Abstract
Rationale:
Primary central nervous system histiocytic sarcoma (PCNSHS) is a rare lymphohematopoietic tumor with a histiocytic cell origin. To our knowledge, only 28 cases have been published in English and 2 cases in Chinese.
Patient concerns:
A 49-year-old Asian female presented to the hospital with a 2 month history of hypomnesia, odynophagia, and gait disorder. Physical examination demonstrated decreased lower extremity muscle strength. The patient denied a history of malignancy.
Diagnoses:
Radiology demonstrated a lesion in parietal lobe with uniformenhancement. Histologic analysis showed pleomorphic tumor cells with a loose arrangement, effacing the normal brain tissue. The tumor cells exhibited abundant eosinophilic cytoplasm, highly atypical nuclei and predominant nucleoli. Immunohistochemistry revealed positive immunoreactivity for CD45, lysozyme, CD68, and CD163, and negative for pan-cytokeratin (CK), epithelial membrane antigen (EMA), glial fibrillary acidic protein (GFAP), CD3, CD20, CD1a, CD79a, CD138, oligodendrocyte transcription factor (olig2), CD15, melan-A, CD30, CD21, CD35, Human Melanoma Black-45 (HMB45), and anaplastic lymphoma kinase-1 (ALK-1). The diagnosis of PCNSHS was rendered.
Interventions:
The patient underwent complete surgical resection and adjuvant radiotherapy.
Outcomes:
Follow-up information shows the patient died 8 months following the initial diagnosis.
Lessons:
PCNSHS is extremely rare with an aggressive clinical course. Immunohistiochemistry is necessary to make this diagnosis and to exclude other primary intracranial and lymphohematopoietic tumors. Further research is required to improve the outcome of patients with PCNSHS.
Keywords: differential diagnosis, histiocytic sarcoma, morphology, primary central nervous system, treatment
1. Introduction
Histiocytic sarcomas (HS) are rare malignant lymphohematopoietic tumors with morphologic and immunophenotypic features of mature tissue histiocytes. This diagnosis accounts for < 1% of non-Hodgkin's lymphoma,[1] and most frequently arises in lymph nodes, skin, and the intestinal tract. Primary central nervous system histiocytic sarcoma (PCNSHS) is extremely rare. To our knowledge, only 30 total cases have been published in English and Chinese literature. We report a case of a 49-year-old Asian female with PCNSHS, as well as a thorough discussion of the differential diagnosis of PCNSHS and a review of the clinical and pathological features of previously published cases.
2. Case presentation
A 49-year-old Asian female presented to the hospital with a 2 month history of hypomnesia, odynophagia, and gait disorder. She denied a history of fever, pruritis, and a personal or family history of malignancy. Physical examination revealed stable vital signs, decreased memory, and a decreased lower extremity muscle strength of 3/5. Laboratory findings demonstrated normal a complete blood count, renal function, liver function tests, thyrotropin, and erythrocyte sedimentation. Serum tumor markers including carcinoembryonic antigen (CEA) and a-fetoprotein (AFP) were within normal limits. Computerized tomography (CT) scan and magnetic resonance imaging (MRI) of the brain demonstrated a 2.5 cm, well-circumscribed, uniformly enhancing lesion in the parietal lobe. Staging bone marrow and positron emission tomography/CT showed no evidence of disease outside of the central nervous system (CNS). A gross total resection of the tumor by imaging criteria was performed. On gross examination, the tumor was 2.5 × 2.2 × 2 cm with a soft texture and gray to red color. Microscopic examination revealed marked disruption of the normal brain architecture by numerous tumor cells with easily identified foci of necrosis (Fig. 1A). At high magnification, the tumor cells were highly pleomorphic and exhibited eosinophilic cytoplasm. The nuclei were medium to large in size with a distinct oval to kidney shape, thickened nuclear membranes and granular chromatin. Prominent single nucleoli and multiple small nucleoli were observed (Fig. 1B). The background consisted of inflammatory cells, hemorrhage and congested vessels. Polynuclear tumor cells were occasionally identified (Fig. 1C and D). Calcification was present, but was not a predominant feature (Fig. 1E). Vascular invasion was easily identified (Fig. 1F). The tumor cells demonstrated strong cytoplasmic immunoreactivity for lysozyme, CD68, and CD163 (Fig. 2A and B), focal cytoplasmic and nuclear immunoreactivity for S100, membrane immunoreactivity for CD45 (Fig. 2C), and the Ki-67 proliferation index was approximately 60% (Fig. 2D). The tumor cells were negative for pan-CK, EMA, GFAP, CD3, CD20, CD1a, CD79a, CD138, olig2, and CD15, melan-A, CD30, CD21, CD35, HMB45, ALK-1. Both morphology and immunophemotype confirm the diagnosis of PCNSHS. The patient received 45 Gray of adjuvant external beam radiation to the tumor bed with generous margins. Unfortunately, she died 8 months after her initial diagnosis.
