Key Points
In the phase 3 TOWER study, exposure-adjusted AE rates were lower for blinatumomab vs SOC chemotherapy in Ph− B-cell r/r ALL patients.
These data further support the role of blinatumomab as an efficacious and well-tolerated treatment option for B-cell r/r ALL patients.
Abstract
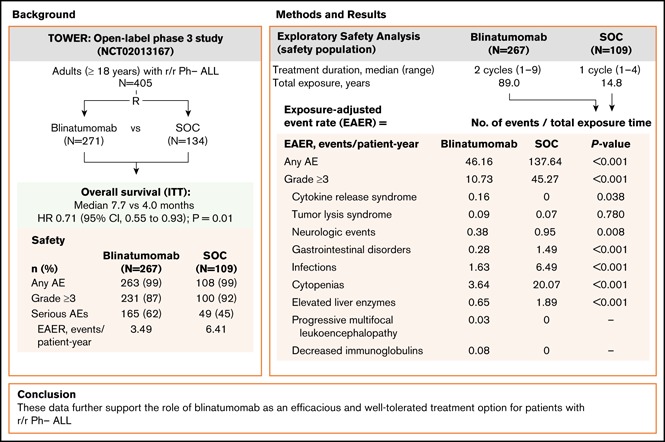
In the phase 3 TOWER study, blinatumomab demonstrated an overall survival benefit over standard-of-care chemotherapy (SOC) in adults with relapsed or refractory (r/r) Philadelphia chromosome–negative (Ph−) B-precursor acute lymphoblastic leukemia (ALL). Nearly all patients in both treatment arms experienced an adverse event (AE), and the incidence rate of serious AEs was higher for blinatumomab. However, as treatment exposure differed between the 2 arms, we conducted an exploratory safety analysis comparing exposure-adjusted event rates (EAERs) of blinatumomab vs SOC. Analyses were conducted for all patients who received therapy (safety population). Patients received a median (range) of 2 cycles (1-9) of blinatumomab (N = 267) vs 1 cycle (1-4) of SOC (N = 109). Grade ≥3 AE rates were generally higher in cycle 1 of blinatumomab than in cycle 2 (76% vs 37%). After adjusting for time on treatment, EAERs of grade ≥3 were significantly lower for blinatumomab vs SOC overall (10.73 vs 45.27 events per patient-year; P < .001) and for events of clinical interest, including infections (1.63 vs 6.49 events per patient-year; P < .001), cytopenias (3.64 vs 20.07 events per patient-year; P < .001), and neurologic events (0.38 vs 0.95 events per patient-year; P = .008). The EAER of grade ≥3 cytokine-release syndrome was higher for blinatumomab than for SOC (0.16 vs 0 events per patient-year; P = .038). These data further support the role of blinatumomab as an efficacious and well-tolerated treatment option for patients with r/r Ph− ALL. This trial was registered at www.clinicaltrials.gov as #NCT02013167.
Visual Abstract
Introduction
In adults with Philadelphia chromosome–negative (Ph−) B-precursor acute lymphoblastic leukemia (ALL), cure rates remain relatively low, particularly in patients who relapse or have primary refractory disease.1-3 Historically, remission rates in relapsed/refractory (r/r) ALL range from 40% to 45% with median overall survival (OS) of up to 9 months.4-7 Outcomes are particularly poor in patients who relapse after allogeneic hematopoietic stem cell transplantation (alloHSCT) or within 12 months of remission, in patients who are primary refractory to induction, and in patients who have received multiple lines of therapy.4,6-9 In these patients, remission rates are generally 30% or less, and median OS is 3 to 6 months.6-9 Furthermore, multiagent chemotherapy regimens for ALL can be associated with significant toxicity.2,10 Because of the myelosuppressive nature of some regimens and the underlying disease, patients are susceptible to serious and sometimes fatal infections.11-13 Thus, prolonged therapy with multiagent chemotherapy for patients with r/r ALL remains a clinical challenge due to the potential for cumulative toxicity.
Blinatumomab, a bispecific T-cell engager antibody construct that redirects CD3+ T cells to lyse CD19+ B cells,14 is indicated for the treatment of patients with r/r B-precursor ALL. The efficacy and safety of blinatumomab in r/r Ph− ALL was first demonstrated in single-arm phase 2 studies15,16 and was recently confirmed in the randomized phase 3 TOWER study comparing blinatumomab with standard-of-care chemotherapy (SOC),17 which demonstrated significantly longer OS with blinatumomab (median 7.7 vs 4.0 months; hazard ratio, 0.71; 95% confidence interval, 0.55-0.93; P = .01). During the study, nearly all patients (99%) in both treatment arms experienced an adverse event (AE); rates of grade ≥3 AEs were similar (87% vs 92%), but the rate of serious AEs was higher in the blinatumomab arm (62% vs 45%). However, duration of treatment exposure varied between the 2 arms.18,19 Specifically, patients received a median of 2 cycles (range, 1-9) of blinatumomab compared with a median of 1 cycle (range, 1-4) of SOC. After adjusting for treatment exposure time, the exposure-adjusted event rate (EAER) for serious AEs was lower for blinatumomab (349.4 vs 641.9 events per 100 patient-years of exposure).17
To better characterize the safety of blinatumomab, we conducted an exploratory analysis of AE data from the phase 3 TOWER study, comparing AEs in patients treated with blinatumomab vs SOC after adjusting for varying treatment exposure times. We report that EAERs were significantly lower for blinatumomab vs SOC overall, and for AEs of clinical interest, including neurologic events, gastrointestinal disorders, infections, and cytopenias.
Methods
The TOWER study was an international, randomized, open-label, phase 3 study of blinatumomab vs SOC in adults (≥18 years of age) with Ph− r/r B-cell precursor ALL. Eligible patients had received at least 1 prior intensive multiagent chemotherapy regimen and were refractory to primary induction or salvage therapy, had experienced a relapse <12 months after first remission, or had relapsed after a second or later remission or after alloHSCT. Other eligibility criteria included >5% bone marrow blasts, Eastern Cooperative Oncology Group performance status of ≤2, and adequate organ function. Key exclusion criteria included clinically relevant central nervous system pathology; isolated extramedullary disease; chemotherapy within 2 weeks of study treatment, immunotherapy within 4 weeks, autologous HSCT within 6 weeks, or alloHSCT within 12 weeks; and acute (grade 2 or higher) or chronic graft-versus-host disease.
