Supplemental Digital Content is available in the text
Keywords: bone graft substitutes, fusion implant, interbody cage, lumbar fusion, spine surgery, surgical practice, survey
Abstract
Lumbar fusion surgery is an established procedure for the treatment of low back pain. Despite the wide set of alternative fusion techniques and existing devices, uniform guidelines are not available yet and common surgical trends are scarcely investigated.
The purpose of this UK-based study was to provide a descriptive portrait of current surgeons’ practice and implant preferences in lumbar fusion surgery.
A UK-based in-person survey was designed for this study and submitted to a group of consultant spinal surgeons (n = 32). Fifteeen queries were addressed based on different aspects of surgeons’ practice: lumbar fusion techniques, implant preferences, and bone grafting procedures. Answers were analyzed by means of descriptive statistics.
Thirty-two consultant spinal surgeons completed the survey. There was clear consistency on the relevance of a patient-centered management (82.3%), along with a considerable variability of practice on the preferred fusion approach. Fixation surgery was found to be largely adopted (96.0%) and favored over stand-alone cages. With regards to the materials, titanium cages were the most used (54.3%). The geometry of the implants influenced the choice of lumbar cages (81.3%). Specifically, parallel-shape cages were mostly avoided (89.2%) and hyperlordotic cages were preferred at the lower lumbar levels. However, there was no design for lumbar cages which was consistently favored. Autograft bone graft surgeries were the most common (60.0%). Amongst the synthetic options, hydroxyapatite-based bone graft substitutes (76.7%) in injectable paste form (80.8%) were preferred.
Current lumbar fusion practice is variable and patient-oriented. Findings from this study highlight the need for large-scale investigative surveys and clinical studies aimed to set specific guidelines for certain pathologies or patient categories.
1. Introduction
Low back pain caused by spinal disorders such as degenerative disc diseases, congenital deformities and trauma affects over 80% of the population worldwide.[1] Spinal fusion surgery is an established and widely adopted procedure for the treatment of low back pain and has recently experienced a substantial growth among the major developed countries. In the United States, the number of spinal fusion procedures increased by 77% between 2002 and 2011.[2] In the United Kingdom, recent data highlighted a similar trend between 2005 and 2015 with an increase of 63% of spinal fusion procedures.[3] Within National Health Service (NHS), spinal surgery was also reported as the largest single component of expenditure for the management of low back pain, with direct costs estimated to be over £1.6 billion per year.[4,5] The spinal fusion market is expected to grow further from $4775m, in 2013, to $6982m by 2020 across United States, France, Germany, Italy, Spain, United Kingdom, Japan, Brazil, India, and China.[6] Such worldwide increase might be partly explained by the recent advances in spinal implants and the development of less-invasive surgical methods.[7] Moreover, novel solutions in terms of biocompatible materials and osteoconductive bone graft substitutes have been shown to reduce pseudarthrosis.[8,9] Currently there are 4 main different approaches to lumbar interbody fusion: anterior lumbar interbody fusion (ALIF), posterior lumbar interbody fusion (PLIF), extreme lateral or direct lumbar interbody fusion (XLIF, DLIF), and transforaminal lumbar interbody fusion (TLIF). There is still no evidence that one surgical approach is clinically superior to the alternatives and indications vary based on the surgeon and the patient.[10–12] The ALIF approach provides with a clear visualization and efficient access to the intervertebral space, thus allowing for a complete discectomy and the placement of a large interbody fusion implant to maximize the contact area.[13,14] Moreover, lumbar lordosis and sagittal balance can be efficiently restored with anterior hyperlordotic cages.[15,16] These advantages have been shown to contribute to a reduced perioperative morbidity and higher fusion rates compared to other traditional approaches.[17] ALIF is particularly favored for the treatment of degenerative disc disease, chronic pain, instability, and deformity at the lumbosacral junction; whilst contraindications include spondylolisthesis greater than grade II, severe vascular disease or inappropriate vascular anatomy.[10,18,19] Stand-alone ALIF cages may be a suitable surgical option in cases with single-level degenerative disc disease; however, posterior fixation may be required for more difficult surgical cases.[14,20] The PLIF approach is one of the most traditional and familiar techniques for spinal surgeons and have been related with an acceptable degree of fusion and a relatively low complication rate.[21,22] Indeed, the posterior approaches avoid vascular complications associated with the anterior approach. However, because of the posterior surgical access, disadvantages of the technique include nerve root retraction and possible associated neurological complications.[23] The traditional PLIF approach utilizes 2 small interbody implants and it is typically followed by posterior fixation. PLIF has been recommended for spondylolisthesis, retrolisthesis, spinal stenosis, and lumbar disc herniation.[24,25] XLIF or DLIF, also referred as lateral lumbar interbody fusion (LLIF), is recognized as a less invasive technique compared to ALIF and PLIF, which has been correlated with reduced operation time and shorter hospital stays.[26,27] Despite these advantages, XLIF has been also associated with a considerably higher frequency of neurological complications and other major morbidity.[28] XLIF usually utilizes a single ovoid implants and is anatomically indicated from the lumbar levels L1/L2 to L4/L5, whilst it is avoided at the L5/S1 level.