Abstract
Introduction
Apraxia is common in neurodegenerative dementias but underrepresented in clinical workup for differential diagnoses.
Methods
Praxis-profiles were assessed with the Dementia Apraxia Test in 93 patients with early stages of biologically supported Alzheimer's disease or frontotemporal lobar degeneration: semantic primary-progressive aphasia, nonfluent primary-progressive aphasia, and behavioral variant frontotemporal dementia. Associations with core cognitive deficits of the dementia subtypes (i.e., visuospatial, sociocognitive, and semantic-linguistic) were explored.
Results
Patients showed significant apraxia compared with healthy controls but also disease-specific praxis-profiles. Using only the Dementia Apraxia Test, all four dementia subtypes could be correctly discriminated in 64.4% of cases, and in 78.2% when only distinguishing Alzheimer's disease versus frontotemporal lobar degeneration. Praxis-profiles showed consistent associations with core cognitive impairments of the different dementia subtypes.
Discussion
The Dementia Apraxia Test is a valid, time-efficient and versatile cognitive marker to delineate variants of frontotemporal lobar degeneration and Alzheimer's disease in clinical routine, facilitating differential diagnoses of dementia subtypes in early disease stages.
Keywords: Apraxia, Primary-progressive aphasia, Frontotemporal dementia, Semantic dementia, Frontotemporal lobar degeneration, Alzheimer's disease, Differential diagnosis, neuropsychology
Highlights
-
•
AD and FTLD variants share common deficits in standard cognitive domains.
-
•
Apraxia is common in these diseases but rarely used as a clinical marker.
-
•
Praxis was assessed in 93 patients with AD and clinical FTLD variants.
-
•
Specific praxis-profiles emerged for AD, bvFTD, svPPA and nfPPA.
-
•
AD and FTLD variants may be clinically differentiated by praxis-profiles.
1. Background
Apraxia relates to a neuropsychological deficit regarding imitation or pantomiming of limb or face postures despite intact sensorimotor skills and task comprehension [1], [2]. Impairments in praxis may occur early in a range of neurocognitive disorders and can be used as a cognitive marker for early neurodegenerative dementias [3], [4], [5]. Despite its recent inclusion as a basic cognitive domain in the Diagnostic and Statistical Manual of Mental Disorders (5th Edition), praxis assessment is underrepresented in both routine neuropsychological workup and current diagnostic criteria for neurocognitive disorders [6], [7].
Disease progression, rate of functional decline, caregiver burden, and therapeutic approaches differ profoundly between neurodegenerative dementia subtypes [8], [9]. A correct differential diagnosis in early disease stages is thus essential for patients, caregivers, and health practitioners.
In the absence of ready-to-use biomarkers for frontotemporal lobar degeneration (FTLD), clinical criteria for the behavioral variant frontotemporal dementia (bvFTD) and the two language variants, nonfluent primary progressive aphasia (nfPPA) and semantic variant primary progressive aphasia (svPPA), have been consecutively refined, improving their diagnostic accuracy [10], [11]. Nevertheless, neither unambiguous operationalizations of diagnostic core features (e.g., “loss of empathy” for bvFTD; “semantic memory dysfunction” for svPPA) nor straightforward and brief clinical tests with sufficient differential value to discriminate between these dementia subtypes simultaneously are available. The correct application of the clinical criteria and a clinical differentiation of the FTLD variants from each other and from Alzheimer's disease (AD) thus remains challenging, particularly in nonexpert settings and for patients in early disease stages [12], [13], [14].
Although standardized neuropsychological testing is recommended to differentiate between the different dementia types in early disease stages, a range of former neuropsychological principles have recently been questioned or shown to be invalid for a reliable differentiation between FTLD and early stage AD: Impairments in verbal memory tests may occur to similar degrees in patients with bvFTD and AD either due to confounding executive influences or due to AD-like hippocampal atrophy in subsamples of patients with bvFTD [15], [16]. Similarly, cognitive domains that place high demands on language (including verbal memory tests) are frequently confounded with deficits in task comprehension and/or semantic memory in patient with PPA [17], [18]. Taken together, a considerable overlap regarding performance in standard neuropsychological domains exists between patients with different underlying neurodegenerative etiologies, particularly when only time-efficient screening tests are available [9], [19], [20].
