Key Points
Lenalidomide penetrates ventricular CSF and is active as monotherapy in relapsed CNS lymphomas.
Maintenance lenalidomide is feasible and may potentiate response duration after salvage in relapsed PCNSL and delay WBRT.
Abstract
There is an unmet need for effective biological therapies for relapsed central nervous system (CNS) lymphoma. Lenalidomide is active in activated B-cell type diffuse large B-cell lymphoma and rituximab is effective in CNS lymphoma. These observations are the basis for this first trial of an immunomodulatory drug as monotherapy in CNS lymphoma, and, in patients with inadequate responses to lenalidomide, with rituximab. In an independent cohort, we evaluated lenalidomide maintenance after salvage with high-dose methotrexate or focal irradiation in relapsed primary CNS lymphoma (PCNSL). We determined safety, efficacy, and cerebrospinal fluid (CSF) penetration of lenalidomide at 10-, 15-, and 20-mg dose levels in 14 patients with refractory CD20+ CNS lymphoma. Nine subjects with relapsed, refractory CNS lymphoma achieved better than partial response with lenalidomide monotherapy, 6 maintained response ≥9 months, and 4 maintained response ≥18 months. Median progression-free survival for lenalidomide/rituximab was 6 months. In the independent cohort, response duration with lenalidomide maintenance after complete responses 2 through 5 were significantly longer than response durations after standard therapy. The CSF/plasma partition coefficient of lenalidomide was ≥20% at 15- and 20-mg dose levels. Change in CSF interleukin-10 at 1 month correlated with clinical response and response duration to lenalidomide. Metabolomic profiling of CSF identified novel biomarkers, including lactate, and implicated indoleamine-2,3 dioxygenase activity with CNS lymphoma progression on lenalidomide. We conclude that lenalidomide penetrates ventricular CSF and is active as monotherapy in relapsed CNS lymphomas. We provide evidence that maintenance lenalidomide potentiates response duration after salvage in relapsed PCNSL and delays whole brain radiotherapy (WBRT). This trial was registered at www.clinicaltrials.gov as #NCT01542918.
Visual Abstract
Introduction
Despite advances in dose-intensive chemotherapy for primary and secondary central nervous system (CNS) lymphomas, there likely exists a plateau in progression-free survival with strategies based on established genotoxic agents.1-3 In virtually every clinical series, 20% to 30% of primary CNS lymphoma (PCNSL) patients experience tumor progression within the first 6 months of treatment. Relapse within the CNS remains a significant cause of death in systemic non-Hodgkin lymphoma (NHL).4 Moreover, high-dose genotoxic therapy has limited relevance for older patients, a subgroup at risk for severe complications of chemotherapy and whole brain radiotherapy.5 Given that PCNSL increasingly affects an older population,6 there is a need for selective agents that target key pro-tumor survival pathways and have minimal collateral toxicity to the patient.
Lenalidomide is a second-generation immunomodulatory agent with pleiotropic antitumor effects including stimulation of natural killer and T-cell expansion.7-10 Lenalidomide enhances the antibody-dependent cell-mediated cytotoxicity of rituximab and may overcome rituximab resistance in NHL.11-13 Lenalidomide also has cell-autonomous cytotoxic effects on lymphoid tumors, including antagonism of IRF4 and MYC pro-survival signals.14,15 IRF4 is relevant to PCNSL given that >90% of diagnostic specimens are IRF4/MUM1-positive.16 Similarly, ∼50% of CNS lymphomas express MYC or activation of MYC pathways.17
We and others reported single-agent activity of lenalidomide in refractory secondary CNS diffuse large B-cell lymphoma (DLBCL) as well as mantle cell lymphoma involving the CNS.18,19 Given reports of neurotoxicity associated with lenalidomide and other immunomodulatory drugs in the setting of myeloma and other lymphoid malignancies,20,21 that the direct binding protein and mediator of cellular activity of lenalidomide, cereblon, is highly expressed in neurons in the brain,22,23 and that it is well-established that CNS lymphoma patients are at high risk for neurocognitive deficits and neurotoxicity, we designed a phase 1 investigation to determine a safe, maximum tolerated dose (MTD) of lenalidomide for this patient population. The rationale for this approach is also supported by the fact that CNS lymphoma patients often have a poor performance status and are particularly vulnerable because of immune suppression.
Based on evidence for synergy between lenalidomide and rituximab13 and prior investigations that demonstrated safety and activity of intraventricular as well as intravenous rituximab in relapsed CNS lymphoma,24,25 to maximize delivery of rituximab to the brain tumor microenvironment, we also performed a pilot analysis of combined intraventricular plus intravenous rituximab in patients that did not respond to lenalidomide.
The primary and secondary objectives of this study were to determine safety, identify MTD, demonstrate cerebrospinal fluid (CSF) penetration of lenalidomide at 3 dose levels, and obtain evidence for activity of lenalidomide in CNS and intraocular lymphomas. Additional goals were to gain experience with lenalidomide with combined intraventricular plus intravenous rituximab in patients who progressed on lenalidomide monotherapy, and to test the hypothesis that the CSF metabolome contains novel prognostic biomarkers that also provide insight into new therapeutic targets.
Finally, given that CNS lymphomas are rare neoplasms that are difficult to study, especially with novel agents in a phase 1 setting, we analyzed outcomes of a retrospective cohort of relapsed, refractory PCNSL patients in which maintenance lenalidomide was administered after salvage high-dose methotrexate or focal irradiation strategies. Inclusion of this series complements the phase 1 trial results in providing evidence for activity, feasibility, and safety of lenalidomide in this disease setting and is relevant to the design of future clinical trials.
Materials and methods
Patients
Eligibility required age ≥18 years, recurrent and/or refractory CD20+ NHL involving brain, CSF/meninges and/or intraocular compartments, HIV negativity, anticipated survival >1 month, granulocytes >1.5 × 109/L, platelets >50 × 109/L, total bilirubin <1.5× upper limit of normal (ULN), transaminases <3× ULN, and creatinine clearance ≥60 mL/min. In patients receiving glucocorticoids, dose could not increase 96 hours before initiation of therapy. No patient could receive methotrexate, thiotepa, cytarabine, investigational agents, or rituximab within 30 days of treatment. All patients received thromboprophylaxis with aspirin and/or warfarin. An Ommaya reservoir was used to facilitate rituximab dosing and restaging in each patient. All patients, both in the phase 1 study and the retrospective analysis, signed informed consent indicating that they were aware of the investigational nature and potential risks and benefits of the study in accordance with national regulatory and review boards, the University of California San Francisco institutional review board/Committee on Human Research, and the Declaration of Helsinki.
Study design
The phase 1 study incorporated 2 treatment modules to assess safety, feasibility, and CSF penetration of lenalidomide as monotherapy (treatment 1) and in combination with intraventricular plus intravenous rituximab (treatment 2) in recurrent CNS NHL. Lenalidomide safety and activity was evaluated in the absence of rituximab, using 3 + 3 dose-limiting toxicity (DLT) assessments, to enable clear attribution of response and toxicity symptoms. The original design was to evaluate safety and activity of lenalidomide monotherapy at 3 dose levels: 10, 20, and 30 mg, administered orally daily for 3 weeks (days 1-21) followed by 1 week off, during which patients were restaged (days 22-28). The design planned for 3 patients to be enrolled at this dose level. If no DLT was observed through the first month of lenalidomide monotherapy (cycle 1), then the plan called for 3 patients to be enrolled at the next higher dose level of 20 mg/d (dose level 2). Treatment was escalated to the next higher dose level if no DLT occurred during the first month of therapy; however, if 1 drug-related DLT occurred in these 3 patients, the cohort was expanded to 6 patients to continue to assess that dose level. If the toxicity rate reached 33% in a cohort (≥2 of 6 patients), this dose level was determined a priori to be considered the nontolerated dose. The next lower dose level, the MTD, was determined to be the recommended phase 2 dose (as long as the toxicity rate was <33%).
Each patient was examined and evaluated for toxicity on a weekly basis for at least the first 4 months of protocol-based therapy. Baseline and repeat Mini-Mental Status Examinations26 were performed monthly (supplemental Table 2). Each patient on trial was discussed on a weekly basis at the University of California San Francisco Helen Diller Family Comprehensive Cancer Center Phase 1 Trial Committee to review adverse events, their attribution, and to review and evaluate responses.
DLT was determined during the first month of treatment 1 with the goal to identify the MTD at which fewer than one-third of patients experience a DLT. Evaluation for DLT required that patients receive >75% of planned lenalidomide dose level during the first month. DLT was defined as any grade 3 neurologic toxicity or grade 4 neutropenia related to lenalidomide lasting >7 days despite growth factor support or thrombocytopenia <25 × 109/L despite platelet transfusion during treatment 1. Additionally, any grade 3 or 4 nonhematologic toxicity considered related to lenalidomide was considered DLT, with exclusion of fatigue, rash, thrombosis, or constipation.
