Abstract
The purpose of our study was to determine accuracy of CT texture analysis (CTTA) for differentiating benign from malignant pulmonary nodules, and well-differentiated from poorly differentiated lung cancers, with histology as the standard of reference.
In this IRB-approved study, 175 adult patients (average age 66 ± 12 years; age range 27–89 years, male 82: female 93) who underwent a noncontrast chest CT examination prior to CT-guided biopsy of pulmonary nodules were included. There were 57 benign (24 tumors or tumor-like lesions; 33 inflammatory conditions) and 120 malignant (29 well-differentiated adenocarcinomas, 48 poorly differentiated adenocarcinomas, and 43 squamous cell carcinomas) diagnoses on pathology. CTTA was performed on the prebiopsy noncontrast CT images using a commercially available software (TexRAD limited, UK). The CTCA features analyzed included mean HU values, percent positive pixels (PPP), mean value of positive pixels (MPP), standard deviation (SD), normalized SD, skewness, kurtosis, and entropy.
The ROC analyses showed that normalized SD [AUC: 0.63, (CI: 0.55–72), P = .003] had moderate accuracy for differentiating between benign and malignant lesions. For differentiating among well-differentiated and poorly differentiated tumors, the ROC analysis showed that except skewness all other parameters were statistically significant The AUC values of other CTTA parameters were: mean (AUC: 0.73–0.76, P = .001– < .0001).
CT texture analyses can reliably predict well- and poorly differentiated lung malignancies. However, inflammatory lung lesions with tissue heterogeneity negatively affect the performance of CTTA when it comes to differentiation between benign and malignant pulmonary nodules.
Keywords: benign and malignant lung nodules, CT texture analysis, lung biopsy, well- and poorly differentiated lung malignancies
1. Introduction
Indeterminate pulmonary nodules are ubiquitous in chest CT, whether performed for cancer screening, staging, or surveillance. Characterization of pulmonary nodules into benign and malignant etiologies affects treatment and prognosis. Semantic characteristics of pulmonary nodules such as size, attenuation, and margins are often insufficient for characterization. This inadequacy necessitates longitudinal chest CT for pulmonary nodules to establish stability (favors benignancy), or to assess change (favors malignancy) over time. Follow-up CT add cost and radiation burden for the patients, in addition to patient anxiety over long waiting time to know the outcome.[1–3]
For characterization, nodules are biopsied with open surgery, percutaneous-image-guided biopsy, or transbronchial tissue sampling. Such invasive procedures are reserved for larger nodules, and those with suspicious findings (irregular margins, mixed attenuation, and growth over time). These tissue biopsy approaches of pulmonary nodules are associated with nontrivial complications such as pneumothorax, chest tube placement, pulmonary hemorrhage, and risks associated with sedation or general anesthesia.[1–3]
Prior studies have demonstrated that radiomics or CT texture analysis (CTTA) can differentiate tumor grades and genetic mutations.[4–13] CTTA uses statistical distribution of pixel values within the tumor as surrogate marker of tumor heterogeneity. This heterogeneity is a recognized feature of malignancy, tumor aggressiveness, and treatment response in patients with cancer. Increased tumor heterogeneity on CTTA is associated with an adverse tumor biology, and is an independent predictor of patient survival for several malignancies.[4–13] We hypothesized that tissue heterogeneity with CTTA can differentiate benign and malignant lung nodules. If successful, CTTA can reduce, and in some cases, replace tissue biopsies for characterization of pulmonary nodules. The purpose of our study was to determine accuracy of CTTA for differentiating benign from malignant pulmonary nodules, and well differentiated from poorly differentiated lung cancers, with histology as the standard of reference.
2. Materials and methods
The Institutional Review Board (IRB) approved the study. The study was compliant with the Health Insurance Portability and Accountability Act (HIPAA) guidelines. We do not have any pertinent financial disclosures related to this study.
