Abstract
Retrospective Cross-Sectional Study.
The purpose of this study was to investigate the accuracy of magnetic resonance imaging (MRI) for distinguishing between pyogenic spondylitis and brucellar spondylitis.
Although pyogenic spondylodiscitis (PS) and brucellar spondylitis (BS) are common causes of spinal infections, the variety of their clinical manifestations complicates differential diagnosis. MRI may be helpful in differential diagnosis and treatment.
MRI images of 64 patients who underwent MRI of the spine and with confirmed spondylitis were retrospectively reviewed. After referring to the related medical literature, we compared 32 patients with pyogenic spondylitis and 32 patients with brucellar spondylitis regarding MRI findings. Statistical analysis was performed with the chi-square test. Statistical significance was defined as P < .05.
The significant differences between PS and BS on MRI findings are listed as follows (P < .05): diffuse, partial and fan-shaped hyperintense signals on middle sagittal fat-suppressed weighted images (PS: 51, 11, 3/65 vs BS:35, 18, 19/72); focal endplate destruction (PS: 9/43 vs BS:27/35); extensive end plate destruction (PS: 29/43 vs BS:8/35); ballooning change of the intravertebral space (PS: 7/32 vs BS:0/32); an inflammatory reaction line from the end plate (PS: 30/65 vs BS: 1/72); a disc invasion sign (PS: 1/28 vs BS:12/33); an inflammatory reaction line in the disc (PS: 5/28 vs BS:25/33); and 8) severe intravertebral space destruction (PS: 17/28 vs BS:12/33);
MRI imaging provides useful information for the differentiation between pyogenic spondylitis and brucellar spondylitis.
Keywords: brucellar spondylitis, brucellar spondylitis, diagnosis, MRI, pyogenic spondylitis
1. Introduction
Infectious spondylitis is defined as an infection by a specific organism, in which vertebral bodies, intervertebral discs, paraspinal soft tissues, epidural spaces, meninges, and the spinal cord may be involved.[1,2] The diagnosis of spinal infections has been a challenge for physicians for many years. It has been especially important to differentiate pyogenic spondylitis (PS) from brucellar spondylitis (BS) in recent years, because proper treatment of these different types can reduce the rate of disability and functional impairment.
Pyogenic spondylitis (PS) is the most common spinal infection. The most common cause of both pyogenic spondylitis and discitis is hematogenous spread of Staphylococcus aureus, which is seen in 55% to 90% of cases, followed by Enterobacter, Salmonella, Pseudomonas, and Serratia species.[3] It was reported that delays in diagnosis could lead to increased morbidity and mortality.[4]
Brucellosis (BS) is the most common bacterial zoonosis, with more than 500,000 cases reported annually worldwide, which is one of the infectious diseases transmissible between animals and humans.[5–7] The disease is caused by small, nonmotile Gram-negative facultative intracellular coccobacilli of the genus Brucella.[5,8] Consumption of or contact with nonpasteurized milk or milk products, body fluids, or pregnancy material from infected animals is the main reason.[5,6,9,10] The disease remains the world's most common bacterial zoonosis, particularly in Mediterranean areas, the south and the center of the Americas, Africa, the Indian subcontinent and the Middle East, with over half a million new cases annually and prevalence rates in some countries exceeding ten cases per 100,000 population. [7,9,11] Brucellosis can involve many organs, and the spine is the most common site of musculoskeletal involvement.[12,13] The incidence of spinal involvement in brucellosis is 2% to 65%.[5,10]
PS and BS have similar clinical presentations with nonspecific symptoms and signs (fever, night sweats, asthenia, insomnia, anorexia and headache).[3,10] Laboratory examination alone is insufficient for a correct diagnosis. Mildly elevated sedimentation rates, higher C-reactive protein levels and white blood cell counts, and lower hemoglobin levels are frequently found in most infections, rendering it difficult to make a diagnosis.[3] A standard tube agglutination test and Rose Bengal test can be helpful for diagnosis of BS; however, some false positive rates persist.[8] The sensitivity of blood cultures is low.[3,8,10,14] The sensitivities of blood culture have been published as high as 80% and 90% in acute cases, respectively, but as low as 15%, 30% and 70%, respectively, in chronic cases.[15,16] Biopsy reveals higher positive results in 50% to 90%.[3] Perhaps it is worthwhile to look for a way to improve the final diagnosis rate. Because of its excellent soft tissue imaging, MRI should be considered first.[3]
The purpose of this study was to determine the value of MRI in distinguishing between pyogenic spondylitis and brucellar spondylitis.
2. Materials and methods
2.1. Patients
This study included all consecutive patients in our institution who had proven hematogenous pyogenic and brucellar spinal infections. Shandong Provincial Hospital Affiliated to Shandong University approved the study and informed consent was given by patients involved in this study. All of the patients underwent MRI examination of the spine between January 2012 and April 2016 at Shandong Provincial Hospital in Jinan, China. All pyogenic spinal infections were proved in all of the patients with positive blood culture (S aureus: 8 vs Escherichia coli: 5) or histologic examination results from either computed tomography (CT)-guided percutaneous biopsy samples (S aureus: 9 vs E coli: 8) or surgical biopsies (S aureus: 2 vs E coli: 0). Diagnosis of BS was based on a positive standard tube agglutination titer test (n = 29) with standard tube agglutination titer tests ≥1:160 or the isolation of brucella species from blood, bone marrow, or tissue (n = 3). All of the MRI were obtained before the surgical biopsy, or either computed tomography (CT)-guided needle biopsy or antibiotic treatment was performed. The exclusion criteria omitted patients with proven tuberculous spinal infections or with previous tuberculous infections of the lung or other body regions, those with postoperative spinal infections, and those with standard tube agglutination titer tests ≤1:160. MRI of 32 PS patients (12 women, 20 men; mean age, 55.5 years old; age range, 22–78 years) and 32 BS patients (8 women, 24 men; mean age, 57.3 years old; age range, 30–74 years) were retrospectively analyzed. The most common pyogenic organism was S aureus (n = 19), followed by E coli (n = 13). The mean interval from presentation to MRI imaging was 6 weeks (range 1–3 months) in patients with pyogenic spondylitis and 9 weeks (range 1–5 months) in patients with brucellar spondylitis.