Patients were randomized 2:1 to receive either blinatumomab or SOC (investigator’s choice of 1 of 4 regimens). Randomization was stratified by age (<35 years or ≥35 years), prior salvage therapy (yes or no), and prior alloHSCT (yes or no). The primary efficacy end point was OS.
The study was conducted in accordance with the International Conference on Harmonisation Good Clinical Practice and the Declaration of Helsinki, and the study protocol was approved by the institutional review boards of participating institutions. All patients provided written informed consent. Additional details of the study design, patient selection, and methods have been published previously.17
Treatment
Patients received blinatumomab as a continuous IV infusion at fixed stepwise doses in 6-week cycles: 4 weeks on (9 µg per day for days 1-7 of cycle 1 and then 28 µg per day thereafter) and 2 weeks off for each cycle. Patients received up to 2 induction cycles and up to 3 consolidation cycles, provided that bone marrow blasts were ≤5% after induction. Patients with ≤5% blasts after consolidation could continue on maintenance therapy with blinatumomab for up to 12 months. Blinatumomab (28 µg per day) maintenance was administered in a 12-week cycle (4 weeks on and 8 weeks off). Dexamethasone premedication (20 mg IV) was required prior to each infusion and dose step to prevent cytokine-release syndrome (CRS). Dexamethasone pretreatment (10-24 mg/m2 per day oral or IV) was also required for patients with high tumor burden (>50% blasts or ≥15 × 109/L peripheral blast count). Inpatient administration was required for the first 9 days of cycle 1, the first 2 days of cycle 2, and for any dose step; outpatient administration was permitted thereafter. Further details on protocol-specified dose interruptions, reductions, and discontinuations have been described previously.17
Patients assigned to the SOC arm received 1 of 4 regimens based on investigator’s choice prior to randomization: fludarabine, cytarabine, and granulocyte colony-stimulating factor plus or minus anthracycline; a high-dose cytarabine−based regimen; a high-dose methotrexate–based regimen; or a clofarabine-based regimen (see “Standard-of-care chemotherapy [SOC] regimens” in supplemental Data for additional details). Dose adjustments were permitted for patients older than 60 years of age. Patients received up to 2 cycles of induction and could continue SOC if bone marrow blasts were ≤5% after induction. Dose interruptions, reductions, and discontinuations were allowed, but not required, for specific AEs.
Prior to the start of treatment and following each induction and consolidation cycle, all patients received intrathecal central nervous system prophylaxis treatment (eg, methotrexate, cytarabine, dexamethasone, or hydrocortisone) according to institutional or national guidelines. Patients could discontinue study treatment any time after cycle 1 and proceed to alloHSCT, per investigator discretion.
Safety assessments
Safety end points included the incidence of treatment-emergent AEs, serious and fatal AEs, and changes in select vital signs and laboratory parameters. Patients were monitored for treatment-emergent AEs throughout the treatment period and up to 30 days after their last study treatment, or prior to undergoing transplant. Based on prior studies of blinatumomab,15,16 AE categories of interest included neurologic events, CRS, infections, elevated liver enzymes, tumor lysis syndrome, acute pancreatitis, cytopenias, decreased immunoglobulins, and leukoencephalopathy; gastrointestinal disorders were included for SOC. AEs were classified according to Medical Dictionary for Regulatory Activities (version 18.1) terminology and were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE; version 4.0).20
Statistical analyses
Safety analyses were conducted with the safety population, defined as all patients who received any protocol-specified therapy who were analyzed according to their received treatment. Exposure adjustment of the safety data was an exploratory analysis. EAERs from each treatment arm were estimated by dividing the number of events reported by the total exposure time of the patients in the treatment arm. Total exposure time was the summation of exposure (time from first dose until either last dose date plus 30 days or the data cutoff date) from all patients in a treatment group converted to patient-year unit of time. To compare EAERs between treatment arms, a Poisson regression model was used, with number of events as the dependent variable and log (exposure time) as the offset. All statistical tests were 2-sided with P < .05 used to identify significance; there were no adjustments for multiple comparisons. Statistical analyses were conducted with SAS software (version 9.4 or later). All study data were available to all authors.
Results
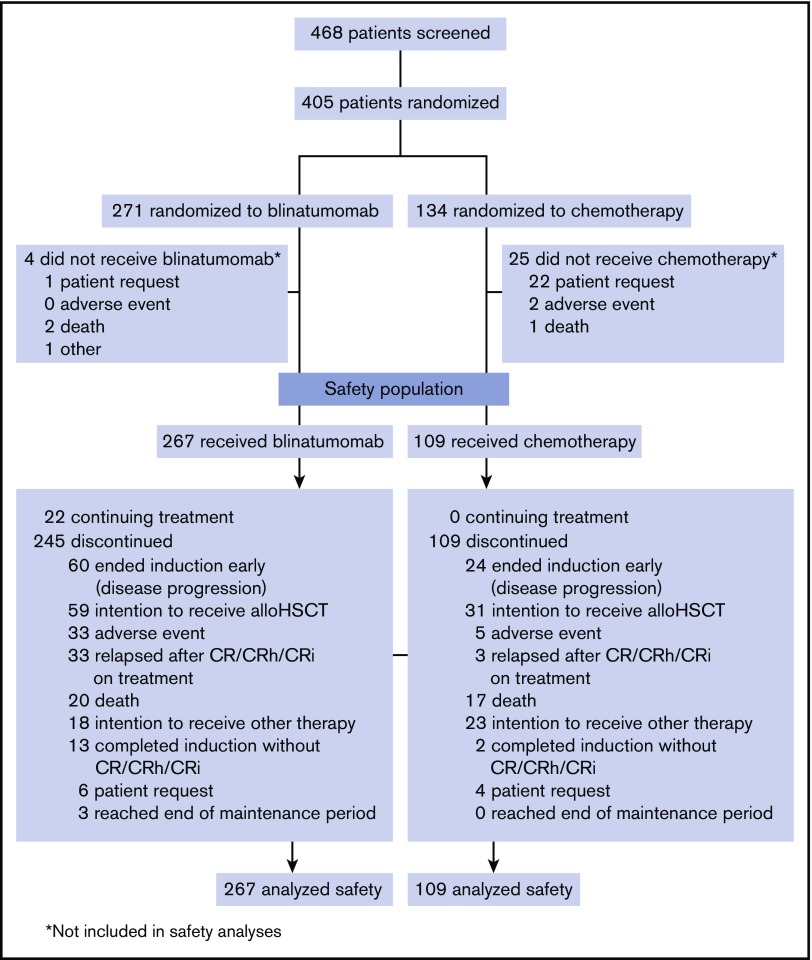
Between January 2014 and September 2015, 405 patients were randomized to blinatumomab and SOC, with 267 and 109 patients, respectively, receiving study treatment and comprising the safety population (Figure 1). Demographic and baseline characteristics for the safety population were balanced between both treatment arms (Table 1) and similar to those of the intention-to-treat population.17 This was a heavily pretreated population with high tumor burden. More than 75% of patients had ≥50% bone marrow blasts, and approximately half either had received 1 or more prior salvage regimens or were refractory to primary or salvage therapy.