Figure 1.
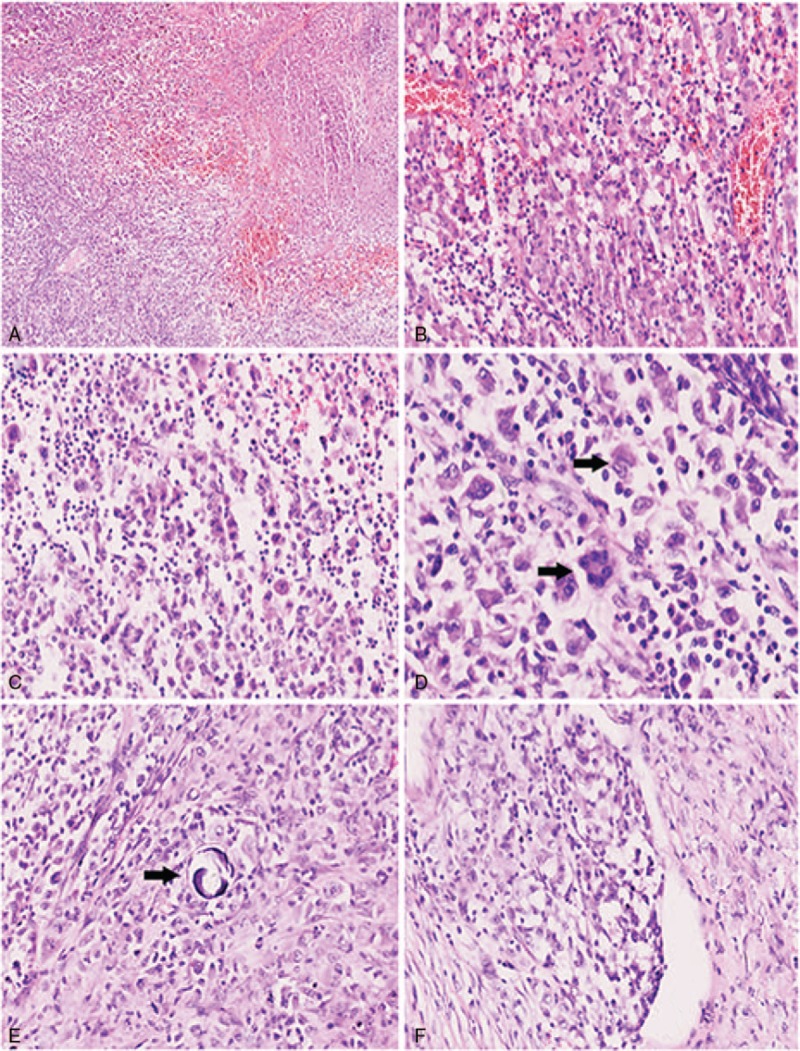
Numerous tumor cells with easily identified foci of necrosis (A, 40×); the background consisted of inflammatory cells, hemorrhage and congested vessels, tumor cells were highly pleomorphic and exhibited eosinophilic cytoplasm (B, 200×); neoplastic cells show loose arrangement (C, 200×); the nuclei were medium to large in size with a distinct oval to kidney shape (black arrow), thickened nuclear membranes and granular chromatin, polynuclear tumor cells could be identified (black arrow, D, 400×); calcification rarely present (E, 200×); vascular invasion was easily identified (F, 200×).
Figure 2.