2.2. MRI imaging
A 1.5 T MRI system (Siemens Avanto, Erlangen, Germany) was used for all of the patients. The imaging protocol consisted of the following: sagittal T2-weighted (3340 ms repetition time, TR, 116 ms echo time, TE, 40 cm FOV for cervicothoracic, and 28 cm FOV for lumbosacral); sagittal T2STIR (4500 ms TR, 69 ms TE, and 40 cm FOV) of the whole spine acquired separately with overlap; sagittal T1-weighted (590 ms TR, 13 ms TE, 40 cm FOV for cervicothoracic, and 28 cm FOV for lumbosacral) of the involved part of the spine; axial T2 (4440 ms TR, 97 ms TE, 24e26 cm FOV); axial T1-weighted (668 ms TR, 11 ms TE, 24e26 cm FOV); axial T2 STIR (7580 ms TR, 62 ms TE, 24e26 cm FOV); and coronal T2 STIR at the site of involvement.
2.3. Imaging evaluation
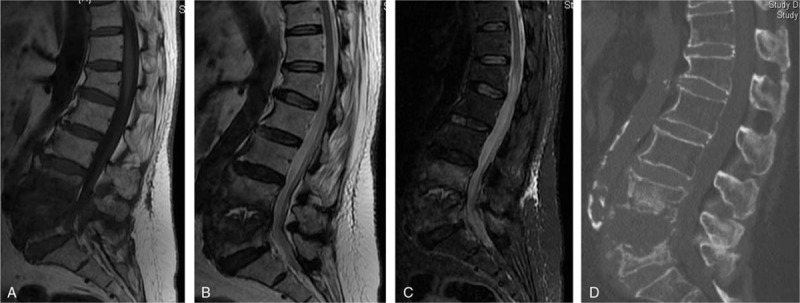
Two musculoskeletal radiologists reviewed the MRI images blindly, and any disagreement that arose was discussed and resolved by consensus. Signal morphology characteristic of involved vertebral bodies was categorized as diffuse, partial, and fan-shaped signals on the sagittal view.[17] Fan-shaped signals were noted as infected portions that started at the anterior or posterior edge of the endplate and then spread into the vertebra in a fan shape (Figs. 1A–C and 2A and B). A diffuse signal was defined as an area of an abnormal signal greater than 50% of the whole area of the abnormal body on middle sagittal views (Fig. 3A–C). A partial signal indicated that the abnormal part was less than 50% of the whole area of the infected body, surrounding the disc and parallel to the endplate (Fig. 4A–C).
Figure 1.
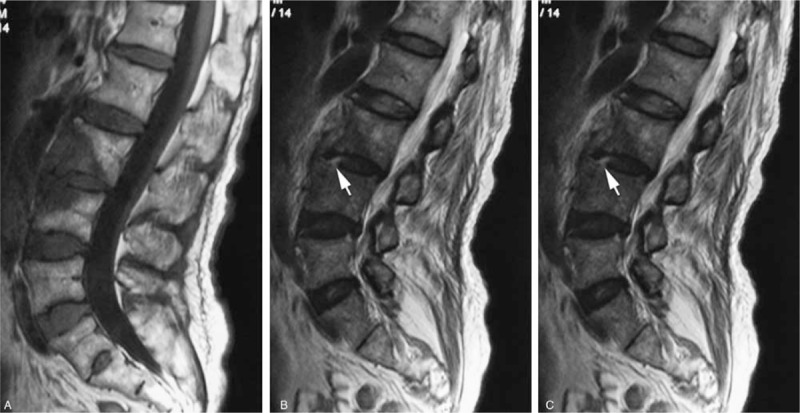
A 69-year-old woman with a brucella infection for 2.5 months without any previous antibiotic treatment. (A) T1-weighted image shows hypointensity of the L3-L4 vertebral bodies, showing evident front fan-shape shade. (B and C) T2-weighted and FS MRIs show infection starting from the anterior of the endplate in both L3 and L4 and then involving the disc from L4 vertebra, which we called the Disc Invasion Sign (arrow), and invading the vertebral body (in fan shape).
Figure 2.