Figure 1.
Patient disposition. Data cutoff was 4 January 2016. CR, complete remission; CRh, complete remission with partial hematologic recovery; CRi, complete remission with incomplete hematologic recovery.
Table 1.
Patient characteristics for the safety population
| Characteristic | Blinatumomab, N = 267 | SOC, N = 109 |
|---|---|---|
| Mean age (range), y | 40.7 (18-80) | 41.0 (18-76) |
| Male sex, n (%) | 159 (60) | 64 (59) |
| ECOG performance status, n (%) | ||
| 0 | 95 (36) | 41 (38) |
| 1 | 133 (50) | 48 (44) |
| 2 | 39 (15) | 19 (17) |
| Unknown | 0 (0) | 1 (1) |
| Key inclusion criteria, n (%) | ||
| Refractory to primary or salvage therapy | 112 (42) | 43 (39) |
| First relapse, first remission <12 mo | 76 (28) | 30 (28) |
| Untreated second or greater relapse* | 31 (12) | 14 (13) |
| Relapsed after alloHSCT* | 46 (17) | 22 (20) |
| No criteria met | 2 (1) | 0 (0) |
| Prior alloHSCT, n (%) | ||
| Yes | 93 (35) | 35 (32) |
| No | 173 (65) | 73 (67) |
| Unknown | 1 (<1) | 1 (1) |
| First relapse with remission duration <12 mo, n (%) | ||
| Yes | 108 (40) | 37 (34) |
| No | 158 (59) | 72 (66) |
| Unknown | 1 (<1) | 0 (0) |
| Salvage status, n (%) | ||
| First | 112 (42) | 55 (50) |
| Second | 91 (34) | 34 (31) |
| Third or later | 64 (24) | 20 (18) |
| Bone marrow blasts (central/local), n (%) | ||
| >5% to <10% | 9 (3) | 4 (4) |
| 10% to <50% | 60 (22) | 19 (17) |
| ≥50% | 198 (74) | 86 (79) |
| Peripheral blasts, n (%) | ||
| 0 | 116 (43) | 43 (39) |
| 1 to 5 × 109/L | 70 (26) | 40 (37) |
| >5 × 109/L | 31 (12) | 12 (11) |
| Unknown | 50 (19) | 14 (13) |
| White blood cells at diagnosis, n (%) | ||
| <30 × 109/L | 141 (53) | 50 (46) |
| ≥30 × 109/L | 69 (26) | 34 (31) |
| Unknown | 57 (21) | 25 (23) |
| Platelets, mean (range), × 109/L | 71.3 (2-454) | 93.1 (8-580) |
| ALT or AST >3× ULN, n (%) | 35 (13) | 10 (9) |
| Patients with history of events of clinical interest, n (%) | ||
| Neutropenias | 58 (22) | 15 (14) |
| Thrombocytopenias | 76 (28) | 34 (31) |
| Leukopenias | 15 (6) | 4 (4) |
| Acute and chronic pancreatitis | 2 (1) | 3 (3) |
| Injection and infusion site reaction | 1 (<1) | 2 (2) |
ALT, alanine aminotransferase; AST, aspartate aminotransferase; ECOG, Eastern Cooperative Oncology Group; ULN, upper limit of normal.
Patients who met none of the above inclusion criteria.
At the time of data cutoff (4 January 2016), patients in the blinatumomab arm had received a median of 2 cycles (range, 1-9) of treatment, with 59 patients initiating consolidation and 27 initiating maintenance (6 or more cycles), with 3 completing the maintenance phase. The median duration of treatment was 54.1 days (range, 0-258) and the median cumulative dose was 1264.9 μg (range, 4-6935). In the SOC arm, patients had received a median of 1 cycle (range, 1-4) of treatment. The most frequently used chemotherapy regimen was fludarabine, cytarabine, and granulocyte colony-stimulating factor with or without anthracycline (45% [n = 49]), followed by high-dose methotrexate (20% [n = 22]), and high-dose cytarabine and clofarabine-based regimens (17% [n = 19] for each). Three patients initiated 3 to 4 cycles of SOC, with no patients receiving ≥5 cycles. The total treatment exposure was longer in the blinatumomab arm than in the SOC arm (89.0 vs 14.8 patient-years).
Incidence rates of treatment-emergent AEs
Nearly all patients in both treatment arms experienced at least 1 treatment-emergent AE of any grade (99% in each arm) (supplemental Table 1). Among AE categories of interest, relative incidence rates of any grade were higher in the blinatumomab arm compared with SOC for CRS (16% vs 0%), neurologic events (61% vs 50%), and tumor lysis syndrome (4% vs 1%), but were lower for cytopenias (60% vs 72%). Overall, the incidence of gastrointestinal disorders, including diarrhea, nausea, and vomiting, was lower in the blinatumomab arm compared with the SOC arm (56% vs 80%). The rate of grade ≥3 AEs was also similar between treatment arms (87% vs 92%). Grade ≥3 neutropenia (18% vs 27%) and infections (34% vs 52%) occurred at a lower rate with blinatumomab than with SOC, whereas CRS was higher (5% vs 0%) (Table 2).
Table 2.