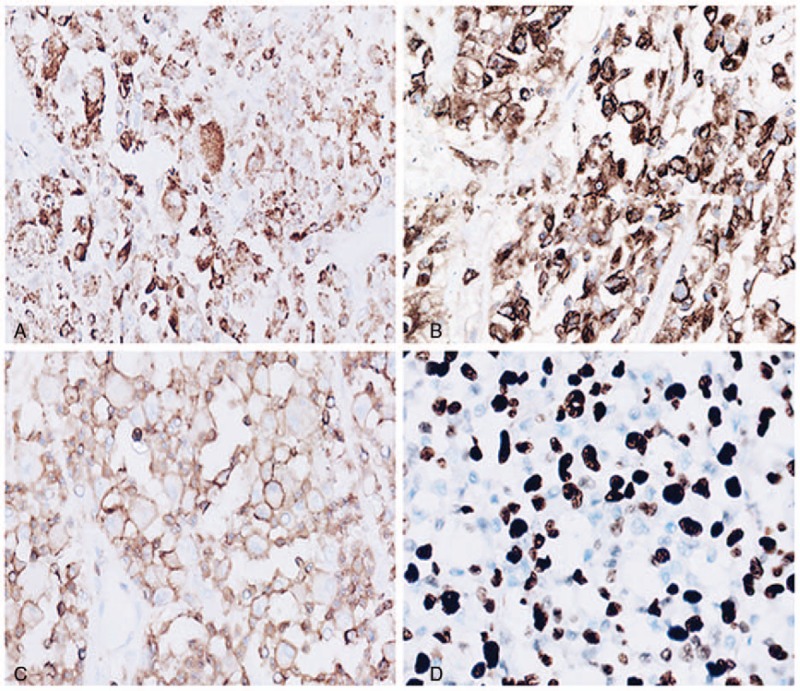
Tumor cells were strong positive for CD68 (A, 200×); tumor cells were strong positive for CD163 (A, 200×); membrane immunoreactivity for CD45 (C, 200×); hot sport show Ki-67 proliferation index is approximately 60% (D, 200×).
3. Discussion
PCNSHS is a rare and difficult diagnosis due to the heterogeneous and somewhat nonspecific histopathologic appearance. To our knowledge, 28 cases of PCNSHS have been published in English and 2 cases in Chinese. The average age at diagnosis is 42.97 ± 19.66 (range: 17 months to 71 years), the ratio of male: female is 16:15, indicating no gender predilection. The size of reported cases of PCNSHS ranges from 0.7 to 6.5 cm3. PCNSHS presents as single or multiple lesions, and can involve the cerebrum, cerebellum, meninges and spinal cord.
According to the World Health Organization (WHO), the diagnosis of HS requires verification of the histiocytic lineage and exclusion of other lymphohematopoetic malignancies.[2] Histologically, PCNSHS demonstrates large, pleomorphic cells, with abundant eosinophilic cytoplasm, irregular nuclei with prominent nucleoli, mitotic figures, and necrosis. PCNSHS may have tumor giant cells, erythrophagocytosis, or focal spindling.[3–8] Prominent acute and chronic inflammation was found in approximately 60% cases,[3] and could be a useful clue for the diagnosis. The morphology of the present case meets the criteria and histopathologic findings of previously published cases of PCNSHS.
Immunohistochemistry plays an important role in the diagnosis of PCNSHS, and a comprehensive workup is warranted, including pan-CK and EMA to rule out carcinoma and meningioma; GFAP and olig2 for glial-derived tumors (especially pleomorphic xanthoastrocytoma); and S100 for melanoma, interdigitating dendritic cell sarcoma, and Rosai–Dorfman disease. However, S100 is positive in approximately 33% of HS, therefore, a positive S100 does not rule out HS,.[9] In our case, pan-CK, EMA, GFAP, olig2 were negative but CD45 was positive, suggesting a lymphohematopoietic cell lineage.