[7] Indications for XLIF combined with posterior fixation include lumbar spinal stenosis and adjacent segment disease.[29,30] Another posterior fusion approach is TLIF, which was proposed to reduce the extent of neural retraction required during the traditional PLIF approach. Accordingly, TLIF has been associated with less blood loss and reduced risk of injury to the neural and vascular structures, as well as shorter operating time.[31] During TLIF, a unilateral access to the intervertebral space is achieved by performing a unilateral laminectomy and a partial facectomy to allow the insertion of a single “banana”-shaped or rectangular interbody fusion implant. Posterior fixation is usually performed to provide immediate segmental stability and potentially enhance the fusion outcomes.[32] TLIF has been indicated for the treatment of low back pain associated with grade I or II spondylolisthesis, degenerative disc disease, recurrent disc herniation, and revision surgery.[33] All the fusion approaches described above can now be performed using minimally invasive surgery (MIS) techniques, which have been shown to reduce intraoperative blood loss and shorten hospital stay.[34,35] Long-term data are needed to examine the clinical effectiveness of MIS versus open LIF procedures.[36,37] As an alternative to lumbar fusion, total disc replacement (TDR) is a motion-preserving technique which has been indicated for the treatment of symptomatic lumbar degenerative disc disease, although superiority compared to fusion has not yet been proved.[38,39] Whilst the number of available surgical options increases, the current practice related to lumbar fusion surgery has been scarcely investigated and no comprehensive information on fusion implant trends is available in the literature.[5,40–42] With this UK-based study, we aim to provide a preliminary investigation of current surgeon practice and implant preferences in lumbar fusion surgery, by specifically focusing on the relevant factors which influence surgeons’ decision making process.
2. Methods
Data were obtained from consultant spinal surgeons to investigate the various factors defining the choice of a specific surgical procedure and interbody fusion implant. This was performed by designing a questionnaire survey which was submitted to a group of eligible participants.
2.1. Survey design and sample size
An in-person survey was conducted at BritSpine 2016 (April 6–8, 2016), in Nottingham, United Kingdom. Eligibility was limited to consultant spinal surgeons with at least 1 year experience as postholder. Eligibility was verified at a preliminary stage of the interviews before the submission of the survey.
The questionnaire was developed by the authors of this paper taking into account the perspectives of both academic researchers and clinicians. At the design stage, we incorporated feedbacks from researchers with survey experience and phrased the most pertinent questions to assure survey effectiveness. The survey was designed to be completed in approximately five minutes. The questionnaire included 15 questions (12 multiple-choice and 3 Likert scale) and consisted of 4 sections aiming to address the following aspects: (A) surgeon's background and experience; (B) choice of surgical procedure for lumbar fusion; (C) factors influencing implant choice; (D) use of bone graft materials.
Section A included general demographic questions on surgeons’ specialty and experience as postholders.
Section B enquired about the number of spinal fusion surgeries performed in the last year of surgeons’ practice. A Likert scale (1–5 ranking) question was included to investigate the relevance of patient-specific evaluation and spinal segment for the choice of the surgical procedure. Questions on the preferred fixation surgeries were also included in this part of the survey.
Section C comprised 2 Likert scale queries (1–5 ranking) on the importance of clinical, market-related factors, and geometrical parameters for the choice of the fusion implant. A multiple-choice question addressed the correlation between cage lordotic angle and affected spinal segment. Queries on the preferred implant material, structure and design were also included. The latter was investigated by asking which design resembled surgeons’ favorite implant. Specifically, 6 different profiles of cage endplate geometry and 6 different designs for bone grafting area were shown.
Finally, section D aimed to investigate the current bone grafting procedures and the potential use of synthetic graft substitutes. The questionnaire is available as the Supplementary information to this manuscript.
2.2. Data collection and analysis
An in-person methodology was selected for this survey to ensure the maximum response rate and minimize the chance of incomplete responses. During the conference, 32 eligible consultant spine surgeons were asked to complete a questionnaire presented in paper form. Data were collected anonymously and entered into a datasheet based on a randomized ID. Due to the nature of this study, no assessment by a medical ethics review board was required. All data were analyzed using descriptive statistics using GraphPad Prism Sofware, by combining simple graphical and tabular descriptive summaries. Frequencies were depicted as percentages of valid responses.
3. Results
3.1. Demographics
Thirty-two questionnaires were returned: 22 fully completed, 10 with an average completion rate of 91.4%. Demographics of the participants were as follow: 87.5% of respondents were orthopedic spine surgeons, whereas only 12.5% of respondents were neurosurgeons. Surgeons’ medical specialties and experience as consultants are reported in Table 1.
Table 1.
Surgeons’ background and experience (n = 32).

3.2. Surgical procedures
3.2.1. Fusion approaches
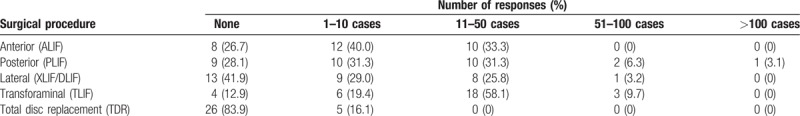
The majority (58.1%) of surgeons performed 11 to 50 cases of TLIF. Accordingly, the most frequently applied fusion technique was TLIF (40.4%), followed by PLIF (28.1%), ALIF (15.8%), and XLIF or DLIF (14.0%). Table 2 shows the occurrences of surgical cases categorized according to each surgical procedure.