2.1. Human subjects
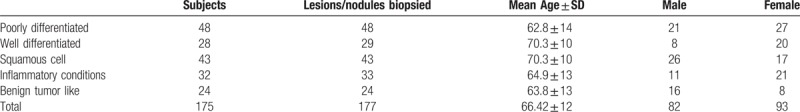
All 175 adult patients (average age 66 ± 12 years; age range 27–89 years, Table 1) included in our study underwent a noncontrast chest CT examination prior to CT-guided biopsy of pulmonary nodules between January 2011 and December 2013. The study cohort comprised 93 women and 82 men.
Table 1.
Type of benign and malignant tumors, number of biopsied lesions/nodules, and demographics.
Only patients with solid pulmonary nodules and definitive benign or malignant diagnosis on pathology were included in the study. For malignant diagnosis, only patients with lung adenocarcinoma (well differentiated and poorly differentiated) and squamous cell cancers were included. We excluded patients with subsolid or part-solid nodules, lesions outside of the lung parenchyma originating in mediastinum, chest wall, or pleura, as well as those with nonprimary lung malignancies. Patients who underwent multiple biopsies were included only if the procedures were performed for biopsy of different lung nodules.
A total of 177 lung nodule biopsies were performed in the included 175 patients. Two patients underwent biopsies of 2 different lung nodules. There were 57 benign and 120 malignant diagnoses on pathology. The benign diagnoses included 24 tumors or tumor-like lesions such as hamartoma, fibroelastic scar, amyloid, and schwannoma; 33 inflammatory conditions like abscess and granulomatous disease. Among 120 malignant primary lung cancers, there were 29 well-differentiated adenocarcinomas, 48 poorly differentiated adenocarcinomas, and 43 squamous cell carcinomas.
The lung lesions were divided into 5 groups based on their histologic diagnosis: benign tumors and tumor-like conditions; inflammatory and infectious processes; well-differentiated adenocarcinoma; poorly differentiated adenocarcinoma; and squamous cell carcinoma.
2.2. Scanning techniques
All pre-biopsy noncontrast CT examinations were performed on a 4-slice, multidetector-row CT (GE LightSpeed QX, GE Healthcare, Waukesha, WI). Scanning parameters included 120 kV, 150 mA, 0.5 second rotation time, 6:1 slice pitch, and 15 mm detector width. The scanning was performed in either prone or supine position, depending on the nodule location and planned biopsy approach. The reconstructed section thickness was 2.5 mm with standard soft tissue reconstruction kernel. Images were reconstructed with filtered back projection technique.
2.3. CTTA
CTTA was performed on the pre-biopsy noncontrast CT images using a commercially available software (TexRAD limited, UK). For each patient, a region of interest (ROI) was drawn on a single image with the maximal nodule dimension in the transverse plane (Fig. 1). We applied a filter with a threshold of −50 HU, to exclude areas of cavitation containing air. Regions with macroscopic calcifications were also excluded from the ROIs. Artifacts related to motion and beam hardening, if present, were avoided. A single co-investigator (RLG) drew the ROIs in all CT examinations.
Figure 1.
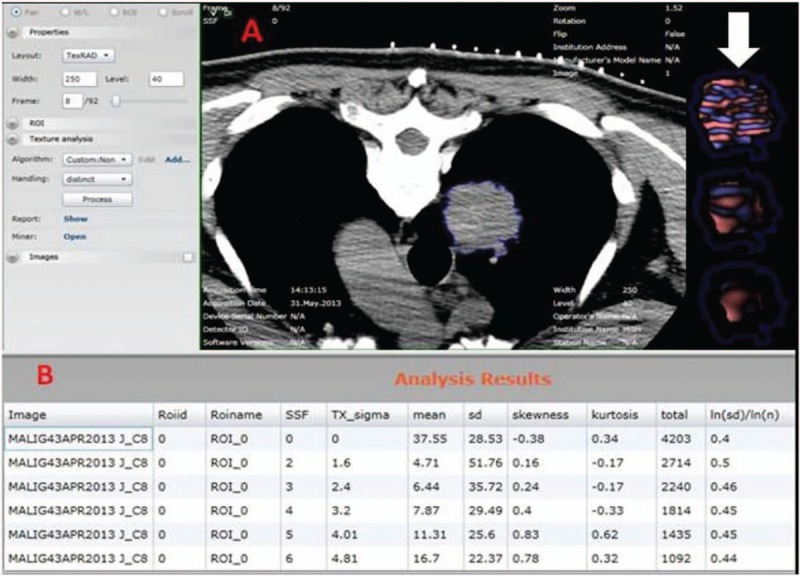
Noncontrast chest CT of a patient with poorly differentiated adenocarcinoma of the right lung was outlined with a ROI using CTTA software. The colored image (arrow) of the tumor with in the ROI demonstrates increased tissue heterogeneity. CTTA = CT texture analysis, ROI = region of interest.