Patients with stable disease or better after 1 month of treatment 1 proceeded to a second month of lenalidomide monotherapy (Figure 1). After 2 months of lenalidomide monotherapy at the assigned dose, patients with stable disease, partial, or complete responses (CRs) continued lenalidomide with restaging every 4 weeks, and if stable beyond 4 months, with restaging every 2 months. Patients who exhibited disease progression during treatment 1 proceeded to treatment 2. Patients with disease progression during treatment 2 went off study.
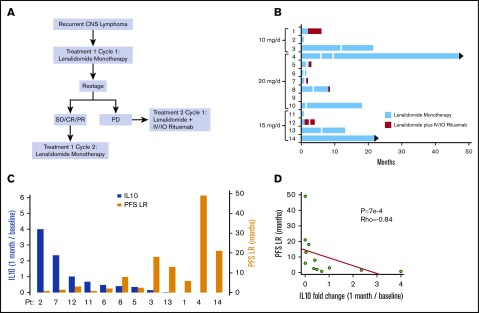
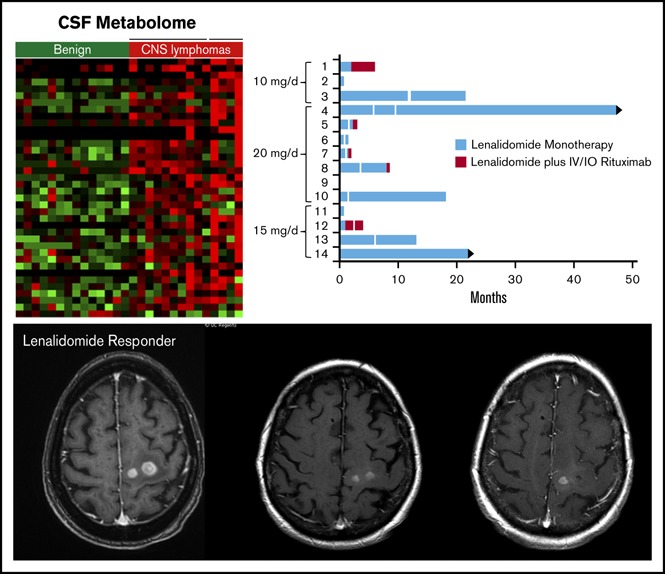
Figure 1.
Phase 1 design and response duration to lenalidomide, lenalidomide/rituximab, and CSF IL-10 as a pharmacodynamic biomarker of response. (A) Protocol schema. (B) Swimmer plot of response duration to lenalidomide monotherapy (treatment 1) and to lenalidomide plus rituximab (treatment 2). Y-axis, study subjects and assigned dose level; x-axis, months on protocol; vertical white lines, dose reduction of lenalidomide. Only 3 of the 9 patients that responded to lenalidomide monotherapy received concomitant dexamethasone. Median OS for the 14 patients is 15.5 months. (C) Y-axis (left in blue), change in CSF IL-10 at 1 month compared with baseline; y-axis (right in yellow), PFS on lenalidomide plus rituximab, in months. There was an inverse correlation between the magnitude of change of IL-10 in CSF at 1 month restaging compared with baseline with response duration to lenalidomide. CSF concentration of IL-10 was determined at pretreatment baseline by enzyme-linked immunosorbent assay (Becton Dickenson) and at 1 month restaging. IL-10 was detected in pretreatment CSF specimens in 13 of 14 patients (92.8%). (CSF IL-10 was not detected at baseline in patient 10, who had CNS DLBCL transformed from CLL.) (D) The inverse correlation between magnitude of change of IL-10 in CSF with response duration to lenalidomide is significant, as demonstrated by Spearman correlation P < .0007 and ρ = −0.84. IO, Intra-Ommaya; LR, lenalidomide/rituximab; OS, overall survival; PFS, progression-free survival; PD, progressive disease; PR, partial response; SD, stable disease.
In treatment 2, cycle 1, lenalidomide was provided on days 1 through 21 with combined intravenous plus intraventricular rituximab administered on days 1, 8, 15, and 22. Intraventricular rituximab (25 mg/dose)24 was administered via an Ommaya reservoir 1 hour after initiation of intravenous rituximab infusion. During subsequent monthly cycles of treatment 2, patients received lenalidomide on days 1 through 21 plus intraventricular rituximab only, every 2 weeks, until disease progression. Restaging was performed monthly. Response criteria were as defined previously.27
Lenalidomide pharmacokinetic analysis
Blood and CSF pharmacokinetic samples were obtained before lenalidomide dose at days 1, 15, and 22 for pharmacokinetic studies. CSF (atraumatic, from Ommaya reservoir) and blood were processed by centrifugation and supernatants stored immediately at −80°C for tandem liquid chromatography mass spectrometry. The analytical methods of lenalidomide quantification are as described elsewhere.28,29 Lenalidomide standard curves were linear (r2 > 0.98) from 0.5 to 100 ng/mL and 5 to 1000 ng/mL in CSF and plasma, respectively. All pharmacokinetic samples were analyzed at Quest Pharmaceutical Services.
CSF metabolomic analysis
To identify candidate metabolomic biomarkers of CNS lymphoma, CSF from 14 control subjects without brain tumors and 14 subjects with CNS lymphoma were processed as described previously and subjected to metabolite identification/quantification using ultra high-performance liquid chromatography or gas chromatography and tandem mass spectrometry with Metabolon.30 CSF lactate in phase 1 trial subjects was quantified by Beckman Coulter Unicell Dxc 800 Clinical Chemistry Analyzer. Quantification of kynurenine and tryptophan in CSF in trial subjects was performed as described previously.31
Results
Phase 1 trial patient characteristics
Baseline characteristics of the 14 phase 1 trial subjects are summarized in Table 1. Median age was 66 years (range, 47-79), including 5 males and 9 females. Median Eastern Cooperative Oncology Group level was 2 (range, 1-4). Subjects had disease involvement in each relevant CNS compartment: 5 had intraocular involvement, 7 had CSF/leptomeningeal disease, and 10 had brain parenchymal involvement. Six had relapsed PCNSL (DLBCL) and 8 had relapsed secondary CNS lymphoma, including 3 with active systemic lymphoid malignancy. Two had concomitant chronic lymphocytic leukemia (CLL), including 1 with extensive leptomeningeal plus systemic involvement (peripheral lymphocyte count, 103 × 109 cells/L) plus secondary DLBCL involving the CSF and optic nerves. One patient had marginal zone lymphoma with dissemination in brain, cauda equina, lymph nodes, bone marrow, and spleen. Subjects in this study experienced disease progression after a median of 3 prior treatment regimens (range, 2-7).
Table 1.
