Abstract
Rationale:
Ticagrelor, a new type of P2Y12 receptor antagonist, has been highly recommended to be used in acute coronary syndrome by the latest guideline, but its side effects are not well-known. We seek to illustrate a potential fatal condition, thrombotic thrombocytopenic purpura (TTP), caused by ticagrelor.
Patient concerns:
An 87-year-old man who had been prescribed with ticagrelor for 2 months after ST-elevation myocardial infarction (STEMI), presented with severe thrombocytopenia, anemia, renal and liver dysfunction, heart failure and fever.
Diagnoses:
Peripheral blood smear showed schistocytosis, and a disintegrin and metalloproteinase with thrombospondin motifs 13 (ADAMTS13) activity is low, with normal initial coagulation tests, which were compatible with a diagnosis of TTP.
Interventions:
After cessation of ticagrelor and initiation of therapeutic plasma exchange, our patient recovered.
Outcomes:
Re-administration of ticagrelor aggravated TTP and led the patient to death.
Lessons:
Clinicians should be aware of the possibility of ticagrelor-induced TTP in patients with a history of recent myocardial infarction; It is of crucial significance to discontinue and never reuse ticagrelor as long as it is suspected to be implicated in TTP.
Keywords: heart failure, myocardial infarction, thrombotic thrombocytopenic purpura, ticagrelor
Key messages
-
1.
Physicians should be aware of ticagrelor-induced TTP even after 2 months prescription of ticagrelor.
-
2.
Acute heart failure may be the primary symptom of TTP, especially in patients who had myocardial infarction before.
1. Introduction
Thrombotic thrombocytopenic purpura (TTP) is a rare and life-threatening occlusive disorder of the microcirculation that is characterized by thrombocytopenia, microangiopathic hemolytic anemia, neurologic changes, renal dysfunction, and fever.[1] It is caused by severely reduced activity of the von Willebrand factor (vWF)-cleaving protease ADAMTS13 (a disintegrin and metalloproteinase with thrombospondin motifs 13). TTP often presents with an acute onset and is almost always fatal if appropriate management is not initiated promptly. Therapeutic plasma exchange (TPE) is the cornerstone of TTP management, which leads to impressive improvement in the prognosis of TTP.[2]
Many drugs, such as thienopyridine derivatives (ticlopidine, clopidogrel, prasugrel), antineoplastic agents (gemcitabine, cyclosporine, mitomycin C), antibiotics (penicillin, cephalosporin), oral contraceptives, and quinine, have been demonstrated to be related to TTP.[2] Ticagrelor, a new type of P2Y12 receptor blocker, is recently preferred to clopidogrel in treating acute coronary syndrome. To our knowledge, only one case of ticagrelor-induced TTP has been diagnosed clinically and reported.[3] Herein, we provided a relatively confirmed case of ticagrelor-induced TTP, which occurred in a patient with ST-elevation myocardial infarction (STEMI) after taking ticagrelor for 2 months. Although the patient recovered with prompt treatment of plasma exchange afterwards, re-administration of ticagrelor led to a fatal consequence. This case alerted us the significance of recognizing ticagrelor as a cause of TTP.