Table 2.
Number of lumbar surgeries performed in the last year (n = 30–32, multiple choice allowed).
The majority of surgeons agreed that the choice of surgical procedure (84.4%) and fusion approach (87.5%) was based on a patient-specific evaluation. The spinal segment was also considered as a relevant parameter for the selection of the surgical procedure and fusion approach by the 78.1% and 81.3% of surgeons, respectively (Fig. 1).
Figure 1.
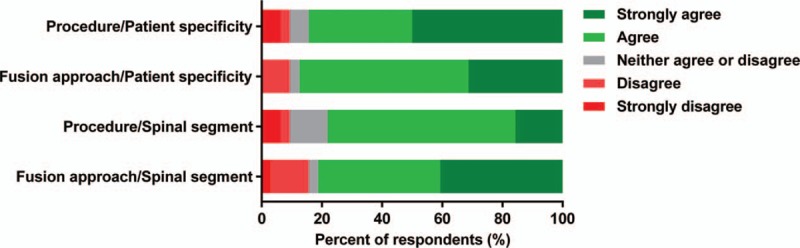
Relevance of a patient-specific evaluation and affected spinal segment for the selection of surgical procedure (e.g., fusion or disc replacement surgery) and fusion approach (e.g., anterior or posterior interbody fusion) (n = 32).
3.2.2. Fixation procedures
Questions on the preferred fixation choices were completed by all the 32 respondents. The preferred fixation procedure involved the insertion of pedicle screws, according to the 46% of total responses. Conversely, the 24% and 16% of participants’ responses favored anterior screws fixation or a combination of anterior and posterior fixation, respectively. The use of fusion cages equipped with anterior fixation plates was reported as first choice for the 10% of responses. Stand-alone cages were selected in only 4% of responses.
3.3. Implant choice
3.3.1. Clinical and market-related factors
Patient specific evaluation and evidence of clinical outcomes turned out to be most relevant factors for the choice of fusion implants, according to the 75.0% and 81.3% of respondents, respectively. The majority of surgeons (81.3%) also considered implant geometry as a crucial parameter influencing implant choice, whilst product price and manufacturer were recognized as important by the 68.8% and 37.5% of surgeons, respectively (Fig. 2).
Figure 2.
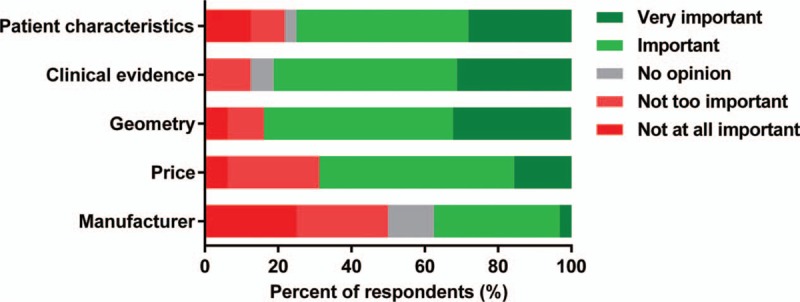
Relevance of generic parameters, such as patient evaluation, clinical evidence, implant geometry, price, and manufacturer which may influence the selection of fusion implants (n = 32).
3.3.2. Material choice
Twenty-eight surgeons answered the questions related to the material structure. The most frequently used material for fusion implants was found to be titanium, according to the 54.3% of responses, followed by tantalum and polyetheretherketone (PEEK) with 28.6% and 17.1% of responses, respectively. Overall, porous/trabecular implants were the first choice for 54.8% of responses, whilst 41.9% were nonporous materials with rough/threaded topology. Implants with smooth surface were consistently disregarded.
3.3.3. Implant geometry
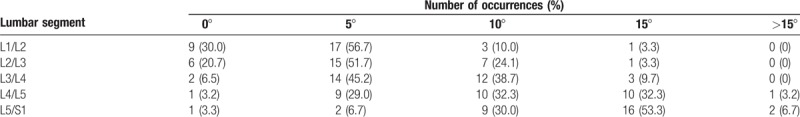
Cage width (90.7%), length (87.5%), thickness (93.8%), and lordotic angle (93.8%) were all considered important variables by the majority of respondents. The majority of surgeons (56.8%) favored implants with double-side angle, whilst 32.4% and 10.8% of respondents chose one-side angled cages and parallel-shape cages, respectively. The correlation between cage lordotic angle and affected spinal segment is shown in Table 3. The most frequently used implant at the L1/L2 (56.7%), L2/L3 (51.7%), and L3/L4 (45.2%) levels was a 5° angled cage, whereas a 15° angled cage was preferred at the L5/S1 segment. At the L4/L5 level, surgeons similarly favored 5° (29.0%), 10° (32.3%), and 15° (32.3%) angle cages.
Table 3.
Cage lordotic angle mostly used according to the lumbar segment (n = 29–31).
Cage endplate designs d and e were favored equally according to the 33.3% of responses, followed by design f at 19.4% (Fig. 3). For bone grafting area, design c with 2 bone graft voids was the preferred profile based on 35.5% of responses (Fig. 4). Instead, the 22.6% of participants’ responses favored a single-hole design b, whereas the 16.1% (5) of responses depicted a complex design f.