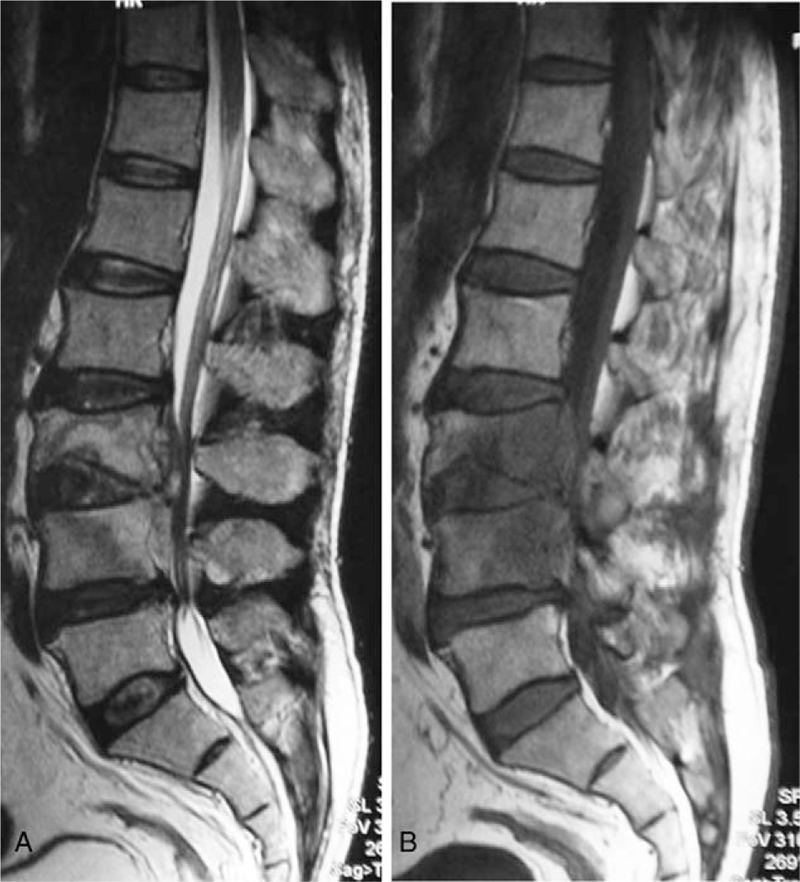
A 55-year-old man with a brucella infection for 3 months without any previous antibiotic treatment. (A) T1-weighted image shows a hypointense signal from L3-L4. (B) T2-weighted image shows a hyperintense signal from L3 to L4; both (A) and (B) present posterior fan shape spreading to the vertebral body.
Figure 3.
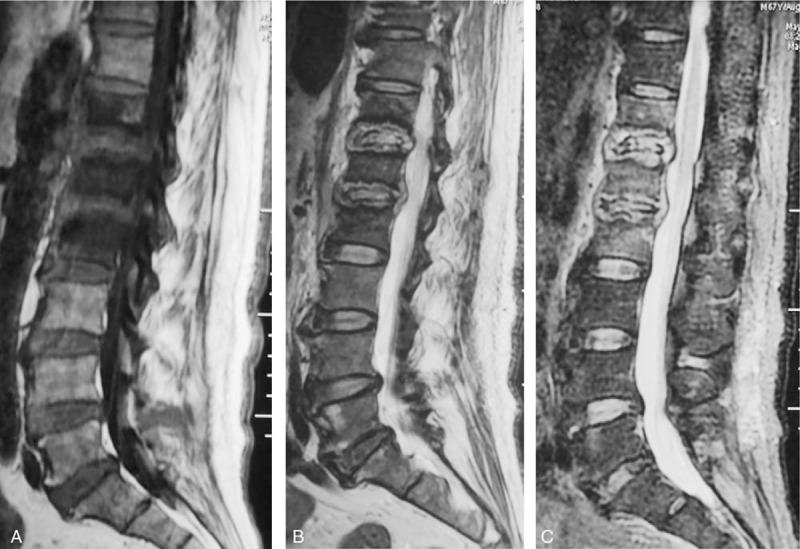
A 67-year-old man with a pyogenic infection (S aureus) for 2 months without any previous antibiotic treatment. A sagittal T1-weighted image (A) shows hypointensity on T12, L1 and L2. T2-weighted image (B) and FS MRIs (C) show the “inflammatory reaction line from the end plate” and the involved discs of T12, L1 and L2, which show hyperintense signals. The endplates were destroyed extensively. This infected disc and endplate comprise a typical “eye sign.” The infected disc can be thought of as an eyeball and the infected endplate can be regarded as an eyelid.
Figure 4.
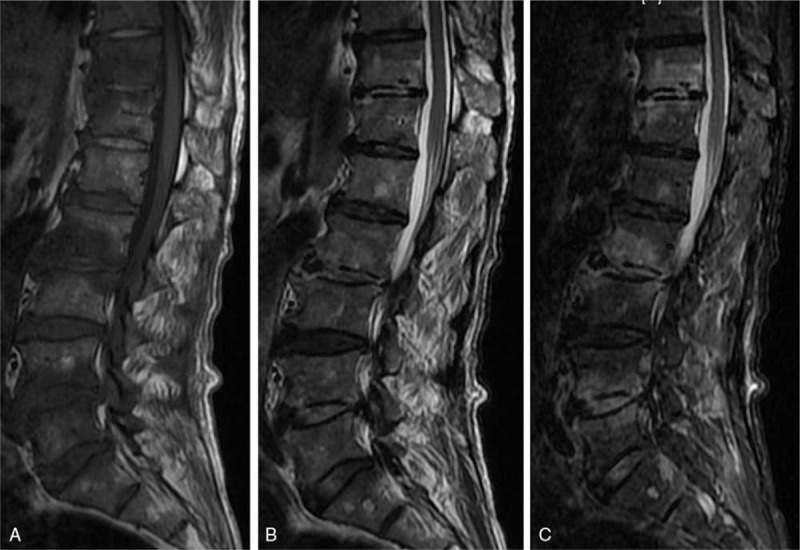
A 61-year-old man with a brucella infection for 2 months without any previous antibiotic treatment. A multiple-level involvement case. (A) T1-weighted image shows a hypointense signal from the T11-L5 vertebrae. (B) T2-weighted image shows a heterogeneous signal from the T11-L5 vertebrae. (C) FS MRI shows a hyperintense signal from the involved vertebrae. An inflammatory reaction line in the disc was found from the T11/12 and L2/3 discs. Focal endplate destruction was demonstrated from the inferior endplate of the T11 vertebra.