Phase 1 trial patient characteristics and responses
| Pt | Age/sex | Original dx | CNS lymphoma progression after prior therapies | Response duration initial therapy, mo | Initial dexamethasone dose/d | On-study ECOG | IOL status | IOL response | Brain status | Brain response | CSF status | CSF response | OR | Overall PFS, mo |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 57/F | PCNSL/IOL | Initial: IF-RT | 9 | None | 1 | Yes | PR (L/R) | No | NA | No | NA | PR | 6 |
| (DLBCL) | Salvage: MT-R, pemetrexate, ASCT (Bu/Cy) | |||||||||||||
| Intraocular MTX + R | ||||||||||||||
| 2 | 63/M | PCNSL | Initial: MT-R, EA | 2 | None | 2 | No | NA | Yes | PD | No | NA | PD | 1 |
| (DLBCL) | ||||||||||||||
| 3 | 58/F | PCNSL | Initial: MT-R, EA | 18 | None | 1 | Yes | PR | No | NA | No | NA | PR | 21 |
| (DLBCL) | ||||||||||||||
| 4 | 69/F | PCNSL | Initial: MT-R, EA | 8 | None | 1 | Yes | CR | No | NA | No | NA | CR | 48+ |
| (DLBCL) | ||||||||||||||
| 5 | 68/F | PCNSL | Initial: MT-R | Primary refractory | 16 mg | 3 | No | NA | Yes | PR | Yes | CR | PR | 2.5 |
| (DLBCL) | Salvage: HD-MTX + R | |||||||||||||
| 6 | 47/F | Stage IV SCNSL | Initial: MT-R + CHOP + EA | 2 | None | 1 | Yes | SD | Yes | CR | Yes | SD | PR | 1.75 |
| (DLBCL) | ||||||||||||||
| 7 | 77/M | PCNSL | Initial: R-MPV, IT-MTX + HD-AraC | 7 | 8 mg | 2 | No | NA | Yes | PR | Yes | SD | PR | 1.5 |
| (DLBCL) | Salvage: ocular IF-RT, intraocular R | |||||||||||||
| MT-R, IF-RT | ||||||||||||||
| 8 | 54/F | PCNSL/IOL | Initial: MPV | 14 | None | 1 | Yes | PR | Yes | CR | No | NA | PR | 9 |
| (DLBCL) | Salvage: MT-R, HD-AraC, WBRT | |||||||||||||
| 9 | 70/F | SCNSL | Initial: R-EPOCH | 7 | 8 mg | 2 | No | NE | Yes | NE | Yes | NE | NE | NE |
| (DLBCL) | Salvage: R-DHAP, IFOS/VP16 | |||||||||||||
| ASCT (BEAM) | ||||||||||||||
| 10 | 64/F | SCNSL | Initial: R-MPV + WBRT + HD-AraC | 5 | None | 1 | No | NA | Yes | CR | No | NA | CR | 18 |
| (CLL + DLBCL) | ||||||||||||||
| 11 | 53/M | SCNSL | Initial: R-CHOP | 14 | 8 mg | 4 | No | NA | Yes | PD (mixed) | Yes | SD | PD | 1 |
| (DLBCL) | Salvage: MT-R, SRS, EA, HD-AraC | |||||||||||||
| 12 | 77/F | SCNSL | Initial: MT-R | Primary refractory | 8 mg | 2 | No | NA | Yes | PD | Yes | SD | PD | 3 |
| (DLBCL) | Salvage: IF-RT | |||||||||||||
| 13 | 79/M | SCNSL | Initial: R-CHOP + IF-RT | 12 | 4 mg | 2 | No | NA | No | NA | Yes | CR | CR | 13 |
| (CLL + DLBCL) | Salvage: R-Bendamustine | |||||||||||||
| 14 | 71/M | Stage IV | Initial: IV Rituximab | Primary refractory | None | 2 | No | NA | Yes | PR | No | NA | PR | 21+ |
| Marginal zone NHL | Salvage: R-CVP, FCR, R-bendamustine, HD-MTX |
Responses indicated were to lenalidomide monotherapy unless otherwise specified. Patient 10, who had systemic CLL with nodal involvement and documented brain parenchymal secondary CNS lymphoma (DLBCL), experienced stable peripheral lymphadenopathy with lenalidomide. Patient 13 had active leptomeningeal CLL plus concomitant DLBCL in CSF, as assessed by flow cytometry, and lymphoma in optic nerves, as assessed by MRI scan and neuroophthalmologic examinations. This patient’s systemic disease exhibited a PR to lenalidomide with >50% decrease in peripheral lymphocyte count compared with baseline counts prelenalidomide.
ASCT, autologous stem cell transplant; BEAM, BCNU, etoposide, cytarabine, melphalan; Bu/Cy, busulfan/cyclophosphamide; CHOP, cyclophosphamide, Adriamycin, vincristine, prednisone; dx, diagnosis; EA, etoposide/cytarabine; ECOG, Eastern Cooperative Oncology Group; F, female; FCR, fludarabine, cyclophosphamide, rituximab; HD-MTX + R, high-dose methotrexate plus rituximab; IF-RT, involved field radiotherapy; IFOS/VP16, ifosfamide/etoposide; IOL, intraocular lymphoma; IT, intrathecal; M, male; MT-R, high-dose methotrexate, temozolomide, rituximab; L, lenalidomide; NA, not applicable; NE, not evaluable; OR, overall response; Pt, patient; R, rituximab; R-CVP, rituximab, cyclophosphamide, vincristine, prednisone; R-DHAP, rituximab, dexamethasone, high-dose cytarabine, cisplatin; R-EPOCH, rituximab, etoposide, prednisone, vincristine, cyclophosphamide, Adriamycin; R-MPV, rituximab, methotrexate, prednisone, vincristine; SRS, stereotactic radiosurgery; WBRT, whole-brain radiotherapy.
Phase 1 trial toxicities
In general, lenalidomide was well-tolerated as monotherapy and with rituximab, with no unexpected toxicities (supplemental Table 1). Two toxicities met DLT criteria at the 20-mg dose. Patient 6 experienced grade 3 confusion, possibly related to lenalidomide, and patient 10, with concomitant CLL, developed Streptococcus pneumoniae bacteremia (grade 4 infection without neutropenia). Ultimately, this patient’s CNS lymphoma responded to lenalidomide after dose reduction to 10 mg/d; protocol-based therapy was continued for 14 months.
Because 2 DLT were detected at 20 mg/d, a dose level at which each patient exhibited clinical responses to lenalidomide, and because we had determined that CSF penetration of lenalidomide at 20 mg/d was superior to the 10 mg/d dose level, the protocol was modified to evaluate an intermediate dose, 15 mg/d, at which there were no DLT. Overall, during the course of treatment 1 administrations among 14 patients, there were 3 ≥grade 3 infections: 1 recurrent urinary tract infection, 1 S pneumoniae, and 1 grade 3 zoster. Two of these infections involved patients with transformed CLL.
Five patients progressed on lenalidomide monotherapy and received treatment 2 for a total 30 administrations of intraventricular rituximab, 18 with concomitant intravenous rituximab. Treatment 2 was associated with only 1 serious adverse event: recurrent pneumonia that resolved with antibiotics and prompted dose reduction of lenalidomide from 15 to 10 mg/d and of intraventricular rituximab to 10 mg/d (patient 12).
There were no thromboembolic events, and although 2 patients succumbed to progression of lymphoma within 30 days of treatment, there were no treatment-related deaths. Patient 9 discontinued therapy at 1 week because of persistent frailty that made weekly transportation to the clinic impossible.
CSF penetration of lenalidomide
A total of 34 time-matched plasma-CSF sample pairs were collected at trough time points (∼16 hours after lenalidomide dose). Plasma lenalidomide concentration ranged from 0 to 220 ng/mL, consistent with its rapid clearance and plasma half-life of 3 to 4 hours.32 Lenalidomide was detected in ventricular CSF in 10 of 13 patients and in 65% of CSF specimens. CSF concentration of lenalidomide ranged from 0 to 16.68 ng/mL, with a mean trough concentration of 4.9 ng/mL.
The CSF/plasma ratio, calculated based on the 23 matched plasma-CSF pairs in which lenalidomide concentration was >0 in plasma, ranged from 0% to 49%. Although the highest concentrations of lenalidomide were detected at the 15-mg dose level, this is explained by the 25% to 60% interindividual variation in plasma area under the curve that has been established in normal individuals.32 We also observed dose-dependent increases in CSF penetration of lenalidomide. Lenalidomide was detected in CSF in 1 patient in the 10-mg cohort, yielding an estimated CSF/plasma partition coefficient of 10%. At 15 mg, mean trough CSF/plasma partition coefficient for lenalidomide was 20.4%, based on 11 time-matched plasma and CSF specimens obtained from each of 4 patients. The mean trough CSF/plasma partition coefficient for lenalidomide at 20 mg was similar, 25.5%, based on 12 matched plasma and CSF specimens obtained in which lenalidomide was detected in CSF in 5 of 6 patients (Table 2).
Table 2.
Lenalidomide trough concentrations in CSF and plasma (16 h after dose)
| Dose, mg/d | Plasma concentration, ng/mL | CSF concentration, ng/mL | Ratio, % |
|---|---|---|---|
| 10 | 2.07 (2.94); n = 10 | 0.05 (0.16); n = 10 | 2.42 (4.84); n = 4 |
| 15 | 48.7 (45.2); n = 11 | 7.37 (3.04); n = 11 | 20.4 (8.20); n = 11 |
| 20 | 24.8 (59.1); n = 13 | 2.97 (3.03); n = 13 | 25.5 (19.6); n = 8 |
Data in the table are shown as mean (standard deviation) with n = number of samples. Concentration below the detection limit is assigned as 0 for plasma and for CSF samples. Ratio is calculated based on the time-matched blood and CSF samples from the same subject.