Standardized assessment of praxis-profiles is time efficient, reliable, and places comparatively little demands on potentially confounding cognitive influences such as working memory or language comprehension. It may thus serve as a versatile neuropsychological tool to differentiate clinically heterogeneous dementia subtypes in early disease stages. However, although mentioned as a basic cognitive domain, apraxia is largely neglected in clinical or neuropsychological routine examinations. Here, we explored patterns of praxis disturbances and tested the clinical feasibility of a single praxis screening for the differential diagnosis between early stages of AD and the three most frequent clinical variants of FTLD (bvFTD, svPPA, and nfPPA). We hypothesized that specific praxis-profiles (operationalized by divergent performance in different praxis domains) are associated with core cognitive deficits of the different dementia syndromes (i.e., visuospatial deficits in AD, linguistic-semantic deficits in PPA, and social cognitive impairment in bvFTD).
2. Methods
2.1. Participants
A total of 93 patients with early (<3 years after the first symptom-onset) neurodegenerative diseases were enrolled in the memory disorder unit at the Department of Neurology at the University Hospital Münster, Germany. The initial diagnostic workup was conducted at our inpatient clinic and included neurological examination, history taking with patients and caregivers, consultation of medical records, comprehensive neuropsychological testing as presented in detail elsewhere [19], motor and speech assessment, and analysis of cerebrospinal fluid (CSF) for dementia biomarkers. Structural T1- and fluid-attenuated inversion recovery–magnetic resonance imaging images of the brain were available from all patients. In addition, 18-Fluorodeoxyglucose Positron Emission Tomography scans were available in 70% (46/66) of patients with suspected FTLD. All recruited patients matched the current criteria for probable AD, probable bvFTD, or imaging-supported PPA evaluated by a multidisciplinary team of senior neurologists and neuropsychologists [10], [11], [21]. Briefly, patients with probable AD (N = 27) presented with memory decline objectified in episodic memory tests and had high or at least intermediate evidence for the pathophysiological process of AD based on neuroimaging and biomarker constellation [21]. Patients with probable bvFTD (N = 31) presented with symptom constellations of social conduct decline, apathy, loss of empathy, and/or executive dysfunction in neuropsychological assessment as well as a consistent frontal and/or anterior temporal atrophy or hypometabolism [10]. Patients with svPPA (N = 21) showed prominent naming and fluency deficits but circumlocutory speech as initial symptoms, whereas patients with nfPPA (N = 14) initially presented with effortful, halting speech either with or without agrammatism. All patients with PPA showed signs of either brain atrophy or hypometabolism consistent with clinical diagnosis (imaging supported). Patients with mixed PPA or logopenic variant were not included due to their high clinical and pathologic heterogeneity [22]. Dementia biomarker constellation was not indicative of AD in patients with PPA or bvFTD diagnosis. Exclusionary criteria for all patients were as follows: history of stroke, brain tumor, traumatic brain injury, major psychiatric disorders, severe vascular lesion load (Fazekas score ≥2), and inflammatory CSF or motor impairment (including parkinsonism).
Healthy age-matched control subjects (HC; N = 34) were relatives of patients, members of the hospital staff, or community-dwelling elderly screened for neurological and psychiatric disorders. HCs with a Mini–Mental State Examination (MMSE [23]) < 28 were excluded. The study was approved by the local ethics committee (2012-365-f-S), and participants gave written informed consent.
