Supplemental Digital Content is available in the text
Keywords: antibiotic sensitivity, bacteria, dacryocystitis, microbiological characteristics
Abstract
The aim of the study was to review the distribution, current trends, and microbiological characteristics of bacterial pathogens isolated from dacryocystitis patients in China during the last 15 years.
This is a retrospective multiple-center noncomparative case series. The medical records of 15,452 consecutive patients from 7 cities diagnosed as having dacryocystitis between 2002 and 2016 were reviewed. The patients’ demographics, microbiological data, and antibiotic sensitivity were reviewed and analyzed.
A total of 3344 lacrimal sac content cultures were taken (21.6%) during the study period. A pathogen was identified in 1996 samples (59.7%), with bacterial isolates accounting for 1902 of the positive cultures (95.3%). Gram-positive isolates, gram-negative isolates, and anaerobic bacteria were found in 1218 (61.0%), 607 (30.4%), and 285 (14.3%) samples, respectively. An increase in gram-positive isolates over the study duration was found (P = .003). The predominant isolates were coagulase negative Staphylococci (485, 25.5%), Staphylococcus aureus (186, 9.8%), Pseudomonas aeruginosa (184, 9.7%), and Haemophilus influenzae (152, 9.0%). There was a trend toward increasing resistance to erythromycin from 10.5% during the first 5 years of the study to 20.7% during the last 5 years (P < .001). Antimicrobial susceptibility testing showed that gatifloxacin was the most effective drug against most of gram-positive, gram-negative, and anaerobic bacteria.
The microbial culture rate of dacryocystitis in China is low. There was an increase in the percentage of gram-positive bacteria over time. The sensitivity of gram-positive isolates to tested antibiotics is relatively low compared with that of gram-negative isolates. Our data show that the empiric use of fourth-generation fluoroquinolones in refractory dacryocystitis may be justified.
1. Introduction
Dacryocystitis is an infection of the lacrimal excretory system that can result in severe morbidity. It commonly occurs in 2 discrete age categories: infants and adults >40 years of age.[1] Initial treatments for dacryocystitis involve the application of local warmth and oral antibiotics; however, incision and drainage may be considered if the infection produce an abscess in the perilacrimal soft tissue. Dacryocystorhinostomy is ususally recommended for patients with chronic dacryocystitis.
Bacterial dacryocystitis accounts for 60.8% to 94.9% of all lacrimal sac infections.[2–4] Early identification of the causative bacterial isolates and knowledge of their sensitivities to available antibiotics are important for effective treatment. Broad-spectrum antibiotics are usually administered as initial treatments. Currently, many community-based ophthalmologists in China use monotherapy with a second- or third-generation fluoroquinolone as empiric therapy, with or without microbial cultures, for the treatment of chronic dacryocystitis.
With widespread use of fluoroquinolones, a corresponding change in ocular surface flora and susceptibility to antibiotics may occur, particularly in gram-positive bacteria.[5,6] The emergence of resistance has been most significant in methicillin-resistant Staphylococcus aureus (MRSA) and methicillin-resistant coagulase-negative Staphylococci (MRCNS), based on reports from both the United States and China, which showed increasing prevalence of these isolates in the community.[7,8] Regional differences exist in the microbial isolates encountered in dacryocystitis, as well as in their pattern and magnitude of resistance to available antibiotics. Such differences are due to the distribution of types of resistant bacterial strains and the antimicrobial agents used.[1] Therefore, epidemiologic studies are essential for providing evidence-based therapy for bacterial dacryocystitis.
This study attempted to identify and define microbiological characteristics of bacterial dacryocystitis and its antibiotic susceptibility patterns, based on data from 7 major Chinese cities. Trends in bacterial isolates and their susceptibility to common antibiotics over the last 15 years were also explored.