2. Case presentation
An 87-year-old Chinese man, who was a civil servant before retirement, went to a local hospital because of chest discomfort and dyspnea for 7 hours on June 10, 2017. He had a history of hypertension, type 2 diabetes, coronary artery disease, and prostate cancer. His personal, family, and psychosocial histories were unremarkable. A 12-lead electrocardiogram (ECG) showed sinus rhythm at 71 beats per minute; ST-segment elevation of 2 to 3 mm in leads V2 through V4, and of 1 to 2 mm in leads V1, V5 and V6; T-wave inversion in leads II, III, and aVF. Prompt coronary angiography was performed after aspirin and clopidogrel administration. Angiography revealed 95% stenosis in left anterior descending (LAD) coronary artery, 40% stenosis in left circumflex coronary artery, and 50% stenosis in right coronary artery. A drug-eluting stent (PROMUS Element 2.5 mm by 12.0 mm) was implanted in LAD coronary artery. Antiplatelet therapy with aspirin 100 mg and clopidogrel 75 mg daily was prescribed for 5 days, and changed to aspirin 100 mg daily and ticagrelor 90 mg twice a day thereafter. Other concomitant medications including perindopril, atorvastatin, furosemide, spironolactone, and repaglinide were continually prescribed. The patient recovered after treatment and discharged from local hospital on June 19, 2017. Twenty-eight days later, the patient complained of palpitation and was admitted to geriatric department of our hospital on July 17, 2017. ECG showed paroxysmal atrial fibrillation, which could be reversed to normal sinus rhythm with amiodarone. The patient was in stable situation until being found to have scattered hemorrhagic spots on bilateral lower extremities on August 14, 2017. He had no other complaints. Vital signs and other physical examination were normal. Thrombocytopenia, normocytic anemia and leukopenia were found by a complete blood count. Aspirin and ticagrelor were suspended to prevent major bleeding. Supportive therapies like platelets transfusion and recombinant human interleukin-11 (subcutaneous injection, daily) were also initiated. Intravenous immunoglobulin was administered to suppress potential autoimmune processes. The patient had a fever of 38.5°C the next day, without complaints of cough, sputum, abdominal pain, diarrhea, or odynuria. Physical examination failed to find any signs of infection. During the next 3 days, although thrombocytopenia recovered slightly, renal function was deteriorating and serum lactate dehydrogenase (LDH) level was escalating. The laboratory data was summarized in Table 1, which showed dynamic alternations before and after treatment. The patient was transferred to ICU for further investigation and treatment on August 18, 2017.
Table 1.
Dynamic laboratory data.
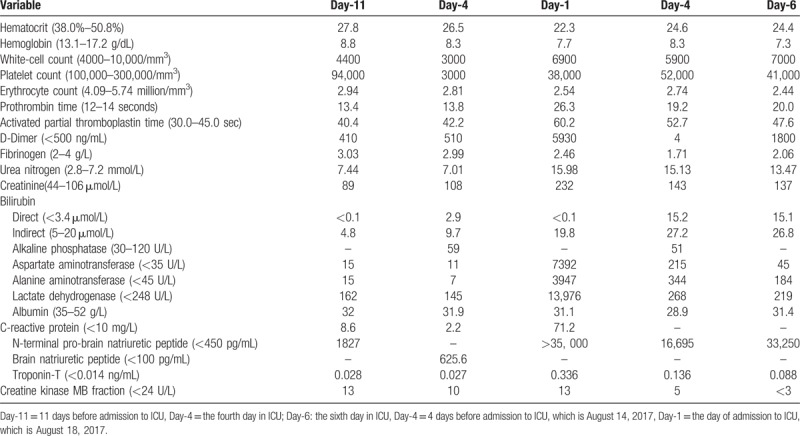
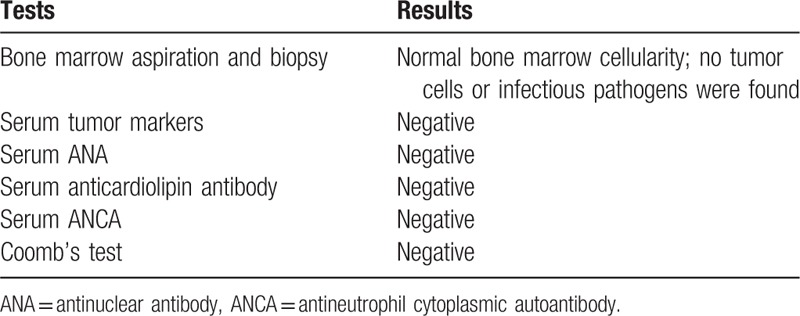