Analyses comprised an initial image filtration step followed by texture quantification within the filtered image. The image filtration is based on the size of the imaging features, and is denoted by the spatial scaling factor (SSF). The SSF ranges between object radii of 0, 2, 3, 4, 5, and 6 mm.
2.4. Quantitative analysis
The CCTA software analyzed the following features: mean HU values defined as the average value of the pixels within the region of interest; standard deviation (SD) defined as variation or “dispersion” from the average (mean value). A low SD indicates that data points are closer to the mean; a high SD suggests data points spread over a large range of values. Percent positive pixels (PPP) represent number of pixels (out of total) with a positive CT attenuation value. The mean positive pixels (MPP) are pixels greater than 0, within the region of interest. Skewness measures asymmetry of the histogram, and can be positive or negative. Kurtosis is a measure of the pointiness of the distribution. A positive kurtosis indicates a histogram with higher peak than a Gaussian (normal) distribution. A negative kurtosis represents flatter histogram than a Gaussian (normal) distribution. In general, kurtosis is a measure of blood vessels, whereas skewness measures nonvascular structures. Entropy measures irregularity or complexity of a structure. The ln(SD)/ln(n) [= ln(SD)/ln (total number of pixel within the ROI)] is called “normalized SD" and relates to the ratio of Log of SD to the log of total pixels (n). It represents potential variability of region size. SD/ln(n) is another variant of normalized SD, which is also linked to entropy. It is particularly useful for smaller ROIs. By selecting among 6 different types of spatial scaling factors (0, 2, 3, 4, 5, 6), the filtration threshold could be changed.
2.5. Statistical analysis
The data were analyzed with SPSS statistical software (SPSS version 21, IBM, Armonk, NY). Independent Sample t test was used for comparing various CTTA quantitative parameters of benign and malignant lesions. A P-value of .05 with a 95% confidence interval was considered statistically significant. We performed univariate and multivariate logistic regression analyses to determine significant CTTA predictors of benign and malignant histologic diagnoses. We also generated receiver operating characteristic (ROC) curves for various quantitative CTTA parameters. Data were analyzed with multivariate logistic regression and receiver operating characteristic (ROC) analyses.
3. Results
Respective longest and orthogonal dimensions (average ± standard deviation) of the analyzed lesions were: 26 ± 19 mm and 19 ± 17 mm for benign tumors or tumor-like lesions; 30 ± 18 mm and 22 ± 12 mm for inflammatory conditions like abscess and granulomatous disease; 34 ± 25 mm and 25 ± 22 mm for well-differentiated adenocarcinomas; 42 ± 25 mm and 31 ± 22 mm for poorly differentiated adenocarcinomas; 40 ± 19 mm and 30 ± 17 mm for squamous cell carcinomas.
3.1. Malignant vs benign lesions
In the 120 primary lung cancers, there were 29 well-differentiated adenocarcinomas, 48 poorly differentiated adenocarcinomas, and 43 squamous cell carcinomas. The 57 benign lesions comprised 24 benign tumor-like lesions and 33 inflammatory lesions.
The independent sample t test demonstrated that 2 CTTA parameters, normalized SD and SD/ln(n), were significantly different between the malignant and the benign lesions (P = .02 and .004, respectively). No significant differences were noted for the remaining CTTA parameters, including mean (P = .7), SD (P = .2), skewness (P = .08), kurtosis (P = .8), entropy (P = .3), PPP (P = .08), and MPP (P = .7).
