Abstract
Pancreatic lipidosis (nonalcoholic fatty pancreas disease, NAFPD) causes insulin resistance and dysfunction of pancreatic β-cells, with the risk of type 2 diabetes mellitus (T2DM). However, the prevalence and pathogenic factors associated with NAFPD are not clear. The aim of the study was to explore the prevalence of NAFPD in a Chinese adult population, and investigate factors associated with NAFPD aggravation.
This was a cross-sectional study; 4419 subjects were enrolled for NAFPD screening and were divided into NAFPD (n = 488) and without NAFPD (n = 3930) groups. The sex, age, related concomitant diseases, general physical parameters, and serum glucose and lipid metabolism were compared between the 2 groups.
The overall NAFPD prevalence was 11.05%, but increased with age. In those <55 years NAFPD prevalence was lower in females than males (P < .05), but prevalence was similar >55 years. Nonalcoholic fatty liver disease (NAFLD), T2DM, homeostasis model assessment-insulin resistance index, total cholesterol, triglyceride, lipoprotein, adiponectin, and glucagon-like peptide 1 (GLP-1) were the independent risk factors for NAFPD (P < .05). Analaysis of mild NAFPD (MN) and severe NAFPD (SN) subgroups, according to the extent of fat deposition, suggested that NAFLD, triglyceride, lipoprotein, and adiponectin were independent risk factors for NAFPD aggravation (P < .05).
The NAFPD prevalence was about 11% in Chinese adults. Its development and progression was related to NAFLD, T2DM, insulin resistance, dyslipidemia, and GLP-1 levels. Severe NAFPD was associated with NAFLD and dyslipidemia.
Keywords: dyslipidemia, nonalcoholic fatty pancreas disease, type 2 diabetes mellitus
1. Introduction
Globally, diabetes mellitus (DM) is the third most common noncommunicable disease, after cardiovascular diseases and cancer. The World Health Organization (WHO) showed that there were 371 million DM patients in the world in 2012.[1] Changes in dietary patterns and lifestyles have made diabetes one of the major public health problems in China.[2] Pancreatic β-cell dysfunction and insulin resistance are 2 key links in the pathogenesis of diabetes, and their occurrence is closely related to the ectopic deposition of pancreatic lipids.[3–5] Pancreatic fat ectopic deposition disease, also known as nonalcoholic fatty pancreas disease (NAFPD), is a disease characterized by pancreatic fat infiltration or pancreatic islet cell steatosis. NAFPD is an independent risk factor for the pathogenesis of type 2 diabetes mellitus (T2DM) and impaired glucose regulation (IGR). But its etiology has not yet been determined. Animal and clinical studies have shown that a high-fat diet could cause NAFPD, resulting in impaired β-cell function, decreased insulin secretion, insulin resistance, and abnormal adipokine secretion.[3,6–8] Also, patients with NAFPD have an increased risk of developing T2DM.[9–11]
Studies concerning NAFPD, so far, have investigated different stages of glucose metabolism (prediabetes and diabetes) to find associations between the diseases.[9–11] But the severity of pancreatic fat deposition had not been studied. Therefore, this study explored the prevalence of NAFPD, and factors related to NAFPD and its severity in a Chinese population. We measured general characteristics, diseases, indicators of glucose and lipid metabolism, adipokines, inflammatory factors, and glucagon-like peptide-1 (GLP-1) levels in the population and measured the level of pancreatic fat deposition, attempting to find any relationship between them.
2. Methods
2.1. Patients
This study was a cross-sectional study from January 2015 to October 2017 in Ningbo Chinese Medical Hospital Affiliated to Zhejiang Chinese Medical University (Ningbo Chinese Medical Hospital). This study followed all applicable ethical rules and was approved by the Ethics Committee of Ningbo Chinese Medical Hospital. All subjects signed the informed consent form.
The inclusion criterium for the study was any adult (>18 years old) who attended the Ningbo Chinese Medical Hospital Affiliated to Zhejiang Chinese Medical University for medical examination or an outpatient visit who had responded to an advertisement from January 2015 to October 2017.
The exclusion criteria were as follows: pancreatic exocrine diseases, pancreatic lesions, and other pancreatic diseases; heavy drinkers, defined as drinking >280 g alcohol/wk for men and >140 g/wk for women;[10] patients using lipid-lowering drugs, such as statins and fibrates,[12–14] and patients using antidiabetic drugs that affect serum adipocytokine or endogenous GLP-1 levels, such as metformin, thiazolidinediones, GLP-1 receptor agonists and dipeptidyl peptidase 4 (DPP4) inhibitors; patients with various conditions such as infection, trauma, serious bleeding, severe brain, and kidney complications; and patients with a variety of acute complications of diabetes such as diabetic ketosis, hypertonic diabetic coma, other diabetic comorbidities (such as cancer, disease of immune system, or hematologic system). (5) Pregnant or lactating, menstrual women.
2.2. Diagnostic criteria
All the ultrasound data were jointly collected by 2 qualified and experienced ultrasound physicians. A high-resolution ultrasound (LOGIQ7, GE Healthcare, USA) and 3.5 MHz linear sensor were used.