CNS lymphoma constitutes a rare group of tumors. The majority of these non-Hodgkin's lymphomas (NHL) are derived from B lymphocytes.[10] Considering the positive CD45 immunohistochemistry and morphologic findings, the main differential diagnosis was diffuse large B cell lymphoma (DLBCL) and anaplastic large cell lymphoma (ALCL). Following immunohistochemistry showed negative immunoreactivity for CD20, CD79a, PAX5, CD3, CD15, CD30 and ALK-1, effectively excluding B and T cell lymphomas. CD1a was negative, excluding Langerhans cell neoplasms; CD21, CD35, and CD23 were negative excluding follicular dendritic cell sarcoma; CD138 negativity excluded plasma cell neoplasms; negativity for MPO, CD117, and CD34 excluded myeloid sarcoma. The tumor cells stained positive for CD68, lysozyme, and CD163 suggesting a histiocytic origin. The Ki-67 proliferation index of reported cases ranges from 10% to 90%. The Ki-67 proliferation index of our case was 60%, indicating a high grade malignancy.[11]
PCNSHS has a very aggressive clinical course with a poor prognosis. The median survival is 7 ± 0.98 months (95%CI: 5.08–8.92) and the average survival is 24.07 ± 5.1 months (95%CI: 14.08–34.06) (Table 1, Fig. 3).[1–9,11–32] Brown et al reported the longest survival at greater than 60 months. The most common treatment reported was a combination of surgery, radiotherapy and chemotherapy (Table 1). Currently, no standardized treatment protocol exists. For patients with a single tumor, surgery is the preferred treatment strategy. Due to the limited number of the cases, we are unable to reach a conclusion regarding if chemotherapy or radiotherapy can effectively control the disease. Chemotherapy regimens including ICE (cyclophosphamide, carboplatin, and etoposide) and CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisone) did not show obvious prognostic improvement. No clear dose radiotherapy has been established.
Table 1.
Summary of clinical features of primary CNS HS in the literature.
Figure 3.
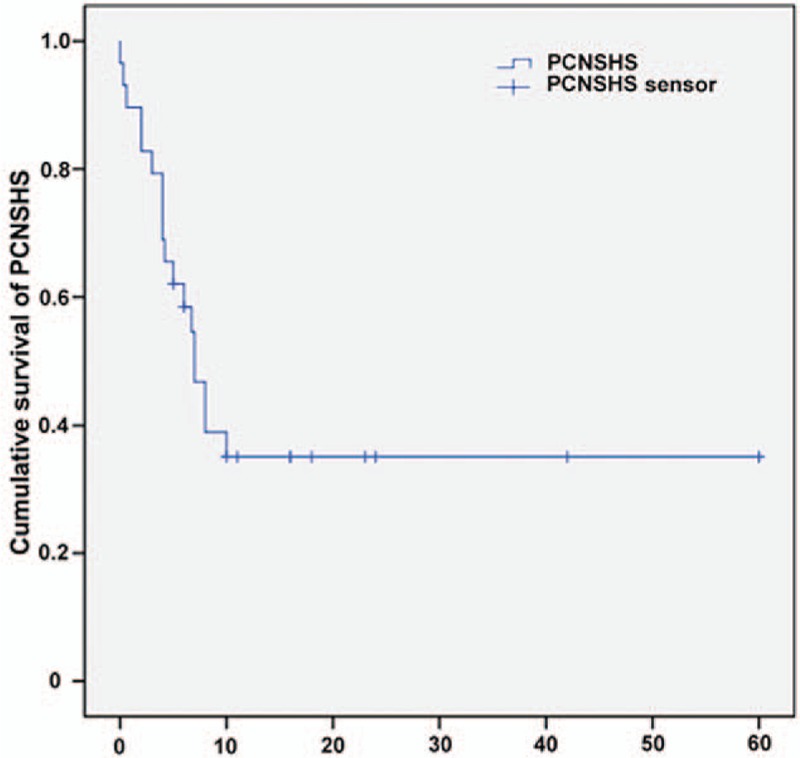
Kaplan–Meier curve demonstrate a poor outcome of PCHSHS's patients. PCHSHS = primary central nervous system histiocytic sarcoma.
4. Conclusion
In conclusion, we present a case of PCNSHS, including the histopathologic and immunohistochemical findings, and the aggressive clinical course. We reviewed all previous publications and conclude that PCNSHS is a rare, aggressive malignancy which most frequently involves brain parenchymal. Due to the rarity and large differential diagnosis, a full immunohistochemical work up is warranted. More research is required to determine the best treatment options. We encourage physicians to publish cases of PCNSHS in order to compile additional data that will assist in developing optimal treatment regimens.
Author contributions
Formal analysis: Hong-Tao Xu.
Methodology: Wan-Lin Zhang, Shuai Shen, Hong-Tao Xu.
Resources: Lian-He Yang.
Supervision: Endi Wang.
Writing – original draft: Shuang Ma.
Writing – review & editing: Michael Schild, Diana Tran, Xuefeng Zhang, Lian-He Yang, Endi Wang.