Phase 1 trial clinical responses
Lenalidomide had significant activity in relapsed, refractory primary and secondary CNS lymphoma patients. By intention to treat, the overall response rate to lenalidomide monotherapy was 64%: 9 of 14 patients who received lenalidomide exhibited regression of CNS lymphoma. Among patients with brain parenchymal lymphoma, response rate was 60% (6 of 10 patients). Among patients with leptomeningeal disease, CR to lenalidomide was 33% (2 of 6 patients), confirmed by ≥3 consecutive, weekly repeat CSF analyses using both flow cytometry and cytology (supplemental Figure 4). Lenalidomide monotherapy was active in intraocular lymphoma in 2 of 4 patients, each with biopsy-proven vitreoretinal disease, who experienced durable regressions of lymphoma, without steroids, as confirmed by complete ophthalmologic examinations. Notably, 6 of 9 patients who had regression of relapsed CNS lymphoma with lenalidomide experienced response durations ≥9 months; in 4 of these patients, the response duration to lenalidomide monotherapy exceeded 18 months (Figure 1)
Although treatment 2, administered to 5 patients after lenalidomide failure, yielded only 1 partial response lasting 4 months (patient 1), 3 patients in this cohort (patients 5, 7, and 12) had previously demonstrated primary resistance to rituximab-containing regimens in absence of lenalidomide.
CSF IL-10 as a pharmacodynamic biomarker of lenalidomide
We and others previously demonstrated that high CSF interleukin-10 (IL-10; >45 pg/mL) is associated with adverse prognosis in CNS lymphoma at diagnosis and that IL-10 concentration serially correlates with clinical response and/or progression.33,34 We therefore evaluated this biomarker in the lenalidomide/rituximab trial. CSF IL-10 was detected by enzyme-linked immunosorbent assay in ventricular CSF in 13 of 14 patients at pretreatment baseline at a median concentration of 165 pg/mL (range, 0-5212 pg/mL). Objective changes in CSF IL-10 concentration compared with pretreatment baseline reassuringly corroborated the clinical assessment of response to lenalidomide (including changes in IL-10 in the CSF that were in agreement with clinical response within the intraocular compartment) made by magnetic resonance imaging (MRI) scan, ophthalmologic examination, or detection of lymphoma cells in CSF. We also noted a significant inverse correlation between the fold-change of IL-10 concentration at 1 month compared with baseline with time to progression to lenalidomide and/or lenalidomide/rituximab (P < .0007; ρ = −0.84; Spearman correlation) (Figure 1) This finding supports CSF IL-10 as a novel, early pharmacodynamic biomarker of response to lenalidomide in CNS lymphomas.
Identification of CSF metabolomic biomarkers
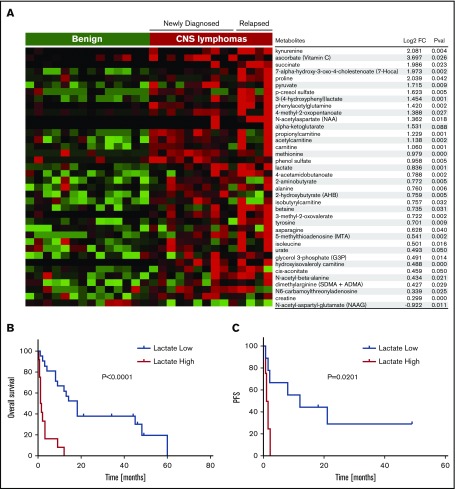
To gain insight into pathogenesis of CNS lymphoma and to identify novel prognostic biomarkers, we performed differential metabolomic profiling of CSF of 14 control subjects with nonmalignant conditions and 14 independent patients with CNS lymphoma (Figure 2; supplemental Table 3). Of 145 metabolites analyzed, 36 were significantly upregulated in CNS lymphoma (P < .05); these were principally involved in energy metabolism pathways, including lactate; purine metabolism, including 5-methylthioadenosine; and amino acid metabolism, including kynurenine. Although the highest CSF levels of lactate, 5-methylthioadenosine, and kynurenine were in the subset of patients with relapsed CNS lymphoma with leptomeningeal involvement, each of these metabolites was significantly elevated in the cohort of CSF collected from PCNSL patients at diagnosis compared with CSF from control subjects without neoplastic conditions.
Figure 2.
Identification of candidate CSF metabolic biomarkers in CNS lymphoma. (A) Relative quantification of metabolites in CSF in 14 control subjects without brain tumors and 14 independent patients with CNS lymphoma are presented in the heat map. Of 145 metabolites analyzed, 36 were significantly upregulated in CNS lymphoma (P < .05, Wilcoxon test). Notably, among the upregulated metabolites in CSF in CNS lymphoma, lactate and kynurenine were most significantly increased in patients with active leptomeningeal lymphoma. In addition, CSF from CNS lymphoma patients contained an increased ratio of kynurenine/tryptophan, indicative of activation of indoleamine-2,3 dioxygenase in CNS lymphoma (fold-change 2.3, P < .039). Using a standardized quantitative assay for CSF lactate (Beckman Coulter Unicell Dxc 800 Clinical Chemistry Analyzer), we confirmed upregulated CSF lactate by more than twofold in an independent validation set of 8 CNS DLBCL vs 8 nonneoplastic controls (P < .0015) (supplemental Figure 2). We also determined that elevated baseline CSF lactate (>ULN 2.8 mmol/L), correlated with short PFS (P = .0129) as well as OS (P = .004) in relapsed CNS lymphoma patients treated with lenalidomide monotherapy. We determined lactate concentrations in ventricular CSF in pretreatment specimens in 33 patients with relapsed CNS lymphoma that participated in 3 independent phase 1 immunotherapy trials involving rituximab (8/10 pretreatment specimens),24 methotrexate plus rituximab (11/14 pretreatment specimens),25 and lenalidomide plus rituximab (all 14 pretreatment specimens). Median baseline lactate concentration in ventricular CSF in these patients was 2.4 mmol/L (range, 1.2-9). Patients with elevated lactate in ventricular CSF (>2.8 mmol/L, ULN of CSF lactate) experienced shorter overall survival in each of these individual trials compared with patients with normal lactate at baseline. In aggregate, patients with elevated CSF lactate (12 patients) exhibited significantly shorter OS compared with patients with low CSF lactate (22 patients) P < .0001. (B) High CSF lactate also correlated with shorter progression-free survival in relapsed CNS lymphoma subjects treated with lenalidomide monotherapy. (C) By contrast, serum LDH at baseline did not significantly correlate with PFS or OS (not shown). Notably, pretreatment baseline, matched CSF, and plasma lactate concentrations were compared in 7 lenalidomide/rituximab trial subjects: in 5, the concentration of lactate in CSF was higher (median, 1.9-fold higher) than the concentration of lactate in plasma.
Given the importance of Warburg metabolism in the pathogenesis of lymphoma,35 we evaluated the prognostic significance of CSF lactate in the context of relapsed CNS lymphoma. We determined lactate concentrations in ventricular CSF in pretreatment specimens in 33 patients with relapsed CNS lymphoma that participated in 3 independent phase 1 immunotherapy trials involving rituximab,24 methotrexate plus rituximab,25 and lenalidomide plus rituximab. Median baseline lactate concentration in ventricular CSF in these patients was 2.4 mmol/L (range, 1.2-9). Patients with elevated lactate in ventricular CSF (>2.8 mmol/L, ULN of CSF lactate36,37) experienced shorter overall survival in each of these individual trials compared with patients with normal lactate at baseline. In aggregate, relapsed CNS lymphoma patients with elevated CSF lactate exhibited significantly shorter overall survival: P < .0001 (Figure 2B). High CSF lactate also correlated with shorter progression-free survival in relapsed CNS lymphoma subjects treated with lenalidomide monotherapy (Figure 2C).
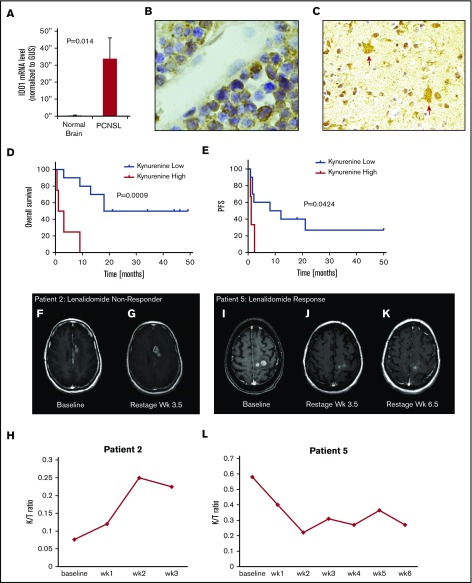
Given that kynurenine is a metabolite of l-tryptophan, via degradation by indoleamine-2,3 dioxygenase (IDO), and that IDO activity mediates suppression of T-cell function,38 we evaluated this pathway in CNS lymphomas and in the context of the lenalidomide trial. We first demonstrated marked upregulation of IDO1 transcripts in diagnostic specimens of PCNSL and identified IDO1 protein expression by lymphoma and tumor-associated macrophages in 19 of 20 cases of newly diagnosed PCNSL (Figure 3A-C; supplemental Table 4).
Figure 3.