2.2. Praxis assessment
We used the Dementia Apraxia Test (DATE) as a screening for praxis impairment as previously described [24]. The test was recently recommended by the European Neurodegenerative Diseases Working Group, and it provides good psychometric properties, standardized stimulus material, and instructions as well as a reliable clinical rating system [6]. It provides a total apraxia score (DATE total) composed of five subscales (two subscales for limb apraxia and three for buccofacial apraxia): for limb apraxia (DATE 1), the subscales imitation of meaningless hand postures (limb imitation) and pantomiming of common objects (object pantomime) are tested. For buccofacial apraxia (DATE 2), the test assesses imitation of face postures (face imitation), emblematic buccofacial postures (e.g., “show me how you clear your throat”; buccofacial emblems), and repetition of pseudowords to test for apraxic speech. Each subscale of the DATE can be described with regard to two dimensions: demands on stimulus-specific semantic knowledge (e.g., knowledge about a common tool vs. novel stimuli) and the involved body part (face vs. limbs). For example, whereas imitation of meaningless hand postures and repetition of pseudowords represent novel stimuli and place relatively little demands on semantic memory, pantomiming of common objects or emblematic buccofacial postures crucially depend on semantic knowledge about the respective stimuli and/or gesture.
2.3. Neuropsychological background testing
To evaluate the overall cognitive status and frontal-executive impairment, we used the MMSE and the Frontal Assessment Battery, respectively [25]. To screen for specific neuropsychological dysfunctions, we used the Language Aphasia Screening Test [26] for semantic-linguistic abilities, the subtest “number location” from the Visual Object and Space Perception Battery [27] for visuospatial impairment, and the Emotion Recognition subtest of the Mini-Social Emotional Assessment [28] to explore sociocognitive deficits. Moreover, a standardized caregiver questionnaire for abnormal behavior, also tapping into social cognition, the Frontal Behavioral Inventory [29] was employed. All test procedures were administered and scored by neuropsychologists in accordance with the respective manual.
2.4. Statistics
Statistical analyses were performed using SPSS, version 25 (IBM). Group differences in demographic data, disease severity scores, and neuropsychological background tests were analyzed using analysis of variances. Group differences in praxis-profiles (DATE) were explored using a multivariate analysis of variance. Normality of distribution was confirmed by visual inspection and Kolmogorov-Smirnov tests. Multivariate discriminant function analysis was performed to determine how well dementia subtypes can be distinguished based on the DATE. Before this, the variance-covariance matrix was checked for strong inhomogeneity. To explore associations between neuropsychological background tests (see Section 2.3) and praxis performance, we computed Kendalls-Tau-b rank correlations for ordinal data using the whole patient sample.
3. Results
3.1. Demographic data, disease severity scores, and neuropsychological background tests
Table 1 summarizes demographics, disease severity scores, and clinical characteristics of the patients and HC. Significant between-group differences were found for age (F = 2.705, df = 4, P = .03) and sex (χ2 = 23.306, df = 4, P < .000). Patients with AD were significantly older than patients with bvFTD, and there was a significantly higher proportion of males among bvFTD compared with the other groups, reflecting typical demographic differences between AD and bvFTD. Groups did not differ regarding years of education or disease duration. Comparison of CSF biomarker profile revealed lower total amyloid-β and CSF levels in the AD group compared with all other dementia groups. Total tau was significantly higher in the AD group than bvFTD. Performance in MMSE and Frontal Assessment Battery was significantly lower in all dementia subtypes compared with HC. Between dementia subtypes, MMSE scores were significantly lower in svPPA versus bvFTD patients, and scores in the Frontal Assessment Battery were lower in AD versus nfPPA. The average Frontal Behavioral Inventory score was significantly higher in patients with bvFTD than in patients with AD and nfPPA, whereas no difference between svPPA and bvFTD was seen. As expected, both PPA groups had significantly lower scores than all other dementia patients in the Language Aphasia Screening Test, but no differences emerged between nfPPA and svPPA. Patients with AD reached significantly better results than all other dementia subtypes in the Mini-Social Emotional Assessment emotion recognition score. For the Visual Object and Space Perception Battery “number location” subtest, no significant group differences were found. Taken together, although significant differences between specific groups on specific neuropsychological background tests were found, no single screening test was able to reliably differentiate between all four dementia subtypes.
