Abstract
Objective:
To examine whether obesity/overweight is a risk predictor for breast cancer recurrence and death by menopausal status in a retrospective study.
Methods:
We performed a retrospective analysis of 1017 breast cancer patients treated in our hospital from January 2004 to December 2012. Three groups were divided according to body mass index (BMI) when breast cancer diagnosis: normal weight, BMI < 25.0 kg/m2; overweight, 25.0≤BMI < 30.0 kg/m2; and obesity, BMI≥30.0 kg/m2. The clinicopathological characteristics and clinical outcomes of patients within 5 years following breast cancer diagnosed were analyzed. Subgroup analyses of BMI on breast cancer prognosis were analyzed according to the menopausal status when breast cancer diagnosis. The Cox proportional hazards model was used to estimate hazard ratios (HRs) and 95% confidence intervals (CIs).
Results:
Overweight and obesity groups were associated with larger size tumors, older age, increased proportion of postmenopausal patients and less patients choosing anthracycline and/or taxane regimen. The 5-year disease-free survival (DFS) and overall survival (OS) decreased in overweight and obese patients (P < .001), and both overweight and obesity were independent predictors for increased risks of breast cancer relapse and death (P < .001). When stratified by menopausal status, both overweight and obesity were associated with reduced 5-year DFS and OS in postmenopausal patients (P < .050), and multivariate analysis showed that the risk of relapse and breast cancer mortality in these 2 groups also increased (P < .050). Among premenopausal patients, the risks of relapse and death were significantly increased in obesity group rather than overweight group by multivariate analysis.
Conclusion:
Overweight and obesity might be independently associated with poorer prognosis for breast cancer patients, and the effects of overweight on the breast cancer prognosis seem to be related to menopausal status.
Keywords: body mass index, breast cancer, menopausal, mortality, obesity, overweight, relapse
1. Introduction
Nowadays, obesity or overweight has become an emerging health concern worldwide with over 500 million adults were obese and 958 million were overweight in 2008,[1,2] and overweight or obesity was also reported to be a risk factor for increased incidence of various forms of cancer.[3,4] Breast cancer remains the most common malignant neoplasm among women,[5] and being overweight or obese in adults is correlated with a greater risk of breast cancer.[6] Besides its established role as a risk factor, there is now widespread consensus on the importance of obesity or overweight as a negative prognostic factor for breast cancer.[7,8]
In 2004, Berclaz et al[9] have reported that obesity or overweight is associated with a poor prognosis after breast cancer treatment, and other studies also suggested that obesity at the time of cancer diagnosis or pre-diagnosis is associated with poor prognosis for breast cancer patients.[10,11] In addition, it has been demonstrated that breast cancer patients with higher body mass index (BMI) estimated as obesity or overweight have a worse prognosis disease regardless of tumor subtype.[12] However, a large retrospective study including stage I-III triple-negative breast cancer (TNBC) patients found that overweight (BMI 25–29.9 kg/m2) or obesity (BMI ≥ 30 kg/m2) at diagnosis did not affect disease-free survival (DFS) or overall survival (OS),[13] and Kwan et al[14] also did not find any association between BMI and risk of breast cancer recurrence or mortality after nearly 8-year follow-up.
According to previous study, the effects of higher BMI on prognosis of breast cancer may be associated with menopausal status.[15] Berclaz et al[9] demonstrated that elevated BMI is significantly associated with a worse prognosis of breast cancer, especially for premenopausal and perimenopausal patients, and Kawai et al[16] found that higher BMI (BMI ≥25.8 kg/m2) was associated with an increase in mortality for premenopausal patients. In a cohort study, obese postmenopausal women at diagnosis were at increased risk of breast cancer mortality compared to normal weight women after 6-year follow-up, while being overweight did not affect survival.[17] Another study found that both obesity and overweight were associated with a non-significant higher risk of recurrence and breast cancer death for premenopausal women, and obesity was associated with a significant higher risk of breast cancer death (not for recurrence), but overweight was not a prognosis predictor for postmenopausal women.[18]
To date, the relationship between obesity/overweight and outcomes of breast cancer patients has not been well defined. To our knowledge, the association between obesity/overweight and risk of breast cancer recurrence and death in Chinese women according to menopausal status is less well understood mechanistically. In our study, we presented the analysis of the link between BMI and prognosis of breast cancer, and the stratification analysis was also conducted according to menopausal status when breast cancer diagnosis.