In ICU, the patient seemed distressed with tachypnea, tachycardia, and oliguria. His vital signs were as follows: temperature, 36.5°C; respiratory rate, 24 breaths/min; pulse, 105 beats/min; blood pressure, 114/65 mm Hg; oxygen saturation, 95% with oxygen therapy by a face mask (5 L/min). He was mentally conscious and bilateral pulmonary rales could be auscultated. Serum N-terminal pro-Brain natriuretic peptide (NT-proBNP) was more than 35,000 pg/mL (normal range: <450 pg/mL), and creatinine 232 μmol/L (normal range: 40–106 μmol/L). Chest computed tomography (CT) indicated bilateral pleural effusion, perihilar ground glass opacification, and cardiomegaly. ECG was unchanged compared with the previous one. Echocardiography showed left ventricular enlargement with a 40% ejection fraction. A diagnosis of heart failure and renal failure was made, diuretics was administered to relieve fluid retention. Further studies showed that 39.5% schistocytes on peripheral blood smear (Fig. 1A) and negative Coomb's test. The diagnosis of microangiopathic hemolytic anemia (MAHA) was definite. As clinical presentations of TTP like fever, MAHA, thrombocytopenia, renal failure, and heart failure were displayed in the current case, a diagnosis of TTP was established clinically and therapeutic plasma exchange (TPE) was conducted instantly. His ADAMTS13 activity was measured the next day after one fresh frozen plasma exchange, which showed moderate reduction of activity (48.1%, normal range: 68–131%). After TPE therapy for 4 times, the patient markedly improved with dyspnea relief, decreased body temperature to normal level, a reduction in the percentage of schistocytes to 5.4% (Fig. 1B), a recovery of platelet count to 52 × 103 per mm3, an obvious decrease in serum LDH level from 13976 to 268 U/L (Table 1 and Fig. 2), a downregulation of NT-proBNP level from more than 35,000 to 16,695 pg/mL, and a decline in serum creatinine level from 232 to 143 μmol/L. TTP could be induced by various precipitating factors, including infection, autoimmune diseases, malignancy, pregnancy, bone marrow transplantation, neurologic diseases, and drugs. Since the patient had a history of prostate cancer, metastasis infiltrating bone marrow was a leading consideration in this case. Other causes including autoimmune diseases and infection were also suspected. Further diagnostic tests, such as bone marrow aspiration and biopsy, tumor markers, antinuclear antibody (ANA), anticardiolipin antibody, antineutrophil cytoplasmic autoantibody (ANCA), and Coomb's test were performed.
Figure 1.
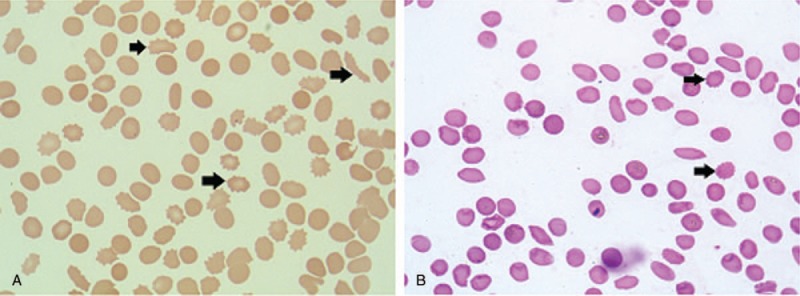
Representative photograph and percentage of peripheral-blood smear in the patient before and after plasma exchange. Peripheral-blood smear was performed on the day after ICU admission before plasma exchange (A) and on the fifth day after ICU admission after 4 times of plasma exchange (B). After plasma exchange treatment, the percentage of schistocytes was markedly decreased from 39.5% to 5.4%. The arrowhead indicates a typical schistocyte (Wright-Giemsa stain, ×1000).
Figure 2.