2. Methods
This study conformed to the provisions of the Declaration of Helsinki and was approved by the Institutional Research Ethics Board of Shanghai Eye, Ear, Nose and Throat Hospital, Fudan University. All patients with acute or chronic dacryocystitis from January 1, 2002, through December 31, 2016, from 7 major Chinese cities (East China: Shanghai, Taixing, Rugao, and Yangzhong; North China: Beijing; South China: Guiyang and Ningbo) were included in the study. The study was divided into 3 periods for analysis: 2002 to 2006, 2007 to 2011, and 2012 to 2016. Acute dacryocystitis was diagnosed in patients with tenderness, erythema, and swelling in the lacrimal sac area.[4] Chronic dacryocystitis was diagnosed in patients with regurgitation of mucoid or mucopurulent discharge with the application of pressure over the lacrimal sac area or with irrigation of the lacrimal drainage system lasting 2 or more weeks.[4]
If applicable, an antiseptic with 5% povidone-iodine was applied to the conjunctival sac, and the patients’ lacrimal sac contents were then collected with sterile cotton wool swabs by applying pressure over the lacrimal sac and examining the area for the presence of bacteria and fungus. Selected samples were also inoculated into 5% sheep blood agar within an anaerobic jar. All samples were inoculated into plates of 5% sheep blood agar, a chocolate agar plate, and a thioglycollate broth, and were then incubated aerobically. All inoculated media were incubated at 35°C for 3 days.[4] Gram staining was routinely prepared. Bacteria and fungi were identified by standard phenotypic methods. In vitro susceptibility of bacteria was determined routinely by the Kirby-Bauer disk diffusion method according to the Clinical and Laboratory Standards Institute guidelines.
Statistical analysis was performed using SPSS software, version 20 (SPSS Inc., Chicago, IL), and Graphpad Prism, version 5 (GraphPad Software, San Diego, CA). Means and standard deviations were used for continuous variables; rates and percentages were used for categorical variables. The χ2 test and Fisher exact test were used to test the qualitative distributions. The Spearman correlation coefficient was used to test for trends. A P value of <.05 was considered statistically significant.
3. Results
3.1. Patients’ demographics
During the 15-year period of the study from 2002 through 2016, 3,344 lacrimal sac content samples were taken from 15,452 dacryocystitis patients. As shown in Table 1, among the dacryocystitis patients investigated for microorganism cultures, 2,384 (71.3%) were female, and ages ranged from 2 to 96 years of age, with a median age of 61.1 years. The majority of patients were retired (55.2%). Most patients were diagnosed with chronic dacryocystitis (69.4%). Most of the patients enrolled were from East China (71.4%). Although the culture rate was low in dacryocystitis patients (3,344 of 15,452, 21.6%), there were no differences in age (P = .563), sex (P = .879), duration of symptoms (P = .295), subtypes of dacryocystitis (P = .540), or patient origin (P = .415) between sampled patients and total patients. There was, however, a significant difference with respect to previous antibiotic usage (P = .003) between patients investigated for the microorganism culture and total patients (supplemental Table 1). Patients without previous antibiotic use were more likely to have undergone examination of the lacrimal sac contents for bacterial culture. The mean number of lacrimal sac content samples per year was 223 ± 48 (Fig. 1 and supplemental Table 2). A significant increase in the number of cultures per year was noted during the 15 years of the study (P < .001).
Table 1.
Clinical characteristics of dacryocystitis patients investigated for microorganism cultures in China from 2002 to 2016 (n = 3344).
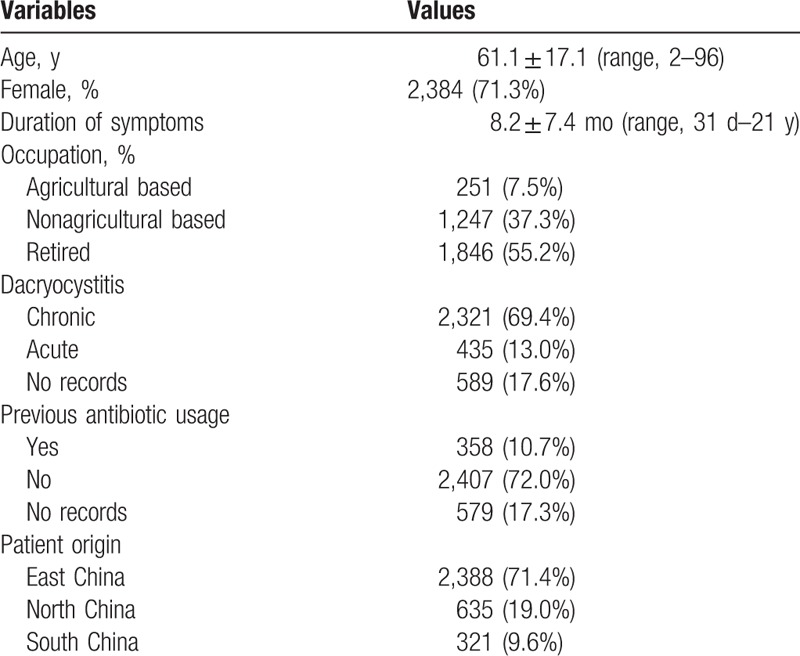
Figure 1.