2. Methods
2.1. Patient selection
Data in the study were extracted from the hospital recording statistics in the Affiliated Changzhou No. 2. People's Hospital with Nanjing Medical University from January 2004 to December 2012. We collected primary and adjuvant treatment from medical records: surgery, radiation, chemotherapy, and specific hormonal therapy. We examined a number of potential confounding variables including age when breast cancer diagnosis, stage at diagnosis, tumor size, lymph node evaluation, estrogen receptor (ER) or progesterone receptor (PR) expression, human epidermal growth factor receptor-2 (HER2) expression, pathological subtype, and histological grade. Survival data, which included date of breast cancer diagnosis, surgery, relapse, death and last follow-up, were collected in this cohort. The diagnostic of breast cancer was based on the pathological diagnosis. Patients who did not perform surgery were excluded. We further excluded patients who were without complete medical records, dying from other causes within 5 years of breast cancer diagnosis and lost follow-up. A total of 1344 breast cancer patients were treated with surgery in our hospital. We further excluded 327 patients for the following reasons: without complete medical records (n = 146), unable to determine the menopausal status (n = 42), death for other reasons (n = 63), and lost to follow-up (n = 76). The final analytic cohort consisted of 1017 women.
All study participants provided written informed consent and the study protocol and procedures were approved by the institutional review boards at the Affiliated Changzhou No. 2 People's Hospital with Nanjing Medical University.
2.2. Group definitions
We calculated BMI as weight in kilograms divided by height in square meters. BMI was categorized according to WHO standard: normal weight, BMI < 25.0 kg/m2; overweight, 25.0≤BMI < 30.0 kg/m2; and obesity, BMI≥30.0 kg/m2. Patient's weight and height were recorded before surgery. Three groups were divided according to BMI when breast cancer diagnosis: normal weight group (474), overweight group (351), and obesity group (192). Furthermore, a stratified analysis about relationship between BMI and prognosis of breast cancer was conducted in line with menopausal status when breast cancer diagnosed. Menopausal status was defined by 1 year of amenorrhea, or previous bilateral oophorectomy. In the present study, a total of 416 breast cancer patients were postmenopausal, and 601 breast cancer patients were premenopausal. The differences in clinicopathologic characteristics and 5-year breast cancer outcomes between 3 groups were compared.
2.3. Follow-up and outcomes
Follow-up has been maintained by reviewing clinical charts and by contacting patients via telephone or mail. Events used for the analysis were 5-year mortality of breast cancer or relapse including local, regional and contralateral breast cancer or distant breast cancer recurrence. Survival status was censored at the date of last contact or 31 December 2017 (last follow-up). DFS defined as the time of diagnosis to development of first evidence of recurrence (distant metastasis or local regional recurrence) or date of last follow-up. OS was defined as from the time of diagnosis to last follow-up or time of mortality from breast cancer (patients dying from other causes within 5 years of breast cancer diagnosis were excluded).
2.4. Statistical analysis
In our study, the associations between different BMI groups and clinicopathologic characteristics of breast cancer patients were analyzed by the chi-square test. The Kaplan–Meier methods were used to calculate 5-year DFS and OS, and the significance was tested by log-rank test. Factors with P < .10 in univariate analysis were included in the multivariate Cox model for multivariate analysis. Multivariable Cox proportional hazard models were used to estimate the adjusted hazard ratios (HRs) of different BMI groups. In multivariate analysis, the normal weight group was regarded as a reference for calculating the HR of BMI. Stratified analyses were conducted to explore whether the effects of obesity or overweight on prognosis of breast cancer were modified by menopausal status. All statistical analyses were carried out with SPSS software (version 17.0), and P < .05 were considered statistically significant.
3. Results
3.1. Clinical characteristics of patients in study groups
The median follow-up time for this study was 80 months (13–140 months). Of the 1017 breast cancer patients, 474 were normal weight, 351 patients were overweight, and 192 were obesity. The clinicopathological features of these 3 groups were summarized in Table 1. The older age (≥66 years) tended to distribute in overweight (14.0%) and obesity (16.1%) groups compared to normal weight group (6.8%) (P < .001). Besides, the larger size of tumors and postmenopausal patients were more likely to distribute (P < .001) in overweight and obesity groups, and less patients chose anthracycline and/or taxane to be chemotherapy regimen in these 2 groups (P = .036). Lymph node metastasis, pathological subtype, histological grade, ER and PR status, HER2 status, the percentages of patients receiving radiotherapy, and hormone therapy were not significantly different among these 3 groups (Table 1).
Table 1.
Comparison of clinicopathological characteristics among breast cancer patients according to the body mass index (kg/m2).

3.2. 5-Year DFS and OS
In total, 180 patients suffered from breast cancer relapse within 5 years after breast cancer diagnosis, and the number of recurrent patients was 60, 71, and 49 in the normal weight group, overweight group, and obesity group, respectively. The 5-year DFS and breast cancer relapse rate for the whole cohort were 82.3% (837/1017) and 17.7% (180/1017), respectively. A total of 115 patients died of breast cancer, 34 patients were in the normal weight group, 46 cases were in the overweight group, and 35 cases were in the obesity group. The 5-year OS and breast cancer mortality rate were 88.7% (902/1017) and 11.3% (115/1017), respectively. The Kaplan–Meier analysis showed that there was a significant difference in the DFS and OS between the three groups (Table 2). The DFS was 87.3, 79.8, 74.5% for normal weight, overweight, and obesity groups, respectively (P < .001) (Table 2, Fig. 1a). The OS was 92.8, 86.9, 81.8% for normal weight, overweight, and obesity groups, respectively (P < .001) (Table 2, Fig. 1B). The 5-year DFS in the premenopausal and postmenopausal groups were 83.4% and 80.8% (P = .311) (Table 2). The 5-year OS in the premenopausal and postmenopausal groups were 89.7% and 87.3% (P = .222) (Table 2).