Treatment-emergent AEs of clinical interest grade ≥3: incidence rates
| No. of patients (%) | ||
|---|---|---|
| Event | Blinatumomab, N = 267 | SOC, N = 109 |
| Any AE | 231 (87) | 100 (92) |
| Cytokine release syndrome | 13 (5) | 0 |
| Cytokine release syndrome | 9 (3) | 0 |
| Hematophagic histiocytosis | 4 (1) | 0 |
| Cytokine storm | 0 | 0 |
| Tumor lysis syndrome | 8 (3) | 1 (1) |
| Acute pancreatitis | 1 (<1) | 1 (1) |
| Neurologic events | 25 (9) | 9 (8) |
| Headache | 1 (<1) | 3 (3) |
| Insomnia | 1 (<1) | 0 |
| Tremor | 1 (<1) | 0 |
| Dizziness | 1 (<1) | 0 |
| Somnolence | 3 (1) | 0 |
| Seizure | 2 (1) | 3 (3) |
| Gastrointestinal disorders | 19 (7) | 15 (14) |
| Diarrhea | 3 (1) | 1 (1) |
| Nausea | 0 | 3 (3) |
| Constipation | 0 | 0 |
| Vomiting | 0 | 1 (1) |
| Stomatitis | 4 (1) | 2 (2) |
| Abdominal pain | 4 (1) | 3 (3) |
| Dyspepsia | 0 | 0 |
| Infections | 91 (34) | 57 (52) |
| Cytopenias | 141 (53) | 74 (68) |
| Febrile neutropenia | 57 (21) | 38 (35) |
| Neutropenia | 47 (18) | 29 (27) |
| Thrombocytopenia | 39 (15) | 30 (28) |
| Decreased platelets | 11 (4) | 13 (12) |
| Decreased white blood cells | 12 (4) | 6 (6) |
| Decreased neutrophils | 10 (4) | 11 (10) |
| Leukopenia | 8 (3) | 5 (5) |
| Decreased lymphocytes | 3 (1) | 4 (4) |
| Lymphopenia | 1 (<1) | 0 |
| Elevated liver enzymes | 34 (13) | 16 (15) |
| Progressive multifocal leukoencephalopathy | 2 (1) | 0 |
| Decreased immunoglobulins | 7 (3) | 0 |
| Other AEs of interest | ||
| Pyrexia | 19 (7) | 5 (5) |
| Anemia | 53 (20) | 38 (35) |
Data are summarized for all patients who received at least 1 dose of study treatment.
Treatment interruption due to AEs occurred in 32% (n = 86) of patients in the blinatumomab arm and in 6% (n = 6) in the SOC arm, and discontinuations due to AEs occurred in 12% (n = 33) and 8% (n = 9), respectively. The most common AEs leading to treatment discontinuation were infections (3% [n = 9] for blinatumomab and 5% [n = 5] for SOC). Discontinuation due to neurologic events was 3% (n = 8) in the blinatumomab arm and 1% (n = 1) in the SOC arm.
Fatal AE incidence rates were similar between blinatumomab and SOC (19% [n = 51] vs 17% [n = 19]). The most common fatal AEs in both arms were infections (blinatumomab, 11% [n = 30]; and SOC, 12% [n = 13]), which included sepsis (3% [n = 8] and 4% [n = 4]). Fatal AEs considered related to blinatumomab (3% [n = 8]) included respiratory failure (n = 1), acute respiratory failure (n = 1), bronchopulmonary aspergillosis (n = 1), bacterial infection (n = 1), neutropenic sepsis (n = 1), sepsis syndrome (n = 1), and sepsis (n = 2). In the SOC arm, fatal AEs considered treatment related (7% [n = 8]) included Pseudomonas infection (n = 1), systemic candidiasis (n = 1), fungal pneumonia (n = 1), enterococcal infection (n = 1), bacteremia (n = 1), sepsis (n = 2), and acute kidney injury (n = 1).
Incidence rates of treatment-emergent AEs during cycles 1 and 2 of treatment
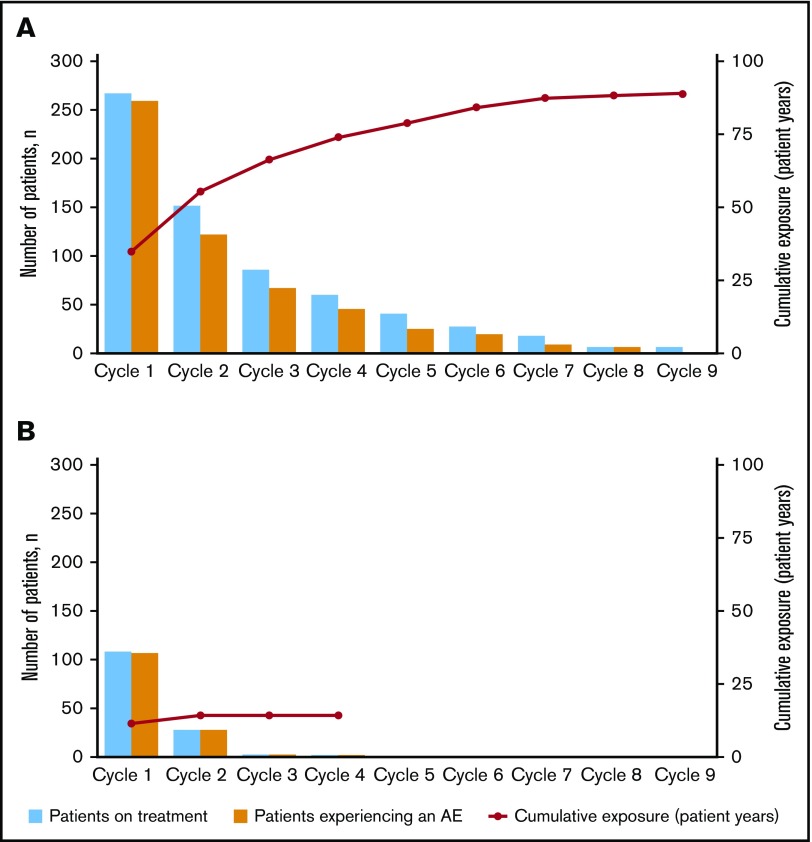
The majority of AEs occurred during induction in both treatment arms (Figure 2). In the blinatumomab arm, the incidence rate for any grade AE decreased from cycle 1 to cycle 2 (97% vs 81%, respectively) (supplemental Table 2). This drop corresponded to a decrease in the rate of blinatumomab treatment interruptions due to AEs from cycle 1 to 2 (24% [n = 65] and 6% [n = 15]) and discontinuations due to AEs (10% [n = 26] and 1% [n = 3]). For individual AEs of any grade, incidence rates decreased between cycle 1 and 2 for febrile neutropenia (22% and 4%), thrombocytopenia (16% and 3%), headache (23% and 9%), and CRS (13% and 3%). The incidence rate of grade ≥3 AEs also decreased from cycle 1 to cycle 2 (76% vs 37%) in the blinatumomab arm, with decreases for cytopenias (44% and 16%), infections (23% and 11%), neurologic events (7% and 3%), and CRS (4% and 1%) (Table 3).
Figure 2.