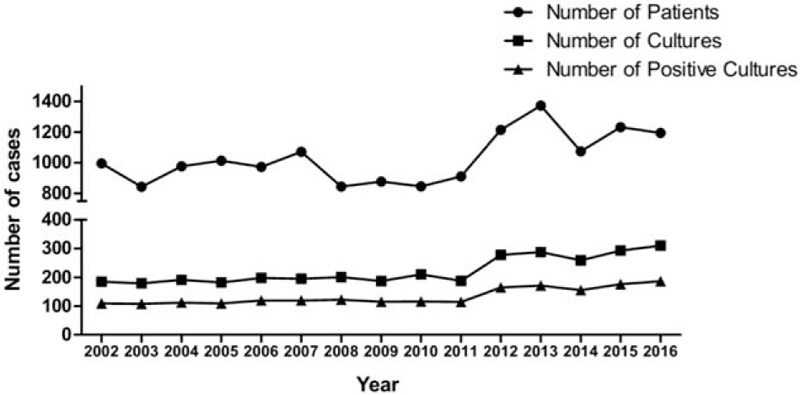
Number of patients, lacrimal sac content samples, and positive cultures per year.
3.2. Bacterial isolates and current trends
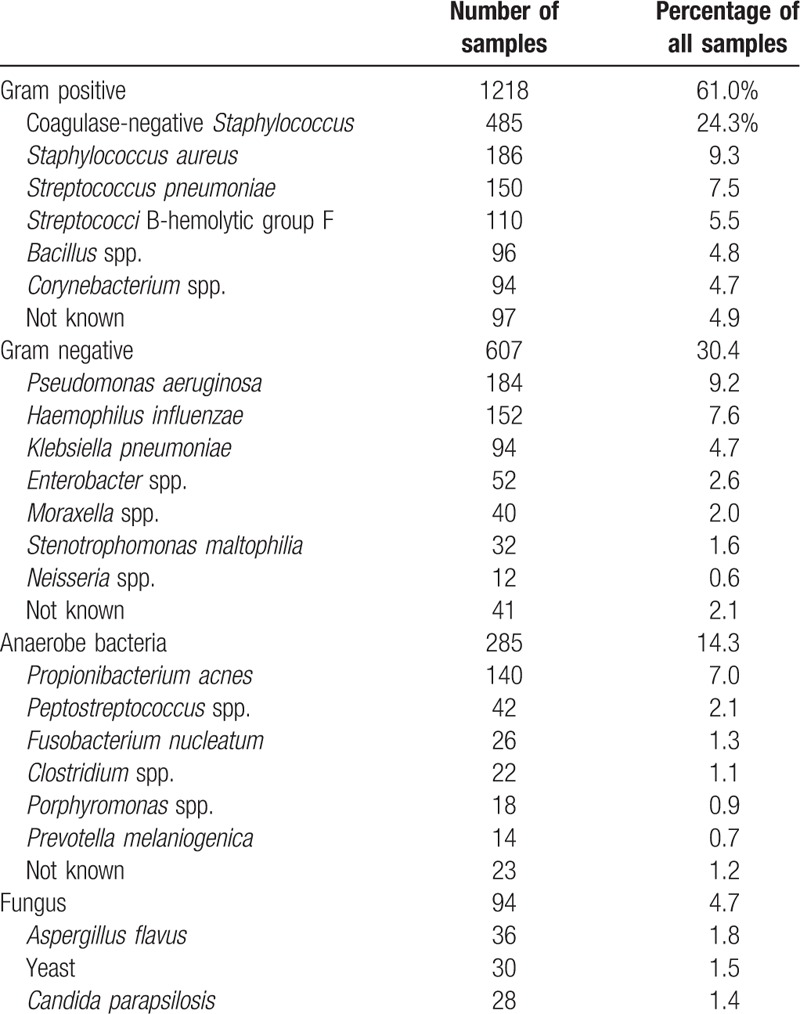
Among the 3344 samples sent for microbiological examination, positive cultures were obtained from 1996 samples (59.7%). The percentage of positive cultures per year ranged from 55.2% to 61.5% (supplemental Table 2). Among them, 10.4% had 2 or more infections. Of the culture-positive samples, the majority of microorganisms were gram-positive bacteria (1218 samples, 61.0% of all positive culture samples), whereas gram-negative bacteria, anaerobic bacteria, and fungi were found in 607 (30.4%), 285 (14.3%), and 94 (4.7%) samples, respectively (Table 2).
Table 2.
Organisms identified in dacryocystitis patients (n = 1996).