Table 2.
Univariate analysis of 5-year survival for all patients.
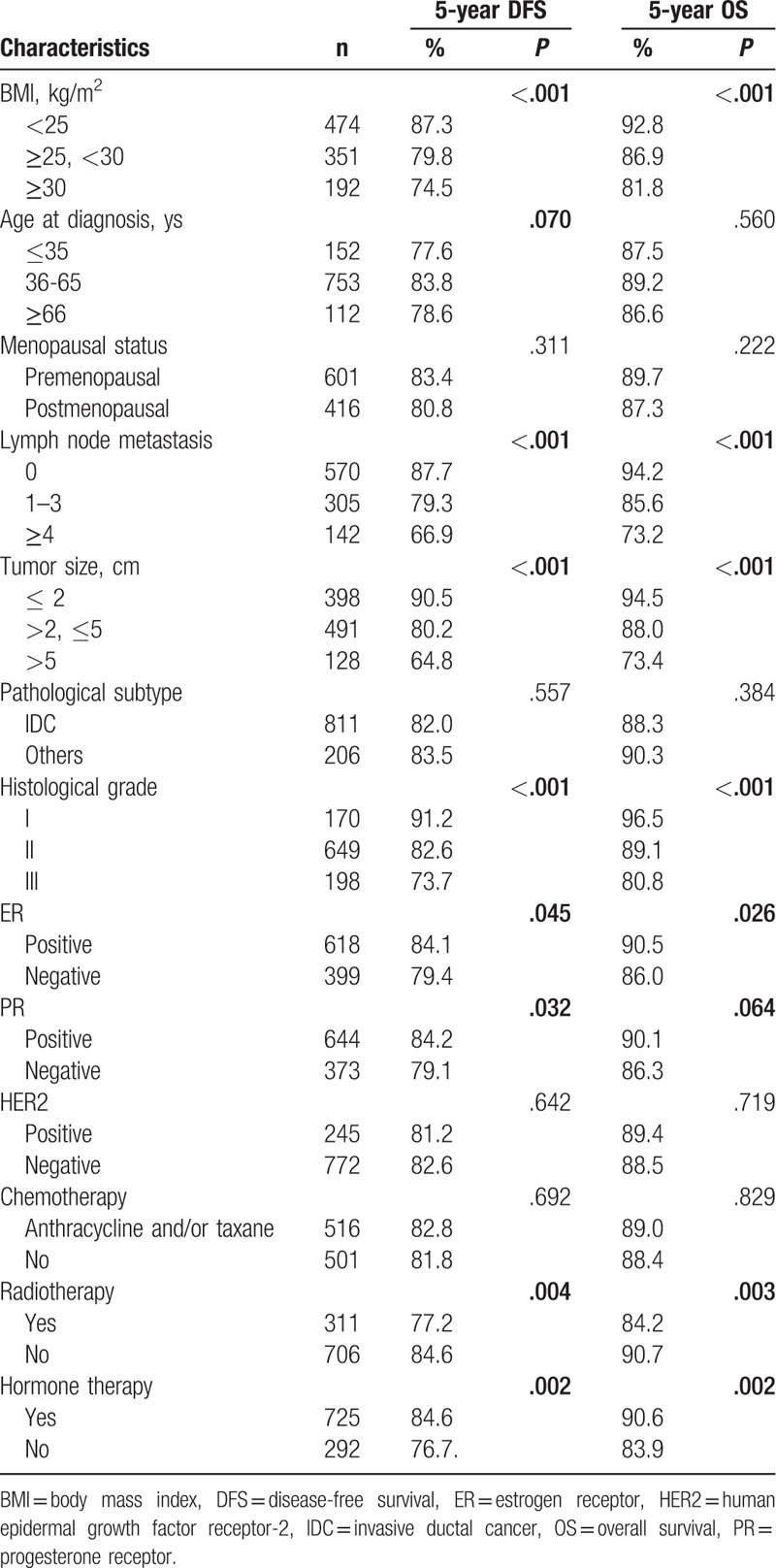
Figure 1.

Kaplan–Meier analysis of 5-year survival outcomes for breast cancer patients according to BMI. A, Disease-free survival. B, Overall survival. Statistically significant differences between the groups were estimated by log-rank test.