Expression of IDO1 in PCNSL and IDO activity and response to lenalidomide in relapsed CNS lymphoma. (A) Quantitative reverse transcription polymerase chain reaction demonstrated increased expression of IDO1 transcripts in 10 cases of PCNSL (DLBCL) compared with 3 cases of nonneoplastic brain (P < .014, Wilcoxon test). Immunohistochemistry using α-IDO-1 antibody (Abcam clone 55305, with diaminobenzidine detection, hematoxylin counterstain) demonstrated IDO-1 expression in 19/20 cases of newly diagnosed PCNSL (supplemental Table 4). In 12 of these, IDO1 immunoreactivity was exhibited by (B, ×1000 >40% of lymphoma cells) and (C, ×400) tumor-associated macrophages. These displayed dendritic morphology and coexpressed CD163, a marker of alternative activation (not shown). Elevated baseline CSF kynurenine concentration (1.5 μM) correlated with shorter overall survival among the 14 phase 1 trial subjects as well as shorter progression-free survival with lenalidomide. (D-E) We also detected increasing CSF kynurenine/tryptophan ratios in 3 patients who had low CSF kynurenine at baseline and in whom CSF kynurenine levels exceeded the high-risk benchmark of 1.5 μM at tumor progression. Serial quantification of the K/T ratio in CSF in lenalidomide/rituximab trial subjects demonstrated that changes in IDO activity correlated with early progression and/or response to lenalidomide in 10 of 13 evaluable patients. Representative examples are shown: axial-T1 postgadolinium MRI scan demonstrating CNS lymphoma progression during first month of lenalidomide in patient 2: pretreatment (F) at baseline and (G) 1 month. The CSF K/T ratio at 1 month increased by nearly threefold compared with baseline during this interval. (H) MRI scan showing progressive regression of CNS lymphoma in response to lenalidomide monotherapy in patient 5: pretreatment (I) at baseline, (J) at 1 month, and (K) 2 months. (L) The CSF K/T ratio declined by >50% during the first 2 months of lenalidomide therapy in this patient. mRNA, messenger RNA.
High serum kynurenine and increasing kynurenine/tryptophan (K/T) ratios are established biomarkers of IDO activity and correlate with tumor aggressiveness in a variety of malignancies.39-43 In particular, in aggressive lymphomas, baseline elevated kynurenine concentration in serum has reproducibly been demonstrated to be an independent adverse prognostic factor associated with short overall survival in both systemic DLBCL (kynurenine concentration, >1.5 μM)43,44 and in adult T-cell leukemia/lymphoma (kynurenine concentration, >2 μM).45 We performed serial analysis of kynurenine and tryptophan concentrations in ventricular CSF in phase 1 subjects treated with lenalidomide. Median pretreatment kynurenine concentration was 1 μM (range, 0.3-3.6). Four patients (patients 5, 9, 11, and 12) had baseline pretreatment CSF kynurenine concentrations >1.5 μM (range, 2.8-3.6). Overall survival for this cohort was shorter than for CNS lymphoma patients whose baseline CSF kynurenine concentration was <1.5 μM (P = .0009), in agreement with kynurenine prognostic cut-points established in systemic aggressive lymphomas. We also detected increasing CSF K/T ratios in 3 patients who had low CSF kynurenine at baseline and in whom CSF kynurenine levels exceeded the serum kynurenine high-risk benchmark of 1.5 μM at the time of tumor progression on lenalidomide monotherapy: within the first 2 months (patients 6 and 7) and at 21 months (patient 3) (Figure 3D-E). Although the sample size is small, taken together, these results agree with previous observations that elevated kynurenine concentration as a biomarker correlates with short survival in aggressive lymphoma and suggest that IDO activity in the CNS lymphoma microenvironment may contribute to therapeutic resistance to lenalidomide.
Lenalidomide maintenance after salvage in recurrent CNS lymphoma
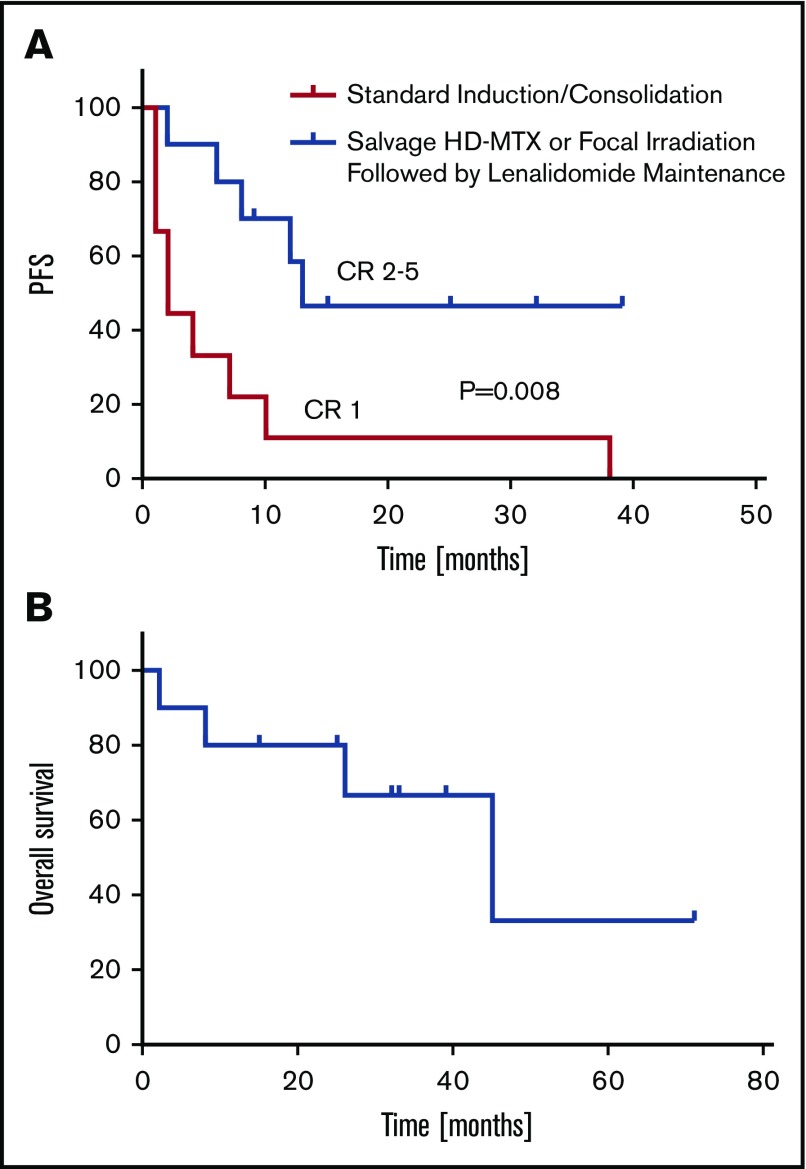
Beginning in 2010, as an alternative to whole brain irradiation or dose-intensive chemotherapy, we began to provide low-dose lenalidomide maintenance (5-10 mg/d) after salvage therapy for patients with relapsed, refractory CNS DLBCL (Table 3). Retrospective analysis of an independent cohort of 10 consecutive relapsed PCNSL patients (median age, 61.5 years; range, 45-81) reveals that the median response duration in CR 2 through 5 (after 1-4 previous relapses) with lenalidomide maintenance were ≥6 times longer than response duration after CR 1 with standard induction therapies (P = .008) (Figure 4). Before lenalidomide, each of these patients received salvage therapy with either repeat intravenous methotrexate, at the same doses used during prior induction, or with focal (not whole brain) irradiation, an unconventional salvage approach that is usually considered inadequate in PCNSL because of its multicentric and infiltrating nature, particularly at relapse.46,47 Low-dose lenalidomide maintenance after salvage has been well-tolerated with no infectious complications and survival for this cohort has been encouraging. Thus far, only 1 patient has progressed to receive whole brain irradiation and 4 have succumbed to DLBCL (Figure 4).
Table 3.