Incidence of treatment-emergent AEs by treatment cycle. For (A) blinatumomab and (B) SOC.
Table 3.
Treatment-emergent AEs of clinical interest grade ≥3: incidence rates in cycles 1 and 2
| Blinatumomab, N = 267 | SOC, N = 109 | |||
|---|---|---|---|---|
| Cycle 1, n = 267 | Cycle 2, n = 151 | Cycle 1, n = 109 | Cycle 2, n = 28 | |
| Any AE | 202 (76) | 56 (37) | 98 (90) | 20 (71) |
| Cytokine release syndrome | 11 (4) | 1 (1) | 0 | 0 |
| Cytokine release syndrome | 8 (3) | 1 (1) | 0 | 0 |
| Hematophagic histiocytosis | 3 (1) | 0 | 0 | 0 |
| Cytokine storm | 0 | 0 | 0 | 0 |
| Tumor lysis syndrome | 8 (3) | 0 | 1 (1) | 0 |
| Acute pancreatitis | 1 (<1) | 0 | 0 | 1 (4) |
| Neurologic events | 19 (7) | 4 (3) | 9 (8) | 1 (4) |
| Headache | 1 (<1) | 0 | 3 (3) | 0 |
| Insomnia | 1 (<1) | 0 | 0 | 0 |
| Tremor | 1 (<1) | 0 | 0 | 0 |
| Dizziness | 1 (<1) | 0 | 0 | 0 |
| Somnolence | 3 (1) | 0 | 0 | 0 |
| Seizure | 2 (1) | 0 | 3 (3) | 0 |
| Gastrointestinal disorders | 11 (4) | 3 (2) | 12 (11) | 3 (11) |
| Diarrhea | 2 (1) | 1 (1) | 1 (1) | 0 |
| Nausea | 0 | 0 | 3 (3) | 0 |
| Constipation | 0 | 0 | 0 | 0 |
| Vomiting | 0 | 0 | 0 | 1 (4) |
| Stomatitis | 0 | 2 (1) | 2 (2) | 0 |
| Abdominal pain | 3 (1) | 0 | 2 (2) | 1 (4) |
| Dyspepsia | 0 | 0 | 0 | 0 |
| Infections | 61 (23) | 17 (11) | 52 (48) | 8 (29) |
| Cytopenias | 118 (44) | 24 (16) | 74 (68) | 17 (61) |
| Febrile neutropenia | 52 (19) | 5 (3) | 36 (33) | 9 (32) |
| Neutropenia | 28 (10) | 14 (9) | 28 (26) | 7 (25) |
| Thrombocytopenia | 37 (14) | 0 | 29 (27) | 9 (32) |
| Decreased platelets | 10 (4) | 1 (1) | 11 (10) | 3 (11) |
| Decreased white blood cells | 10 (4) | 2 (1) | 5 (5) | 1 (4) |
| Decreased neutrophils | 7 (3) | 1 (1) | 9 (8) | 5 (18) |
| Leukopenia | 8 (3) | 2 (1) | 4 (4) | 1 (4) |
| Decreased lymphocytes | 2 (1) | 0 | 4 (4) | 0 |
| Lymphopenia | 1 (<1) | 0 | 0 | 0 |
| Elevated liver enzymes | 32 (12) | 2 (1) | 14 (13) | 3 (11) |
| Progressive multifocal leukoencephalopathy | 1 (<1) | 0 | 0 | 0 |
| Decreased immunoglobulins | 0 | 3 (2) | 0 | 0 |
| Other AEs of interest | ||||
| Pyrexia | 15 (6) | 2 (1) | 4 (4) | 1 (4) |
| Anemia | 51 (19) | 1 (1) | 37 (34) | 9 (32) |
Values are n (%).
First onset of CRS and neurologic events usually occurred within cycle 1 of blinatumomab treatment. Median time to first onset was 2 days (range, 1-254) for any grade CRS, 4 days (range, 1-254) for grade ≥3 CRS, 7 days (range, 1-190) for neurologic events of any grade, and 18 days (range, 1-401) for grade ≥3 neurologic events. The median time to first onset of infection was more delayed at 17 days (range, 1-246) for any grade and 24 days (range, 1-246) for grade ≥3.
EAERs for treatment-emergent AEs
A total of 4108 AEs (955 events grade ≥3) were reported among the 267 patients who received blinatumomab, compared with 2037 events (650 events grade ≥3) among the 109 patients who received SOC. After adjusting for treatment exposure time, the overall EAER was significantly lower for blinatumomab vs SOC for any grade AEs (46.16 vs 137.64 events per patient-year; P <.001), for grade ≥3 AEs (10.73 vs 45.27 events per patient-year; P <.001), and for fatal AEs (0.57 vs 1.28 events per patient-year; P = .005). There was no statistically significant difference between treatment arms for exposure-adjusted treatment interruptions due to AEs (1.64 vs 1.22 events per patient-year; P = .21), whereas exposure-adjusted treatment discontinuations due to AEs were lower in the blinatumomab arm (0.45 vs 0.88 events per patient-year; P = .048).
Among AE categories of interest, differences between blinatumomab and SOC were observed, particularly EAERs of any grade for gastrointestinal disorders (4.52 vs 22.91; P < .001), cytopenias (5.25 vs 23.99; P < .001), and infections (4.36 vs 12.16; P < .001) (supplemental Table 3). For grade ≥3 events, EAERs were lower for blinatumomab compared with SOC for neurologic events (0.38 vs 0.95 events per patient-year; P = .008), gastrointestinal disorders (0.28 vs 1.49 events per patient-year; P < .001), infections (1.63 vs 6.49 events per patient-year; P < .001), cytopenias (3.64 vs 20.07 events per patient-year; P < .001), and elevated liver enzymes (0.65 vs 1.89 events per patient-year; P < .001) (Table 4). The EAER was higher in the blinatumomab arm for grade ≥3 CRS (0.16 vs 0 events per patient-year; P = .038). There was no statistically significant difference between treatment arms for grade ≥3 tumor lysis syndrome (0.09 vs 0.07 events per patient-year; P = .780). EAERs were generally lower with blinatumomab for cytopenias of grade ≥3.
Table 4.