Table 1.
Demographic and clinical data of the sample
| HC (n = 34) | AD (n = 27) | bvFTD (n = 31) | svPPA (n = 21) | nfPPA (n = 14) | Group differences∗ | |
|---|---|---|---|---|---|---|
| Demographics | ||||||
| Sex, male:female | 15:19 | 11:16 | 29:2 | 12:9 | 6:8 | bvFTD > (AD, svPPA, nfPPA, HC)† |
| Age, years | 68 ± 6 | 71 ± 10 | 64 ± 8 | 67 ± 9 | 68 ± 11 | bvFTD < AD |
| Education, years | 12 ± 1 | 11 ± 1 | 11 ± 2 | 12 ± 2 | 11 ± 1 | ND |
| Disease severity scores | ||||||
| Disease duration, months | NA | 25 ± 24 | 28 ± 18 | 34 ± 23 | 24 ± 10 | ND |
| CSF Aβ, pg/mL | NA | 418 ± 157 | 929 ± 465 | 855 ± 422 | 1066 ± 394 | AD < (bvFTD, svPPA, nfPPA) |
| CSF total tau, pg/mL | NA | 732 ± 266 | 360 ± 190 | 567 ± 304 | 482 ± 394 | AD > bvFTD |
| MMSE, max. 30 | 29 ± 1 | 23 ± 3 | 25 ± 4 | 22 ± 5 | 24 ± 5 | HC > (bvFTD, AD, nfPPA, svPPA), bvFTD > svPPA |
| FAB-D, max. 18 | 17 ± 1 | 13 ± 2 | 12 ± 4 | 11 ± 3 | 10 ± 4 | HC > (bvFTD, AD, nfPPA, svPPA) AD > nfPPA |
| Neuropsychological background tests | ||||||
| Aphasia screening LAST, max. 15 | 15 ± 0 | 14 ± 1 | 15 ± 1 | 12 ± 2 | 12 ± 2 | HC > (AD, nfPPA, svPPA) (AD, bvfTD) > (svPPA, nfPPA) |
| Visuospatial screening VOSP 7, max. 10 | 9 ± 1 | 8 ± 2 | 9 ± 1 | 8 ± 3 | 7 ± 2 | ND |
| Mini-SEA-Emotion Recognition, max. 15 | 11.9 ± 1.1 | 11.2 ± 2 | 9.3 ± 2.6 | 8.8 ± 2.5 | 8.5 ± 2.3 | HC > (bvFTD, svPPA, nfPPA) AD > (bvFTD, svPPA, nfPPA) |
| Frontal Behavioral Inventory, max. 72 | NA | 9 ± 7 | 27 ± 10 | 18 ± 14 | 13 ± 8 | (AD, nfPPA) < bvFTD |
Abbreviations: HC, healthy controls; AD, Alzheimer's disease; bvFTD, behavioral variant of frontotemporal dementia; svPPA, semantic variant of primary-progressive aphasia; nfPPA, nonfluent primary-progressive aphasia; CSF, cerebrospinal fluid; Aβ, amyloid β; tau, tau protein; MMSE, Mini–Mental State Examination; ND, no differences; NA, not available; FAB-D, Frontal Assessment Battery; LAST, Language Aphasia Screening Test; VOSP, Visual Object and Space Perception Battery; Mini-SEA, Mini Social Cognition and Emotional Assessment.
NOTE. Results are expressed as mean ± SD.
Significant differences (P < .05) between groups by statistical tests (i.e., analysis of variance if not otherwise specified) in post hoc comparisons (Games-Howell Tests).
χ2(4) = 23.306, P < .05.