The ROC analyses showed that normalized SD (values when 1 = benign/0 = malignant, area under curve (AUC): 0.63, (CI: 0.55–72), P = .003) had moderate accuracy for differentiating between benign and malignant lesions. The AUC values of other CTTA parameters were lower compared to normalized SD: mean (AUC: 0.58, P = .056), SD (AUC: 0.47, P = .6), skewness (AUC: 0.45, P = .3), kurtosis (AUC: 0.50, P = .8), entropy (AUC: 0.58, P = .06), PPP (AUC: 0.58, P = .08), MPP (AUC: 0.56, P = .2), (SD)/ln(n) (AUC: 0.41, P = .07).
Univariate logistic regression analyses showed that only normalized SD was the predictor for differentiating benign versus malignant tumors (P = .006, Nagelkerke R2 = 0.6). The normalized SD had 60% variance in differentiating benign and malignant, and correctly classified 68% of lesions as benign or malignant. Once again, the remaining CTTA parameters were not significant predictors of benign or malignant histology: mean (P = .7), SD (P = .2), skewness (P = .09), kurtosis (P = .9), entropy (P = .4), PPP (P = .09), and MPP (P = .7).
Both forward and backward conditional logistic regression models were statistically significant for normalized SD (forward: conditional P = .006, and backward: conditional P = .011). The forward model correctly classified 68.0% of lesions and backward model correctly classified 69% of lesions.
3.2. Malignant vs benign tumor like
For this analysis, we compared 120 patients with lung malignancy and 24 patients with benign tumor like histology. Multivariate logistic regression analysis (Forward and Backward: Conditional) was performed by adding all CTTA parameters in the regression model to different malignant versus benign tumor like lesions/tumors. The forward: conditional logistic regression model was statistically significant for ln(SD)/ln(n) or normalized SD (P = .006, Nagelkerke R2 = 0.12). The forward model correctly classified 83.0% of tumors. The backward: conditional logistic regression model was also statistically significant for normalized SD (P = .023), PPP (P = .018), and entropy (P = .031) (Nagelkerke R2 = 0.22). The backward model explained 22.0% of the variance (due to independent factors: normalized SD, PPP, and entropy) in differentiating benign tumor-like lesions from malignant tumors, and correctly classified 83.0% of lesions/tumors.
3.3. Well-differentiated vs poorly differentiated tumors
For this analysis, we divided malignant lung lesions/tumors into 2 groups: well-differentiated group (29 total: well-defined adenocarcinomas), and poorly differentiated group (91 total: 48 poorly differentiated adenocarcinomas and 43 squamous cell carcinomas).
The ROC analysis showed that except skewness all other parameters were statistically significant among well-differentiated and poorly differentiated tumors. The AUC values of other CTTA parameters were: mean (AUC: 0.70, P = .001), SD (AUC: 0.63, P = .032), skewness (AUC: 0.61, P = .069), kurtosis (AUC: 0.73, P < .0001), entropy (AUC: 0.65, P = .014), PPP (AUC: 0.76, P < .0001), MPP (AUC: 0.64, P = .025), (SD)/ln(n) (AUC: 0.73, P < .0001), and ln(SD)/ln(n) (AUC: 0.73, P < .0001) (Fig. 2, Table 2).
Figure 2.
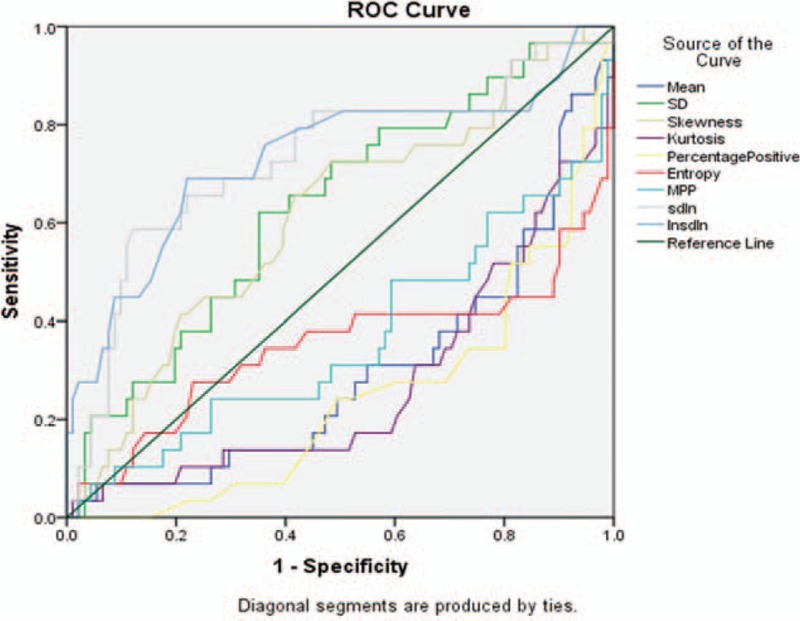
Area under the curve (AUC) analysis graph for well-differentiated vs poorly differentiated tumors. AUC = area under curve.