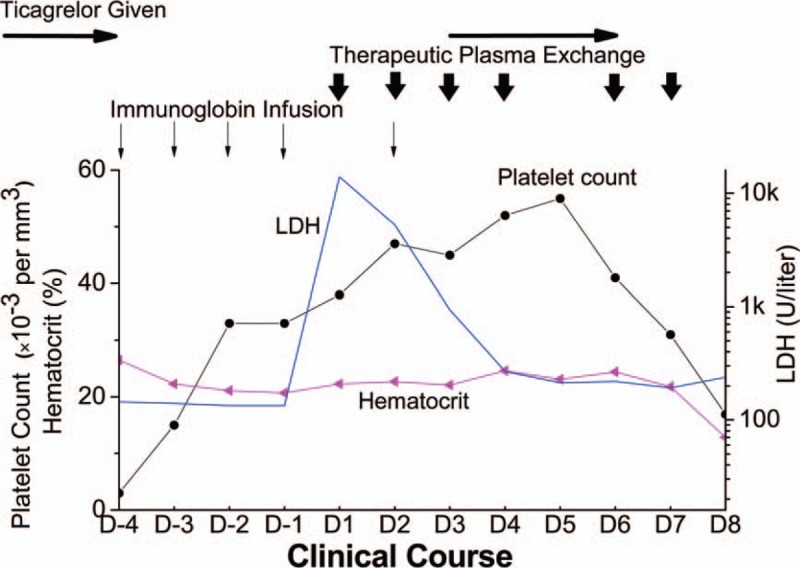
Dynamic changes of platelet count, hematocrit and lactate dehydrogenase (LDH) level during his hospitalization. Platelet count and lactate dehydrogenase recovered with the treatment of therapeutic plasma exchange, and worsened after re-administration of ticagrelor. D-1 to D-4: 1 to 4 days prior to admission to ICU. D1 to D8: the first to eighth day in ICU. Immunoglobin infusion was denoted by vertical thin arrows, therapeutic plasma exchange by vertical short arrows, ticagrelor given by horizontal thin arrows. LDH = lactate dehydrogenase.
While waiting for the results of these diagnostic tests (Table 2), ticagrelor was resumed with a dose of 45 mg twice a day on August 20, 2017 to prevent restenosis in coronary artery or stent after platelet count recovered to 45 × 103 per mm3. Nevertheless, after taking ticagrelor for 3 days, the patient's condition started to deteriorate. He presented with dyspnea, hypoxemia, and hypotention with obvious rales over bilateral lung fields. A significant drop of platelet count was observed from 52 × 103 to 16.9 × 103 per mm3, accompanied with a decrease in hemoglobin from 83 to 56 g/L (Fig. 2). A significant elevation of serum proBNP level was also found from 16695 to 33250 pg/mL, while serum creatinine and troponin level were barely changed. In addition, there was no change in ECG. Recurred acute heart failure with probable MAHA, which suggested exacerbation of TTP, was considered. Hence, ticagrelor was discontinued again. Other treatment, such as continuous renal replacement therapy, tracheal intubation and vasoactive agents, were also used. However, the patient's condition continued to deteriorate. The patient and his family rejected further investigation and therapy. He finally died on the eighth day in ICU.
Table 2.
Diagnostic tests.
3. Discussion
One case of ticagrelor-induced TTP has been reported very recently.[3] However, the causal association of ticagrelor with TTP in that case was not definite according to the criteria established for drug-induced thrombotic microangiopathy.[4,5] Thus we presented here an interesting and relatively confirmed case of TTP, which presented with acute heart failure besides other classic findings of TTP, occurred after administration of ticagrelor for as long as 2 months, and recurred rapidly and incurably with reuse of ticagrelor.
3.1. Diagnosis and differential diagnosis of TTP
This patient manifested anemia with a negative Coomb's test and a large number of schistocytes on peripheral blood smear, so the diagnosis of microangiopathic hemolytic anemia (MAHA) was definite. Causes of MAHA include TTP, disseminated intravascular coagulation (DIC), hemolytic-uremic syndrome (HUS), severe vitamin B12 deficiency, malignant hypertension, malfunctioning prosthetic cardiac valve, and pregnancy-related diseases (preeclampsia, eclampsia, and HELLP syndrome).[6,7] Since our patient was an old man with well-controlled hypertension, and without prosthetic cardiac valve implanted, the differential diagnosis of MAHA, such as TTP, HUS, DIC, and severe vitamin B12 deficiency should be considered.