The total number of bacterial isolates over the 15-year period of the study was 1902 (95.1%). The number of bacteria cultures per period was 528, 563, and 811, for 2002 to 2006, 2007 to 2011, and 2012 to 2016, respectively. The number and percentage of gram-positive, gram-negative, and anaerobic bacteria per period are shown in Figure 2 and Table 3. There was an increase in the percentage of gram-positive isolates (P = .003) but not of gram-negative isolates (P = .662) or anaerobic bacteria (P = .574).
Figure 2.
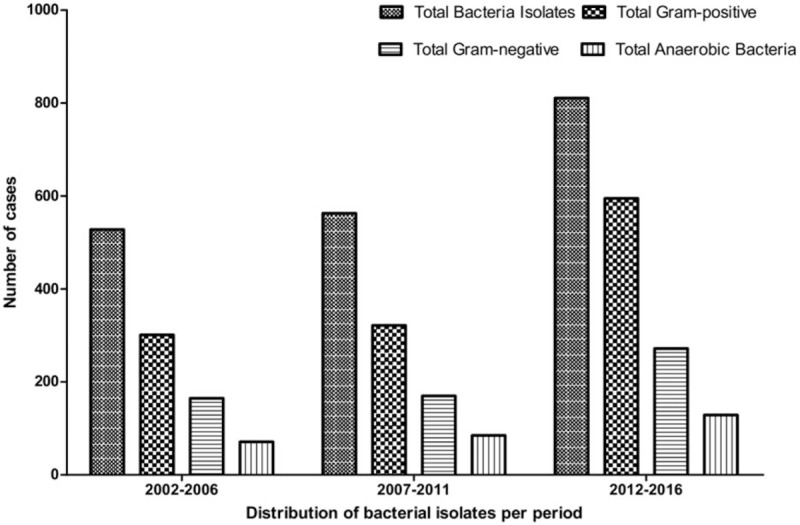
Number of gram-positive, gram-negative, and anaerobic bacteria isolates per period.
Table 3.
Most commonly isolated bacteria per period.
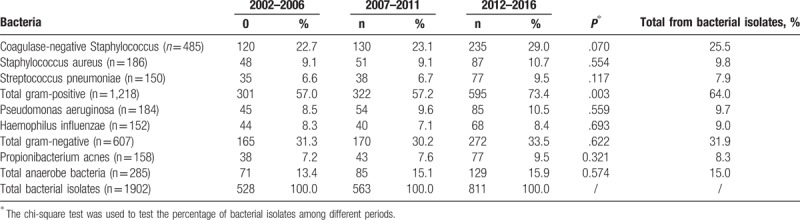
As shown in Table 3, the most commonly isolated bacteria were coagulase-negative staphylococci (CNS; 485 samples, 25.5%), S. aureus (186, 9.8%), Streptococcus pneumoniae (150, 7.9%), Pseudomonas aeruginosa (184, 9.7%), Haemophilus influenzae (152, 9.0%), and Propionibacterium acnes (158, 8.3%), respectively. We detected no changes in percentages of these common isolated bacteria during the 15 years of the study.
3.3. Sensitivity to antibiotics of gram-positive microorganisms
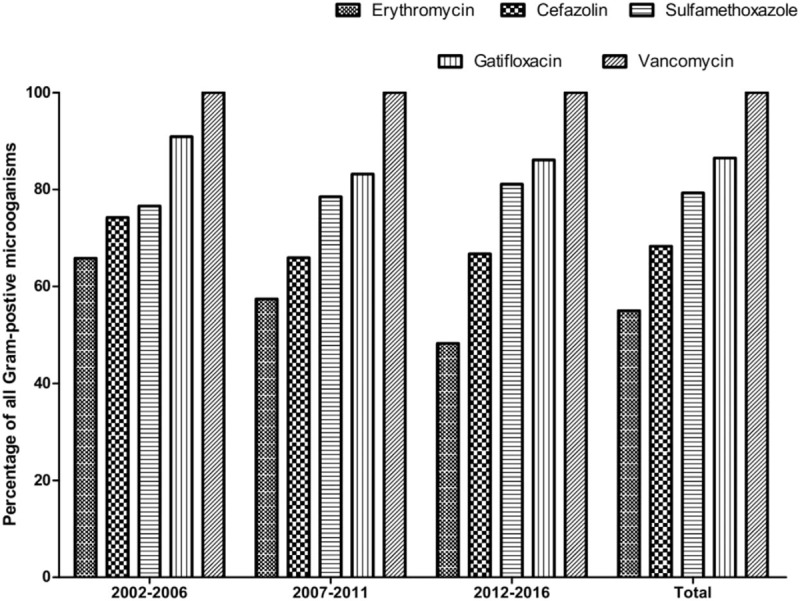
The sensitivity of gram-positive microorganisms to erythromycin was 55.0% (range, 45.3%–65.8% per period) with a significant decrease in antibiotic, sensitivity over time (P < .001) No such decrease was observed for cefazolin (P = .064), sulfamethoxazole (P = .108), gatifloxacin (P = .152), or vancomycin. The sensitivities of the isolates tested to cefazolin, sulfamethoxazole, and gatifloxacin were 68.3% (range, 65.9%–74.2%), 79.3% (range, 76.6%–81.1%), and 86.5% (range, 83.2%–90.9%), respectively. All gram-positive isolates were sensitive to vancomycin (Fig. 3).