2.3. NAFPD
The criteria for diagnosis of NAFPD were based on the literature:[15] the parenchymal echogenicity of the pancreas was compared with the spleen as a control. NAFPD was diagnosed if the pancreas showed a plump morphorlogy, with increased volume and fuzzy edges. The echogenicity for the head, body, and tail of the pancreas were similar and stronger than that of the spleen. The area of enhanced echogenicity was >80%, and pancreatic fibrosis was excluded. The 2 grades of NAFPD were as follows: mild—parenchymal echogenicity of the pancreas was moderately to extremely enhanced; the margin of the pancreatic body was moderately fuzzy; and the border of the pancreatic ducts were moderately fuzzy. Severe—parenchymal echogenicity of the pancreas was extremely enhanced; the margin of the pancreatic body was extremely fuzzy; and the border of the pancreatic ducts were extremely fuzzy.
2.4. NAFLD
The diagnosis of NAFLD was according to the 2007 Asia-Pacific guidelines on NAFLD:[16] Two or more of the following abnormal findings were diagnosed as fatty liver: the local echogenicity of the liver was increased and the distant echogenicity was decreased. The parenchymal echogenicity of the liver was dense, and stronger than that of the kidney. Intrahepatic vessels and biliary structures were unclear.
2.5. T2DM
The American Diabetes Association standards (2010) were adopted for diagnosis of T2DM:[17] fasting plasma glucose (FPG) ≥7.0 mmol/L, plasma glucose of 2 hours post glucose-load (2 hPG) ≥11.1 mmol/L.
2.6. Hypertension
Hypertension was diagnosed according to the 2010 Chinese guidelines for the management of hypertension,[18] if the systolic pressure was ≥140 mm Hg and (or) diastolic pressure was ≥90 mm Hg at the time of visit without taking any antihypertensive drugs.
2.7. Overweight and obestiy
The 2009 International Diabetes Federation (IDF) standard was used for diagnosis of obesity [19] and central obesity.[20] The body mass index (BMI) was calculated as body weight (kg) × height (m)−219 and overweight was defined as 25 kg/m2≤BMI <28 kg/m2, obesity was defined as BMI ≥28 kg/m2. Central obesity was defined as Chinese men with a waist ≥85 cm, or women with a waist ≥80 cm.
2.8. Clinical data collection
The patients’ sex and age were recorded. Their height and weight were measured at fasting state by 2 independent specialists in the physical examination center and the mean value was adopted. Regular physical exercise was recorded if undertaken ≥3 times/wk. Current smoking was defined as at least 1 pack per month, for half a year.
2.9. Laboratory data collection
Four milliliters of fasting blood were collected after 12 hours of fasting from the enrolled subjects, and the serum was obtained. The total cholesterol (TC), triglyceride (TG), high-density lipoprotein (HDL), low-density lipoprotein (LDL), and fasting blood glucose (FBG) were determined by an automatic biochemical analyzer (AU5400, Olympus, USA). An enzyme-linked immunosorbent assay (ELISA) was used to measure the fasting serum C-peptide (DRG, EIA1293), serum leptin (LP, R&D MAB398, BAM398), adiponectin (APN, R&D MAB10651, BAM1065), tumor necrosis factor-α (TNF-α, R&D MAB610, BAF210), and fasting active GLP-1 level (abcam ab23472, ab48291).
Two milliliters of blood was taken and the glycosylated hemoglobin (HbA1c) [21] was determined by affinity chromatography microcolumn assay.
The homeostasis model assessment-insulin residence (HOMA-IR) index [22] was calculated according to the value of FBG and fasting C peptide levels using the HOMA2 calculator 2.2.3 version (provided by the Diabetes Center at Oxford University, UK).
2.10. Statistical analysis
Statistical analysis was performed using SPSS 23.0 (IBM Corporation, Armonk, NY). Normality of distribution was evaluated using the Kolmogorov–Smirnov test. Variables with a normal distribution were compared using analysis of variance (ANOVA) and post hoc testing, and values are presented as means ± standard deviation. For variables with an abnormal distribution, the Kruskal–Wallis test was used for comparisons, and values are presented as medians (interquartile range). Categorical variables are presented as frequencies and were analyzed using the χ2 or Fisher exact test, as appropriate. Logistic regression analysis was used for univariate analysis. The variants of α = 0.05 were entered into the equation, and the variants of α = 0.1 were eliminated. Two-sided P values ≤.05 were considered to be statistically significant.
3. Results
3.1. Prevalence of NAFPD
There were 4418 subjects enrolled on the study. A total of 488 NAFPD cases were diagnosed, and 3930 subjects were without NAFPD. This was a detection rate of 11.05%. After excluding various patients from the analysis, 473 subjects were in the NAFPD group and 3895 in the without NAFPD group as shown in the flow chart (Fig. 1).
Figure 1.
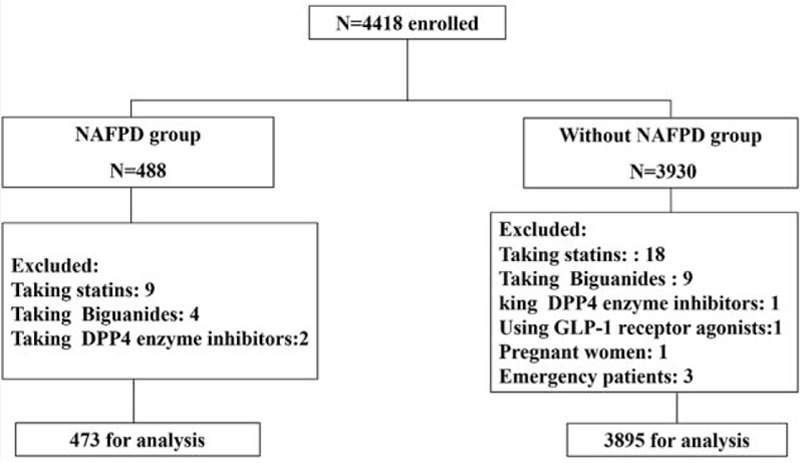
Flow chart of the enrolled patients.