Figure 3.
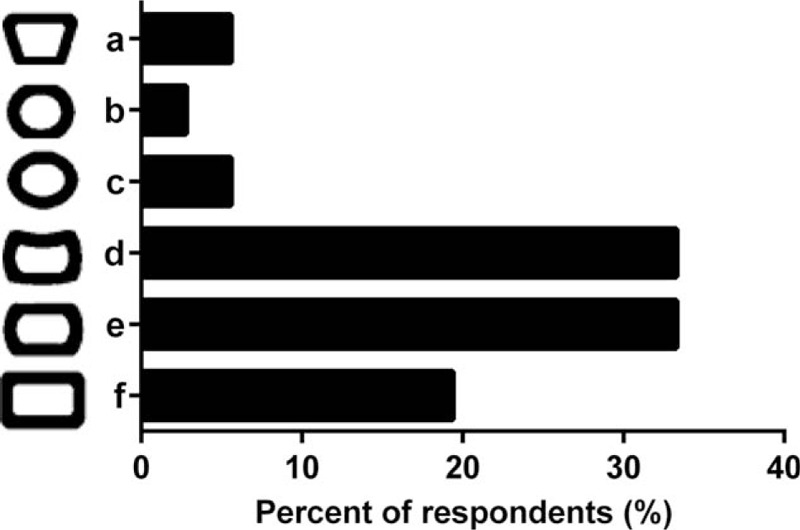
Selection of an endplate geometry of implant by surgeons during fusion surgery procedures (n = 31).
Figure 4.
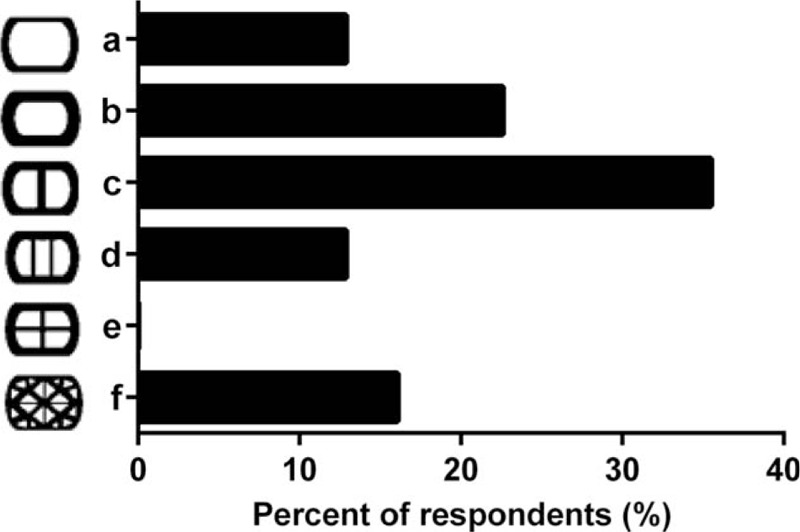
Selection of implant design based on available bone grafting area by spinal surgeons (n = 26).
3.4. Bone graft materials
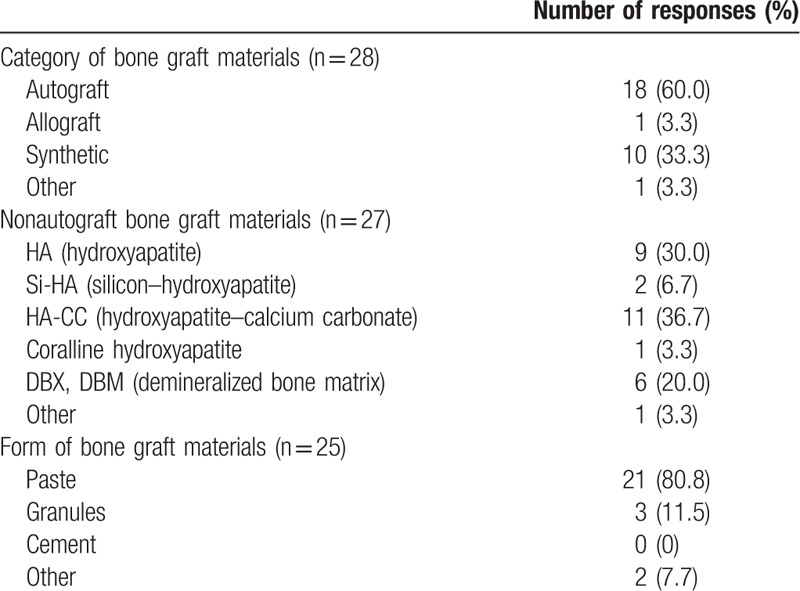
Most interviewed surgeons preferred autograft bone grafting procedures according to the 60.0% of responses, whilst the 33.3% of responses indicated synthetic bone graft substitutes (Table 4). Within the nonautograft bone graft solutions, the preferred materials were synthetic hydroxyapatite (HA)-based composites according to the 76.7% of responses. The preferred form for bone graft substitutes was injectable paste according to the 80.8% of responses.
Table 4.
Preferred bone graft materials (multiple choice allowed).