3.3. BMI and breast cancer relapse and mortality risks for all patients
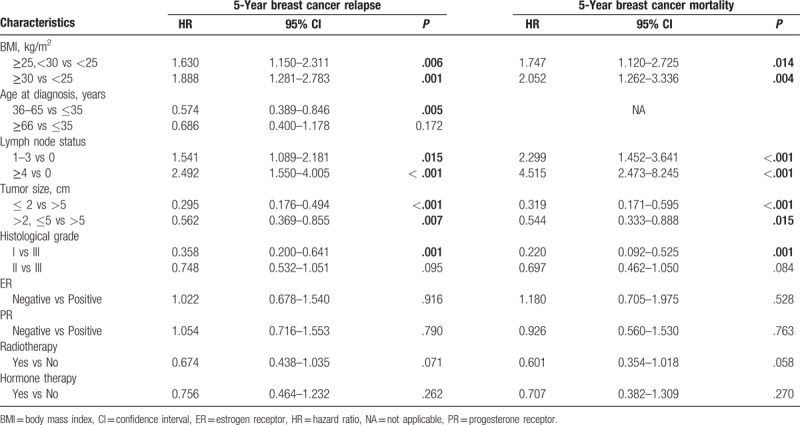
All factors were included in the univariate analysis, and the 5-year DFS and OS were significantly different in terms of lymph node status, tumor size, histological grade, expression of ER and PR, radiotherapy, and hormone therapy (P < .05) (Table 2). In multivariate analysis, when compared with normal weight patients, the risks of 5-year breast cancer relapse (HR, 1.630; 95% confidence interval [CI], 1.150–2.311; P = .006) and mortality (HR, 1.747; 95% CI, 1.120–2.725; P = .014) for the overweight patients were significantly increased after adjusted for other variables, and the 5-year risks of breast cancer relapse (HR, 1.888; 95% CI, 1.281–2.783; P = .001) and mortality (HR, 2.052; 95% CI, 1.262–3.336; P = .004) were also significantly increased in obesity group after adjusting for age, lymph node status, tumor size, histological grade, ER, PR, radiotherapy, and hormone therapy (Table 3).
Table 3.
Multivariate Cox proportional hazards model for survival in all patients.
3.4. BMI and prognosis of breast cancer for premenopausal patients
In univariate analysis, both the 5-year DFS and OS were significantly different among the 3 groups for premenopausal patients. The DFS was 86.7, 81.9, 75.5% for normal weight, overweight, and obesity groups, respectively (P = .026) (Fig. 2A), and the OS was 92.4, 88.3, 83.7% for normal weight, overweight, and obesity groups, respectively (P = .034) (Fig. 2B) (Table 4). Besides, the 5-year DFS and OS were significantly different in terms of age, lymph node status, tumor size, histological grade, radiotherapy, and hormone therapy (Table 4). By multivariate analysis, the 5-year risks of breast cancer relapse (HR, 1.824; 95% CI, 1.096–3.037; P = .021) and mortality (HR, 1.948; 95% CI, 1.029–3.687; P = .041) were significantly increased in obesity group compared to normal weight group after adjusting for confounding factors. However, the 5-year risks of relapse (HR, 1.462; 95% CI, 0.926–2.308; P = .103) and mortality (HR, 1.619; 95% CI, 0.904–2.898; P = .105) were non-significantly increased for overweight group after adjusting for age, lymph node status, tumor size, histological grade, radiotherapy, and hormone therapy (Table 5).
Figure 2.

Kaplan–Meier analysis of 5-year survival outcomes for premenopausal breast cancer patients according to BMI. A, Disease-free survival. B, Overall survival. Statistically significant differences between the groups were estimated by log-rank test.
Table 4.
Univariate analysis of 5-year survival for premenopausal patients (n = 601).

Table 5.
Multivariate Cox proportional hazards model for survival in premenopausal patients.

3.5. BMI and prognosis of breast cancer for postmenopausal patients
When compared to the normal weight group, the 5-year DFS and OS of overweight and obesity groups for postmenopausal patients were significantly decreased. As shown in Table 6, the 5-year DFS was 88.7, 77.3, 73.4% for normal weight, overweight, and obesity groups, respectively (P = .003) (Fig. 3A); the 5-year OS was 93.7, 85.3, 79.8% for normal weight, overweight, and obesity groups, respectively (P = .003) (Fig. 3B). Additionally, the 5-year DFS and OS were significantly different in terms of lymph node status, tumor size, histological grade, PR expression, and hormone therapy (P < .05) (Table 6). By multivariate analysis, 5-year risks of relapse (HR, 1.884; 95% CI, 1.066–3.331; P = .029) and mortality (HR, 2.210; 95% CI, 1.040–4.696; P = .039) were increased for overweight group after adjusting for lymph node status, tumor size, histological grade, ER, PR, chemotherapy, and hormone therapy (Table 7). The 5-year risks of breast cancer relapse (HR, 2.031; 95% CI, 1.091–3.782; P = .026) and mortality (HR, 2.493; 95% CI, 1.117–5.564; P = .026) were also increased in obesity group after adjusting confounding factors (Table 7).