DIC was less likely the primary diagnosis in this patient because the initial coagulation tests including APTT, PT, fibrinogen, and D-dimer were almost within the normal range.[8] HUS can result in thrombocytopenia, acute renal failure, and MAHA, as platelet aggregation and fibrin formation predominantly occurs in glomerular microcirculation. However, typical HUS is frequently preceded by hemorrhagic enterocolitis.[9] Our patient had no preceding abnormal bowel movement or stomachache, which made HUS less likely. Megaloblastic anemia due to vitamin B12 deficiency can also be ruled out, as his serum vitamin B12 level was within normal range. Our patient had 4 symptoms of the TTP classic pentad (fever, MAHA, thrombocytopenia and renal failure). Hence, he was empirically treated for TTP based on the clinical and basic laboratory findings.
TTP was first described by Moschcowitz[10] in 1924 as a fatal thrombotic microangiopathy, and then a pentad of fever, thrombocytopenia, MAHA, neurological disorders and renal dysfunction was put forward to diagnose TTP in clinical practice.[11] Acute heart failure is a rare presentation of TTP, occurs only in 9.5% of patients with TTP.[12] The mechanisms of heart failure in TTP are partly due to myocardial ischemia and microinfarctions resulting from diffuse platelet thrombi in the cardiac microcirculation.[13] Despite the fact of thrombi occlusion, demonstrable ischemic changes may not be present in these patients.[14] Renal failure contributing to fluid overload may also be a cause of heart failure.[12] In the present case, acute heart failure occurred twice, the aetiology of which may be multifactorial, with a combination of hemolytic anaemia, fluid overload due to acute renal failure and probable platelet plugging in cardiac microcirculation. As there were no symptoms of chest pain or marked electrocardiogram changes in our patient, coronary angiography was not performed. Acute heart failure appeared in TTP is usually life-threatening, and therapeutic options are usually limited due to hemorrhagic complications associated with the use of mechanical device. [15] The reason why our patient was more susceptible to acute heart failure may be related to his recent attack of STEMI. Even smaller and lesser occlusion of cardiac microcirculation may result in the obvious manifestation of heart failure in this patient.
The mechanism of TTP was later found to be associated with a deficiency of ADAMTS13, which was a protein with vWF-processing activity. Lack of ADAMTS13 activity leads to the persist existence of ultra-large vWF multimers, which bind to, accumulate and active platelets to form the vWF-rich microthrombi. These microthrombi scavenge platelets and mechanically destroy red blood cells, resulting in thrombocytopenia and schistocytes. At the same time, microcirculation would be occluded by those microthrombi, which causes organ ischemia and dysfunction. Further studies showed that ADAMTS13 activity <10% is specific for TTP, and could be used to differentiate TTP from HUS and other thrombotic microangiopathy.[16] Nevertheless low ADAMTS13 levels are only specific for congenital and idiopathic TTP, variable ADAMTS13 levels are found in secondary TTP.[15] ADAMTS13 activity of our patient was measured after one time of fresh frozen plasma exchange and several times of immunoglobin therapy. Usually, serum ADAMTS13 activity would increase in TTP patients after plasma exchange.[17] Thus, the moderate reduction in ADAMTS13 activity in the current case might be due to the previous treatments of plasma exchange/immunoglobin therapy and/or secondary TTP caused by ticagrelor. Although ADAMTS13 activity assessment is crucial in evaluating a patient with acute MAHA, it is not always available, and treatment of TTP should not be postponed due to the high mortality rate of the disease.
3.2. Causes of TTP
TTP could be induced by various precipitating factors, including infection, autoimmune diseases (such as systemic lupus erythematous and antiphospholipid syndrome), malignancy, pregnancy, bone marrow transplantation, neurologic diseases and drugs.[1] Although our patient had a history of prostate cancer, his cancer was in stable condition with PSA level within the normal range and without signs of metastasis in bone marrow or other organs. In terms of autoimmune diseases, this patient had no rash, arthralgia or scleroderma, and laboratory tests such as ANA, anticardiolipin antibody, anti-Scl 70 antibody, and Coomb's test were negative. Besides, there were no signs of infection in this patient. Therefore, drug-induced TTP was suspected.