Figure 3.
Sensitivity of gram-negative microorganisms to the tested antibiotics per period.
In the subgroup analysis, sensitivity of CNS to erythromycin was found in 52.4% of the isolates; cefazolin was effective in only 56.4% of the cases. A relatively high-sensitivity profile was found with sulfamethoxazole, demonstrating 71.3% sensitivity, and with gatifloxacin, for which the sensitivity was 90.4%. In addition, we found that 64.9% of S. aureus isolates were sensitive to erythromycin, whereas 78.1%, 87.6%, and 90.7% were sensitive to cefazolin, sulfamethoxazole, and gatifloxacin, respectively. Finally, S. pneumoniae was sensitive to erythromycin, cefazolin, sulfamethoxazole, and gatifloxacin in 76.9%, 87.9%, 71.3%, and 90.3% of the cases.
MRSA was present in 8.1% (15/186) of the S. aureus isolates, whereas MRCNS was present in 52.0% (252/485) of the tested isolates, with no significant increasing trend in either microorganism over time (MRSA,P = .968; MRCNS, P = .532; supplemental Figures 1 and 2). All MRSA and MRCNS isolates were 100% sensitive to vancomycin.
P. acnes, a common anaerobic bacteria isolate, was found to be sensitive to erythromycin, cefazolin, sulfamethoxazole, and gatifloxacin in 60.9%, 75.9%, 64.8%, and 94.3% of the cases. None of these isolates were resistant to vancomycin.
3.4. Sensitivity to antibiotics of gram-negative microorganisms
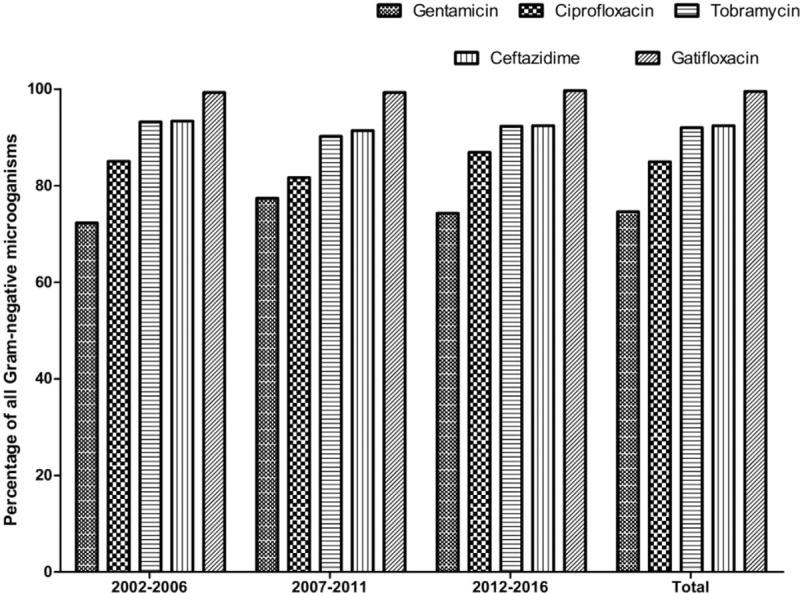
Of the gram-negative isolates, 74.6% (range, 72.3%–77.4% per period) were sensitive to gentamicin. The sensitivity to ciprofloxacin was 85.0% (range, 81.7%–86.0% per period). Tobramycin and ceftazidime had similar sensitivities of 92.0% (range, 90.2%–93.2% and 91.4%–93.4%, respectively), whereas gatifloxacin was most effective, with sensitivity observed in 99.5% of cases (range, 99.3%–99.7%). None of the previously mentioned antibiotics showed any significant trend in sensitivity patterns (Fig. 4).
Figure 4.
Sensitivity of gram-negative microorganisms to the tested antibiotics per period.
