & Technology of Chengdu (No. 2015-HM01-00431-SF).
YW, YT, and YL contributed equally to this work.
The authors have no conflicts of interest to disclose.
Supplemental Digital Content is available for this article.
References
- [1].Rupasree Y, Naushad SM, Rajasekhar L, et al. Association of estrogen receptor 1 (ESR1) haplotypes with risk for systemic lupus erythematosus among South Indians. Indian J Exp Biol 2015;53:714–8. [PubMed] [Google Scholar]
- [2].Molina-Ruiz AM, Lasanta B, Barcia A, et al. Drug-induced systemic lupus erythematosus in a child after 3 years of treatment with carbamazepine. Australas J Dermatol 2017;58:e20–2. [DOI] [PubMed] [Google Scholar]
- [3].Maroz N, Segal MS. Lupus nephritis and end-stage kidney disease. Am J Med Sci 2013;346:319–23. [DOI] [PubMed] [Google Scholar]
- [4].Liu Z, Xue L, Liu Z, et al. Tumor necrosis factor-like weak inducer of apoptosis accelerates the progression of renal fibrosis in lupus nephritis by activating SMAD and p38 MAPK in TGF-beta1 signaling pathway. Mediators Inflamm 2016;2016: 8986451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Orlicka K, Barnes E, Culver EL. Prevention of infection caused by immunosuppressive drugs in gastroenterology. Ther Adv Chronic Dis 2013;4:167–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Hahn BH, McMahon MA, Wilkinson A, et al. American College of Rheumatology guidelines for screening, treatment, and management of lupus nephritis. Arthritis Care Res (Hoboken) 2012;64:797–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].IEEE Xplore: Available at: http://ieeexplore.ieee.org (2017). Accessed 05 Oct 2017. [Google Scholar]
- [8].Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum 1997;40:1725–34. [DOI] [PubMed] [Google Scholar]
- [9].Bombardier C, Gladman DD, Urowitz MB, et al. Derivation of the SLEDAI. A disease activity index for lupus patients. The Committee on Prognosis Studies in SLE. Arthritis Rheum 1992;35:630–40. [DOI] [PubMed] [Google Scholar]
- [10].Austin HA, III, Muenz LR, Joyce KM, et al. Diffuse proliferative lupus nephritis: identification of specific pathologic features affecting renal outcome. Kidney Int 1984;25:689–95. [DOI] [PubMed] [Google Scholar]
- [11].Wang Y, Zhou B, Zhao Y, et al. Association of plasma IL-32 levels and gene polymorphisms with systemic lupus erythematosus in Chinese Han Population. Dis Markers 2016;2016: 2460206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Vanithamani S, Shanmughapriya S, Narayanan R, et al. Lipopolysaccharide specific immunochromatography based lateral flow assay for serogroup specific diagnosis of leptospirosis in India. PLoS ONE 2015;10:e0137130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Meng XM, Nikolic-Paterson DJ, Lan HY. Inflammatory processes in renal fibrosis. Nat Rev Nephrol 2014;10:493–503. [DOI] [PubMed] [Google Scholar]
- [14].Yap DY, Tang CS, Ma MK, et al. Survival analysis and causes of mortality in patients with lupus nephritis. Nephrol Dial Transplant 2012;27:3248–54. [DOI] [PubMed] [Google Scholar]
- [15].Chi S, Yu Y, Shi J, et al. Antibodies against C1q are a valuable serological marker for identification of systemic lupus erythematosus patients with active lupus nephritis. Dis Markers 2015;2015: 450351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Zhang S, Lu X, Shu X, et al. Elevated plasma cfDNA may be associated with active lupus nephritis and partially attributed to abnormal regulation of neutrophil extracellular traps (NETs) in patients with systemic lupus erythematosus. Intern Med 2014;53:2763–71. [DOI] [PubMed] [Google Scholar]
- [17].Qi C, Wang L, Zhang M, et al. Serum renalase levels correlate with disease activity in lupus nephritis. PLoS ONE 2015;10:e0139627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Gupta R, Yadav A, Misra R, et al. Urinary sCD25 as a biomarker of lupus nephritis disease activity. Lupus 2015;24:273–9. [DOI] [PubMed] [Google Scholar]
- [19].Susianti H, Wijaya JW, Rastini A, et al. urinary neutrophil gelatinase-associated lipocalin to monitor lupus nephritis disease activity. Biomark Insights 2015;10:81–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Abulaban KM, Fall N, Nunna R, et al. Relationship of cell-free urine MicroRNA with lupus nephritis in children. Pediatr Rheumatol Online J 2016;14:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Navarro JF, Milena FJ, Mora C, et al. Renal pro-inflammatory cytokine gene expression in diabetic nephropathy: effect of angiotensin-converting enzyme inhibition and pentoxifylline administration. Am J Nephrol 2006;26:562–70. [DOI] [PubMed] [Google Scholar]
- [22].Alzawawy A, Zohary M, Ablordiny M, et al. Estimation of monocyte-chemoattractantprotein-1 (Mcp-1) level in patients with lupus nephritis. Int J Rheum Dis 2009;12:311–8. [DOI] [PubMed] [Google Scholar]
- [23].Abujam B, Cheekatla S, Aggarwal A. Urinary CXCL-10/IP-10 and MCP-1 as markers to assess activity of lupus nephritis. Lupus 2013;22:614–23. [DOI] [PubMed] [Google Scholar]
- [24].Marks SD, Shah V, Pilkington C, et al. Urinary monocyte chemoattractant protein-1 correlates with disease activity in lupus nephritis. Pediatr Nephrol 2010;25:2283–8. [DOI] [PubMed] [Google Scholar]
- [25].Wang T, Zhou R, Gao LB, et al. Clinical assessment of the specificity of an adipsin rapid test for the diagnosis of preeclampsia. Hypertens Pregnancy 2016;35:420–5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