More than 70 drugs have been reported to be associated with thrombotic microangiopathy (TMA), including TTP and HUS. Twenty-two drugs were confirmed to have a definite causal association with TMA,[4] which was established if 4 criterions were met. These 4 criterions include: Therapy with the candidate drug preceded TMA, and recovery from TMA was complete and sustained with the drug discontinued. The candidate drug was the only drug used before the onset of TMA, or other drugs were continued or reintroduced after discontinuation of therapy with the candidate drug. Other causes for TMA were excluded. Re-exposure to the candidate drug resulted in recurrent TMA.[4,5] Drugs used in our patient, which have been reported to cause TTP, were clopidogrel [17] and ticagrelor.[3] The recent prescription of clopidorel was from 10th to 14th June 2017, which was more than 2 months before onset of TTP. However, according to the published data, the onset of TTP following administration of clopidogrel was < 2 weeks.[17] In addition, until 4 months ago, the patient had been taking clopidogrel for several years without any side effects. Therefore, it was very unlikely that clopidogrel was the drug to induce the occurrence of TTP in the current case. On the contrary, ticagrelor was the only new drug that the patient was taking when TTP was suspected. The time between drug initiation and TMA onset varies according to different mechanisms and categories of drugs. Toxicity-mediated drug-induced TMA (DITMA) is dose-dependent, which may occur acutely with a high dose, and gradually over several months with a lower dose. In typical immune-mediated DITMA, symptoms occur within 21 days of initiation of a drug taken daily, or within 24 hours of exposure to a drug administered intermittently.[4,18] Ticlopidine, an antiplatelet drug of the thienopyridine family, is a common cause of immune-mediated DITMA. However, the time from drug exposure to onset of symptoms could be as long as 2 months for ticlopidine.[17] Our patient had been taking ticagrelor for 2 months until TTP occurred. Although the duration of exposure to ticagrelor is longer than the drugs involved in typical immune-mediated DITMA, the connection between ticagrelor and TTP could not be excluded. Besides, following TPE and cessation of ticagrelor, TTP recovered. Re-exposure to ticagrelor resulted in aggregation of TTP presented primarily with heart failure, in spite of continuation of TPE. Thus, ticagrelor might be the most candidate drug to result in TTP in this case.
3.3. Treatment of ticagrelor-induced TTP
Treatment of ticagrelor-induced TTP is similar to other drug-related TTP. Discontinuation of the implicated drug is foremost. TPE, as the cornerstone of management of TTP, should be started as soon as the diagnosis of TTP is suspected, and be performed daily until manifestations related to organ involvement have resolved and thrombocytopenia recovered stably.[19] In our case, initiation of TPE significantly improved the patient's condition, which in return assisted in the diagnosis of TTP of this patient. Due to the autoimmune nature of ticagrelor-induced TTP, steroids may be efficacious as an adjunctive treatment to TPE.[20] Nevertheless, level of proof about steroid efficacy in treating TTP is still very low.[21] Rituximab, as anti-CD20 monoclonal antibody, could stop autoantibody production, and is recommended to be used in patients with suboptimal response to TPE.[22] Other immunomodulators, such as vincristine and cyclosporine A, is reserved only for patients refractory to other therapies.[1] Moreover, new drugs stemming from further understanding of TTP pathophysiology may complete the management of TTP in the future.[23].
3.4. Recurrence of TTP
The second attack of heart failure in this patient was very likely due to aggravation of TTP. Since after TPE therapy, the patient was getting better with recovering renal function. When he presented with symptoms of heart failure, there were no signs of infection, fluid overload, arrhythmia or obvious myocardial infarction. Only decreased serum platelet count and hemoglobin were found. Therefore, acute heart failure was in all probability due to recurrence of TTP, and resulted from hemolytic anemia and platelet occlusion in cardiac microcirculation.