When performing the subgroup analysis, we found that P. aeruginosa and H. influenzae were 100% susceptible to gatifloxacin. P. aeruginosa was sensitive to gentamicin, ciprofloxacin, tobramycin, and ceftazidime in 84.8%, 89.9%, 94.2%, and 95.3% of cases, respectively. In addition, 86.4%, 90.3%, 95.1%, and 97.1% of H. influenzae were sensitive to gentamicin, ciprofloxacin, tobramycin, and ceftazidime, respectively.
4. Discussion
To the best of our knowledge, no prior study included a larger number of lacrimal sac content cultures than this 15-year study (n = 3344). Bacterial isolates, especially gram-positive microorganisms, accounted for the majority of positive cultures in dacryocystitis. There was a trend of increasing gram-positive infection. We have demonstrated reducing erythromycin sensitivity. MRSA and MRCNS were noted in our series, and all were sensitive to vancomycin. Among the antibiotics tested, gatifloxacin showed the best antimicrobial action on bacterial isolates.
Our positive culture rate was 59.7%. The rate of positive cultures in previous reports has varied widely, ranging from 8.3% to 100%.[1,4,9,10] These studies have collected samples from dacryocystitis patients using different procedures, including placing pressure on the lacrimal sac,[11] irrigating the lacrimal drainage system,[12] puncturing and aspirating of the lacrimal sac,[13] and taking samples from the lacrimal sac during dacryocystorhinostomy.[14] In our study, we used an antiseptic treatment to reduce the risk of contamination during the sample collection.
With regard to common bacteria, our results were in agreement with recent studies from the United States,[13] Nepal,[3] Thailand,[4] India,[15] and Ethiopia,[16] which have found S. aureus and S. pneumoniae to be the most common among gram-positive, and H. influenzae, Serratia marcescens, and P. aeruginosa the most common gram-negative bacteria. The immunization of H. influenzae vaccine for children is not mandatory in China, which may contribute to its infection. In addition, similar to other report,[12,17] CNS was the most common bacteria in dacryocystitis, present in 25.5% of cases. In this study, anaerobic bacteria were found in 14.3% of all samples, mostly by P. acnes. Our results confirmed previous studies, which have found anaerobic bacteria in between 13% and 32% of samples, with Propionibacterium species were predominating.[17–19]
We found a significant increase in the percentage of gram-positive microorganisms over the past 15 years. This trend has been reported in previous series, in which as high as 71% of all bacterial isolates were gram-positive.[15] As shown in Table 3, the increasing trend toward gram-positive bacteria in lacrimal sac infection may possibly be the result of the increasing pathogenic potential of CNS, although it was previously considered a nonvirulent pathogen. Usually, as one element of normal conjunctival flora, CNS is washed away by the tear flow after entering the lacrimal sac. However, obstruction of the nasolacrimal duct can block this process and create a microenvironment facilitating the growth of bacteria.
Previous studies showed that erythromycin is among the antibiotics to which the majority of lacrimal isolates are sensitive.[1,18] However, our results demonstrate a decrease in gram-positive sensitivity to erythromycin over the study period. This might be attributable to inappropriate use of antibiotics, lack of microbial cultures, and unavailability of guidelines on empiric treatment options. In China, community physicians widely use erythromycin eye drops for treating ocular-surface infectious diseases, and the drops are even available in the pharmacy without a prescription. Our findings suggest that erythromycin should not be recommended for prophylaxis or therapy in dacryocystitis when gram-positive infection is suspected.
All gram-positive bacteria were sensitive to vancomycin, and 86.5% were sensitive to gatifloxacin. Meanwhile, nearly all gram-negative bacteria were sensitive to gatifloxacin (99.5%), and 92% were sensitive to tobramycin and ceftazidime. Thus, gatifloxacin was the most effective agent against all gram-positive, gram-negative, and anaerobic isolates in our study. Our findings therefore suggest that, because of its relatively high activity for most common bacterial isolates, gatafloxacin could be reserved for dacryocystitis patients unresponsive to other antibiotics.
Few reports are available on the correlations between MRSA and MRCNS and dacryocystitis.[20] These 2 isolates have been reported as important ocular pathogens resistant to multiple antibiotics.[8,21] MRSA infection has tended to occur in acute dacryocystitis.[13] We found a small increase in the percentage of MRSA and MRCNS isolates during the study period, which was not significant. Similar to previous studies,[21,22] all MRSA and MRCNS isolates were sensitive to vancomycin. Because more than half of MRSA and MRCNS isolates may be resistant to fourth-generation fluoroquinolones,[8] physicians should suspect these infections when dacryocystitis patients are unresponsive to gatifloxacin.