Table 2.
Area under the curve (AUC) values for well-differentiated vs poorly differentiated tumors.
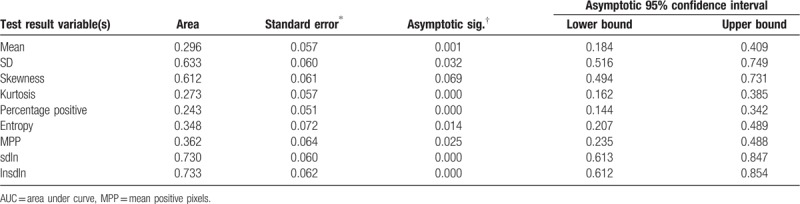
Univariate logistic regression analyses showed that mean, (SD)/ln, normalized SD, entropy, PPP were the predictor for differentiating well- and poorly differentiated lung cancers (P = .005– < .0001). Multivariate logistic regression analysis showed that the forward: conditional logistic regression model was statistically significant for ln(SD)/ln(n) or normalized SD (P < .0001, Nagelkerke R2 = 0.23). The forward model correctly classified 79.0% of well- and poorly differentiated lung cancers. However, the backward: conditional logistic regression model was also statistically significant for kurtosis (P = .037) and entropy (P = .005) (Nagelkerke R2 = 0.3). The backward model explained 30.0% of the variance (due to independent factors: kurtosis and entropy) in differentiating well differentiated like versus poorly differentiated malignant tumors and correctly classified 83.0% of noduless/tumors, slightly higher the forward conditional model.
4. Discussion
Many patients with lung nodules require tissue sampling for diagnosis.[14–23] Despite high sensitivity, specificity, and accuracy of percutaneous image-guided biopsy for diagnosis of malignancy, benign diagnoses can only be made in 20% to 50% of cases.[21–23] Patients with nonspecific benign and nondiagnostic results require further workup; a review of 74 cases of percutaneous biopsy with either nonspecific benign or nondiagnostic finding on biopsy revealed an eventual malignancy rate of 18%. The false-negative rate of percutaneous biopsy is variable, ranging between 6% to –54%.[14–23]
Asymptomatic benign lung lesions are generally managed conservatively and surgery is performed only in selected cases. Pulmonary abscesses or infections are treated pharmacologically; surgery is reserved for complications such as empyema or fistula formation. When possible, malignant nodules are treated aggressively with complete anatomical resection such as segmentectomy, lobectomy, and pneumonectomy.[14–23] With awareness and emphasis on tumor biology and histology, some low-grade malignancies are being treated with less aggressive nonanatomical surgeries such as wedge resections. A multicenter clinical trial demonstrated that low-grade adenocarcinomas can be successfully treated with wedge resection for T1a tumors, and with segmentectomy for T1b tumors. Several studies have shown that tumor grading, alone and in multivariate analysis, is a significant predictor of treatment outcome, cure rate, overall and disease-free survival and might also have a significant impact on choosing optimal treatment.[14–23] Prior studies have employed CTTA to differentiate benign and malignant tumor along with tumor aggressiveness, prognosis, disease risk-stratification, and treatment-response prediction.[4–13]
Although our study concurs with existent CTTA literature with regards to distinction between well-differentiated and poorly differentiated lung malignances (AUC 0.73–0.76), CTTA had limited accuracy (normalized SD, AUC 0.63, sensitivity 69%, specificity 56%) for distinguishing presence of tissue necrosis and heterogeneity within these inflammatory conditions, likely rendering most CTTA parameters ineffective since these characteristics are also hallmarks of malignancies. Our findings are contradictory to Bayanati et al,[7] who reported 81% sensitivity and 80% specificity (AUC = 0.87, P < .0001) for differentiating benign and malignant mediastinal lymph nodes with CTTA. Discordance may be explained on the different patient subtypes in the two studies or differences between inflammatory responses between lung lesions in our study and mediastinal lymph nodes in the previous study from Bayanti et al.[7] The sensitivity of normalized SD increased to 83%, once inflammatory lung lesions (such as abscesses and granulomatous diseases) were removed from the benign group. Such decisions may not be possible on a prospective basis in a routine clinical practice.