Failure to recognize a drug as the cause of TMA, especially immune-mediated DITMA, may be fatal if the patient is re-exposed to the implicated drug.[4] In our case, ticagrelor was discontinued on account of thrombocytopenia. Lack of awareness about ticagrelor-induced TTP led to re-administration of ticagrelor after platelet count recovered, which ultimately resulted in the death of our patient. Hence, it is of significance to identify ticagrelor or other potential drugs in causing TTP and avoid reuse of these implicated drugs.
3.5. Strengths and limitations
Strengths in the management of this case are the early diagnosis of TTP, and prompt treatment of TTP with therapeutic plasma exchange, which reverses the course of TTP.
The limitations of the present case report are as follows: we did not detect the antibody to the ADAMTS13 or ticagrelor, and the direct correlation between ticagrelor and TTP, as well as the underlying mechanism of ticagrelor-induced TTP have not yet been identified. Besides, reuse of ticagrelor is fatal in patients with ticagrelor-induced TTP.
4. Conclusions
Although use of ticagrelor may benefit patients with ACS, cardiologists and intensive care physicians should be aware of the possibility of ticagrelor-induced TTP even after 2 months prescription of ticagrelor. Besides, acute heart failure may also be the primary symptom of TTP, especially in patients who had myocardial infarction before.
Author contributions
XYW and SFZ contributed to the acquisition and analysis of the data and the initial draft writing of this paper. LQL, JJH, LZ, and LBL contributed to the collection and interpretation of data. GSZ contributed to the concept of the study, the revision of this paper, and the final approval of the version to be published. All authors read and approved the final manuscript.
Conceptualization: Xiaoya Wang, Gensheng Zhang.
Data curation: Xiaoya Wang, Shufang Zhang, Leiqing Li, Junjie Hua, Lei Zhu, Libin Li.
Formal analysis: Xiaoya Wang.
Supervision: Gensheng Zhang.
Validation: Leiqing Li, Junjie Hua, Lei Zhu, Libin Li.
Writing – original draft: Xiaoya Wang, Shufang Zhang.
Writing – review & editing: Gensheng Zhang.
Footnotes
Abbreviations: ADAMTS13 = a disintegrin and metalloproteinase with thrombospondin motifs 13, STEMI = ST-elevation myocardial infarction, TTP = thrombotic thrombocytopenic purpura.
XW and SZ contributed equally to this work.
Compliance with ethical standard: The present work was approved by the Ethics Committee of the Second Affiliated Hospital, Zhejiang University School of Medicine (Hangzhou, China).
Informed consent: Written informed consent was obtained from the patient's son for the publication of this manuscript.
This work was supported in part by grants from the Medical and Health Research Program of Zhejiang Province (Core Talents Plan) (No. 2016RCA014, GSZ) and the Medical and Health Research Program of Zhejiang Province (No. 2018KY094, SFZ). The remaining authors have disclosed that they do not have any conflict of interest.