Characteristics of the 10 patients with relapsed PCNSL that received maintenance lenalidomide in CR 2 through 5, after salvage therapy with either high-dose methotrexate or focal irradiation
| Pt | Age/sex | Original dx | Disease site at dx and relapse | ECOG | Therapy | Response duration, mo |
|---|---|---|---|---|---|---|
| 1 | 45/F | PCNSL | Dx: occipital mass | 3 | MT-R + EA | CR: 2 |
| Progression 1: basal ganglia mass | 2 | γ Knife + lenalidomide maintenance (10 mg/d) | CR: 12 | |||
| 2 | 81/F | PCNSL | Dx: frontal mass | 3 | MT-R (5 g/m2 HD-MTX) | CR: 1 |
| Progression: Frontal DLBCL Mass | 3 | M-R (5 g/m2 × 4 doses HD-MTX) + lenalidomide maintenance (5 mg/d) | CR: 39+ | |||
| 3 | 75/M | PCNSL/IOL | Dx: bilateral IOL + CSF | 3 | MT-R + EA | CR: 38 |
| Progression 1: frontal lobe mass | 3 | MT-R (×8 doses HD-MTX) + lenalidomide maintenance (5 mg/d) | CR: 8 | |||
| 4 | 55/M | PCNSL | Dx: thalamic mass | 2 | MT-R + EA | CR: 7 |
| Progression 2: thalamus/basal ganglia masses | 2 | MT-R (×8 doses HD-MTX) | CR: 8 | |||
| Progression 3: thalamus/basal ganglia | 2 | MT-R (×8 doses HD-MTX) | CR: 3 | |||
| Progression 4: thalamus/basal ganglia | 3 | MT-R (×8 doses HD-MTX) | PR: 2 | |||
| Progression 5: thalamus/basal ganglia | 3 | IFRT 30.6 Gy/17 fx + lenalidomide maintenance (5 mg alternating with 10 mg/d) | CR: 32+ | |||
| 5 | 38/F | PCNSL | Dx: multifocal brain lesions | 3 | MT-R + EA | CR: 2 |
| Progression 1: IOL | 1 | M-R X 3 doses + lenalidomide maintenance (10 mg/d) | CR: 9 | |||
| 6 | 48/F | PCNSL | Dx: basal ganglia | 2 | MT-R + EA | CR: 4 |
| Progression 1: thalamus/basal ganglia | 2 | MT-R + ASCT (TT, Carbo, VP16) | CR: 3 | |||
| Progression 2: Thalamus/basal ganglia | 3 | M-R 4 cycles + lenalidomide maintenance (10 mg/d) | CR: 13 | |||
| 7 | 61/M | PCNSL | Dx: left temporal lobe mass | 2 | MT-R + EA | CR: 10 |
| Progression 1: thalamus mass | 3 | M-R × 8 doses | PR: 1 | |||
| Progression 2: thalamus mass | 3 | IFRT followed by lenalidomide maintenance (5 mg/d) | PR: 2 | |||
| 8 | 78/F | PCNSL | Dx: frontal mass | 2 | MT-R (4 g/m2) | CR: 1 |
| Progression 1: frontal mass | 2 | GK + lenalidomide maintenance (5 mg/d) | CR: 6 | |||
| 9 | 61/F | PVRL | Dx: B IOL | 2 | MT-R + EA, vitrectomy | CR: 1 |
| Progression 1: B IOL | 2 | IFRT + HD-MTX × 3 doses HD-MTX | CR: 9 | |||
| Progression: 2: frontal mass | 2 | GK followed by lenalidomide maintenance (5 mg alternating with 10 mg QOD) | CR: 25+ | |||
| 10 | 73/F | PCNSL | Dx: temporal lobe | 2 | R-MPV + EA (3.5 mg/m2) | CR: 10 |
| Progression 1: bilateral IOL | 2 | M-R (1.5-3 mg/m2 × 6 doses HD-MTX) followed by lenalidomide maintenance (5 mg/d) | CR: 15+ |
Carbo, carboplatin; IF-RT, involved field radiotherapy; TT, thiotepa.
Figure 4.
Lenalidomide maintenance after salvage in recurrent PCNSL. (A) Retrospective analysis of cohort of 10 relapsed PCNSL patients (median age, 61 years), reveals that the median response duration in CR 2-5 (after 1-4 previous relapses) with lenalidomide maintenance (5-10 mg/d; days 1-21/cycle) is ≥6 times longer than response duration after CR 1 in these patients with disease relapsing after standard induction therapies (P < .008; 95% confidence interval of ratio, 2.2-19.4). For 8 of these 10 patients with relapsed PCNSL, the longest response duration achieved with any intervention has been with lenalidomide maintenance. Five patients received salvage treatment with focal irradiation only, before lenalidomide (supplemental Figure 3), and 5 were retreated at last salvage with high-dose methotrexate at the same doses used during induction, before lenalidomide (Table 3). (B) Median overall survival is 45 months. With a median follow-up of 12.5 months, thus far, only 1 patient has progressed on lenalidomide to receive whole brain irradiation and 4 have succumbed to DLBCL. Patients on maintenance lenalidomide were closely monitored and examined in the clinic on an at least monthly basis for the first year, with restaging studies performed as indicated and with similar frequency as after previous therapies. Restaging MRI scans of the brain were performed in all 10 patients with a mean frequency of every 3.65 months; ophthalmologic examinations were performed for 6 patients at highest risk for ocular relapse (patients 1, 3-5, and 9-10) with a mean frequency of every 4 months (supplemental Table 5).
Discussion
To our knowledge, this is the first study to demonstrate significant activity of lenalidomide monotherapy in relapsed, refractory CNS lymphomas. Responses to lenalidomide were detected at each dose level in 9/13 evaluable patients and in brain, CSF, and intraocular compartments. Six patients maintained responses to lenalidomide for ≥9 months and 4 maintained responses ≥18 months. Although 3 responding patients received lenalidomide in combination with dexamethasone, each had previously experienced CNS lymphoma progression with dexamethasone with prior chemotherapies. Toxicity, including neurotoxicity, was as anticipated for lenalidomide in lymphoid malignancy, and manageable, although the Mini-Mental Status Examination has limited sensitivity compared with more extensive neurocognitive batteries.48
Notably, the CSF penetration of lenalidomide did not appear to require a disrupted blood–brain barrier, because lenalidomide was detected in ventricular CSF in 2 patients (treated at 10- and 20-mg dose levels) who had no radiographic or cytologic evidence of brain or leptomeningeal disease; each had normal CSF protein concentration. This information may be relevant to the management of patients with systemic aggressive lymphoma who are at risk for CNS relapse, as well as for patients with plasma cell neoplasms. Given the similar trough CSF concentrations for lenalidomide at 15- and 20-mg/d dose levels, and that DLT was observed at 20 mg dose, our study supports the recommended starting dose of lenalidomide 15 mg/d in treatment of relapsed CNS NHL.
Our rationale to carefully investigate the safety and CSF penetration of lenalidomide via 3 + 3 dose escalation as monotherapy in the CNS lymphoma patient population is supported by the experiences of other recent phase 1/2 studies of targeted agents in relapsed CNS lymphoma. For example, a potentially lethal and unanticipated toxicity of ibrutinib monotherapy in CNS lymphoma has reproducibly been demonstrated in each prospective investigation of ibrutinib in the CNS lymphoma population the development of invasive aspergillosis involving the brain and lungs.49,50 We did not detect invasive aspergillosis in association with lenalidomide in either the phase 1 trial or in the retrospective lenalidomide maintenance cohort. In addition, toxicities experienced in a recent phase 2 study of temsirolimus, an mTOR inhibitor, were considerable, with 13.5% treatment-related mortality and an 18.9% rate of severe infections,51 notably higher than the 9% rate of severe infections detected with temsirolimus in mantle cell lymphoma.52
We demonstrate that CSF lactate is a novel metabolic biomarker of CNS lymphoma that may be valuable in discriminating CNS lymphoma patients likely to benefit from lenalidomide. We hypothesize that high lactate may impair antitumor immune responses mediated by infiltrating myeloid, natural killer, and T cells within the tumor microenvironment.53-55 (supplemental Figure 1). Given that determination of lactate concentration in CSF is a clinically validated and inexpensive test, incorporation of CSF lactate measurements could be integrated in future clinical investigation of CNS lymphoma (and other brain tumors) for prognostication, risk stratification, and interstudy comparisons.
In addition, we provide evidence for the activation of the IDO pathway in CNS lymphomas, raising the possibility that IDO may contribute to early resistance to lenalidomide. Notably, IDO1 expression is adversely prognostic in systemic DLBCL43,44 and IDO activity antagonizes CD19 CART cell therapy.56 Given the emergence of IDO inhibitors in immuno-oncology,57 these data suggest their potential integration in future studies in combination with immunomodulatory drugs, CD19-CART cells, and/or checkpoint blockade in CNS lymphoma. Additional immunosuppressive metabolites and peptides identified and/or characterized in the CNS lymphoma microenvironment in this study and that represent potential targets include IL-10,58-60 5-methylthioadenosine,61,62 and dimethylarginine.63,64
Although the biomarker and response data need to be considered in the context of small sample size of an early-phase trial, the combined results from phase 1 investigation plus a parallel analysis of low-dose lenalidomide maintenance in independent patients support the relevance of these data to the design of future studies. Notably, based on the lenalidomide maintenance data presented in this study, 2 randomized phase 2 studies are in development to evaluate low-dose lenalidomide maintenance in older PCNSL patients after induction, in lieu of intensive consolidation. In addition, given the potential of PD1 checkpoint blockade in CNS lymphoma,65 a study is in development to evaluate IDO antagonism in combination with anti-PD1 therapy. Further validation of CSF lactate, the IDO pathway, and other candidate targets are key correlatives of this study.