Table 6.
Univariate analysis of 5-year survival for postmenopausal patients (n = 416).

Figure 3.
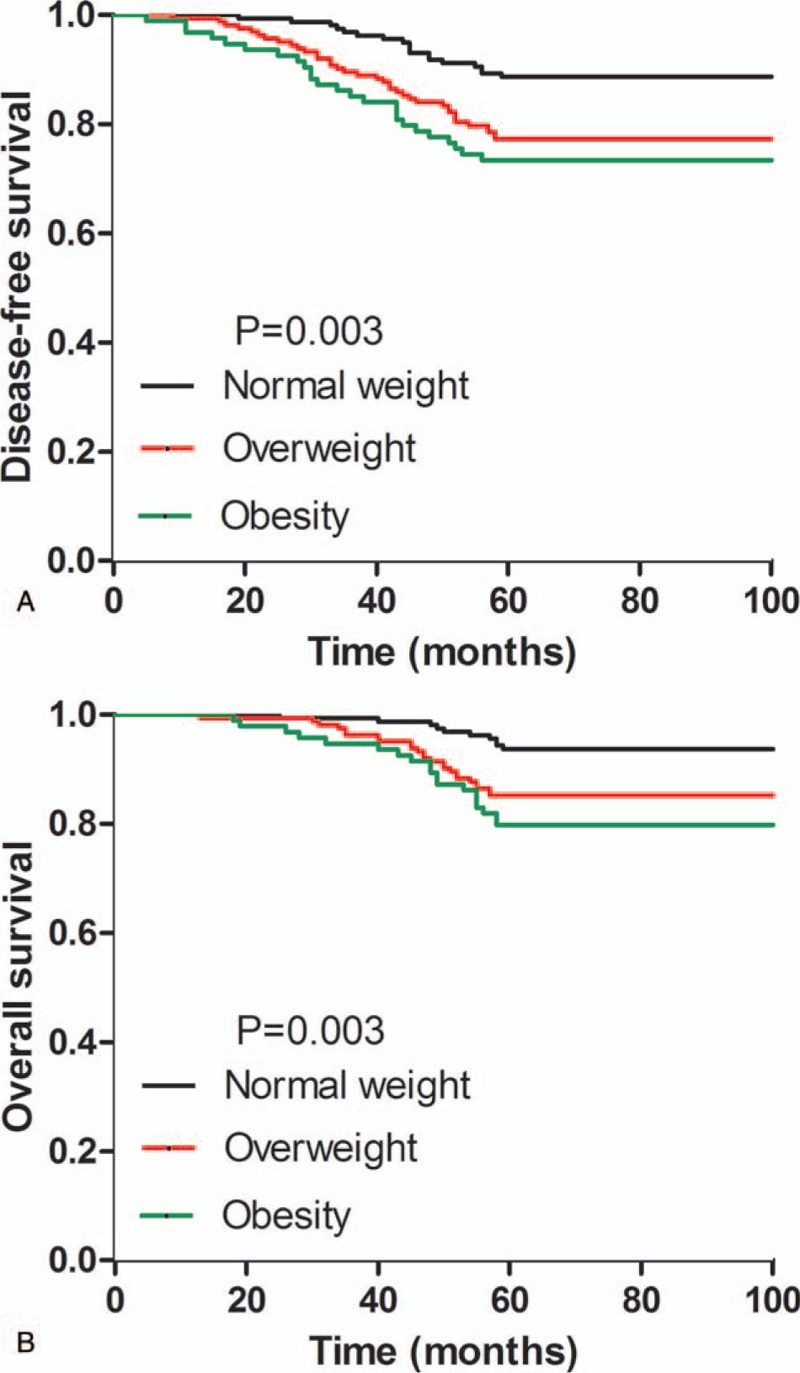
Kaplan–Meier analysis of 5-year survival outcomes for postmenopausal breast cancer patients according to BMI. A, Disease-free survival. B, Overall survival. Statistically significant differences between the groups were estimated by log-rank test.
Table 7.
Multivariate Cox proportional hazards model for survival in postmenopausal patients.

4. Discussion
Obesity and overweight in adults have been reported to be correlated with a greater risk of breast cancer,[6,19] while the studies that evaluated influence of overweight and obesity on breast cancer survival have yielded mixed findings.[20,21] In an effort to address this gap, we conducted a retrospective study to elucidate the relationship between obesity and breast cancer prognosis.