Overall, 2287 subjects were male, and 2131 subjects were female, with a male/female ratio of 1.07:1. Among them, 1433 cases were <45 years, and 1492 cases were 45 to 54 years old; the number of males in these 2 age groups was higher than females (P < .05), 1493 cases were >54 years old, there was no significant difference between males and females in this age group (P >.05), as shown in Table 1. The 488 subjects with NAFPD included 277 males and 211 females. The prevalence in males was higher than that of females (P < .05). When analyzed as different age groups, we found that under 55 years, the prevalence of NAFPD was higher in males than females, but the prevalence in females increased after 55 years, so above 55 years there was no significant difference in prevalence between the 2 sexes.
Table 1.
Comparison of NAFPD prevalence in males and female of different ages and with different concomitant diseases.
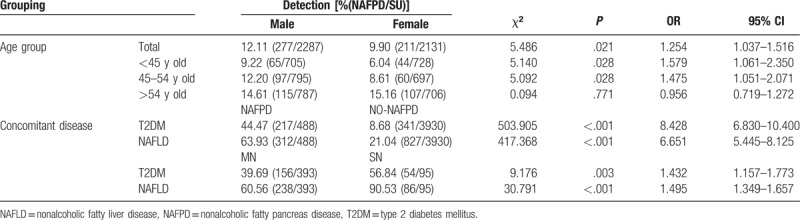
The age of the subjects ranged from 35 to 66 years, and the average age of the NAFPD patients was 49.51 ± 9.15 years, the average age of without NAFPD subjects was 47.21 ± 6.93 years, the average age of the NAFPD patients was higher than the without NAFPD subjects, and the difference was statistically significant (t = −5.300, P < .001). The prevalence of NAFPD was 7.61% (109/1433) in the group <45 years, 10.19% (152/1492) in the group 45 to 54 years old, and 15.20% (227/1493) in the group >54 years, indicating that the prevalence of NAFPD increased with increasing age of the subjects, as shown in Figure 2A
Figure 2.
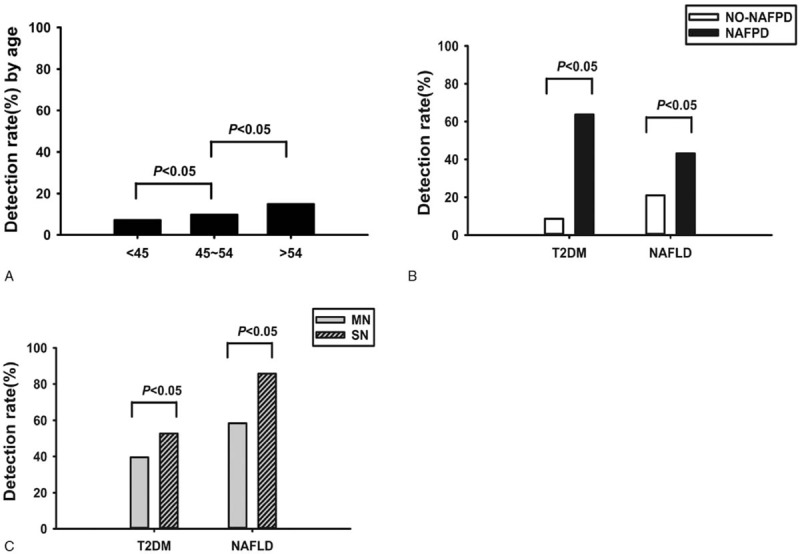
Comparison of NAFPD diagnoses between different age groups (A); the prevalence of T2DM and NAFLD in the 2 different groups (B); and in the mild NAFLD (MN) and severe NAFLD (SN) subgroups (C).
The rates of T2DM and NAFLD were both higher in NAFPD patients than in without NAFPD subjects, and the differences were statistically significant (P < .05). There were 393 cases (80.8%) with mild pancreatic fat deposits and 95 cases (19.2%) with severe pancreatic fat deposits in NAFPD patients, which were subdivided into mild NAFPD group (MN) and severe NAFPD group (SN). The rates of T2DM and NAFLD in the MN group were lower than in the SN group, and the differences was statistically significant (P < .05), as shown in Table 1 and Figure 2B–C.