4. Discussion
This UK-based pilot study provides a descriptive portrait of current surgical practice in lumbar fusion surgery. To-date, only few studies have described the current practice for the treatment of low back pain.[5,41,43] However, none of them focused also on surgeons’ implant decision making process. Hence, the purpose of our survey was to provide not only a snapshot of the current treatment of choice in the United Kingdom, but also to explore the rationale behind each choice.
Amongst the possible surgical procedures, lumbar fusion is the most prevalent surgery. Indeed, the majority of respondents (83.9%) performed no cases of lumbar artificial disc replacement over their last year of clinical practice. There is some variability of practice related to the possible fusion approaches, which might confirm the agreement for a patient-centered care and individual patient management.[5] Despite this variability, TLIF is the most performed fusion approach rather than PLIF, ALIF, or XLIF/DLIF. However, there is no convincing clinical evidence in the literature which supports the choice of a specific surgical technique for a certain population of patients.[11,44,45] Our findings illustrate that spinal instrumentation is widely adopted as an adjunct to lumbar fusion. Accordingly, fixation surgery is largely favored (96.0%) over the use of stand-alone cages. Posterior fixation with pedicle screws is the most common procedure. However, there is no clear uniformity of opinion as it pertains to fixation surgery, which is in accordance with the variety of possible fusion approaches. Importantly, our findings consistently highlight the relevance of a patient-oriented management for the choice of the surgical procedure (84.4%), fusion approach (87.5%), and associated interbody fusion implant (75.0%).
With regards to the shape and materials of the device, there is limited evidence in the literature concerning the current fusion implant trends among surgeons’ practice. Interbody fusion cages are in fact available in a wide range varying materials, shapes, and architectures. However, it does not appear clear which parameter is currently the most important.[46,47] According to a recent survey conducted in the Netherlands, neurosurgeons use cages made in polyether–ether–ketone (PEEK) more often than titanium cages, whereas orthopedic surgeons prefer titanium cages.[41] In our study, the majority of respondents were orthopedic spinal consultants (87.5%) and, overall, titanium cages were the most common (54.3%). Additionally, implants with smooth surface are generally discarded, whilst porous/trabecular or rough/threaded implants seem to be the favorite choice. Overall, our findings suggest uniformity of opinion concerning the relevance of implant geometry for the choice of the fusion implant (81.3%). Design factors such as cage width, length, height, and lordotic angle are all considered relevant. Parallel-shape cages are avoided in most cases (89.2%) and hyperlordotic cages are preferred at the lumbosacral level. Indeed, in order to correct sagittal alignment and balance, a 5° to 6° angle was proven to be sufficient at L4–L5 level and above, but not enough at L5–S1 level.[48] Furthermore, our results show that some implant designs are more common than others but there is uncertainty regarding which design should be preferred. A specific fusion technique limits the width of the surgical access and, in turn, the possible sizes and shapes of the implant are restricted. Hence, the established variety of practice well reflects the diversity of opinion on implant design. Several clinical and biomechanical studies have been focused on evaluating the performance of different profiles for lumbar cages.[42,49–51] However, clinical evidence is still inadequate and comparisons are limited to few different designs. Based on this survey, surgeons have shown to value a patient-oriented care, nevertheless, there is limited scientific literature evidencing uniform guidelines for some patient categories or specific diseases. In this context, patient-specific technology has the potential to alter future spinal surgical practice, by providing perfectly fitting implants tailored on a given situation. This approach has shown great popularity in orthopedics over the past 5 years, however, its applicability to spinal fusion surgery has only recently been explored.[52,53] The feasibility of designing and manufacturing of anatomical-shaped fusion cages using 3D printing technology has been shown.[52,54] However, clinically relevant studies in this area still need to be explored. The current stage of the spinal implant market is not yet patient-specific, but future industrial and academic investments will be likely focus towards the customization of spinal implants.
The last part of the survey aimed to investigate the current practice as it pertains the possible bone grafting procedures. The results of our survey highlight that autograft surgeries were the most common bone grafting procedures in the United Kingdom, still overcoming the use of synthetic substitutes. Contrarily, a slight shift from autologous to substitute grafts was reported in the United States during the past 16 years.[55] Between the nonautograft options, surgeons favor the use of synthetic substitutes, preferable HA-based composite materials (76.7%). Injectable paste form is preferred (80.8%), perhaps for the ease of insertion and possibility to fill small bone cavities.
The pilot nature of this study includes indeed several limitations. First, this survey was conducted at a single event (i.e., BritSpine 2016, April 6–8, 2016, Nottingham, United Kingdom). This event, which is held every 2 years, attracts participants from the British Association of Spinal Surgeons (BASS) which counted 381 memberships at the end of 2016, and includes a large part of the population of spinal consultants in the United Kingdom. The results of our study indeed include a sample of the UK consultant spinal surgeons. Because of the relatively small size of the interviewed group, we were unable to perform any more accurate statistical analysis of subpopulations or correlations among responses. Hence, this may affect the scalability of the results of this research. However, a strength of this study was the fact that we approached only expert spinal surgeons with at least 1 year experience as consultants. Therefore, only surgeons who undertook their own surgical decisions were recruited. Because of the face-to-face recruitment method, we could maximize the response and completion rates of the questionnaire, however, this may have interfered with the in-depth thinking of participants, thus increase the response bias. In addition, no specific distinction between open versus minimally invasive fusion surgeries was made in this study. In the context of such rapidly evolving techniques, it will be useful to continue this investigation by repeating the survey at future meetings and updating the questions accordingly. Such longitudinal analysis will allow us to monitor the progresses in this field. Moreover, surgeons’ indications for specific diseases such as lumbar degenerative disc disease, trauma, and deformity have not been assessed in details. Future investigations should therefore focus on identifying subgroups of cases for whom a specific lumbar fusion approach is contemplated as an effective treatment. As it pertains to the factors influencing implant choice, the correlation between distinctive designs with specific fusion approaches has not been fully addressed in the questionnaire, thus limiting the interpretation of the results. Indeed, the geometry of the interbody fusion implant is a direct function of the surgical approach that it is used for. Finally, a more detailed cost analysis of surgical procedures and implants would also be relevant for the description of surgeons’ decision making process. Despite these limitations, we believe that this pilot study can provide a snapshot of current surgeons’ practice and, at the same time, encourages bigger scale surveys.