In our study, we found that overweight and obese breast cancer patients were associated with larger size tumors compared to normal weight patients. Additionally, patients tended to be older, the proportion of postmenopausal patients was increased, and lesser patients choose anthracycline and/or taxane to be chemotherapy regimen in overweight and obesity groups. There were also several studies demonstrating that obese women develop aggressive breast cancer with a significantly larger size compared to normal weight women,[22,23] and patients with BMI ≥25 kg/m2 tended to be older and to have larger tumor size,[24] postmenopausal patients were more distributed in patients with higher BMI (BMI≥25.8 kg/m2).[16] Interestingly, some authors claimed that women with higher BMI undergo less screening so tend to have more advanced disease at presentation.[25]
We also found that overweight and obesity were independent predictors for increased risks of 5-year breast cancer relapse and mortality for the whole cohort. In a previous study from America, after 5-year follow-up, the authors calculated that HRs for risks of recurrence was 1.18 (95% CI 1.02–1.36) in overweight patients and breast cancer mortality was 1.23 (95% CI 1.00–1.52) in the obese group relative to the normal weight, while overweight did not influence recurrence and obesity wasn’t a predictor for breast cancer mortality.[20] In a study including African TNBC patients, overweight was significantly related to breast cancer mortality (HR 2.903, 95% CI: 1.551–5.432) and recurrence (HR 1.899, 95% CI: 1.05- 3.433) by multivariable analysis.[26] Another study found that the risks of developing distant metastasis were significantly increased for Danish obese patients, while both obesity and overweight women had higher risk of breast cancer mortality relative to normal weight women.[27] However, Kawai et al [18] reported that obesity was an independent risk factor for breast cancer death (HR: 1.47; 95% CI: 1.11–1.93) but not for recurrence, and overweight had no association with breast cancer prognosis. Nevertheless, in another study from America, Kwan et al[14] observed that overweight or obesity was not associated with increased risk of recurrence and breast cancer mortality compared to normal weight, a report from the Korean Breast Cancer Society demonstrated similar results.[21]
Although the association between obesity/overweight and the prognosis of breast cancer patients remains controversial, it has been reported that impact of BMI on prognosis of breast cancer may relate to menopausal status.[15] To identify this issue, we conducted a stratified analysis according to the menopausal status.
In stratified analysis, we found that being overweight was associated with increased risks of breast cancer relapse and mortality within 5 years after breast cancer diagnosis for postmenopausal but not for premenopausal women, and obesity was an independently poor predictor for breast cancer relapse and mortality regardless to menopausal status. Similar to our study, Reeves et al[4] have reported that obesity and overweight were related to increased breast cancer progression and mortality primarily in British postmenopausal women. A meta-analysis of 82 studies that included 213,075 breast cancer patients demonstrated that obesity was associated with higher risk of breast cancer mortality (HR, 1.41; 95% CI, 1.29–1.53) in both premenopausal (HR, 1.75; 95% CI, 1.26–2.41) and postmenopausal (HR, 1.34; 95% CI, 1.18–1.53) women,[28] similar results were found by Niraula et al.[29] A study from Japan found that obesity and overweight were associated with a nonsignificant higher risk of recurrence and breast cancer death for premenopausal women, and obesity but not overweight was associated with a significant higher risk of breast cancer death for postmenopausal women (not for recurrence),[18] results from a cohort study also suggest that obesity (not overweight) was an independent poor prognostic predictor for American postmenopausal breast cancer patients.[17] However, some authors[9] found that overweight or obesity is significantly associated with a shorter OS and DFS for premenopausal and perimenopausal patients. Besides, another study including American breast cancer patients also stated that overweight or obesity was positively associated with recurrence in premenopausal rather than postmenopausal women.[15] Moreover, some studies indicated that overweight is an independent prognostic factor for increased breast cancer mortality and recurrence in premenopausal TNBC women, while similar results were not found in postmenopausal women.[26,30]
Although the effects of BMI on breast cancer prognosis are still controversial, the possible mechanisms have been disclosed as follow. Some authors reported that in postmenopausal patients with higher BMI, increased synthesis of peripheral estrogen in adipose tissue and reduced sex hormone binding globulin might be responsible for the poor breast cancer prognosis due to enhanced aromatase activity may induce and stimulate the growth of abnormal mammary cells,[31,32] and higher BMI women may not fully benefit from aromatase inhibitors in postmenopausal women.[33] Furthermore, women with higher BMI and older age may withstand comorbidities and chemotherapy dose reduction due to concerns about toxicity,[34] and women with higher BMI may present increased levels of insulin, insulin-like growth factor, and hormones with potent mitogenic activity.[35] Besides, paracrine secretion of interleukin-6 and tumor necrosis factor-alpha and the establishment of a pro-inflammatory micro-environment may promote tumor growth, metastasis.[36] Several cytokines produced by obese adipose tissue may promote the progression of breast cancer via upregulating breast cancer stem cells, inhibiting the antitumor immunity and stimulating breast tumor angiogenesis.[37] Thus higher BMI might induce the development of breast cancer.