3.2. Analysis of general body index, serum glucose metabolism index, and lipid metabolism index in NAFPD patients
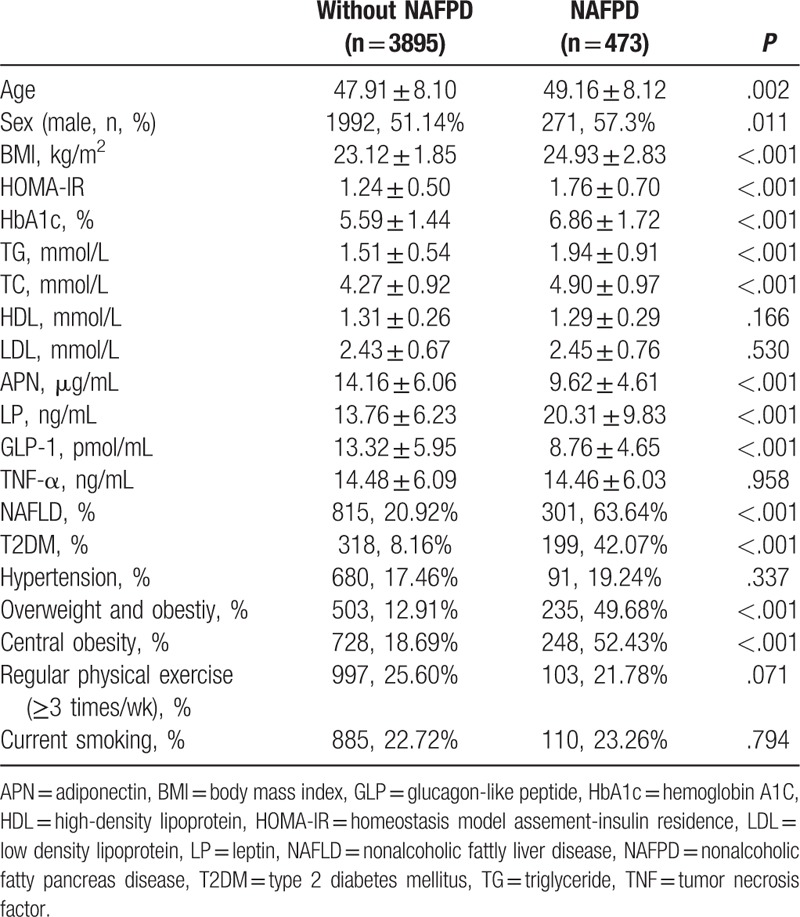
Fifteen subjects were excluded from the 488 cases diagnosed with NAFPD for factors that may have influenced the serum measurements, including 9 subjects using statins, 4 subjects using biguanides, and 2 subjects using DPP4 inhibitors, so a total of 473 NAFPD patients participated in the statistical analysis. Among the without NAFPD group, 33 subjects were excluded (including 18 subjects using statins, 9 subjects using biguanides, 1 subject using DPP4 inhibitor, 1 subject using GLP-1 receptor agonist, 1 pregnant woman, and 3 emergency patients), so a total of 3895 without NAFPD subjects participated in the statistical analysis. There were 271 males and 202 females in the NAFPD group, and 1992 males and 1905 females in the without NAFPD group.
Univariate analysis found that the age, BMI, HOMA-IR, levels of TC, TG, and LP in the NAFPD group were higher than in the without NAFPD group, whereas the levels of APN and GLP-1 were lower than those in the without NAFPD group, and the difference was statistically significant (P < .05). Age and levels of HDL, LDL, and TNF-α were not significantly different from those in the without NAFPD group (P > .05), as shown in Table 2.
Table 2.
Comparison of general conditions and laboratory indexes between the 2 groups.
3.3. Factors related to the pathogenesis of NAFPD
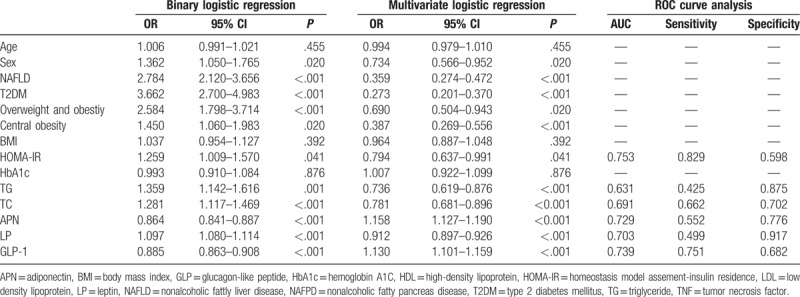
With NAFPD as the dependent variable (NAFPD = 1, without NAFPD = 0), 12 indicators had statistically significant differences between the NAFPD group and without NAFPD group including age, sex, and others as independent variables. After assignment of count data: sex (male = 1, female = 0), occurrence of NAFLD (yes = 1, no = 0), occurrence of T2DM (yes = 1, no = 0), both binary logistic regression analysis and multivariate logistic regression analysis were carried out. The results showed that sex, concomitant NAFLD, concomitant T2DM, HOMA-IR, TC, TG, APN, LP, and GLP-1 entered the regression equation, sex (male), occurrence of NAFLD, occurrence of T2DM, HOMA-IR, TC, TG, and LP were positively correlated with NAFPD (P < .05, OR >1), and APN was negatively correlated with NAFPD (P < .05, OR <1), as shown in Table 3. This indicated that males with NAFLD or T2DM, elevated HOMA-IR index, elevated serum levels of TC, TG, LP, and decreased serum levels of APN and GLP-1 were independent risk factors for the pathogenesis of NAFPD.
Table 3.
Logistic regression analysis for risk factors of NAFPD.