Supplementary Material
The full-text version of this article contains a data supplement.
Acknowledgments
The authors are grateful for the clinical research support of Mallory Kock, Wesley Cheung, and Eseosa Igbinedion.
This research was supported by the Leukemia & Lymphoma Society; National Institutes of Health, National Cancer Institute (R01CA139-83-01A1); Sandler Program for Breakthrough Biomedical Research (J.L.R.); and Cancer Center Support Grant P30CA082103 from the National Institutes of Health, National Cancer Institute.
Footnotes
Presented in part in oral abstract form at the 2016 Annual Meeting of the American Society of Clinical Oncology, Chicago, IL, 5 June 2016, and at the 2015 Annual Meeting of the International Congress of Malignant Lymphoma, Lugano, Switzerland, 20 June 2015.
Authorship
Contribution: J.L.R. designed and performed the research, analyzed the data, and wrote the paper; H.G. analyzed the data and wrote the paper; E.J.F. analyzed the data; P.F. codesigned and performed the research; L.C., J.S., P.K., K.C., J.V., and J.L. performed the research; J.K. performed the research and codesigned the study; C.L., J.H., and P.T. analyzed the data; P.K.S. performed the research; X.W., N.C., and J.G. performed the research, analyzed the data; P.N.M. codesigned the research and analyzed the data; and B.D. performed the research and analyzed the data.
Conflict-of-interest disclosure: J.L.R. receives research support from Celgene and Genentech. The remaining authors declare no competing financial interests.
Correspondence: James L. Rubenstein, University of California, San Francisco, Division of Hematology/Oncology, M1282, Box 1270, San Francisco, CA 94143; e-mail: james.rubenstein@ucsf.edu.
References
- 1.Carnevale J, Rubenstein JL. The challenge of primary central nervous system lymphoma. Hematol Oncol Clin North Am. 2016;30(6):1293-1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kasenda B, Loeffler J, Illerhaus G, Ferreri AJ, Rubenstein J, Batchelor TT. The role of whole brain radiation in primary CNS lymphoma. Blood. 2016;128(1):32-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ferreri AJ, Illerhaus G. The role of autologous stem cell transplantation in primary central nervous system lymphoma. Blood. 2016;127(13):1642-1649. [DOI] [PubMed] [Google Scholar]
- 4.Savage KJ, Slack GW, Mottok A, et al. Impact of dual expression of MYC and BCL2 by immunohistochemistry on the risk of CNS relapse in DLBCL. Blood. 2016;127(18):2182-2188. [DOI] [PubMed] [Google Scholar]
- 5.Panageas KS, Elkin EB, Ben-Porat L, Deangelis LM, Abrey LE. Patterns of treatment in older adults with primary central nervous system lymphoma. Cancer. 2007;110(6):1338-1344. [DOI] [PubMed] [Google Scholar]
- 6.Villano JL, Koshy M, Shaikh H, Dolecek TA, McCarthy BJ. Age, gender, and racial differences in incidence and survival in primary CNS lymphoma. Br J Cancer. 2011;105(9):1414-1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Krönke J, Udeshi ND, Narla A, et al. Lenalidomide causes selective degradation of IKZF1 and IKZF3 in multiple myeloma cells. Science. 2014;343(6168):301-305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lu G, Middleton RE, Sun H, et al. The myeloma drug lenalidomide promotes the cereblon-dependent destruction of Ikaros proteins. Science. 2014;343(6168):305-309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bjorklund CC, Lu L, Kang J, et al. Rate of CRL4(CRBN) substrate Ikaros and Aiolos degradation underlies differential activity of lenalidomide and pomalidomide in multiple myeloma cells by regulation of c-Myc and IRF4. Blood Cancer J. 2015;5(10):e354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lopez-Girona A, Mendy D, Ito T, et al. Cereblon is a direct protein target for immunomodulatory and antiproliferative activities of lenalidomide and pomalidomide [published correction appears in Leukemia. 2012;26:2445]. Leukemia. 2012;26(11):2326-2335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reddy N, Hernandez-Ilizaliturri FJ, Deeb G, et al. Immunomodulatory drugs stimulate natural killer-cell function, alter cytokine production by dendritic cells, and inhibit angiogenesis enhancing the anti-tumour activity of rituximab in vivo. Br J Haematol. 2008;140(1):36-45. [DOI] [PubMed] [Google Scholar]
- 12.Wu L, Adams M, Carter T, et al. lenalidomide enhances natural killer cell and monocyte-mediated antibody-dependent cellular cytotoxicity of rituximab-treated CD20+ tumor cells. Clin Cancer Res. 2008;14(14):4650-4657. [DOI] [PubMed] [Google Scholar]
- 13.Hernandez-Ilizaliturri FJ, Reddy N, Holkova B, Ottman E, Czuczman MS. Immunomodulatory drug CC-5013 or CC-4047 and rituximab enhance antitumor activity in a severe combined immunodeficient mouse lymphoma model. Clin Cancer Res. 2005;11(16):5984-5992. [DOI] [PubMed] [Google Scholar]
- 14.Lopez-Girona A, Heintel D, Zhang LH, et al. Lenalidomide downregulates the cell survival factor, interferon regulatory factor-4, providing a potential mechanistic link for predicting response. Br J Haematol. 2011;154(3):325-336. [DOI] [PubMed] [Google Scholar]
- 15.Yang Y, Shaffer AL III, Emre NC, et al. Exploiting synthetic lethality for the therapy of ABC diffuse large B cell lymphoma. Cancer Cell. 2012;21(6):723-737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Camilleri-Broët S, Crinière E, Broët P, et al. A uniform activated B-cell-like immunophenotype might explain the poor prognosis of primary central nervous system lymphomas: analysis of 83 cases. Blood. 2006;107(1):190-196. [DOI] [PubMed] [Google Scholar]
- 17.Rubenstein JL, Fridlyand J, Shen A, et al. Gene expression and angiotropism in primary CNS lymphoma. Blood. 2006;107(9):3716-3723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rubenstein JL, Treseler PA, Stewart PJ. Regression of refractory intraocular large B-cell lymphoma with lenalidomide monotherapy. J Clin Oncol. 2011;29(20):e595-e597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cox MC, Mannino G, Lionetto L, Naso V, Simmaco M, Spiriti MA. Lenalidomide for aggressive B-cell lymphoma involving the central nervous system? Am J Hematol. 2011;86(11):957. [DOI] [PubMed] [Google Scholar]
- 20.Patel UH, Mir MA, Sivik JK, Raheja D, Pandey MK, Talamo G. Central neurotoxicity of immunomodulatory drugs in multiple myeloma. Hematol Rep. 2015;7(1):5704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rollin-Sillaire A, Delbeuck X, Pollet M, et al. Memory loss during lenalidomide treatment: a report on two cases. BMC Pharmacol Toxicol. 2013;14(1):41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Higgins JJ, Tal AL, Sun X, et al. Temporal and spatial mouse brain expression of cereblon, an ionic channel regulator involved in human intelligence. J Neurogenet. 2010;24(1):18-26. [DOI] [PubMed] [Google Scholar]
- 23.Aizawa M, Abe Y, Ito T, Handa H, Nawa H. mRNA distribution of the thalidomide binding protein cereblon in adult mouse brain. Neurosci Res. 2011;69(4):343-347. [DOI] [PubMed] [Google Scholar]
- 24.Rubenstein JL, Fridlyand J, Abrey L, et al. Phase I study of intraventricular administration of rituximab in patients with recurrent CNS and intraocular lymphoma. J Clin Oncol. 2007;25(11):1350-1356. [DOI] [PubMed] [Google Scholar]
- 25.Rubenstein JL, Li J, Chen L, et al. Multicenter phase 1 trial of intraventricular immunochemotherapy in recurrent CNS lymphoma. Blood. 2013;121(5):745-751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state.” A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189-198. [DOI] [PubMed] [Google Scholar]
- 27.Abrey LE, Batchelor TT, Ferreri AJ, et al. ; International Primary CNS Lymphoma Collaborative Group. Report of an international workshop to standardize baseline evaluation and response criteria for primary CNS lymphoma. J Clin Oncol. 2005;23(22):5034-5043. [DOI] [PubMed] [Google Scholar]
- 28.Chen N, Lau H, Choudhury S, Wang X, Assaf M, Laskin OL. Distribution of lenalidomide into semen of healthy men after multiple oral doses. J Clin Pharmacol. 2010;50(7):767-774. [DOI] [PubMed] [Google Scholar]