Vertebral body destruction was recorded and categorized as endplate destruction, which was further graded as focal and extensive destruction (Table 1) or severe vertebral destruction, indicated that vertebral body was destroyed to more than 50% height of the adjacent normal vertebral body (Fig. 5A–D). The disc destruction was graded as mild or severe. Mild destruction indicated that the height of the involved disc space was more than 50% of the adjacent normal disk height, and severe destruction indicated that the height of the infected disc space was less than 50% of the adjacent normal disk height.[18]
Table 1.
The comparison of the infected vertebral body and disc on MRIs.
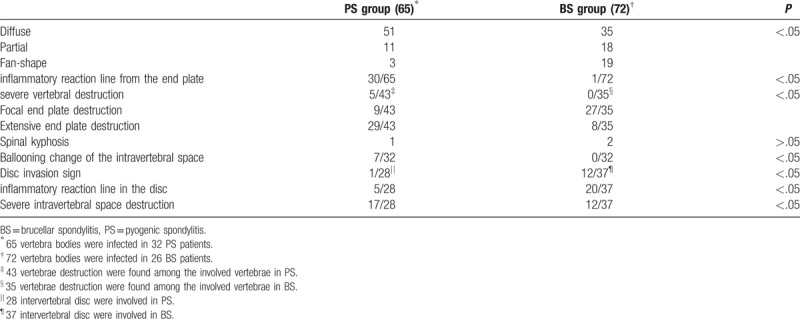
Figure 5.
A 77-year-old woman with a pyogenic infection (E coli) for 1 month. (A) T1-weighted MRI shows a hypointense signal from the interspace of L4/5 and the L4 and L5 vertebrae. (B) isointensity signal are shown from L4 and L5 vertebrae. A heterogeneous signal can be observed from the L4/5 interspace, which indicates abscess formation. (C) FS-MRI shows a hyperintense signal from the L4 and L5 vertebrae. (D) The L5 vertebra has been destroyed severely, and the interspace has expanded, which we can define as “ballooning change of the intravertebral space.”
2.4. Data analysis
The X2 test was used to evaluate the differences between the data of the 2 groups, and P < .05 indicated a significant difference.
3. Results
Of 32 patients with pyogenic spondylitis, 4 had cervical, 2 had thoracic, 5 had thoracolumbar, 19 had lumbar, and 2 had sacral vertebral involvement. Sixty-five vertebrae were infected, and the mean number of involved vertebrae was 2.03 per patient. One patient was found to have multiple-level involvement. No skip lesions were found. Of 32 patients with pyogenic spondylitis, 0 had cervical, 2 had thoracic, 4 had thoracolumbar, 24 had lumbar and, 2 had sacral vertebral involvement. Seventy-two vertebrae were infected, and 2.25 vertebrae were involved per patient. Five patients were found with multiple-level involvement. No significant differences were founded in the following: sex composition, the onset age range, urban and rural proportions, favorite site, or skip involvement. Multiple-level involvement was more frequently seen in BS than in PS.
As Table 1 demonstrates, 4 types of vertebral signal changes were recorded: diffuse/partial/fan-shape/inflammatory reaction line from the end plate on FS images. Among the infected vertebrae in PS, 78% showed diffuse signals, and 46% demonstrated inflammatory reaction lines from the end plate (Figs. 3B and C and 6A and B). Among the involved vertebrae in BS, 25% and 26% were recorded, respectively, as partial and fan-shaped abnormal signals. Only 1 vertebra was found with an inflammatory reaction line from the end plate. Only one case of severe vertebral destruction due to PS was found in both groups (Fig. 5A–D). The destruction of the endplate was recorded as focal end plate destruction and extensive end plate destruction. A total of 76% of infected vertebrae due to PS showed extensive end plate destruction (Fig. 3B and C); 77% of involved vertebrae due to BS demonstrated focal end plate destruction (Fig. 4B and C). Five cases among PS patients and 0 cases among BS patients were found with ballooning changes in the intervertebral space (Fig. 5A–C). One case showed the disc invasion sign in PS and 12 cases in BS (Fig. 1B and C). An inflammatory reaction line in the disc was found in 25% and 61%, respectively, of PS and BS patients (Figs. 4B and C and 6A and B). Around 61% of involved discs due to PS were recorded as severe intervertebral disc spaces, and 29% were founded among the BS cases.
Figure 6.