Footnotes
Abbreviations: AFP = a-fetoprotein, ALCL = anaplastic large cell lymphoma, ALK-1 = anaplastic lymphoma kinase-1, CEA = carcinoembryonic antigen, CHOP = cyclophosphamide, doxorubicin, vincristine, and prednisone, CK = cytokeratin, CNS = central nervous system, CT = computed tomography, DLBCL = diffuse large B-cell lymphoma, EMA = epithelial membrane antigen, GFAP = glial fibrillary acidic protein, HMB45 = Human Melanoma Black-45, HS = histiocytic sarcoma, ICE = cyclophosphamide, carboplatin, etoposide, MPO = myeloperoxidase, MRI = magnetic resonance imaging, NHL = non-Hodgkin's lymphomas, olig2 = oligodendrocyte transcription factor, PCHSHS = primary central nervous system histiocytic sarcoma, WHO = World Health Organization.
Competing interests: The authors declare that they have no competing interests.
Ethical review: Approval was provided by Medical Research Ethics Committee of China Medical University. Written informed consent was obtained from the patient for publication of this Case report and any accompanying images.
Funding: This study was supported by National Natural Science Foundation of China (Grant No. 81301930 to LHY, Grant No. 81372497 to HTX), General project of Education Department of Liaoning Province (Grant No. L2015595 to LHY) and Students’ Innovation and Entrepreneurship Training Program (Grant No. 201610159000036 to LHY).
The authors have no conflicts of interest to disclose.
References
- [1].Wang J, Li T, Chen H, et al. A case of primary central nervous system histiocytic sarcoma. Clin Neurol Neurosurg 2012;114:1074–6. [DOI] [PubMed] [Google Scholar]
- [2].Takahashi E, Nakamura S. Histiocytic sarcoma: an updated literature review based on the 2008 WHO classification. J Clin Exp Hematop 2013;53:1–8. [DOI] [PubMed] [Google Scholar]
- [3].Zanelli M, Ragazzi M, Marchetti G, et al. Primary histiocytic sarcoma presenting as diffuse leptomeningeal disease: case description and review of the literature. Neuropathology 2017;37:517–25. [DOI] [PubMed] [Google Scholar]
- [4].Ueno T, Nishijima H, Kurotaki H, et al. An unusual case of chronic meningitis due to histiocytic sarcoma of the central nervous system with meningeal dissemination. Neurol Sci 2016;37:1875–7. [DOI] [PubMed] [Google Scholar]
- [5].So H, Kim SA, Yoon DH, et al. Primary histiocytic sarcoma of the central nervous system. Cancer Res Treat 2015;47:322–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Foster M, Kamaly-Asl I, Stivaros S, et al. Primary cerebral histiocytic sarcoma in childhood: a case report of protracted survival and review of the literature. Childs Nerv Syst 2015;31:2363–8. [DOI] [PubMed] [Google Scholar]
- [7].Chen CJ, Williams EA, McAneney TE, et al. Histiocytic sarcoma of the cavernous sinus: case report and literature review. Brain Tumor Pathol 2015;32:66–71. [DOI] [PubMed] [Google Scholar]
- [8].Moulignier A, Mikol J, Heran F, et al. Isolated III cranial nerve palsies may point to primary histiocytic sarcoma. BMJ Case Rep 2014;2014:bcr2014204663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Nangal JK, Kapoor A, Narayan S, et al. A case of CD68 negative histiocytic sarcoma of axilla masquerading as metastatic breast cancer. J Surg Case Rep 2014;2014:rju071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Hochberg FH, Miller DC. Primary central nervous system lymphoma. J Neurosurg 1988;68:835–53. [DOI] [PubMed] [Google Scholar]
- [11].Sun W, Nordberg ML, Fowler MR. Histiocytic sarcoma involving the central nervous system: clinical, immunohistochemical, and molecular genetic studies of a case with review of the literature. Am J Surg Pathol 2003;27:258–65. [DOI] [PubMed] [Google Scholar]
- [12].Torres CF, Korones DN, Powers JM, et al. Primary leptomeningeal histiocytic lymphoma in a young child. Med Pediatr Oncol 1996;27:547–50. [DOI] [PubMed] [Google Scholar]