Conversely, in malignant nodules, several CTTA parameters had greater success in separating well-differentiated and poorly differentiated lung malignancies (Figs. 3 and 4). These included PPP, kurtosis, entropy, and normalized SD which had up to 83% sensitivity and 69% specificity for assessing tumor differentiation. Such findings have been reported in previous studies, for example, Sacconi et al[10] reported substantial correlation between CTTA parameters (such as mean, SD, skewness, and entropy) and epidermal growth factor receptor (EGFR) mutations as well as survival rates in patients with lung adenocarcinoma.
Figure 3.
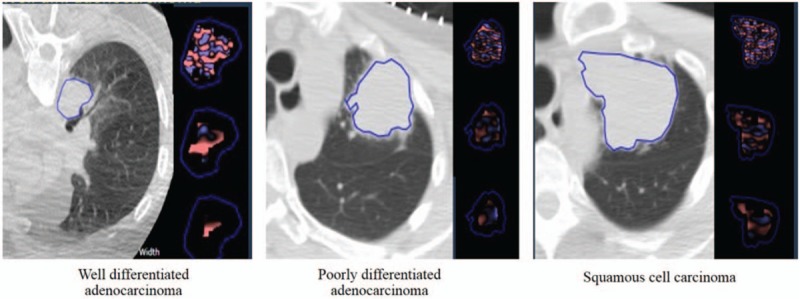
Patients with poorly differentiated adenocarcinoma and squamous cell carcinoma showed increased tissue heterogeneity compared to a patient with well-differentiated adenocarcinoma.
Figure 4.
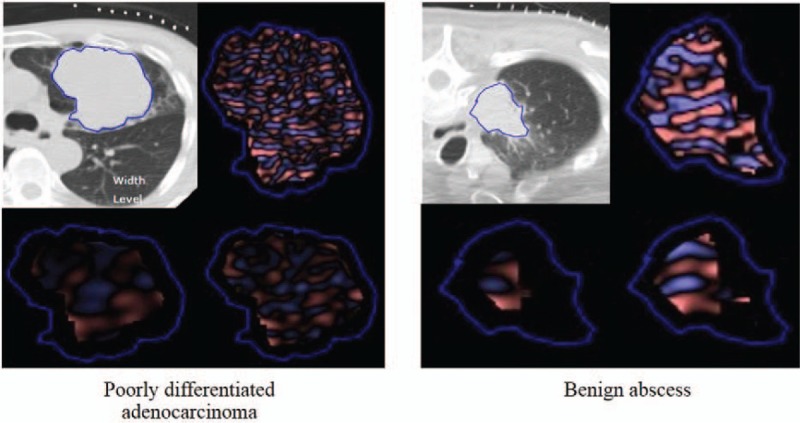
Patient with poorly differentiated adenocarcinoma showed increased tissue heterogeneity compared to patient with a benign abscess.
The primary implication of our study is that lesion aggressiveness, whether in benign or malignant nodules, impact the behavior of CTTA parameters. Aggressive or heterogenous benign lung lesions, like on semantic, subjective interpretation of images, negatively influence accuracy of CTTA. Although not observed in our study, it is conceivable that presence of superimposed pneumonitis associated with malignant lesions can render CTTA less effective in assessment of tumor aggressiveness. Tumor aggressiveness is associated with changes in CTTA parameters. Patients with malignancy undergo several follow-up CT to assess stability, decrease or progression in disease burden. CTTA can be applied on such CT examinations to assess tumor burden and treatment response. If CTTA features demonstrate increased tumor aggressiveness or heterogeneity compared to prior exams, then invasive procedures such as biopsy can be performed. Hence, CTTA can reduce the number of invasive procedures performed in the patients with malignant tumors.