Exposure-adjusted event rates for grade ≥3 treatment-emergent AEs of clinical interest
| Blinatumomab, N = 267 (total treatment exposure, 89.0 y) | SOC chemotherapy, N = 109 (total treatment exposure, 14.8 y) | ||||
|---|---|---|---|---|---|
| No. of events | Exposure-adjusted event rate* | No. of events | Exposure-adjusted event rate* | P† | |
| Cytokine release syndrome | 14 | 0.16 | 0 | 0 | .038‡ |
| Cytokine release syndrome | 9 | 0.10 | 0 | 0 | .096 |
| Hematophagic histiocytosis | 5 | 0.06 | 0 | 0 | — |
| Cytokine storm | 0 | 0 | 0 | 0 | — |
| Tumor lysis syndrome | 8 | 0.09 | 1 | 0.07 | .780 |
| Acute pancreatitis | 1 | 0.01 | 1 | 0.07 | .232 |
| Neurologic events | 34 | 0.38 | 14 | 0.95 | .008§ |
| Headache | 1 | 0.01 | 3 | 0.20 | .006§ |
| Insomnia | 1 | 0.01 | 0 | 0 | — |
| Tremor | 1 | 0.01 | 0 | 0 | — |
| Dizziness | 1 | 0.01 | 0 | 0 | — |
| Somnolence | 3 | 0.03 | 0 | 0 | — |
| Seizure | 2 | 0.02 | 3 | 0.20 | .018§ |
| Gastrointestinal disorders | 25 | 0.28 | 22 | 1.49 | <.001§ |
| Diarrhea | 3 | 0.03 | 1 | 0.07 | .572 |
| Nausea | 0 | 0 | 3 | 0.20 | — |
| Constipation | 0 | 0 | 0 | 0 | — |
| Vomiting | 0 | 0 | 1 | 0.07 | — |
| Stomatitis | 4 | 0.05 | 2 | 0.14 | .240 |
| Abdominal pain | 4 | 0.05 | 5 | 0.34 | .004§ |
| Dyspepsia | 0 | 0 | 0 | 0 | — |
| Infections | 145 | 1.63 | 96 | 6.49 | <.001§ |
| Cytopenias | 324 | 3.64 | 297 | 20.07 | <.001§ |
| Febrile neutropenia | 71 | 0.80 | 48 | 3.24 | <.001§ |
| Neutropenia | 77 | 0.87 | 41 | 2.77 | <.001§ |
| Thrombocytopenia | 71 | 0.80 | 97 | 6.55 | <.001§ |
| Decreased platelets | 33 | 0.37 | 64 | 4.32 | <.001§ |
| Decreased white blood cells | 20 | 0.23 | 8 | 0.54 | .051 |
| Decreased neutrophils | 17 | 0.19 | 22 | 1.49 | <.001§ |
| Leukopenia | 18 | 0.20 | 5 | 0.34 | .335 |
| Decreased lymphocytes | 3 | 0.03 | 4 | 0.27 | .008§ |
| Lymphopenia | 1 | 0.01 | 0 | 0 | — |
| Elevated liver enzymes | 58 | 0.65 | 28 | 1.89 | <.001§ |
| Progressive multifocal leukoencephalopathy | 3 | 0.03 | 0 | 0 | — |
| Decreased immunoglobulins | 7 | 0.08 | 0 | 0 | — |
| Other AEs of interest | |||||
| Pyrexia | 22 | 0.25 | 8 | 0.54 | .076 |
| Anemia | 100 | 1.12 | 81 | 5.47 | <.001§ |
—, not estimable.
Per patient-year.
P value comparing blinatumomab vs SOC from a Poisson regression model using number of AEs as the dependent variable and log(exposure time) as offset.
Favors SOC.
Favors blinatumomab arm.
Overall, 311 AEs in the blinatumomab arm and 95 AEs in the SOC were classified as serious. The EAER profiles of events of interest were generally consistent with those for grade ≥3 events. Infections were the most common serious AEs by system organ class, with an EAER of 1.17 events per patient-year for blinatumomab and 2.91 events per patient-year for SOC. Other serious AEs by system organ class included gastrointestinal disorders (0.10 and 0.20 events per patient-year for blinatumomab and SOC, respectively). Among other serious AEs of interest by preferred term, EAERs for serious febrile neutropenia were 0.32 events per patient-year for blinatumomab and 0.81 events per patient-year for SOC. Serious tumor lysis syndrome occurred at an EAER of 0.03 events per patient-year in the blinatumomab arm, and serious CRS occurred at an EAER of 0.08 events per patient-year; there were no serious tumor lysis syndrome or CRS events in the SOC arm. Of 22 grade ≥3 pyrexia events in the blinatumomab arm, 20 were classified as serious with an EAER of 0.23 events per patient-year, whereas 1 of 8 grade ≥3 pyrexia events was classified as serious in the SOC arm with an EAER of 0.07 per patient-year.
Discussion
In this exploratory analysis of the phase 3 TOWER study in adults with r/r ALL, blinatumomab showed an AE profile that was well tolerated compared with SOC and consistent with that previously reported in r/r ALL.15,16,21 Nearly all patients (99%) in both treatment arms experienced at least 1 AE regardless of causality during treatment. In the blinatumomab arm, the majority of AEs occurred during cycle 1, and AE incidence rates generally decreased during cycle 2, consolidation, and maintenance. Fatal AEs were primarily related to infection in both treatment arms. Exposure-adjusted AE rates were generally lower for blinatumomab compared with SOC, including those for grade ≥3 cytopenias and infections. The EAER rate for CRS was higher in the blinatumomab arm, which is likely related to its mode of action.
Prior single-arm studies with blinatumomab identified AEs of interest, including infection, neurologic events, CRS, and tumor lysis syndrome.15,16,21 Infection has been a clinical challenge in treating patients with r/r ALL and is a common cause of morbidity and mortality with SOC.11-13 There was a notable difference in the EAERs for grade ≥3 infections between blinatumomab and SOC (1.63 vs 6.49 events per patient-year), which corresponded to a lower EAER of fatal AEs for blinatumomab compared with SOC (0.57 vs 1.28 events per patient-year, respectively). In addition, the rate of infections decreased from >50% during induction to ∼40% during blinatumomab maintenance therapy, which corresponded to a similar decrease in cytopenias from >50% to ∼11%. A better understanding of potential correlations between infection and cytopenias, and with decreases in immunoglobulins, may inform patient management strategies.22 An analysis of lymphocyte subpopulations in patients enrolled in a phase 2 study of blinatumomab in ALL showed a drop in both B cells and T cells within a few days of treatment initiation.23 Although B cells continued to be suppressed during treatment, T cells recovered within a few days and expanded over the subsequent 2 to 3 weeks.