References
- [1].Joly BS, Coppo P, Veyradier A. Thrombotic thrombocytopenic purpura. Blood 2017;129:2836–46. [DOI] [PubMed] [Google Scholar]
- [2].Kremer Hovinga JA, Coppo P, Lammle B, et al. Thrombotic thrombocytopenic purpura. Nat Rev Dis Primers 2017;3:17020. [DOI] [PubMed] [Google Scholar]
- [3].Dogan A, Ozdemir B, Bal H, et al. Ticagrelor-associated thrombotic thrombocytopenic purpura. Anatol J Cardiol 2017;17:73–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Al-Nouri ZL, Reese JA, Terrell DR, et al. Drug-induced thrombotic microangiopathy: a systematic review of published reports. Blood 2015;125:616–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].George JN, Raskob GE, Shah SR, et al. Drug-induced thrombocytopenia: a systematic review of published case reports. Ann Intern Med 1998;129:886–90. [DOI] [PubMed] [Google Scholar]
- [6].Kottke-Marchant K. Diagnostic approach to microangiopathic hemolytic disorders. Int J Lab Hematol 2017;39(suppl 1):69–75. [DOI] [PubMed] [Google Scholar]
- [7].Masias C, Vasu S, Cataland SR. None of the above: thrombotic microangiopathy beyond TTP and HUS. Blood 2017;129:2857–63. [DOI] [PubMed] [Google Scholar]
- [8].Toh CH, Alhamdi Y, Abrams ST. Current pathological and laboratory considerations in the diagnosis of disseminated intravascular coagulation. Ann Lab Med 2016;36:505–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Jokiranta TS. HUS and atypical HUS. Blood 2017;129:2847–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Moschcowitz E. Hyaline thrombosis of the terminal arterioles and capillaries: a hither to undescribed disease. Proc N Pathol Soc 1924;24:21–4. [Google Scholar]
- [11].Amorosi EL, Ultmann JE. Thrombotic thrombocytopenic purpura: report of 16 cases and review of the literature. Medicine (Baltimore) 1966;45:139–59. [Google Scholar]
- [12].Gami AS, Hayman SR, Grande JP, et al. Incidence and prognosis of acute heart failure in the thrombotic microangiopathies. Am J Med 2005;118:544–7. [DOI] [PubMed] [Google Scholar]
- [13].Podolsky SH, Zembowicz A, Schoen FJ, et al. Massive myocardial necrosis in thrombotic thrombocytopenic purpura: a case report and review of the literature. Arch Pathol Lab Med 1999;123:937–40. [DOI] [PubMed] [Google Scholar]
- [14].De Landtsheer Q, Labriola L, Houssiau F, et al. Acute heart failure after thrombotic thrombocytopenic purpura successfully treated by ECLS. Transfus Med 2013;23:199–201. [DOI] [PubMed] [Google Scholar]
- [15].Gaddam S, Pablani L, Chainani V, et al. Complete recovery of ischemic cardiomyopathy from thrombotic thrombocytopenic purpura. Clin Med Insights Cardiol 2011;5:29–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Veyradier A, Obert B, Houllier A, et al. Specific von Willebrand factor—cleaving protease in thrombotic microangiopathies: a study of 111 cases. Blood 2001;98:1765–72. [DOI] [PubMed] [Google Scholar]
- [17].Jacob S, Dunn BL, Qureshi ZP, et al. Ticlopidine-, clopidogrel-, and prasugrel-associated thrombotic thrombocytopenic purpura: a 20-year review from the Southern Network on Adverse Reactions (SONAR). Semin Thromb Hemost 2012;38:845–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Page EE, Little DJ, Vesely SK, et al. Quinine-induced thrombotic microangiopathy: a report of 19 patients. Am J Kidney Dis 2017;70:686–95. [DOI] [PubMed] [Google Scholar]
- [19].Scully M, Hunt BJ, Benjamin S, et al. Guidelines on the diagnosis and management of thrombotic thrombocytopenic purpura and other thrombotic microangiopathies. Brit J Haematol 2012;158:323–35. [DOI] [PubMed] [Google Scholar]
- [20].Balduini CL, Gugliotta L, Luppi M, et al. High versus standard dose methylprednisolone in the acute phase of idiopathic thrombotic thrombocytopenic purpura: a randomized study. Ann Hematol 2010;89:591–6. [DOI] [PubMed] [Google Scholar]
- [21].Bell WR, Braine HG, Ness PM, et al. Improved survival in thrombotic thrombocytopenic purpura-hemolytic uremic syndrome. Clinical experience in 108 patients. N Engl J Med 1991;325:398–403. [DOI] [PubMed] [Google Scholar]
- [22].Page EE, Kremer Hovinga JA, Terrell DR, et al. Rituximab reduces risk for relapse in patients with thrombotic thrombocytopenic purpura. Blood 2016;127:3092–4. [DOI] [PubMed] [Google Scholar]
- [23].Tersteeg C, Schiviz A, De Meyer SF, et al. Potential for recombinant ADAMTS13 as an effective therapy for acquired thrombotic thrombocytopenic purpura. Arterioscl Throm Vas 2015;35:2336–42. [DOI] [PubMed] [Google Scholar]