3.4. Reciever-operating characteristic (ROC) curve analysis of APN, LP, and TG in NAFPD patients
The ROC curves of APN, LP, and TG levels in NAFPD and without NAFPD groups are shown in Figure 3, the area under curve (AUC), sensitivity, and specificity are shown in Table 3. The AUC of HOMA-IR, APN, LP, and GLP-1 were all >0.7 (P < .05), indicating that these 4 indicators were discriminative to the occurrence of NAFPD. The AUC of TG and TC were both <0.7 (P < .05), and their discriminative power was not within the acceptable range.
Figure 3.

Receiver-operating characteristic (ROC) curve for HOMA-IR, TG, TC, and LP (A) ROC curve for APN and GLP-1 (B).
3.5. Analysis of indicators of NAFPD with different degrees of pancreatic fat deposition
The NAFPD patients were divided into 2 subgroups according to the extent of pancreatic fat deposition, namely, the MN group and the SN group. There were 224 males and 158 females in the MN group, and 47 males and 44 females in the SN group; there was no significant difference in sex between the 2 groups (χ2 = 1.468, P = .240). Independent samples T tests were performed on relevant factors, and the results showed that levels of BMI, HOMA-IR, HbA1c, TG, and LP in the SN group were higher than those in the MN group, and the levels of APN and GLP-1 were lower than those in the MN group, and the difference was statistically significant (P < .05). There was no significant difference in age, TC, HDL, LDL, and TNF-α level between both groups (P > .05), as shown in Table 4.
Table 4.
Comparison of general conditions and laboratory indexes of NAFPD patients with different extents of pancreatic fat deposition (mean ± SD).

With the extent of NAFPD as a dependent variable (MN = 1, SN = 2), binary logistic regression analysis and multiple logistic regressions were carried out with 8 indicators that had statistically significant differences between the 2 groups such as NAFLD, T2DM, and BMI as dependent variables. Among them, NAFLD, central obesity, and TG were positively correlated with the severity of NAFLD (P < .05, OR >1), and the level of APN was negatively correlated with the severity of NAFPD (P < .05, OR < 1), as shown in Table 5. This indicated that the presence of NAFLD, central obesity, elevated TG levels, and decreased APN levels were independent risk factors for NAFPD exacerbation. ROC curves of APN, LP, and TG values of the MN group and SN group are shown in Figure 4, and the AUC, sensitivity and specificity of the 2 groups are shown in Table 5, the AUC of TG and APN were both <0.7 (P < .05), and the discrimination was not within the acceptable range.
Table 5.
Logistic regression analysis of related factors of NAFPD with different extent of pancreatic fat deposition.

Figure 4.
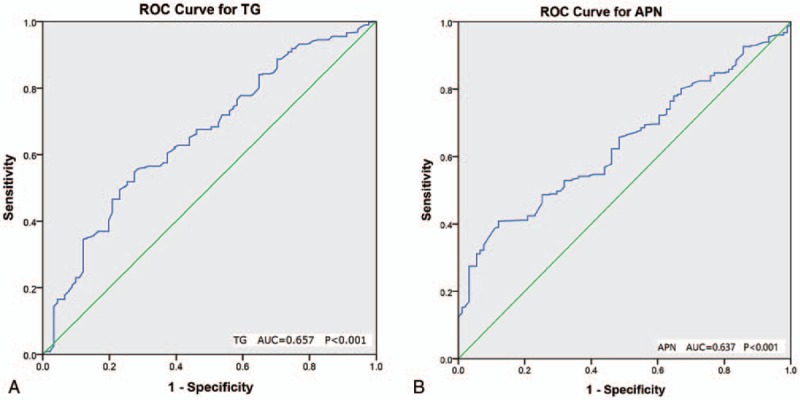
Receiver-operating characteristic (ROC) curve for severity of NAFPD and TG (A) or APN (B).
4. Discussion
The aim of this study was to investigate the prevalence of NAFPD in a Chinese population and to explore factors associated with the risk of NAFPD. The results showed that the overall prevalence of NAFPD was 11%. The prevalence of NAFPD was higher overall in males than in females, but although the prevalence was higher in males in subjects <55 years of age, there was no difference between males and females after 55 years. NAFLD, T2DM, HOMA-IR index, TC, TG, LP, APN, and GLP-1 were independent risk factors for NAFPD. Also, NAFLD, TG, LP, and APN were independent risk factors for NAFPD aggravation.