Limitations of our study include relatively small sample size. In addition, CTTA analyses were performed on a single CT image rather than the entire tumor volume over contiguous images. However, prior studies have reported similar results with single CT images and entire volume.[6,24,25] We assessed CTTA on noncontrast chest CT, our results may not be applicable on contrast enhanced chest CT examinations. This is not a substantial limitation since most chest CT for evaluation of pulmonary nodules are performed without contrast. Finally, we excluded patients with metastatic pulmonary nodules which could have affected accuracy of CTTA parameters in a negative or positive manner.
In summary, CT texture analyses can reliably predict well- and poorly differentiated lung malignancies. However, inflammatory lung lesions with tissue heterogeneity negatively affect the performance of CTTA when it comes to differentiation between benign and malignant pulmonary nodules.
Author contributions
Conceptualization: Subba R. Digumarthy, Mannudeep K. Kalra.
Data curation: Atul M. Padole.
Formal analysis: Atul M. Padole.
Investigation: Subba R. Digumarthy, Mannudeep K. Kalra.
Methodology: Jo-Anne Shepard.
Resources: Atul M. Padole, Ramandeep Singh.
Software: Roberto Lo Gullo.
Supervision: Subba R. Digumarthy, Jo-Anne Shepard, Mannudeep K. Kalra.
Visualization: Ramandeep Singh.
Writing – original draft: Atul M. Padole, Mannudeep K. Kalra.
Footnotes
Abbreviations: AUC = area under curve, CTTA = CT texture analysis, MPP = mean positive pixels, PPP = percent positive pixels, ROC = receiver operating characteristic, ROI = region of interest, SSF = spatial scaling factor.
SRD and AMP equally contributed to this study.
The authors have no conflicts of interest to disclose.
References
- [1].Ozeki N1, Iwano S2, Taniguchi T1, et al. Therapeutic surgery without a definitive diagnosis can be an option in selected patients with suspected lung cancer. Interact Cardiovasc Thorac Surg 2014;19:830–7. [DOI] [PubMed] [Google Scholar]
- [2].Merritt RE, Shrager JB. Indications for surgery in patients with localized pulmonary infection. Thorac Surg Clin 2012;22:325–32. [DOI] [PubMed] [Google Scholar]
- [3].Scott WJ. Surgical treatment of other bronchial tumors. Chest Surg Clin N Am 2033;13:111–2. [DOI] [PubMed] [Google Scholar]
- [4].Miles KA. How to use CT texture analysis for prognostication of non-small cell lung cancer. Cancer Imaging 2016;16:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Yip SSF, Liu Y, Parmar C, et al. Associations between radiologist-defined semantic and automatically computed radiomic features in non-small cell lung cancer. Sci Rep 2017;7:3519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Lubner MG, Malecki K, Kloke J, et al. Texture analysis of the liver at MDCT for assessing hepatic fibrosis. Abdom Radiol (NY) 2017;42:2069–78. [DOI] [PubMed] [Google Scholar]
- [7].Bayanati H, E Thornhill R, Souza CA, et al. Quantitative CT texture and shape analysis: can it differentiate benign and malignant mediastinal lymph nodes in patients with primary lung cancer? Eur Radiol 2015;25:480–7. [DOI] [PubMed] [Google Scholar]
- [8].Andersen MB, Harders SW, Ganeshan B, et al. CT texture analysis can help differentiate between malignant and benign lymph nodes in the mediastinum in patients suspected for lung cancer. Acta Radiol 2016;57:669–76. [DOI] [PubMed] [Google Scholar]
- [9].Ganeshan B, Panayiotou E, Burnand K, et al. Tumour heterogeneity in non-small cell lung carcinoma assessed by CT texture analysis: a potential marker of survival. Eur Radiol 2012;22:796–802. [DOI] [PubMed] [Google Scholar]