3.2. DATE praxis-profiles of dementia subtypes
Table 2 summarizes results of the DATE for dementia subtypes and HC. DATE total score was significantly lower in all dementia subgroups compared with HC. The scale DATE 1–limb apraxia differed significantly between HC and patients with AD or PPA diagnosis. On the scale DATE 2–face apraxia, all dementia subtypes performed significantly lower compared with HC and patients with bvFTD and nfPPA performed lower than patients with AD.
Table 2.
Results of the DATE–apraxia assessment
| HC (n = 34) | AD (n = 27) | bvFTD (n = 31) | svPPA (n = 21) | nfPPA (n = 14) | Group differences∗ | |
|---|---|---|---|---|---|---|
| DATE total score, max. 15 | 13.06 ± 1.07 | 10.28 ± 2.22 | 9.86 ± 2.65 | 8.94 ± 2.41 | 6.59 ± 3.44 | HC > (AD, bvFTD, svPPA, nfPPA) (AD, bvFTD) > nfPPA |
| Scales | ||||||
| DATE 1 limb apraxia, max. 6 | 4.61 ± 0.81 | 3.12 ± 1.09 | 4.15 ± 1.1 | 3.08 ± 1.5 | 2.82 ± 1.64 | (HC, bvFTD) > (AD, svPPA, nfPPA) |
| DATE 2 face apraxia, max. 9 | 8.44 ± 0.83 | 7.51 ± 1.48 | 5.7 ± 1.97 | 5.86 ± 1.51 | 3.76 ± 2.19 | HC > (AD, bvFTD, svPPA, nfPPA) AD > (bvFTD, svPPA, nfPPA), svPPA > nfPPA |
| Subscales | ||||||
| Limb imitation, max. 3 | 2.13 ± 0.36 | 1.19 ± 0.59 | 1.65 ± 0.61 | 1.41 ± 0.72 | 1.21 ± 0.73 | HC > (AD, bvFTD, svPPA, nfPPA) AD < bvFTD |
| Object pantomime, max. 3 | 2.49 ± 0.68 | 1.88 ± 0.92 | 2.52 ± 0.72 | 1.69 ± 1.07 | 1.46 ± 1.22 | (HC, bvFTD) > svPPA |
| Buccofacial emblems, max. 3 | 2.82 ± 0.49 | 2.38 ± 0.96 | 1.98 ± 1.06 | 1.45 ± 1.12 | 1.18 ± 1.20 | HC > (bvFTD, svPPA, nfPPA) AD > (svPPA, nfPPA) |
| Pseudowords, max. 3 | 2.81 ± 0.52 | 2.44 ± 0.86 | 2.02 ± 0.91 | 2.33 ± 0.83 | 0.88 ± 0.96 | HC > (bvFTD, nfPPA) (AD, bvFTD, svPPA) > nfPPA |
| Face imitation, max. 3 | 2.81 ± 0.28 | 2.23 ± 0.63 | 1.77 ± 0.76 | 2.14 ± 0.67 | 1.6 ± 0.74 | HC > (AD, bvFTD, svPPA, nfPPA) |
Abbreviations: HC, healthy controls; AD, Alzheimer's disease; bvFTD, behavioral variant of frontotemporal dementia; svPPA, semantic variant of primary-progressive aphasia; nfPPA, nonfluent primary-progressive aphasia; DATE, Dementia Apraxia Test; ANOVA, analysis of variance.
NOTE. Results are expressed as mean ± SD.
Significant differences (P < .05) between groups by ANOVAs and post hoc comparisons (Games-Howell Tests).
Fig. 1 indicates DATE subscale performance for each group. Significant differences between dementia types were found on all five subscales. Patients with AD showed significantly lower performance in limb imitation than patients with bvFTD. On the subscale object pantomime, results differed significantly between patients with svPPA and bvFTD. Patients with nfPPA were most impaired in face imitation, and bvFTD also showed a statistical trend toward lower performance in face imitation compared with AD patients. On the subscale buccofacial emblems, patients with svPPA and nfPPA scored significantly lower compared with AD. Patients with nfPPA were significantly more impaired on the subscale pseudowords compared with all other dementia subtypes. Taken together, we found group-specific praxis-profiles showing major deficits in limb imitation for AD, major deficits in buccofacial praxis domains for both bvFTD and nfPPA, major impairment in subscales demanding semantic memory for svPPA, and a differential deficit for nfPPA on the subscale measuring apraxic speech. To test how well these between-group differences in the different subscales (i.e., praxis-profiles) could discriminate between groups, we next used a discriminant function analysis on the data.