The result of this study showed a clear sex difference in NAFPD prevalence in subjects <55 years that was lost as the subjects aged. We speculated that this might be related to the role of estrogen in energy regulation and lipid metabolism. Dysfunction of lipid metabolism, caused by decreased estrogen in postmenopausal women, might be the reason of the significant increase in the prevalence of NAFPD in women after 55 years of age.[19] Other studies have also shown that sex differences were associated with the onset of NAFPD.[9,23] Among 4418 subjects, the average age of NAFPD patients was higher than without NAFPD subjects, and the prevalence of NAFPD increased with age. We inferred that this might be related to lipid metabolism dysfunction being aggravated by age-related slowing of metabolism and aggravation of ectopic fat deposition caused by prolonged dyslipidemia. Ectopic fat deposition is likely to be due to excessive TG-induced supersaturated fat storage of cells, preadipocyte differentiation, abnormal lipid synthesis, and degradation in mature adipocytes and the decreased lipid storage capacity of cells, which causes TG to deposit in nonfatty tissues and induce insulin resistance (IR).[24] Lipids are less harmful to health if they are deposited subcutaneously and in other parts of the body.[25,26] An epidemiological study in Asians showed that a simple increase in BMI did not increase the risk of T2DM without NAFLD and IR.[27] So, it could be concluded that the damage caused by obesity to the body occurs mostly in the presence of ectopic fat deposition, which is often accompanied by the appearance of IR. This study showed that T2DM and NAFLD rates were higher in NAFPD patients than those without NAFPD, and the T2DM and NAFLD rates in severe NAFPD patients were higher than in mild NAFPD patients. Thus, it can be seen that aggravation of pancreatic fat deposition is related to increased abnormal glucose metabolism and liver fat deposition. This was consistent with the conclusion of similar studies[4,5] that NAFPD was an independent risk factor for the onset of T2DM. According to the prevalence of NAFLD, we could infer that ectopic lipid deposition caused by abnormal lipid metabolism usually co-occurred in multiple organs, suggesting that IR caused by ectopic fat deposition in multiple organs might lead to further aggravation of glucose metabolic disorder.
We found that patients with NAFPD also had increased insulin resistance, increased obesity (especially central obesity), serum lipid metabolism disorders and decreased GLP-1. With more severe NAFPD, the occurrence of central obesity and lipid metabolism disorder were further increased. So, male gender, NAFLD, T2DM, overweight and obestiy, increased central obesity, increased levels of HOMA-IR, TG, TC, LP, and decreased levels of APN and GLP-1 were independent risk factors for NAFPD. At the same time, NAFLD, increased central obesity, increased TG levels, and decreased APN levels were also independent risk factors for exacerbation of NAFPD. ROC curve analysis showed that HOMA-IR, APN, LP, and GLP-1 were all able to predict the onset of NAFPD, of these HOMA-IR had the highest sensitivity (0.829) and LP had the highest specificity (0.917). The results of this study on serum TG were similar to the results of Lee et al,[7] indicating that high serum TG was an important cause of NAFPD. APN and LP are adipokines that are closely related to IR. APN has an anti-inflammatory effect and was found to be decreased in the serum of obese and T2DM populations, and negatively correlated with IR index,[4] whereas LP is highly expressed in serum and adipose tissue of obese populations, and positively correlated with the increase in volume and number of adipocytes.[28] LP resistance induced by increased LP could cause a decrease in the effect of LP and then lead to enhancement of lipid esterification, which is an important mechanism of ectopic fat deposition. This study found that decreased APN and increased LP were independent risk factors for the onset of NAFPD and exacerbation of NAFPD, which were consistent with the results of similar studies, which further suggested that lipid metabolism disorder and abnormal secretion of adipokines are the basis of the pathogenesis of NAFPD, as well as an important factor that leads to IR and T2DM.
We also found that there was a decrease in serum GLP-1 levels in NAFPD patients compared with normal subjects. GLP-1 is a polypeptide hormone secreted by L cells in the terminal ileum and colon, that can promote insulin secretion through the incretin effect,[29] and can also increase the sensitivity of peripheral tissues to insulin by activating the phosphatidylinositol-4,5-bisphosphate 3-kinase (PI3K) pathway or activating peroxisome proliferator-activated receptor α (PPARα) to reduce the synthesis of apolipoprotein CIII and then improve lipid metabolism,[30] which is closely related to glucose metabolism and lipid metabolism. The incretin effect is decreased in diabetic patients, and one study found that an obese population also had decreased incretin effect,[31,32] but whether this is related to the decrease of endogenous GLP-1 secretion in obese populations, remains in debate.[33,34] This study found that the fasting endogenous GLP-1 (7–37) levels in the NAFPD population was significantly lower than that in the healthy population, and GLP-1 was an independent risk factor for NAFPD. Therefore, we concluded that the decreased endogenous GLP-1 might be more prevalent in populations with ectopic fat deposition than in those with simple obesity. The inconsistent results in previous studies on obese populations might be because ectopic fat deposition was not considered, and data was included from studies of obese populations without ectopic fat deposition.
This study has some limitations. This was a single center study and did not use random sampling based on residents, so these results may not be applicable for the population as a whole. This means the prevalence of the study is potentially higher than actual. As a cross-sectional study, this study could only investigate the prevalence of NAFPD and suggest risk factors; the causal relationship between NAFPD and the risk factors could not be established. The population of subjects without NAFPD would have to be followed-up for a long time to investigate the incidence of NAFPD and the true risk of developing this disease in this population. In addition, this study didn’t include factors such as socioeconomic status, which may also bias the research results.
In summary, our study showed that the occurrence of NAFPD increased with age, and there were gender differences. NAFLD, T2DM, overweight and obestiy, central obesity and HOMA-IR, TC, TG, LP, APN, and GLP-1 were all independent risk factor for the onset of the disease. Among them, NAFLD, central obesity, TG and APN were also independent risk factors for exacerbation of NAFPD. We concluded that lipid metabolism disorder was the basis for the pathogenesis of NAFPD, and the resulting abnormal secretion of adipokines and ectopic fat deposition in other areas could interact to cause IR and glucose metabolism disorder, which resulted in T2DM. Therefore, we should pay close attention to the degree of pancreatic fat deposition in people with central obesity and lipid metabolism disorders. And people who already have pancreatic fat deposition should be carefully screened for ectopic fat deposition in other areas. In addition, the blood glucose of patients with NAFPD should be screened and followed up, to discover prediabetes, diabetes, and other related metabolic diseases.