Our study found that obesity at diagnosis was related to poor prognosis of breast cancer irrespective of menopausal status, while overweight was only associated with prognosis of postmenopausal patients. However, our study has some limitations. Firstly, there is an active debate regarding the limitations of BMI to define obesity and overweight categories for various populations.[38] Secondly, negative effects of weight change on breast cancer prognosis have been reported,[39,40] the association limited to BMI when cancer diagnosis may attenuate over time, but we did not dynamically monitor changes in body weight in breast cancer patients. Thirdly, obese patients are thought to have a higher risk of comorbid conditions, but our study did not include information on comorbidities. Finally, the sample size was small relatively, follow-up period was short, and we did not adjust other potential confounders. The limitations mentioned above may partly bring about the discrepancy in our study.
In conclusion, our study adds to the literature by showing a link of obesity, menopausal status, and breast cancer prognosis. However, further studies with larger sample sizes and more comprehensive design are urgently warranted.
Author contributions
Investigation: Yulan Zhu.
Validation: Qi Qian.
Writing – original draft: Li Sun.
Writing – review & editing: Liming Tang.
Footnotes
Abbreviations: BMI = body mass index, CIs = confidence intervals, DFS = disease-free survival, ER = estrogen receptor, HER2 = human epidermal growth factor receptor-2, HRs = hazard ratios, OS = overall survival, PR = progesterone receptor, TNBC = triple-negative breast cancer.
LT will handle the production process.
The authors declare no conflicts of interest.
References
- [1].Finucane MM, Stevens GA, Cowan MJ, et al. National, regional, and global trends in body-mass index since 1980: systematic analysis of health examination surveys and epidemiological studies with 960 country-years and 9.1 million participants. Lancet 2011;377:557–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Kelly T, Yang W, Chen CS, et al. Global burden of obesity in 2005 and projections to 2030. Int J Obes (Lond) 2008;32:1431–7. [DOI] [PubMed] [Google Scholar]
- [3].Wang J, Yang DL, Chen ZZ, et al. Associations of body mass index with cancer incidence among populations, genders, and menopausal status: a systematic review and meta-analysis. Cancer Epidemiol 2016;42:1–8. [DOI] [PubMed] [Google Scholar]
- [4].Reeves GK, Pirie K, Beral V, et al. Cancer incidence and mortality in relation to body mass index in the Million Women Study: cohort study. BMJ 2007;335:1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin 2015;65:5–29. [DOI] [PubMed] [Google Scholar]
- [6].Neuhouser ML, Aragaki AK, Prentice RL, et al. Overweight, obesity, and postmenopausal invasive breast cancer risk: a secondary analysis of the women's health initiative randomized clinical trials. JAMA Oncol 2015;1:611–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Protani M, Coory M, Martin JH. Effect of obesity on survival of women with breast cancer: systematic review and meta-analysis. Breast Cancer Res Treat 2010;123:627–35. [DOI] [PubMed] [Google Scholar]
- [8].Gevorgyan A, Bregni G, Galli G, et al. Body mass index and clinical benefit of fulvestrant in postmenopausal women with advanced breast cancer. Tumori 2016;102:e11–4. [DOI] [PubMed] [Google Scholar]
- [9].Berclaz G, Li S, Price KN, et al. Body mass index as a prognostic feature in operable breast cancer: the International Breast Cancer Study Group experience. Ann Oncol 2004;15:875–84. [DOI] [PubMed] [Google Scholar]
- [10].Caan BJ, Kwan ML, Hartzell G, et al. Pre-diagnosis body mass index, post-diagnosis weight change, and prognosis among women with early stage breast cancer. Cancer Causes Control 2008;19:1319–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Bao PP, Cai H, Peng P, et al. Body mass index and weight change in relation to triple-negative breast cancer survival. Cancer Causes Control 2016;27:229–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Calle EE, Rodriguez C, Walker-Thurmond K, et al. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med 2003;348:1625–38. [DOI] [PubMed] [Google Scholar]
- [13].Dawood S, Lei X, Litton JK, et al. Impact of body mass index on survival outcome among women with early stage triple-negative breast cancer. Clin Breast Cancer 2012;12:364–72. [DOI] [PubMed] [Google Scholar]
- [14].Kwan ML, Chen WY, Kroenke CH, et al. Pre-diagnosis body mass index and survival after breast cancer in the After Breast Cancer Pooling Project. Breast Cancer Res Treat 2012;132:729–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Warren LE, Ligibel JA, Chen YH, et al. Body mass index and locoregional recurrence in women with early-stage breast cancer. Ann Surg Oncol 2016;23:3870–9. [DOI] [PubMed] [Google Scholar]