Several limitations should be taken into account. First, the actual prevalence of bacterial infection in dacryocystitis is difficult to evaluate in our study because only specimens sent for culture of microbial organisms were included in this report. Second, the results of gram smear were not collected in our study. It may be helpful for clinicians at the beginning of the treatment when the results of culture are not prepared. Third, the possibility cannot be ruled out that bacteria isolated from some cases, especially the anaerobic bacteria, may represent nonpathogenic commensal bacteria from the ocular surface, although we applied the antiseptic treatment before we collected the samples. Fourth, not enough atypical microorganisms were isolated for a further subgroup analysis. Fifth, as a retrospective multi-center study, some information were not available in the current study, for examples, the duration on antibiotics, the clinical response to the antibiotics, and the proportion of polymicrobial infection. In addition, we were not able to analyze the results of lacrimal abscess and conjunctival and nasal mucosal swabs. Finally, clinical presentations of dacryocystitis can vary according to geographical area and the microbiological etiology.[1] Thus, our findings may not be generalizable to other regions or populations.
In conclusion, the microbial culture rate of dacryocystitis in China is low, and bacterial microorganisms are the most common causes of infectious dacryocystitis. We documented an increase in the percentage of gram-positive microorganisms identified in the past 15 years. The sensitivity of gram-negative isolates to tested antimicrobials was excellent, mostly >80% on average. This was not the case for gram-positive isolates, for which the levels of resistance to tested antibiotics were relatively high. Our findings also suggest that the empiric use of topical or systemic gatifloxacin, a fourth-generation fluoroquinolone, in refractory dacryocystitis with monitoring the drug side effects may be justified. If it fails, vancomycin could be administered next.
Author contributions
Conceptualization: Tongsheng Fu, Zhongmou Sun, Jianjiang Xu, Jiaxu Hong.
Data curation: Lijuan Chen, Tongsheng Fu, Hao Gu, Ying Jie, Donghong Jiang, Jibing Yu, Xinxing Zhu, Jianjiang Xu, Jiaxu Hong.
Formal analysis: Jiaxu Hong.
Funding acquisition: Jiaxu Hong.
Investigation: Lijuan Chen, Tongsheng Fu, Ying Jie, Zhongmou Sun, Donghong Jiang, Jibing Yu, Xinxing Zhu, Jianjiang Xu, Jiaxu Hong.
Methodology: Lijuan Chen, Tongsheng Fu, Donghong Jiang, Jibing Yu, Jiaxu Hong.
Resources: Lijuan Chen, Tongsheng Fu, Hao Gu, Ying Jie, Donghong Jiang, Jibing Yu, Xinxing Zhu, Jiaxu Hong.
Software: Jianjiang Xu, Jiaxu Hong.
Supervision: Zhongmou Sun, Jiaxu Hong.
Validation: Hao Gu, Ying Jie, Zhongmou Sun, Donghong Jiang, Jiaxu Hong.
Writing – original draft: Lijuan Chen, Tongsheng Fu, Hao Gu, Jiaxu Hong.
Writing – review and editing: Zhongmou Sun, Jianjiang Xu, Jiaxu Hong.
Supplementary Material
Footnotes
Abbreviations: CNS = coagulase-negative staphylococci, MRCNS = methicillin-resistant coagulase-negative Staphylococci, MRSA = methicillin-resistant Staphylococcus aureus.
LC and TF equally contributed to this paper.
The authors were supported by grants from the National Natural Science Foundation of China (81670820, 81670818); the Young Scientist Excellence Program, Shanghai (2017YQ055); Shanghai Rising-Star Program (18QA1401100); the Guizhou Science and Technology Program (2016–2825, GZWJKJ2018-1-003); the Medical Science and Technology Program of Development Center, National Health Commision (W2017MGD26); and the New Technology Joint Research Project in Shanghai Hospitals (SHDC12014114). The sponsor or funding organization had no role in the design or conduct of this research.
The authors have no conflicts of interest to disclose.
Supplemental Digital Content is available for this article.