Acknowledgments
The authors thank the help of Department of Ultrasound (Ningbo Municipal Hospital of TCM, Ningbo) for their expertise and technical help.
Author contributions
Data curation: Siying Weng.
Investigation: Siying Weng, Jianyang Zhou, Xiabo Chen, Yihong Sun, Zhujun Mao, Kefu Chai.
Methodology: Siying Weng, Jianyang Zhou, Xiabo Chen, Yihong Sun, Zhujun Mao, Kefu Chai.
Supervision: Siying Weng.
Validation: Siying Weng.
Visualization: Siying Weng.
Writing – original draft: Siying Weng, Jianyang Zhou, Xiabo Chen, Yihong Sun, Zhujun Mao, Kefu Chai.
Writing – review and editing: Siying Weng, Jianyang Zhou, Xiabo Chen, Yihong Sun, Zhujun Mao, Kefu Chai.
Footnotes
Abbreviations: AUC = area under curve, BMI = body mass index, DM = diabetes mellitus, ELISA = enzyme-linked immunosorbent assay, GLP-1 = glucagon-like peptide 1, HDL = high-density lipoprotein, HOMA-IR = homeostasis model assessment-insulin residence, IDF = International Diabetes Federation, IGR = impaired glucose regulation, LDL = low-density lipoprotein, MN = mild NAFPD, NAFLD = nonalcoholic fatty liver disease, NAFPD = nonalcoholic fatty pancreas disease, PI3K = phosphatidylinositol-4,5-bisphosphate 3-kinase, PPARα = peroxisome proliferator-activated receptor α, ROC = receiver-operating characteristic, SN = severe NAFPD, T2DM = type 2 diabetes mellitus, TC = total cholesterol, TG = triglyceride.
This study was supported by grants from the Zhejiang Provincial Public Welfare Technology Research Project (no. 2015C33169) and the National Natural Science Foundation of China (no. 81774177) and the Key Medical Subjects Program of Ningbo Integrated Traditional Chinese and Western Medicine Endocrinology Department (no. 2016-Z01).
The authors have no conflicts of interest to disclose.
All authors declare that they have no any conflict of interests.
References
- [1].Dalan R, Jong M, Choo R, et al. Predictors of cardiovascular complication in patients with diabetes mellitus: a 5-year follow-up study in a multiethnic population of Singapore: CREDENCE II study. Int J Cardiol 2013;169:e67–9. [DOI] [PubMed] [Google Scholar]
- [2].Yang W, Lu J, Weng J, et al. Prevalence of diabetes among men and women in China. N Engl J Med 2010;362:1090–101. [DOI] [PubMed] [Google Scholar]
- [3].Lee Y, Lingvay I, Szczepaniak LS, et al. Pancreatic steatosis: harbinger of type 2 diabetes in obese rodents. Int J Obes (Lond) 2010;34:396–400. [DOI] [PubMed] [Google Scholar]
- [4].Tushuizen ME, Bunck MC, Pouwels PJ, et al. Pancreatic fat content and beta-cell function in men with and without type 2 diabetes. Diabetes Care 2007;30:2916–21. [DOI] [PubMed] [Google Scholar]
- [5].van der Zijl NJ, Goossens GH, Moors CC, et al. Ectopic fat storage in the pancreas, liver, and abdominal fat depots: impact on beta-cell function in individuals with impaired glucose metabolism. J Clin Endocrinol Metab 2011;96:459–67. [DOI] [PubMed] [Google Scholar]
- [6].Zyromski NJ, Mathur A, Gowda GA, et al. Nuclear magnetic resonance spectroscopy-based metabolomics of the fatty pancreas: implicating fat in pancreatic pathology. Pancreatology 2009;9:410–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Lee JS, Kim SH, Jun DW, et al. Clinical implications of fatty pancreas: correlations between fatty pancreas and metabolic syndrome. World J Gastroenterol 2009;15:1869–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Maggio AB, Mueller P, Wacker J, et al. Increased pancreatic fat fraction is present in obese adolescents with metabolic syndrome. J Pediatr Gastroenterol Nutr 2012;54:720–6. [DOI] [PubMed] [Google Scholar]
- [9].Wang CY, Ou HY, Chen MF, et al. Enigmatic ectopic fat: prevalence of nonalcoholic fatty pancreas disease and its associated factors in a Chinese population. J Am Heart Assoc 2014;3:e000297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Ou HY, Wang CY, Yang YC, et al. The association between nonalcoholic fatty pancreas disease and diabetes. PLoS One 2013;8:e62561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Della Corte C, Mosca A, Majo F, et al. Nonalcoholic fatty pancreas disease and Nonalcoholic fatty liver disease: more than ectopic fat. Clin Endocrinol (Oxf) 2015;83:656–62. [DOI] [PubMed] [Google Scholar]
- [12].Nie JM, Li HF. Metformin in combination with rosiglitazone contribute to the increased serum adiponectin levels in people with type 2 diabetes mellitus. Exp Ther Med 2017;14:2521–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Osonoi T, Saito M, Hariya N, et al. Add-on therapy with anagliptin in Japanese patients with type-2 diabetes mellitus treated with metformin and miglitol can maintain higher concentrations of biologically active GLP-1/total GIP and a lower concentration of leptin. Peptides 2016;86:118–25. [DOI] [PubMed] [Google Scholar]