- [13].Cheuk W, Walford N, Lou J, et al. Primary histiocytic lymphoma of the central nervous system: a neoplasm frequently overshadowed by a prominent inflammatory component. Am J Surg Pathol 2001;25:1372–9. [DOI] [PubMed] [Google Scholar]
- [14].Qi Z, He M. A case of primary intracranial histiocytic sarcoma. J Clin Radiol 2002;21:556–7. [Google Scholar]
- [15].Cao M, Eshoa C, Schultz C, et al. Primary central nervous system histiocytic sarcoma with relapse to mediastinum: a case report and review of the literature. Arch Pathol Lab Med 2007;131:301–5. [DOI] [PubMed] [Google Scholar]
- [16].Toshkezi G, Edalat F, O’Hara C, et al. Primary intramedullary histiocytic sarcoma. World Neurosurg 2010;74:523–7. [DOI] [PubMed] [Google Scholar]
- [17].RD G. DK Histiocytic sarcoma: a case of a 52-year-old female with two synchronus primary malignancies at presentation. Med Forum 2011;11:31–3. [Google Scholar]
- [18].Bell SL, Hanzely Z, Alakandy LM, et al. Primary meningeal histiocytic sarcoma: a report of two unusual cases. Neuropathol Appl Neurobiol 2012;38:111–4. [DOI] [PubMed] [Google Scholar]
- [19].Devic P, Androdias-Condemine G, Streichenberger N, et al. Histiocytic sarcoma of the central nervous system: a challenging diagnosis. QJM 2012;105:77–9. [DOI] [PubMed] [Google Scholar]
- [20].Gill-Samra S, Ng T, Dexter M, et al. Histiocytic sarcoma of the brain. J Clin Neurosci 2012;19:1456–8. [DOI] [PubMed] [Google Scholar]
- [21].Gomi K, Tanaka M, Yoshida M, et al. Primary cerebellar histiocytic sarcoma in a 17-month-old girl. J Neurosurg Pediatr 2012;10:126–9. [DOI] [PubMed] [Google Scholar]
- [22].Almefty RO, Tyree TL, Fusco DJ, et al. Primary histiocytic sarcoma of the brain mimicking cerebral abscess. J Neurosurg Pediatr 2013;12:251–7. [DOI] [PubMed] [Google Scholar]
- [23].Chalasani S, Hennick MR, Hocking WG, et al. Unusual presentation of a rare cancer: histiocytic sarcoma in the brain 16 years after treatment for acute lymphoblastic leukemia. Clin Med Res 2013;11:31–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Laviv Y, Zagzag D, Fichman-Horn S, et al. Primary central nervous system histiocytic sarcoma. Brain Tumor Pathol 2013;30:192–5. [DOI] [PubMed] [Google Scholar]
- [25].Perez-Ruiz E, Delgado M, Sanz A, et al. Primary leptomeningeal histiocytic sarcoma in a patient with a good outcome: a case report and review of the literature. J Med Case Rep 2013;7:127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Wu W, Tanrivermis Sayit A, Vinters HV, et al. Primary central nervous system histiocytic sarcoma presenting as a postradiation sarcoma: case report and literature review. Hum Pathol 2013;44:1177–83. [DOI] [PubMed] [Google Scholar]
- [27].Bai J, Li G, Shen M, et al. Primary central nervous system histiocytic sarcoma mimicking glioma. Neurol India 2014;62:684–5. [DOI] [PubMed] [Google Scholar]
- [28].Idbaih A, Mokhtari K, Emile JF, et al. Dramatic response of a BRAF V600E-mutated primary CNS histiocytic sarcoma to vemurafenib. Neurology 2014;83:1478–80. [DOI] [PubMed] [Google Scholar]
- [29].Bai J, Sui D, Shen M, et al. Chinese Journal of Minimally Invasive Neurosurgery. Chin J Minim Invasive Neurosurg 2015;20:101–4. [Google Scholar]
- [30].Brown AF, Fan H, Floyd JR, et al. Primary central nervous system histiocytic sarcoma arising after precursor B-cell acute lymphoblastic leukemia. J Neuropathol Exp Neurol 2015;74:1120–6. [DOI] [PubMed] [Google Scholar]
- [31].Mittal N, Aakalu V, Mehendele S, et al. Primary orbital histiocytic sarcoma in a child successfully treated with multiagent chemotherapy. J Pediatr Hematol Oncol 2016;38:653–7. [DOI] [PubMed] [Google Scholar]
- [32].Clifton W, Akinduro OO, Lopez-Chiriboga S, et al. Infection or glioma? the false dilemma of primary central nervous system histiocytic sarcoma. World Neurosurg 2017;106:1053.e1–5. [DOI] [PubMed] [Google Scholar]