- 29.Chen N, Weiss D, Reyes J, et al. No clinically significant drug interactions between lenalidomide and P-glycoprotein substrates and inhibitors: results from controlled phase I studies in healthy volunteers [published correction appears in Cancer Chemother Pharmacol. 2014;74(3):659-660]. Cancer Chemother Pharmacol. 2014;73(5):1031-1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cassol E, Misra V, Dutta A, Morgello S, Gabuzda D. Cerebrospinal fluid metabolomics reveals altered waste clearance and accelerated aging in HIV patients with neurocognitive impairment. AIDS. 2014;28(11):1579-1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gertsman I, Gangoiti JA, Barshop BA. Validation of a dual LC-HRMS platform for clinical metabolic diagnosis in serum, bridging quantitative analysis and untargeted metabolomics. Metabolomics. 2014;10(2):312-323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen N, Zhou S, Palmisano M. Clinical pharmacokinetics and pharmacodynamics of lenalidomide. Clin Pharmacokinet. 2017;56(2):139-152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rubenstein JL, Wong VS, Kadoch C, et al. CXCL13 plus interleukin 10 is highly specific for the diagnosis of CNS lymphoma. Blood. 2013;121(23):4740-4748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sasayama T, Nakamizo S, Nishihara M, et al. Cerebrospinal fluid interleukin-10 is a potentially useful biomarker in immunocompetent primary central nervous system lymphoma (PCNSL). Neuro-oncol. 2012;14(3):368-380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shim H, Dolde C, Lewis BC, et al. c-Myc transactivation of LDH-A: implications for tumor metabolism and growth. Proc Natl Acad Sci USA. 1997;94(13):6658-6663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Body BA, Oneson RH, Herold DA. Use of cerebrospinal fluid lactic acid concentration in the diagnosis of fungal meningitis. Ann Clin Lab Sci. 1987;17(6):429-434. [PubMed] [Google Scholar]
- 37.Kadoch C, Li J, Wong VS, et al. Complement activation and intraventricular rituximab distribution in recurrent central nervous system lymphoma. Clin Cancer Res. 2014;20(4):1029-1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pilotte L, Larrieu P, Stroobant V, et al. Reversal of tumoral immune resistance by inhibition of tryptophan 2,3-dioxygenase. Proc Natl Acad Sci USA. 2012;109(7):2497-2502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Corm S, Berthon C, Imbenotte M, et al. Indoleamine 2,3-dioxygenase activity of acute myeloid leukemia cells can be measured from patients’ sera by HPLC and is inducible by IFN-gamma. Leuk Res. 2009;33(3):490-494. [DOI] [PubMed] [Google Scholar]
- 40.Hascitha J, Priya R, Jayavelu S, et al. Analysis of kynurenine/tryptophan ratio and expression of IDO1 and 2 mRNA in tumour tissue of cervical cancer patients. Clin Biochem. 2016;49(12):919-924. [DOI] [PubMed] [Google Scholar]
- 41.Lucarelli G, Rutigliano M, Ferro M et al. Activation of the kynurenine pathway predicts poor outcome in patients with clear cell renal cell carcinoma. Urol Oncol. 2017;35(7):461.e415-461.e427. [DOI] [PubMed] [Google Scholar]
- 42.Lenzen A, Zhai L, Lauing KL, et al. The kynurenine/tryptophan ratio and glioblastoma patients treated with Hsppc-96 vaccine. Immunotherapy (Los Angel). 2016;2(3):2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yoshikawa T, Hara T, Tsurumi H, et al. Serum concentration of L-kynurenine predicts the clinical outcome of patients with diffuse large B-cell lymphoma treated with R-CHOP. Eur J Haematol. 2010;84(4):304-309. [DOI] [PubMed] [Google Scholar]
- 44.Ninomiya S, Hara T, Tsurumi H, et al. Indoleamine 2,3-dioxygenase in tumor tissue indicates prognosis in patients with diffuse large B-cell lymphoma treated with R-CHOP. Ann Hematol. 2011;90(4):409-416. [DOI] [PubMed] [Google Scholar]
- 45.Masaki A, Ishida T, Maeda Y, et al. Prognostic significance of tryptophan catabolism in adult T-cell leukemia/lymphoma. Clin Cancer Res. 2015;21(12):2830-2839. [DOI] [PubMed] [Google Scholar]
- 46.Deangelis LM, Iwamoto FM. An update on therapy of primary central nervous system lymphoma. Hematology Am Soc Hematol Educ Program. 2006;2006:311-316. [DOI] [PubMed] [Google Scholar]
- 47.Lai R, Rosenblum MK, DeAngelis LM. Primary CNS lymphoma: a whole-brain disease? Neurology. 2002;59(10):1557-1562. [DOI] [PubMed] [Google Scholar]
- 48.Correa DD, Shi W, Abrey LE, et al. Cognitive functions in primary CNS lymphoma after single or combined modality regimens. Neuro-oncol. 2012;14(1):101-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Grommes C, Pastore A, Palaskas N, et al. Ibrutinib unmasks critical role of Bruton tyrosine kinase in primary CNS lymphoma. Cancer Discov. 2017;7(9):1018-1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lionakis MS, Dunleavy K, Roschewski M et al. Inhibition of B cell receptor signaling by ibrutinib in primary CNS lymphoma. Cancer Cell. 2017;31:833-843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Korfel A, Schlegel U, Herrlinger U, et al. Phase II trial of temsirolimus for relapsed/refractory primary CNS lymphoma. J Clin Oncol. 2016;34(15):1757-1763. [DOI] [PubMed] [Google Scholar]
- 52.Hess G, Herbrecht R, Romaguera J, et al. Phase III study to evaluate temsirolimus compared with investigator’s choice therapy for the treatment of relapsed or refractory mantle cell lymphoma. J Clin Oncol. 2009;27(23):3822-3829. [DOI] [PubMed] [Google Scholar]
- 53.Molon B, Calì B, Viola A. T cells and cancer: how metabolism shapes immunity. Front Immunol. 2016;7:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Brand A, Singer K, Koehl GE, et al. LDHA-associated lactic acid production blunts tumor immunosurveillance by T and NK cells. Cell Metab. 2016;24(5):657-671. [DOI] [PubMed] [Google Scholar]
- 55.Colegio OR, Chu NQ, Szabo AL, et al. Functional polarization of tumour-associated macrophages by tumour-derived lactic acid. Nature. 2014;513(7519):559-563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ninomiya S, Narala N, Huye L, et al. Tumor indoleamine 2,3-dioxygenase (IDO) inhibits CD19-CAR T cells and is downregulated by lymphodepleting drugs. Blood. 2015;125(25):3905-3916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sheridan C. IDO inhibitors move center stage in immuno-oncology. Nat Biotechnol. 2015;33(4):321-322. [DOI] [PubMed] [Google Scholar]
- 58.de Vries JE. Immunosuppressive and anti-inflammatory properties of interleukin 10. Ann Med. 1995;27(5):537-541. [DOI] [PubMed] [Google Scholar]
- 59.Mittal SK, Roche PA. Suppression of antigen presentation by IL-10. Curr Opin Immunol. 2015;34:22-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Brooks DG, Lee AM, Elsaesser H, McGavern DB, Oldstone MB. IL-10 blockade facilitates DNA vaccine-induced T cell responses and enhances clearance of persistent virus infection. J Exp Med. 2008;205(3):533-541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Henrich FC, Singer K, Poller K, et al. Suppressive effects of tumor cell-derived 5′-deoxy-5′-methylthioadenosine on human T cells. OncoImmunology. 2016;5(8):e1184802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Haskó G, Cronstein B. Regulation of inflammation by adenosine. Front Immunol. 2013;4:85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hong D, Gao HC, Wang X, et al. Asymmetric dimethylarginine triggers macrophage apoptosis via the endoplasmic reticulum stress pathway. Mol Cell Biochem. 2015;398(1-2):31-38. [DOI] [PubMed] [Google Scholar]
- 64.Pekarova M, Kubala L, Martiskova H, et al. Asymmetric dimethylarginine regulates the lipopolysaccharide-induced nitric oxide production in macrophages by suppressing the activation of NF-kappaB and iNOS expression. Eur J Pharmacol. 2013;713(1-3):68-77. [DOI] [PubMed] [Google Scholar]
- 65.Nayak L, Iwamoto FM, LaCasce A, et al. PD-1 blockade with nivolumab in relapsed/refractory primary central nervous system and testicular lymphoma. Blood. 2017;129(23):3071-3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.