- [14].Kargulewicz A, Szulinska M, Kujawska-Luczak M, et al. Improvement of serum adiponectin and leptin concentrations: effects of a low-calorie or isocaloric diet combined with metformin or orlistat - a prospective randomized open-label trial. Eur Rev Med Pharmacol Sci 2016;20:3868–76. [PubMed] [Google Scholar]
- [15].Yang DM, Kim HC, Ryu JK, et al. Sonographic appearance of focal fatty infiltration of the pancreas. J Clin Ultrasound 2010;38:45–7. [DOI] [PubMed] [Google Scholar]
- [16].Farrell GC, Chitturi S, Lau GK, et al. Guidelines for the assessment and management of non-alcoholic fatty liver disease in the Asia-Pacific region: executive summary. J Gastroenterol Hepatol 2007;22:775–7. [DOI] [PubMed] [Google Scholar]
- [17].American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care 2011;34(Suppl 1):S62–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Liu LS. Writing Group of 2010 Chinese Guidelines for the Management of Hypertension. 2010 Chinese guidelines for the management of hypertension. Zhonghua Xin Xue Guan Bing Za Zhi 2011;39:579–615. [PubMed] [Google Scholar]
- [19].He W, Li Q, Yang M, et al. Lower BMI cutoffs to define overweight and obesity in China. Obesity (Silver Spring) 2015;23:684–91. [DOI] [PubMed] [Google Scholar]
- [20].Zhou BF. Predictive values of body mass index and waist circumference for risk factors of certain related diseases in Chinese adults--study on optimal cut-off points of body mass index and waist circumference in Chinese adults. Biomed Environ Sci 2002;15:83–96. [PubMed] [Google Scholar]
- [21].Hanas R, John G. 2010 consensus statement on the worldwide standardization of the hemoglobin A1C measurement. Diabetes Care 2010;33:1903–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Levy JC, Matthews DR, Hermans MP. Correct homeostasis model assessment (HOMA) evaluation uses the computer program. Diabetes Care 1998;21:2191–2. [DOI] [PubMed] [Google Scholar]
- [23].Wong VW, Wong GL, Yeung DK, et al. Fatty pancreas, insulin resistance, and beta-cell function: a population study using fat-water magnetic resonance imaging. Am J Gastroenterol 2014;109:589–97. [DOI] [PubMed] [Google Scholar]
- [24].Wallace TM, Levy JC, Matthews DR. An increase in insulin sensitivity and basal beta-cell function in diabetic subjects treated with pioglitazone in a placebo-controlled randomized study. Diabet Med 2004;21:568–76. [DOI] [PubMed] [Google Scholar]
- [25].Matsushita Y, Nakagawa T, Yamamoto S, et al. Effect of longitudinal changes in visceral fat area and other anthropometric indices to the changes in metabolic risk factors in Japanese men:the Hitachi Health Study. Diabetes Care 2012;35:1139–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Kwon H, Kim D, Kim JS. Body fat distribution and the risk of incident metabolic syndrome: A Longitudinal Cohort Study. Sci Rep 2017;7:10955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Eshtiaghi R, Esteghamati A, Nakhjavani M. Menopause is an independent predictor of metabolic syndrome in Iranian women. Maturitas 2010;65:262–6. [DOI] [PubMed] [Google Scholar]
- [28].Rossi AP, Fantin F, Zamboni GA, et al. Predictors of ectopic fat accumulation in liver and pancreas in obese men and women. Obesity (Silver Spring) 2011;19:1747–54. [DOI] [PubMed] [Google Scholar]
- [29].Sung KC, Jeong WS, Wild SH, et al. Combined influence of insulin resistance, overweight/obesity, and fatty liver as risk factors for type 2 diabetes. Diabetes Care 2012;35:717–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Liu XX, Liu KY, Li P, et al. Adiponectin is expressed in the pancreas of high-fat-diet-fed mice and protects pancreatic endothelial function during the development of type 2 diabetes. Diabetes Metab 2014;40:363–72. [DOI] [PubMed] [Google Scholar]
- [31].van Meijl LE, Mensink RP. Effects of low-fat dairy consumption on markers of low-grade systemic inflammation and endothelial function in overweight and obese subjects: an intervention study. Br J Nutr 2010;104:1523–7. [DOI] [PubMed] [Google Scholar]
- [32].Holst JJ, Gromada J. Role of incretin hormones in the regulation of insulin secretion in diabetic and nondiabetic humans. Am J Physiol Endocrinol Metab 2004;287:E199–206. [DOI] [PubMed] [Google Scholar]
- [33].Nakamura K, Oe H, Kihara H, et al. DPP-4 inhibitor and alpha-glucosidase inhibitor equally improve endothelial function in patients with type 2 diabetes: EDGE study. Cardiovasc Diabetol 2014;13:110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Knop FK, Aaboe K, Vilsboll T, et al. Impaired incretin effect and fasting hyperglucagonaemia characterizing type 2 diabetic subjects are early signs of dysmetabolism in obesity. Diabetes Obes Metab 2012;14:500–10. [DOI] [PubMed] [Google Scholar]