5. Conclusions
The present survey attempts at investigating the variability in surgeons’ decision making process in spinal fusion surgery. Our findings suggest the relevance of a patient-oriented management, which is confirmed by a wide variability of choices among surgeons’ practice. There is a lack of consensus among spinal surgeons concerning the choice of the fusion approach, fixation surgery, and interbody fusion implant. Various parameters in terms of implant geometry might lead surgeons’ practice; however, current clinical evidence is still inadequate to justify the superiority of one implant over another. Given the importance of a patient-centered care, research needs to examine the outcomes of lumbar fusion surgery for certain pathologies and specific populations of patients.
Acknowledgments
None of the authors have any commercial associations or financial relationships that would create a conflict of interest with the work presented in this article. Authors would like to gratefully acknowledge Ceramisys Ltd for their support throughout this project.
Author contributions
Conceptualization: Elena Provaggi, Claudio Capelli, Julian J.H. Leong, Deepak M. Kalaskar.
Data curation: Elena Provaggi, Claudio Capelli, Deepak M. Kalaskar.
Formal analysis: Elena Provaggi.
Investigation: Deepak M. Kalaskar.
Methodology: Elena Provaggi, Deepak M. Kalaskar.
Project administration: Deepak M. Kalaskar.
Supervision: Claudio Capelli, Julian J.H. Leong, Deepak M. Kalaskar.
Writing – original draft: Elena Provaggi.
Writing – review & editing: Elena Provaggi, Claudio Capelli, Julian J.H. Leong, Deepak M. Kalaskar.
Supplementary Material
Footnotes
Abbreviations: ALIF = anterior lumbar interbody fusion, DLIF = direct lumbar interbody fusion, HA = hydroxyapatite, LIF = lumbar interbody fusion, LLIF = lateral lumbar interbody fusion, MIS = minimally invasive surgery, NHS = National Health Service, PEEK = polyetheretherketone, PLIF = posterior lumbar interbody fusion, TDR = total disc replacement, XLIF = extreme lateral interbody fusion.
Funding: This study was supported through a SLMS IMPACT Studentship Funding Model at University College London (Award no. 170401).
The authors have no conflicts of interest to disclose.
Supplemental Digital Content is available for this article.
References
- [1].Rubin DI. Epidemiology and risk factors for spine pain. Neurol Clin 2007;25:353–71. [DOI] [PubMed] [Google Scholar]
- [2].Audrey J, Weiss AE. Trends in Operating Room Procedures in U.S. Hospitals. 2001–2011. Rockville, MD: Agency for Healthcare Research and Quality; 2014. [PubMed] [Google Scholar]
- [3].The Health and Social Care Information Centre. HES online. Admitted Patient Care, Total procedures interventions: 3 character tables (2004–2005, 2014–2015). Available from: http://www.hesonline.nhs.uk. [Google Scholar]
- [4].Maniadakis N, Gray A. The economic burden of back pain in the UK. Pain 2000;84:95–103. [DOI] [PubMed] [Google Scholar]
- [5].Rushton A, White L, Heap A, et al. Evaluation of current surgeon practice for patients undergoing lumbar spinal fusion surgery in the United Kingdom. World J Orthop 2015;6:483–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].GlobalData. MediPoint: Spinal Fusion - Global Analysis and Market Forecasts. December 2016. Report Code: GDME0246MAR. Available from: http://www.globaldata.com. [Google Scholar]
- [7].Takahashi T, Hanakita J, Ohtake Y, et al. Current status of lumbar interbody fusion for degenerative spondylolisthesis. Neurol Med Chir (Tokyo) 2016;56:476–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Kim HJ, Buchowski JM, Zebala LP, et al. RhBMP-2 is superior to iliac crest bone graft for long fusions to the sacrum in adult spinal deformity: 4- to 14-year follow-up. Spine 2013;38:1209–15. [DOI] [PubMed] [Google Scholar]
- [9].Lee JH, Jang HL, Lee KM, et al. Cold-spray coating of hydroxyapatite on a three-dimensional polyetheretherketone implant and its biocompatibility evaluated by in vitro and in vivo minipig model. J Biomed Mater Res B Appl Biomater 2017;105:647–57. [DOI] [PubMed] [Google Scholar]
- [10].Talia AJ, Wong ML, Lau HC, et al. Comparison of the different surgical approaches for lumbar interbody fusion. J Clin Neurosci 2015;22:243–51. [DOI] [PubMed] [Google Scholar]
- [11].Mobbs RJ, Phan K, Malham G, et al. Lumbar interbody fusion: techniques, indications and comparison of interbody fusion options including PLIF, TLIF, MI-TLIF, OLIF/ATP, LLIF and ALIF. J Spine Surg 2015;1:2–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Lee N, Kim KN, Yi S, et al. Comparison of outcomes of anterior, posterior, and transforaminal lumbar interbody fusion surgery at a single lumbar level with degenerative spinal disease. World Neurosurg 2017;101:216–26. [DOI] [PubMed] [Google Scholar]