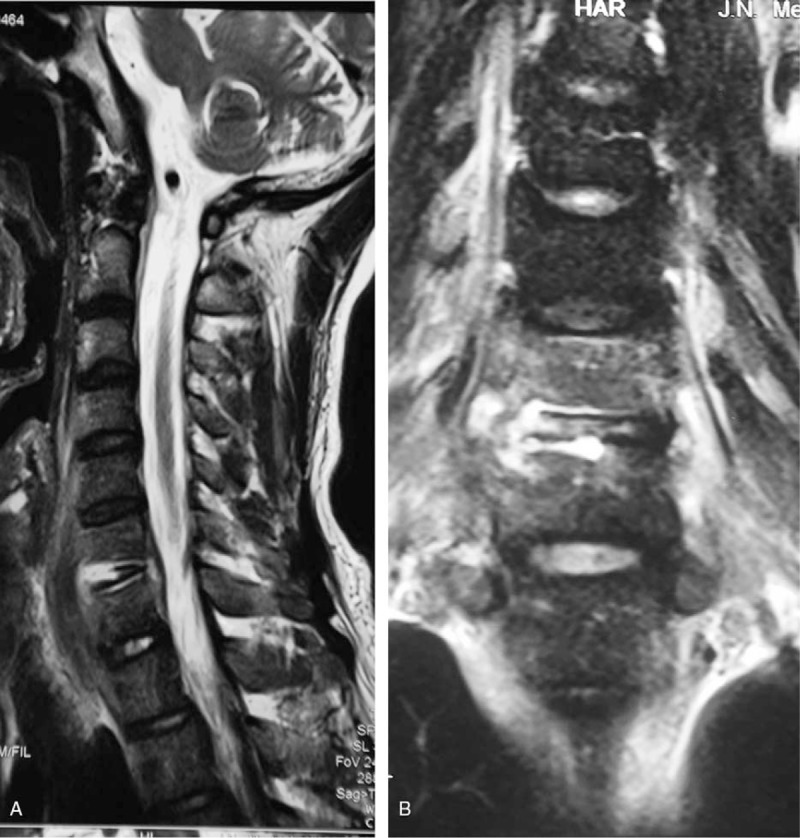
A 37-year-old man with a pyogenic infection (S aureus) for 1.5 months without any previous antibiotic treatment. The sagittal T2-weighted image (A) and coronal T2-weighted image (B) show the “inflammatory reaction line from the end plate” of C6 and C7 and the disc of C6/7 involving the infected endplates.
As Table 2 shows, 10 cases of PS and 14 cases of BS were found with epidural masses. Fourteen PS patients and 15 BS patients showed anterior subligament spread. In terms of anterior subligamentous spread, no significant differences were recorded: 86% and 93% of cases had abscess spread in PS and BS, respectively, with fewer than 3 vertebral levels or even no spread in PS and BS.
Table 2.
The analysis of abscess of the pyogenic spondylitis and brucellar spondylitis.
4. Discussion
Pyogenic spondylitis and brucellar spondylitis are common spinal diseases, of which both may present with a variety of clinical manifestations and complications. Prompt diagnosis and an effective cure become critically important to minimize spinal deformity and permanent neurologic deficiencies. On the basis of the preceding relevant studies, we present our further research into differential diagnosis using MRI.
In both groups, MRI showed hypointensity on T1-weighted images of infected vertebral bodies. Hyperintense signals were found on T2-weighted MRI sequences, with either a homogeneous or a heterogeneous pattern.[19] A high rate of homogeneous hyperintense signals was demonstrated on fat-suppressed images. Signal intensity changes in infected vertebrae were recorded as diffuse, partial, or fan-shaped or an inflammatory reaction line from the end plate. The first 3 signal change modes were reported in our former research.[17] An inflammatory reaction line in the endplate indicates a hyperintense edema line in the endplate, which can be clearly observed on T2/fat-suppressed MRI. Among these signal modes, the “diffuse” and “inflammatory reaction line in the endplate” can be observed more frequently in PS than in BS. However, “fan-shaped” and “partial” signal changes are more often seen in BS, amounted to 50% of cases. The apex of the “fan-shaped” signal mode in BS frequently lies at the superior or inferior of the anterior or posterior edge of vertebral endplate, indicating that the infection starts from this point (Figs. 1A and C and 2A and B). Sharif and Harman found that the first involved point in BS is often located at the anterior edge of the superior endplate because of its rich blood supply, and we further found that the starting point could also begin from the posterior of the endplate.[12,17,20] Unlike in BS, many researchers have reported in their studies that PS always commences at the superior or inferior anterior vertebral body corner adjacent to the discovertebral junction, consistent with our research.[3,19,21–23] Perhaps we can conclude that PS has an acute onset and progresses quickly. When PS patients visit doctors, the infection almost always involves the whole vertebra. The “fan-shaped” and “partial” signal modes result from chronic clinical progression. However, due to the acute progression in PS, we cannot capture these 2 signal modes. Regarding the “inflammatory reaction line in the endplate,” our study found that it is a common imaging feature in pyogenic spondylitis, in accordance with other studies.[24] Hong et al[21] reported that the “inflammatory reaction line in the endplate” and endplate destruction can be found frequently in fungal spondylitis on MRI, which can make it difficult to differentiate it from PS. However, the absence on T2WI of disk hyperintensity and the preservation of the intranuclear cleft in fungus spondylitis may be helpful.[21]
Severe vertebral destruction is uncommon in both groups.[25] By comparing TS (tubercular spondylitis) and PS on MRI, Galhotra et al[19] found that the pyogenic group had relatively mild vertebral destruction, and 20% of cases had loss of cortical definition. Tali et al[5] reported that vertebral collapse is rare in BS. Esendagli-Yilmaz and Ulugolu[26] explained that, in contrast with pyogenic bacteria, brucella does not involve proteinase activity to destroy the disc and vertebra, and they further demonstrated that, in BS, osteoblastic activity is induced, which may partly explain the less prominent bone and disc destruction than in PS. Although vertebral destruction mainly concentrates on the area of the endplate in BS and PS, the differences are still significant.[17,18] In PS, erosion of the endplate often demonstrates extensive destruction. Bone destruction is more serious in PS than in BS. This extensive destruction in PS may result from rapid involvement of the endplate (inflammatory reaction line in the endplate). As the disease progresses in PS, the vertebrae are destroyed increasingly seriously, and the involved intervertebral space becomes increasingly wide, finally becoming a “ballooning change in the intervertebral space” (Figs. 5A–D and 7A–E). However, due to the timely, the effective use of antibiotic treatment, immune enhancement of the host or lower toxicity of pathogenic bacteria, the “ballooning change in the intervertebral space” is rare. Focal destruction is often seen in BS, reported as “mimicking Schmorl's nodules” by Oztekin et al[27] (Fig. 4B and C).[5,28] These 2 different vertebral destruction modes have differential values between PS and BS.