- [10].Sacconi B, Anzidei M, Leonardi A, et al. Analysis of CT features and quantitative texture analysis in patients with lung adenocarcinoma: a correlation with EGFR mutations and survival rates. Clin Radiol 2017;72:443–50. [DOI] [PubMed] [Google Scholar]
- [11].Haider MA, Vosough A, Khalvati F, et al. CT texture analysis: a potential tool for prediction of survival in patients with metastatic clear cell carcinoma treated with sunitinib. Cancer Imaging 2017;17:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Zhang GM, Sun H, Shi B, et al. Quantitative CT texture analysis for evaluating histologic grade of urothelial carcinoma. Abdom Radiol (NY) 2017;42:561–8. [DOI] [PubMed] [Google Scholar]
- [13].Smith AD, Gray MR, del Campo SM, et al. Predicting overall survival in patients with metastatic melanoma on antiangiogenic therapy and RECIST stable disease on initial posttherapy images using CT texture analysis. AJR Am J Roentgenol 2015;205:W283–93. [DOI] [PubMed] [Google Scholar]
- [14].Saji H, Nakamura H, Tsuchida T, et al. The incidence and the risk of pneumothorax and chest tube placement after percutaneous CTguided lung biopsy: the angle of the needle trajectory is a novel predictor. Chest 2002;121:1521–6. [DOI] [PubMed] [Google Scholar]
- [15].Wu CC, Maher MM, Shepard J-AO. Complications of CT-guided percutaneous needle biopsy of the chest: prevention and management. AJR Am J Roentgenol 2011;196:W678–82. [DOI] [PubMed] [Google Scholar]
- [16].Yeow K-M, Su I-H, Pan K-T, et al. Risk factors of pneumothorax and bleeding: multivariate analysis of 660 CT-guided coaxial cutting needle lung biopsies. Chest 2004;126:748–54. [DOI] [PubMed] [Google Scholar]
- [17].Klein JS, Salomon G, Stewart EA. Transthoracic needle biopsy with a coaxially placed 20-gauge automated cutting needle: results in 122 patients. Radiology 1996;198:715–20. [DOI] [PubMed] [Google Scholar]
- [18].Savage C, Walser EM, Schnadig V, et al. Transthoracic image guided biopsy of lung nodules: when is benign really benign? J Vasc Intervent Radiol 2004;15:161. [DOI] [PubMed] [Google Scholar]
- [19].Connor S, Dyer J, Guest P. Image-guided automated needle biopsy of 106 thoracic lesions: a retrospective review of diagnostic accuracy and complication rates. Eur Radiol 2000;10:490–4. [DOI] [PubMed] [Google Scholar]
- [20].Okada M. radical sublobar resection for lung cancer. Gen Thorac Cardivasc Surg 2008;56:151–7. [DOI] [PubMed] [Google Scholar]
- [21].Tsutani Y, Miyata Y, Nakayama H, et al. Appropriate sublobar resection choice for ground glass opacity-dominant clinical stage IA lung adenocarcinoma: wedge resection or segmentectomy. Chest 2014;145:66–71. [DOI] [PubMed] [Google Scholar]
- [22].Barletta JA, Yeap BY, Chirieac LR. Prognostic significance of grading in lung adenocarcinoma. Cancer 2010;116:659–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Chung CK, Zaino R, stryker JA, et al. Carcinoma of the lung: evaluation of histological grade and factors influencing prognosis. Ann Thoracic Surg 1982;33:599–604. [DOI] [PubMed] [Google Scholar]
- [24].Lubner MG, Stabo N, Lubner SJ, et al. CT textural analysis of hepatic metastatic colorectal cancer: pre-treatment tumor heterogeneity correlates with pathology and clinical outcomes. Abdom Imaging 2015;40:2331–7. [DOI] [PubMed] [Google Scholar]
- [25].Ng F, Kozarski R, Ganeshan B, et al. Assessment of tumor heterogeneity by CT texture analysis: can the largest cross-sectional area be used as an alternative to whole tumor analysis? Eur J Radiol 2013;82:342–8. [DOI] [PubMed] [Google Scholar]