- [16].Kawai M, Minami Y, Nishino Y, et al. Body mass index and survival after breast cancer diagnosis in Japanese women. BMC Cancer 2012;12:149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Conroy SM, Maskarinec G, Wilkens LR, et al. Obesity and breast cancer survival in ethnically diverse postmenopausal women: the Multiethnic Cohort Study. Breast Cancer Res Treat 2011;129:565–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Kawai M, Tomotaki A, Miyata H, et al. Body mass index and survival after diagnosis of invasive breast cancer: a study based on the Japanese National Clinical Database-Breast Cancer Registry. Cancer Med 2016;5:1328–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Chlebowski RT. Obesity and breast cancer outcome: adding to the evidence. J Clin Oncol 2012;30:126–8. [DOI] [PubMed] [Google Scholar]
- [20].Jiralerspong S, Kim ES, Dong W, et al. Obesity, diabetes, and survival outcomes in a large cohort of early-stage breast cancer patients. Ann Oncol 2013;24:2506–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Moon HG, Han W, Noh DY. Underweight and breast cancer recurrence and death: a report from the Korean Breast Cancer Society. J Clin Oncol 2009;27:5899–905. [DOI] [PubMed] [Google Scholar]
- [22].Kaviani A, Neishaboury M, Mohammadzadeh N, et al. Effects of obesity on presentation of breast cancer, lymph node metastasis and patient survival: a retrospective review. Asian Pac J Cancer Prev 2013;14:2225–9. [DOI] [PubMed] [Google Scholar]
- [23].Daling JR, Malone KE, Doody DR, et al. Relation of body mass index to tumor markers and survival among young women with invasive ductal breast carcinoma. Cancer 2001;92:720–9. [DOI] [PubMed] [Google Scholar]
- [24].Chen HL, Ding A, Wang ML. Impact of central obesity on prognostic outcome of triple negative breast cancer in Chinese women. SpringerPlus 2016;5:594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Wee CC, McCarthy EP, Davis RB, et al. Obesity and breast cancer screening. J Gen Intern Med 2004;19:324–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Al Jarroudi O, Abda N, Seddik Y, et al. Overweight: is it a prognostic factor in women with triple-negative breast cancer? Asian Pac J Cancer Prev 2017;18:1519–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Ewertz M, Jensen MB, Gunnarsdottir KA, et al. Effect of obesity on prognosis after early-stage breast cancer. J Clin Oncol 2011;29:25–31. [DOI] [PubMed] [Google Scholar]
- [28].Chan DS, Vieira AR, Aune D, et al. Body mass index and survival in women with breast cancer-systematic literature review and meta-analysis of 82 follow-up studies. Ann Oncol 2014;25:1901–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Niraula S, Ocana A, Ennis M, et al. Body size and breast cancer prognosis in relation to hormone receptor and menopausal status: a meta-analysis. Breast Cancer Res Treat 2012;134:769–81. doi:10.1007/s10549-012-2073-x. [DOI] [PubMed] [Google Scholar]
- [30].Hao S, Liu Y, Yu KD, et al. Overweight as a prognostic factor for triple-negative breast cancers in chinese women. PloS One 2015;10:e0129741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Key TJ, Appleby PN, Reeves GK, et al. Body mass index, serum sex hormones, and breast cancer risk in postmenopausal women. J Natl Cancer Inst 2003;95:1218–26. [DOI] [PubMed] [Google Scholar]
- [32].Bulun SE, Chen D, Moy I, et al. Aromatase, breast cancer and obesity: a complex interaction. Trends Endocrinol Metab 2012;23:83–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Folkerd EJ, Dixon JM, Renshaw L, et al. Suppression of plasma estrogen levels by letrozole and anastrozole is related to body mass index in patients with breast cancer. J Clin Oncol 2012;30:2977–80. [DOI] [PubMed] [Google Scholar]
- [34].Griggs JJ, Sorbero ME, Lyman GH. Undertreatment of obese women receiving breast cancer chemotherapy. Arch Intern Med 2005;165:1267–73. [DOI] [PubMed] [Google Scholar]
- [35].Stephenson GD, Rose DP. Breast cancer and obesity: an update. Nutr Cancer 2003;45:1–6. [DOI] [PubMed] [Google Scholar]
- [36].Lorincz AM, Sukumar S. Molecular links between obesity and breast cancer. Endocr Relat Cancer 2006;13:279–92. [DOI] [PubMed] [Google Scholar]
- [37].Picon-Ruiz M, Morata-Tarifa C, Valle-Goffin JJ, et al. Obesity and adverse breast cancer risk and outcome: mechanistic insights and strategies for intervention. CA Cancer J Clin 2017;67:378–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Shah NR, Braverman ER. Measuring adiposity in patients: the utility of body mass index (BMI), percent body fat, and leptin. PloS One 2012;7:e33308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Bradshaw PT, Ibrahim JG, Stevens J, et al. Postdiagnosis change in bodyweight and survival after breast cancer diagnosis. Epidemiology 2012;23:320–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Chen X, Lu W, Zheng W, et al. Obesity and weight change in relation to breast cancer survival. Breast Cancer Res Treat 2010;122:823–33. [DOI] [PMC free article] [PubMed] [Google Scholar]