Figure 7.
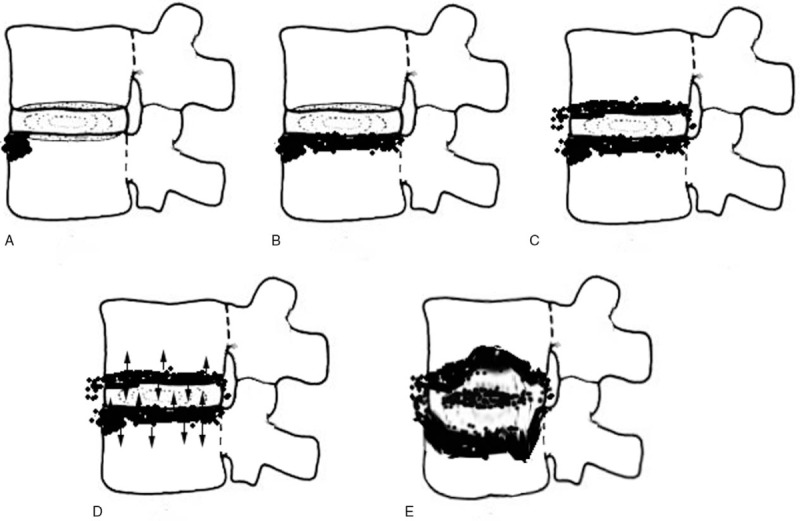
A simulated image of the infection course in PS. (A) The infection usually started from the front edge of the endplate. (B) The endplate was infected in line. (C) The next endplate was infected. (D) Both involved endplates further infected the vertebrae and disc, respectively (arrow). (E) With further destruction, the intravertebral space expands toward a “ballooning change.”
In accordance with previous studies, both PS and BS can involve the disc. Ledermann and Galhotra reported that spondylodiscitis was observed in 89% and 61% of patients with PS, respectively, and G A, A T, and Namiduru showed involved discs in BS.[14,18,19,25] However, it is known from the comparison that significant differences still exist in this abnormal signal. In BS, 29% of the involved discs were recorded as showing the disc invasion sign, indicating a hyperintense signal on sagittal T2-weighted or FS MRI beginning from the initial infection point (frequently the frontal edge of the endplate) and then invading the disc (Fig. 1B and C). In agreement with Tali et al's[5] study, intervertebral discs were affected only after the vertebral bodies in brucellar spondylodiscitis. Our study further showed that disc infection due to BS could be out of accordance with the adjacent involved vertebrae. A vertebra may be infected for a long time; however, a normal disc may exist nearby, and in PS, the disc is usually infected quickly. The disc invasion sign, an inflammatory reaction line in the disc and focal destruction in the endplate linked to the former provided us with the knowledge that the disc with BS was usually destroyed from the inside to the outside and then involved the endplate (Fig. 8A–C). However, in PS, combining the “inflammatory reaction line from the end plate” and “extensive end plate destruction” signs, we can conclude that the disc invasion not only starts from the anterior edge of the endplate, but it also starts from the infected endplate (involved from the outside to the inside) (Fig. 7A–E),[24] which is perhaps one reason why the abnormal signals from infected discs in PS present diffusely. Furthermore, the disc invasion sign and inflammatory reaction line in the disc sign may also exist, but they are difficult to capture because of the acute progression of PS. A total of 61% of involved intervertebral spaces showed severe destruction in PS; however, only 29% of such spaces were found in BS, similar to Ozaksoy et al.[29,30] These results also demonstrated that pyogenic spondylitis is more acute and destructive than brucellar spondylitis.
Figure 8.
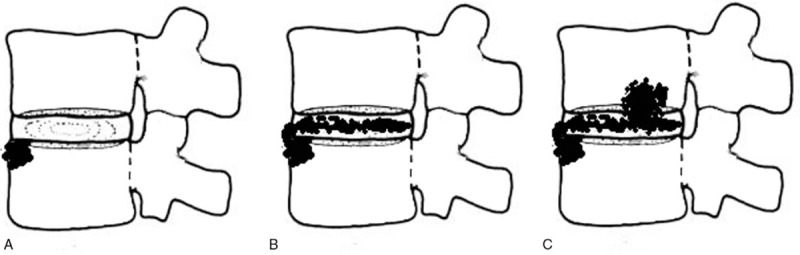
A simulated image of the infection course between the disc and vertebrae in BS. (A) The infection usually started from the front edge of the endplate. The shaded area shows the endplate edema. (B) The infection was involved into the disc (shadow on disc). (C) The infected disc further involves the endplate of next vertebra and causes focal destruction from the endplate.