- [13].Gumbs AA, Bloom ND, Bitan FD, et al. Open anterior approaches for lumbar spine procedures. Am J Surg 2007;194:98–102. [DOI] [PubMed] [Google Scholar]
- [14].Strube P, Hoff E, Hartwig T, et al. Stand-alone anterior versus anteroposterior lumbar interbody single-level fusion after a mean follow-up of 41 months. Clin Spine Surg 2012;25:362–9. [DOI] [PubMed] [Google Scholar]
- [15].Hsieh PC, Koski TR, O'Shaughnessy BA, et al. Anterior lumbar interbody fusion in comparison with transforaminal lumbar interbody fusion: implications for the restoration of foraminal height, local disc angle, lumbar lordosis, and sagittal balance. J Neurosurg Spine 2007;7:379–86. [DOI] [PubMed] [Google Scholar]
- [16].Kadam A, Wigner N, Saville P, et al. Overpowering posterior lumbar instrumentation and fusion with hyperlordotic anterior lumbar interbody cages followed by posterior revision: a preliminary feasibility study. J Neurosurg Spine 2017;27:650–60. [DOI] [PubMed] [Google Scholar]
- [17].Rao PJ, Loganathan A, Yeung V, et al. Outcomes of anterior lumbar interbody fusion surgery based on indication: a prospective study. Neurosurgery 2015;76:7–23. discussion 23–24. [DOI] [PubMed] [Google Scholar]
- [18].Mobbs RJ, Loganathan A, Yeung V, et al. Indications for anterior lumbar interbody fusion. Orthop Surg 2013;5:153–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Quraishi NA, Konig M, Booker SJ, et al. Access related complications in anterior lumbar surgery performed by spinal surgeons. Eur Spine J 2013;22(suppl 1):16–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Kerolus M, Turel MK, Tan L, et al. Stand-alone anterior lumbar interbody fusion: indications, techniques, surgical outcomes and complications. Expert Rev Med Devices 2016;13:1127–36. [DOI] [PubMed] [Google Scholar]
- [21].Lestini WF, Fulghum JS, Whitehurst LA. Lumbar spinal fusion: advantages of posterior lumbar interbody fusion. Surg Technol Int 1994;3:577–90. [PubMed] [Google Scholar]
- [22].Yan D-l, Pei F-x, Li J, et al. Comparative study of PILF and TLIF treatment in adult degenerative spondylolisthesis. Eur Spine J 2008;17:1311–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Okuda S, Miyauchi A, Oda T, et al. Surgical complications of posterior lumbar interbody fusion with total facetectomy in 251 patients. J Neurosurg Spine 2006;4:304–9. [DOI] [PubMed] [Google Scholar]
- [24].Madhu TS. Posterior and anterior lumbar interbody fusion. Curr Orthop 2008;22:406–13. [Google Scholar]
- [25].Satoh I, Yonenobu K, Hosono N, et al. Indication of posterior lumbar interbody fusion for lumbar disc herniation. J Spinal Disord Tech 2006;19:104–8. [DOI] [PubMed] [Google Scholar]
- [26].Ozgur BM, Aryan HE, Pimenta L, et al. Extreme lateral interbody fusion (XLIF): a novel surgical technique for anterior lumbar interbody fusion. Spine J 2006;6:435–43. [DOI] [PubMed] [Google Scholar]
- [27].Rodgers WB, Gerber EJ, Patterson J. Intraoperative and early postoperative complications in extreme lateral interbody fusion: an analysis of 600 cases. Spine 2011;36:26–32. [DOI] [PubMed] [Google Scholar]
- [28].Epstein NE. Extreme lateral lumbar interbody fusion: do the cons outweigh the pros? Sur Neurol Int 2016;7(suppl 25):S692–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Lee BH, Yang JH, Kim HS, et al. Effect of sagittal balance on risk of falling after lateral lumbar interbody fusion surgery combined with posterior surgery. Yonsei Med J 2017;58:1177–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Aichmair A, Alimi M, Hughes AP, et al. Single-level lateral lumbar interbody fusion for the treatment of adjacent segment disease: a retrospective two-center study. Spine 2017;42:E515–22. [DOI] [PubMed] [Google Scholar]
- [31].Fleege C, Rickert M, Rauschmann M. [The PLIF and TLIF techniques. Indication, technique, advantages, and disadvantages]. Orthopade 2015;44:114–23. [DOI] [PubMed] [Google Scholar]
- [32].Cole CD, McCall TD, Schmidt MH, et al. Comparison of low back fusion techniques: transforaminal lumbar interbody fusion (TLIF) or posterior lumbar interbody fusion (PLIF) approaches. Curr Rev Musculoskelet Med 2009;2:118–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Holly LT, Schwender JD, Rouben DP, et al. Minimally invasive transforaminal lumbar interbody fusion: indications, technique, and complications. Neurosurg Focus 2006;20:E6. [DOI] [PubMed] [Google Scholar]
- [34].Spoor AB, Öner FC. Minimally invasive spine surgery in chronic low back pain patients. J Neurosurg Sci 2013;57:203–18. [PubMed] [Google Scholar]