References
- [1].Pinar-Sueiro S, Sota M, Lerchundi TX, et al. Dacryocystitis: systematic approach to diagnosis and therapy. Curr Infect Dis Rep 2012;[Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- [2].Assefa Y, Moges F, Endris M, et al. Bacteriological profile and drug susceptibility patterns in dacryocystitis patients attending Gondar University Teaching Hospital, Northwest Ethiopia. BMC Ophthalmol 2015;15:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Badhu BP, Karki BS, Khanal B, et al. Microbiological patterns of chronic dacryocystitis. Ophthalmology 2006;113: 2377 e2371–2372. [DOI] [PubMed] [Google Scholar]
- [4].Pornpanich K, Luemsamran P, Leelaporn A, et al. Microbiology of primary acquired nasolacrimal duct obstruction: simple epiphora, acute dacryocystitis, and chronic dacryocystitis. Clin Ophthalmol 2016;10:337–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Shalchi Z, Gurbaxani A, Baker M, et al. Antibiotic resistance in microbial keratitis: ten-year experience of corneal scrapes in the United Kingdom. Ophthalmology 2011;118:2161–5. [DOI] [PubMed] [Google Scholar]
- [6].Politis M, Wajnsztajn D, Rosin B, et al. Trends of bacterial keratitis culture isolates in Jerusalem; a 13-y analysis. PLoS One 2016;11:e0165223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Chang VS, Dhaliwal DK, Raju L, et al. Antibiotic resistance in the treatment of staphylococcus aureus keratitis: a 20-year review. Cornea 2015;34:698–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Hong J, Xu J, Hua J, et al. Bacterial keratitis in Shanghai. Ophthalmology 2013;120:647. [DOI] [PubMed] [Google Scholar]
- [9].Pinar-Sueiro S, Fernandez-Hermida RV, Gibelalde A, et al. Study on the effectiveness of antibiotic prophylaxis in external dacryocystorhinostomy: a review of 697 cases. Ophthalmic Plast Reconstr Surg 2010;26:467–72. [DOI] [PubMed] [Google Scholar]
- [10].Owji N, Khalili MR. Normalization of conjunctival flora after dacryocystorhinostomy. Ophthalmic Plast Reconstr Surg 2009;25:136–8. [DOI] [PubMed] [Google Scholar]
- [11].Bharathi MJ, Ramakrishnan R, Maneksha V, et al. Comparative bacteriology of acute and chronic dacryocystitis. Eye 2008;22:953–60. [DOI] [PubMed] [Google Scholar]
- [12].Sun X, Liang Q, Luo S, et al. Microbiological analysis of chronic dacryocystitis. Ophthalmic Physiol Opt 2005;25:261–3. [DOI] [PubMed] [Google Scholar]
- [13].Mills DM, Bodman MG, Meyer DR, et al. Group ADS. The microbiologic spectrum of dacryocystitis: a national study of acute versus chronic infection. Ophthalmic Plast Reconstr Surg 2007;23:302–6. [DOI] [PubMed] [Google Scholar]
- [14].Das JK, Deka AC, Kuri GC, et al. Bacteriology of chronic dacryocystitis in adult population of northeast India. Orbit 2008;27:243–7. [DOI] [PubMed] [Google Scholar]
- [15].Pradeep AV, Patil SS, Koti SV, Arunkumar JS, Garag SS, Hegde JS. Clinico-bacteriological study of chronic dacryocystitis cases in northern karnataka, India. J Clin Diagn Res 2013;7:2502–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Kebede A, Adamu Y, Bejiga A. Bacteriological study of dacryocystitis among patients attending in Menelik II Hospital, Addis Ababa, Ethiopia. Ethiop Med J 2010;48:29–33. [PubMed] [Google Scholar]
- [17].Hartikainen J, Lehtonen OP, Saari KM. Bacteriology of lacrimal duct obstruction in adults. Br J Ophthalmol 1997;81:37–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Chaudhry IA, Shamsi FA, Al-Rashed W. Bacteriology of chronic dacryocystitis in a tertiary eye care center. Ophthalmic Plast Reconstr Surg 2005;21:207–10. [DOI] [PubMed] [Google Scholar]
- [19].Brook I, Frazier EH. Aerobic and anaerobic microbiology of dacryocystitis. Am J Ophthalmol 1998;125:552–4. [DOI] [PubMed] [Google Scholar]
- [20].Kodsi S. Community-acquired methicillin-resistant Staphylococcus aureus in association with chronic dacryocystitis secondary to congenital nasolacrimal duct obstruction. J AAPOS 2006;10:583–4. [DOI] [PubMed] [Google Scholar]
- [21].Lichtinger A, Yeung SN, Kim P, et al. Shifting trends in bacterial keratitis in Toronto: an 11-year review. Ophthalmology 2012;119:1785–90. [DOI] [PubMed] [Google Scholar]
- [22].Elsahn AF, Yildiz EH, Jungkind DL, et al. In vitro susceptibility patterns of methicillin-resistant Staphylococcus aureus and coagulase-negative Staphylococcus corneal isolates to antibiotics. Cornea 2010;29:1131–5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