Paraspinal abscess is a common feature that is hypointense on T1 and hyperintense on T2 in both PS and BS.[20,24,31] There is no significant difference between PS and BS regarding abscess spread. Small abscesses are frequent, in accordance with Tali et al.[5] Around 31% of cases in PS and 47% in BS showed epidural abscesses, often with or without cord compression.[19,24] Our study found that paraspinal abscess had little value in the differential diagnosis between PS and BS on MRI.
This study had several limitations. First, the sample of 32 patients with PS was limited relative to different types of bacteria. A large sample including many types of bacteria causing PS would have rendered this study more persuasive. Second, chronic bacteria may demonstrate some similar features on MRI to those of BS, such as partial, fan-shaped signals from infected vertebrae, focal destruction of the endplate, and the disc invasion sign. Third, in PS, a typical inflammatory reaction line from the end plate can be found, but the reason remains unclear. Last but not least, lack of contrast enhancement on MRI leaves us ignorant about morphology of the abscess wall, homogeneity of signal changes from infected vertebrae, and the enhancement of involved discs, which may have marked value in differentiation between the 2 groups. Furthermore, diffusion-weighted imaging (DWI) has been widely used in recent years, which is used to describe the random motion (or Brownian motion) of water molecules and DWI measures the mobility of water protons in tissues. The application of DWI to the spine has been proved to be a reliable method for the differential diagnosis of benign edema and malignant tumor infiltration of bone marrow, and especially for the differentiation of benign osteoporotic and tumorous vertebral compression fractures.[32,33] Razek and Ashmalla[34] had reported DWI play a valuable rule in differentiating benign from malignant paraspinal neurogenic tumors. O. Oztekin et al[35] had given a conclusion that DWI reveals hyper intensity in the affected vertebrae and paravertebral infectious soft tissue in acute spondylodiscitis, whereas in the chronic stage, it reveals hypointensity. In PS, which usually present as acute infection, the Brownian motion of water may be different from that of BS, which relatively present as chronic infection. So the DWI may have the potential for differentiating PS from BS.
5. Conclusions
In conclusion, due to different progression speeds in PS and BS, many distinguishing features can be found on MRI. We can make a diagnosis respectively based on the following conditions. Brucellar spondylitis: mild destruction of disc; inflammatory reaction line from the disc; focal end plate destruction and the disc invasion sign, partial and fan-shaped hyperintense signals on middle sagittal fat-suppressed weighted images from infected vertebrae. Pyogenic spondylitis: severe destruction of disc; inflammatory reaction line from the end plate; extensive end plate destruction; diffuse signal changes on middle sagittal fat-suppressed weighted images from infected vertebrae and the mode of spread between the disc and endplate.
Author contributions
Conceptualization: Tao Li, Jianmin Sun.
Data curation: Tao Li.
Formal analysis: Wei Li.
Funding acquisition: Yong Du.
Investigation: Tao Li, Meng Gao, Jianmin Sun.
Methodology: Tao Li, Zhensong Jiang, Jianmin Sun.
Resources: Yong Du, Xingang Cui, Jianmin Sun.
Software: Guodong Wang.
Supervision: Tao Li, Jianmin Sun.
Visualization: Tao Li.
Writing – original draft: Tao Li, Xiaoyang Liu.
Writing – review & editing: Tao Li, Yong Du, Haomin Cui.
Footnotes
Abbreviations: ADC = apparent diffusion coefficient, BS = brucellar spondylitis, MRI = magnetic resonance imaging, PS = pyogenic spondylitis.
Disclosure: This article did not receive any funding from NIH or other agencies.
All the authors have given consent to the author sequence.
The device is FDA-approved for this indication.
The authors have no conflicts of interest to disclose.
References
- [1].Baleriaux DL, Neugroschl C. Spinal and spinal cord infection. Eur Radiol 2004;14(suppl 3):E72–83. [DOI] [PubMed] [Google Scholar]
- [2].Tali ET. Spinal infections. Eur J Radiol 2004;50:120–33. [DOI] [PubMed] [Google Scholar]
- [3].Mukherji SK. Spinal infections. Neuroimaging Clin N Am 2015;25:xiii. [DOI] [PubMed] [Google Scholar]
- [4].Gold M. Magnetic resonance imaging of spinal emergencies. Top Magn Reson Imaging 2015;24:325–30. [DOI] [PubMed] [Google Scholar]
- [5].Tali ET, Koc AM, Oner AY. Spinal brucellosis. Neuroimag Clin N Am 2015;25:233–45. [DOI] [PubMed] [Google Scholar]
- [6].Zhong Z, Yu S, Wang X, et al. Human brucellosis in the people's republic of China during 2005–2010. Int J Infect Dis 2013;17:e289–92. [DOI] [PubMed] [Google Scholar]
- [7].Franco MP, Mulder M, Gilman RH, et al. Human brucellosis. Lancet Infect Dis 2007;7:775–86. [DOI] [PubMed] [Google Scholar]