- [35].Zhang D, Mao K, Qiang X. Comparing minimally invasive transforaminal lumbar interbody fusion and posterior lumbar interbody fusion for spondylolisthesis: a STROBE-compliant observational study. Medicine 2017;96:e8011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Goldstein CL, Macwan K, Sundararajan K, et al. Comparative outcomes of minimally invasive surgery for posterior lumbar fusion: a systematic review. Clin Orthop Relat Res 2014;472:1727–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].McGirt MJ, Parker SL, Mummaneni P, et al. Is the use of minimally invasive fusion technologies associated with improved outcomes after elective interbody lumbar fusion? Analysis of a nationwide prospective patient-reported outcomes registry. Spine J 2017;17:922–32. [DOI] [PubMed] [Google Scholar]
- [38].Wei J, Song Y, Sun L, et al. Comparison of artificial total disc replacement versus fusion for lumbar degenerative disc disease: a meta-analysis of randomized controlled trials. Int Orthop 2013;37:1315–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Büttner-Janz K, Guyer RD, Ohnmeiss DD. Indications for lumbar total disc replacement: selecting the right patient with the right indication for the right total disc. Int J Spine Surg 2014;8:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Willems P. Decision making in surgical treatment of chronic low back pain: the performance of prognostic tests to select patients for lumbar spinal fusion. Acta Orthop Suppl 2013;84:1–35. [DOI] [PubMed] [Google Scholar]
- [41].Kersten RFMR, van Gaalen SM, Willems PC, et al. Lumbar spinal fusion: indications, surgical techniques and post-operative management. A survey among spine surgeons in the Netherlands. MOJ Orthop Rheumatol 2016;4: 00155. [Google Scholar]
- [42].Phan K, Mobbs RJ. Evolution of design of interbody cages for anterior lumbar interbody fusion. Orthop Surg 2016;8:270–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Willems P, de Bie R, Öner C, et al. Clinical decision making in spinal fusion for chronic low back pain. Results of a nationwide survey among spine surgeons. BMJ Open 2011;1:e000391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Jacobs WC, Rubinstein SM, Koes B, et al. Evidence for surgery in degenerative lumbar spine disorders. Best Pract Res Clin Rheumatol 2013;27:673–84. [DOI] [PubMed] [Google Scholar]
- [45].Alentado VJ, Caldwell S, Gould HP, et al. Independent predictors of a clinically significant improvement after lumbar fusion surgery. Spine J 2017;17:236–43. [DOI] [PubMed] [Google Scholar]
- [46].Chou Y-C, Chen D-C, Hsieh WA, et al. Efficacy of anterior cervical fusion: comparison of titanium cages, polyetheretherketone (PEEK) cages and autogenous bone grafts. J Clin Neurosci 2008;15:1240–5. [DOI] [PubMed] [Google Scholar]
- [47].Yu CH, Wang CT, Chen PQ. Instrumented posterior lumbar interbody fusion in adult spondylolisthesis. Clin Orthop Relat Res 2008;466:3034–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Barrey C, Darnis A. Current strategies for the restoration of adequate lordosis during lumbar fusion. World J Orthop 2015;6:117–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Postigo S, Schmidt H, Rohlmann A, et al. Investigation of different cage designs and mechano-regulation algorithms in the lumbar interbody fusion process—a finite element analysis. J Biomech 2014;47:1514–9. [DOI] [PubMed] [Google Scholar]
- [50].Bashkuev M, Checa S, Postigo S, et al. Computational analyses of different intervertebral cages for lumbar spinal fusion. J Biomech 2015;48:3274–82. [DOI] [PubMed] [Google Scholar]
- [51].Tsai P-I, Hsu C-C, Chen S-Y, et al. Biomechanical investigation into the structural design of porous additive manufactured cages using numerical and experimental approaches. Comput Biol Med 2016;76:14–23. [DOI] [PubMed] [Google Scholar]
- [52].Serra T, Capelli C, Toumpaniari R, et al. Design and fabrication of 3D-printed anatomically shaped lumbar cage for intervertebral disc (IVD) degeneration treatment. Biofabrication 2016;8:035001. [DOI] [PubMed] [Google Scholar]
- [53].Figueroa-Cavazos JO, Flores-Villalba E, Diaz-Elizondo J, et al. Design concepts of polycarbonate-based intervertebral lumbar cages: finite element analysis and compression testing. Appl Bionics Biomech 2016;2016:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Capelli C, Serra T, Kalaskar D, et al. Computational models for characterisation and design of patient-specific spinal implant. Spine J, 16:S53–S54. [Google Scholar]
- [55].Kinaci A, Neuhaus V, Ring DC. Trends in bone graft use in the United States. Orthopedics 2014;37:e783–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