Neurologic AEs associated with blinatumomab during the TOWER study were consistent with prior studies.15,16,21 Here, the incidence rate of neurologic AEs of any grade was higher in the blinatumomab arm than in the SOC arm (61% vs 50%), as was the rate of discontinuations due to neurologic AEs (3% vs 1%). However, the rate of grade ≥3 neurologic AEs was similar between the treatment arms, and taking into account the differences in time on treatment, the EAER for grade ≥3 neurologic events was lower for blinatumomab than for SOC. In the exposure-adjusted analysis, grade ≥3 seizures were infrequent in both arms and were less frequent with blinatumomab than with SOC. The etiology of blinatumomab-associated neurologic events is unknown, but these events can usually be managed with dexamethasone treatment.15
The rate of CRS events in the current study was consistent with that of the earlier, single-arm phase 2 blinatumomab study.15 The phase 2 study also used the dose step and dexamethasone premedication, which, in addition to the prephase dexamethasone treatment of patients with high tumor burden, has effectively mitigated CRS.15,16 In the phase 2 study, an analysis of lymphocyte subpopulations showed a transient release of cytokines within the first few days of cycle 1 of blinatumomab treatment (dominated by interleukin-10, interleukin-6, and interferon-γ) that declined quickly and was not observed during cycle 2.23 Regardless, CRS requires careful monitoring, as it is associated with symptoms that overlap with pyrexia, neutropenia, infection, and tumor lysis syndrome, but differs in management.24 The rate of serious pyrexia events in the blinatumomab arm of the TOWER study may reflect the concern of investigators for patients developing CRS or infection.
Other AEs of clinical interest were consistent with the mode of action for blinatumomab, including tumor lysis syndrome and a decrease in immunoglobulins, both with low rates of grade ≥3 events. The rate of elevated liver enzymes in the blinatumomab arm was similar to that reported in the phase 2 study.16 Transient elevations in liver enzymes are known to occur with blinatumomab.25 The incidence rate of elevated liver enzymes was similar between the blinatumomab and SOC arms, whereas the EAER for grade ≥3 events was lower for blinatumomab. There were no incidences of drug-induced liver injury with jaundice in either treatment arm.
As longer exposure in a randomized trial also means longer observation time for AEs, exposure adjustment is helpful to better characterize the safety profile of both interventions when the treatment duration in both arms differs strongly. Additionally, unadjusted incidence rates can underestimate the relevance of delayed events.18,19 Here, we have demonstrated that the exposure-adjusted safety profile of blinatumomab supports extended treatment compared with SOC. There were no delayed events that would preclude blinatumomab consolidation or maintenance therapy. A potential limitation of this approach is that rates for acute AEs may be diluted in the arm with greater exposure.18,19,26 To address this possibility, we have provided both incidence rates and exposure-adjusted rates. Future analyses will evaluate safety during blinatumomab maintenance therapy, and the potential impact of inpatient blinatumomab administration on the safety and tolerability profile.
The safety data reported here further characterize the benefit-to-risk profile of blinatumomab. In the primary analysis, blinatumomab achieved a significantly higher composite complete remission (CR) rate (full, partial, or incomplete hematologic recovery) within 12 weeks of treatment initiation compared with SOC (44% vs 25%; P < .001), a significantly higher rate of CR with full hematologic recovery (34% vs 16%; P < .001), and a longer duration of remission (7.3 vs 4.6 months).17 Among patients who achieved CR with or without full hematologic recovery, minimal residual disease negativity was observed in 76% of patients in the blinatumomab arm compared with only 48% in the SOC arm. Consistent with these differential response outcomes, longer OS was observed with blinatumomab as compared with SOC (median 7.7 vs 4.0 months; hazard ratio, 0.71; 95% confidence interval, 0.55 to 0.93; P = .01).
In summary, the data reported here further support the role of blinatumomab as an efficacious and well-tolerated treatment option for patients with r/r Ph− ALL. Compared with SOC, patients receiving blinatumomab showed a notable decrease in rates of AEs as treatment progressed (including decreased CRS and neurologic events), and the types of AEs observed were consistent with those previously reported for blinatumomab.
Supplementary Material
The full-text version of this article contains a data supplement.
Acknowledgments
Medical writing and editorial support were provided by Janice Y. Ahn (Amgen Inc, Thousand Oaks, CA), Ashleigh Pulkoski-Gross (Fishawack Communications, Conshohocken, PA), and Michael Raffin (Fishawack Communications).
This work was supported by Amgen Inc. Medical writing and editorial support were funded by Amgen Inc.
Authorship
Contribution: A.S.S. and M.S.T. contributed to the concept and design of the study; A.S.S., R.A.L., A.C.S., W.S., E.L.-M., and M.S.T. contributed to data acquisition; A.S.S., R.A.L., A.C.S., W.S., E.L.-M., Q.T., Z.Z., W.K., and M.S.T. analyzed and interpreted data; and all authors critically revised the manuscript and reviewed and approved the final version.
Conflict-of-interest disclosure: A.S.S. received consulting fees and is a member of the speaker’s bureau for Amgen Inc. R.A.L received consulting fees from Amgen Inc. A.C.S. received consulting fees from Amgen Inc. W.S. received honoraria from Amgen Inc. E.L.-M. received consulting fees from Amgen Inc, Roche, and Janssen-Cilag. Q.T. is an employee of Amgen Inc. Z.Z. and W.K. are employees of and hold stock in Amgen Inc. M.S.T. received research grants from Amgen Inc, Gilead, Macrogenics, Roche, and Regeneron, as well as consulting fees from Amgen Inc, Roche, and Regeneron.
Correspondence: Max S. Topp, Medizinische Klinik und Poliklinik II, Universitätsklinikum Würzburg, Oberdürrbacher Str 6, 97080 Würzburg, Germany; e-mail: topp_m@ukw.de.