- [8].Hasanjani RM, Ebrahimpour S. Human brucellosis: an overview. Caspian J Intern Med 2015;6:46–7. [PMC free article] [PubMed] [Google Scholar]
- [9].Akpinar O. Historical perspective of brucellosis: a microbiological and epidemiological overview. Infez Med 2016;24:77–86. [PubMed] [Google Scholar]
- [10].Koubaa M, Maaloul I, Marrakchi C, et al. Spinal brucellosis in South of Tunisia: review of 32 cases. Spine J 2014;14:1538–44. [DOI] [PubMed] [Google Scholar]
- [11].Erdem H, Senbayrak S, Gencer S, et al. Tuberculous and brucellosis meningitis differential diagnosis. Travel Med Infect Dis 2015;13:185–91. [DOI] [PubMed] [Google Scholar]
- [12].Sharif HS, Aideyan OA, Clark DC, et al. Brucellar and tuberculous spondylitis: comparative imaging features. Radiology 1989;171:419–25. [DOI] [PubMed] [Google Scholar]
- [13].Zileli M, Ebeoglu A. Brucellar spondylodiscitis. ArgoSpine News J 2011;23:99–104. [Google Scholar]
- [14].Aydin G, Tosun A, Keles I, et al. Brucellar spondylodiscitis: a case report. Int J Clin Pract 2006;60:1502–5. [DOI] [PubMed] [Google Scholar]
- [15].Kokoglu OF, Hosoglu S, Geyik MF, et al. Clinical and laboratory features of brucellosis in two university hospitals in Southeast Turkey. Trop Doct 2016;36:49–51. [DOI] [PubMed] [Google Scholar]
- [16].Mantur BG, Mangalgi SS. Evaluation of conventional castaneda and lysis centrifugation blood culture techniques for diagnosis of human brucellosis. J Clin Microbiol 2004;42:4327–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Gao M, Sun J, Jiang Z, et al. Comparison of tuberculous and brucellar spondylitis on magnetic resonance images. Spine 2017;42:113–21. [DOI] [PubMed] [Google Scholar]
- [18].Ledermann HP, Schweitzer ME, Morrison WB, et al. MR imaging findings in spinal infections: rules or myths? Radiology 2003;228:506–14. [DOI] [PubMed] [Google Scholar]
- [19].Galhotra RD, Jain T, Sandhu P, et al. Utility of magnetic resonance imaging in the differential diagnosis of tubercular and pyogenic spondylodiscitis. J Nat Sci Biol Med 2015;6:388–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Harman M, Unal O, Onbasi KT, et al. Brucellar spondylodiscitis: MRI diagnosis. Clin Imaging 2001;25:421–7. [DOI] [PubMed] [Google Scholar]
- [21].Hong SH, Choi JY, Lee JW, et al. MR imaging assessment of the spine: infection or an imitation? Radiographics 2009;29:599–612. [DOI] [PubMed] [Google Scholar]
- [22].Marquez SP. Spondylodiscitis. Radiologia 2016;58(suppl 1):50–9. [DOI] [PubMed] [Google Scholar]
- [23].Marquez SP. Imaging of spondylodiscitis. Radiologia 2016;58(suppl 1):50–9. [DOI] [PubMed] [Google Scholar]
- [24].Tyler KL. Acute pyogenic diskitis (spondylodiskitis) in adults. Rev Neurol Dis 2008;5:8–13. [PubMed] [Google Scholar]
- [25].Namiduru M, Karaoglan I, Gursoy S, et al. Brucellosis of the spine: evaluation of the clinical, laboratory, and radiological findings of 14 patients. Rheumatol Int 2004;24:125–9. [DOI] [PubMed] [Google Scholar]
- [26].Esendagli-Yilmaz G, Uluoglu O. Pathologic basis of pyogenic, nonpyogenic, and other spondylitis and discitis. Neuroimaging Clin N Am 2015;25:159–61. [DOI] [PubMed] [Google Scholar]
- [27].Oztekin O, Calli C, Adibelli Z, et al. Brucellar spondylodiscitis: magnetic resonance imaging features with conventional sequences and diffusion-weighted imaging. Radiol Med 2010;115:794–803. [DOI] [PubMed] [Google Scholar]
- [28].Kilic T, Ozer AF, Ozgen S, et al. Brucellar spondylitis mimicking lumbar disc herniation. Case report. Paraplegia 1995;33:167–9. [DOI] [PubMed] [Google Scholar]
- [29].Ozaksoy D, Yucesoy K, Yucesoy M, et al. Brucellar spondylitis: MRI findings. Eur Spine J 2001;10:529–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Tali ET. Spinal infections. Neuroimaging Clin N Am 2015;25: xv. [DOI] [PubMed] [Google Scholar]
- [31].Aizawa T, Ozawa H, Koakutsu T, et al. Atypical findings on magnetic resonance imaging in the patients with active pyogenic spondylitis in Japanese university hospitals. Tohoku J Exp Med 2013;231:13–9. [DOI] [PubMed] [Google Scholar]
- [32].Buyn WM. Diffusion-weighted MRI of vertebral bone marrow: differentiation of degenerative spines and spondylitis involving bone marrow adjacent to end plates. Proc Int Soc Magn Reson Med 2001;9:1626. [Google Scholar]
- [33].Raya JG, Dietrich O, Reiser MF, et al. Methods and applications of diffusion imaging of vertebral bone marrow. J Magn Reson Imaging 2006;24:1207–20. [DOI] [PubMed] [Google Scholar]
- [34].Razek AAKA, Ashmalla GA. Assessment of paraspinal neurogenic tumors with diffusion-weighted MR imaging. Eur Spine J 2018;27:841–6. [DOI] [PubMed] [Google Scholar]
- [35].Oztekin O, Calli C, Adibelli Z, et al. Brucellar spondylodiscitis: magnetic resonance imaging features with conventional sequences and diffusion-weighted imaging. La Radiol Med 2010;115:794–803. [DOI] [PubMed] [Google Scholar]