References
- 1.Goldstone AH, Richards SM, Lazarus HM, et al. In adults with standard-risk acute lymphoblastic leukemia, the greatest benefit is achieved from a matched sibling allogeneic transplantation in first complete remission, and an autologous transplantation is less effective than conventional consolidation/maintenance chemotherapy in all patients: final results of the International ALL Trial (MRC UKALL XII/ECOG E2993). Blood. 2008;111(4):1827-1833. [DOI] [PubMed] [Google Scholar]
- 2.Bassan R, Hoelzer D. Modern therapy of acute lymphoblastic leukemia. J Clin Oncol. 2011;29(5):532-543. [DOI] [PubMed] [Google Scholar]
- 3.Nishiwaki S, Inamoto Y, Sakamaki H, et al. Allogeneic stem cell transplantation for adult Philadelphia chromosome-negative acute lymphocytic leukemia: comparable survival rates but different risk factors between related and unrelated transplantation in first complete remission. Blood. 2010;116(20):4368-4375. [DOI] [PubMed] [Google Scholar]
- 4.Fielding AK, Richards SM, Chopra R, et al. ; Eastern Cooperative Oncology Group. Outcome of 609 adults after relapse of acute lymphoblastic leukemia (ALL); an MRC UKALL12/ECOG 2993 study. Blood. 2007;109(3):944-950. [DOI] [PubMed] [Google Scholar]
- 5.Tavernier E, Boiron JM, Huguet F, et al. ; Australasian Leukaemia and Lymphoma Group. Outcome of treatment after first relapse in adults with acute lymphoblastic leukemia initially treated by the LALA-94 trial. Leukemia. 2007;21(9):1907-1914. [DOI] [PubMed] [Google Scholar]
- 6.Oriol A, Vives S, Hernández-Rivas JM, et al. ; Programa Español de Tratamiento en Hematologia Group. Outcome after relapse of acute lymphoblastic leukemia in adult patients included in four consecutive risk-adapted trials by the PETHEMA Study Group. Haematologica. 2010;95(4):589-596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gökbuget N, Stanze D, Beck J, et al. ; German Multicenter Study Group for Adult Acute Lymphoblastic Leukemia. Outcome of relapsed adult lymphoblastic leukemia depends on response to salvage chemotherapy, prognostic factors, and performance of stem cell transplantation. Blood. 2012;120(10):2032-2041. [DOI] [PubMed] [Google Scholar]
- 8.Kantarjian HM, Thomas D, Ravandi F, et al. Defining the course and prognosis of adults with acute lymphocytic leukemia in first salvage after induction failure or short first remission duration. Cancer. 2010;116(24):5568-5574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.O’Brien S, Thomas D, Ravandi F, et al. Outcome of adults with acute lymphocytic leukemia after second salvage therapy. Cancer. 2008;113(11):3186-3191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Terwilliger T, Abdul-Hay M. Acute lymphoblastic leukemia: a comprehensive review and 2017 update. Blood Cancer J. 2017;7(6):e577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Offidani M, Corvatta L, Malerba L, Marconi M, Leoni P. Infectious complications in adult acute lymphoblastic leukemia (ALL): experience at one single center. Leuk Lymphoma. 2004;45(8):1617-1621. [DOI] [PubMed] [Google Scholar]
- 12.Rowe JM, Buck G, Burnett AK, et al. ; MRC/NCRI Adult Leukemia Working Party. Induction therapy for adults with acute lymphoblastic leukemia: results of more than 1500 patients from the international ALL trial: MRC UKALL XII/ECOG E2993. Blood. 2005;106(12):3760-3767. [DOI] [PubMed] [Google Scholar]
- 13.Patel B, Kirkwood AA, Dey A, et al. Pegylated-asparaginase during induction therapy for adult acute lymphoblastic leukaemia: toxicity data from the UKALL14 trial. Leukemia. 2017;31(1):58-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bargou R, Leo E, Zugmaier G, et al. Tumor regression in cancer patients by very low doses of a T cell-engaging antibody. Science. 2008;321(5891):974-977. [DOI] [PubMed] [Google Scholar]
- 15.Topp MS, Gökbuget N, Stein AS, et al. Safety and activity of blinatumomab for adult patients with relapsed or refractory B-precursor acute lymphoblastic leukaemia: a multicentre, single-arm, phase 2 study. Lancet Oncol. 2015;16(1):57-66. [DOI] [PubMed] [Google Scholar]
- 16.Topp MS, Gökbuget N, Zugmaier G, et al. Phase II trial of the anti-CD19 bispecific T cell-engager blinatumomab shows hematologic and molecular remissions in patients with relapsed or refractory B-precursor acute lymphoblastic leukemia. J Clin Oncol. 2014;32(36):4134-4140. [DOI] [PubMed] [Google Scholar]
- 17.Kantarjian H, Stein A, Gökbuget N, et al. Blinatumomab versus chemotherapy for advanced acute lymphoblastic leukemia. N Engl J Med. 2017;376(9):836-847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhou Y, Ke C, Jiang Q, Shahin S, Snapinn S. Choosing appropriate metrics to evaluate adverse events in safety evaluation. Ther Innov Regul Sci. 2015;49(3):398-404. [DOI] [PubMed] [Google Scholar]
- 19.He X, Chen L, Lei L, Xia H, Lee M. A simple method for estimating confidence intervals for exposure adjusted incidence rate and its applications to clinical trials. J Biom Biostat. 2015;6(3):238. [Google Scholar]
- 20.Common terminology criteria for adverse events (CTCAE) v4.0. Bethesda, MD: National Cancer Institute; 2010. [Google Scholar]
- 21.Martinelli G, Boissel N, Chevallier P, et al. Complete hematologic and molecular response in adult patients with relapsed/refractory Philadelphia chromosome-positive B-precursor acute lymphoblastic leukemia following treatment with blinatumomab: results from a phase II, single-arm, multicenter study. J Clin Oncol. 2017;35(16):1795-1802. [DOI] [PubMed] [Google Scholar]
- 22.Lee KJ, Chow V, Weissman A, Tulpule S, Aldoss I, Akhtari M. Clinical use of blinatumomab for B-cell acute lymphoblastic leukemia in adults. Ther Clin Risk Manag. 2016;12:1301-1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Klinger M, Brandl C, Zugmaier G, et al. Immunopharmacologic response of patients with B-lineage acute lymphoblastic leukemia to continuous infusion of T cell-engaging CD19/CD3-bispecific BiTE antibody blinatumomab. Blood. 2012;119(26):6226-6233. [DOI] [PubMed] [Google Scholar]
- 24.Lee DW, Gardner R, Porter DL, et al. Current concepts in the diagnosis and management of cytokine release syndrome [published correction appears in Blood. 2015;126(8):1048]. Blood. 2014;124(2):188-195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nägele V, Kratzer A, Zugmaier G, et al. Changes in clinical laboratory parameters and pharmacodynamic markers in response to blinatumomab treatment of patients with relapsed/refractory ALL. Exp Hematol Oncol. 2017;6(1):14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Siddiqui O. Statistical methods to analyze adverse events data of randomized clinical trials. J Biopharm Stat. 2009;19(5):889-899. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.