Fig. 1.
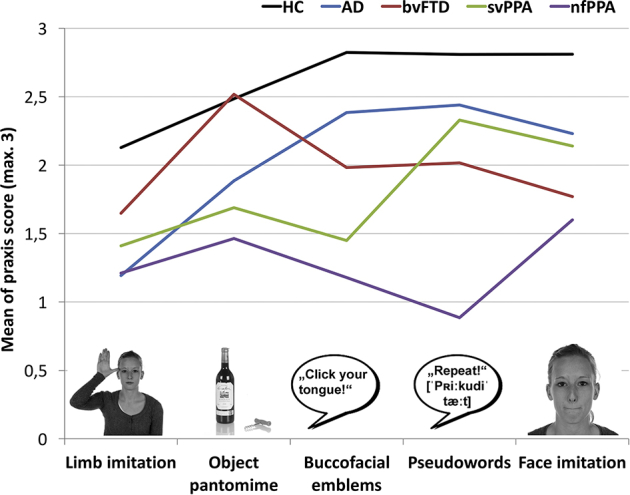
Praxis-profiles of dementia subtypes and healthy controls. X-axis displays praxis subscales of the DATE and a representative item sample for each subscale. Y-axis displays mean of total praxis score (min. 0, max. 3). Profile lines represent mean scores for each diagnostic group in each praxis subdomain. Abbreviations: HC, healthy controls; AD, Alzheimer's disease; bvFTD, behavioral variant of frontotemporal dementia; svPPA, semantic variant of primary progressive aphasia; nfPPA, nonfluent primary progressive aphasia.
3.3. Differentiation of dementia subtypes based on praxis-profiles
Discriminant function analysis, including the dementia subtypes as grouping variable and the five subscales of the DATE as discriminating variables, identified three significant discriminant functions (Fig. 2A). Function 1 (Wilks`λ = 0.443, χ2 = 66.438, P < .000) discriminated between AD versus bvFTD and AD versus nfPPA. The function showed positive correlations with all buccofacial praxis subscales: pseudowords (r = .697), face imitation (r = .493) and buccofacial emblems (r = .355) and negative correlations with limb imitation (r = −.409) and object pantomime (r = −.512), indicating a body part–specific component for limb versus face postures. Function 2 (Wilks`λ = 0.686, χ2 = 30.695, P < .000) discriminated both PPA subtypes from AD and bvFTD. This function correlated positively with object pantomime (r = .546), pseudowords (r = .421), and buccofacial emblems (r = .410). Function 3 (Wilks`λ = 0.875, χ2 = 10.874., P = .012) discriminated between AD and svPPA and more importantly between nfPPA and svPPA. This function showed a positive correlation with buccofacial emblems (r = .686) and a negative correlation with pseudowords (r = −.499). Fig. 2A displays means of the discriminant scores for all three functions grouped by dementia subtype. Regarding the efficiency for differential diagnosis using only the DATE, a total of 64.4% of cases could be correctly classified to the correct dementia subtype. Fig. 2B represents the percentage of classified cases for each dementia subtype to the different groups. Inspection of Fig. 2B shows that some groups were easier to classify than others: In patients with svPPA, a relatively large percentage of cases (20%) was falsely classified as having AD, whereas patients with AD were rarely classified as having FTLD (<15%). In fact, when only FTLD (bvFTD, nfPPA and svPPA merged) versus AD patients were discriminated, the classification accuracy of the DATE raised to 78.2%.
Fig. 2.
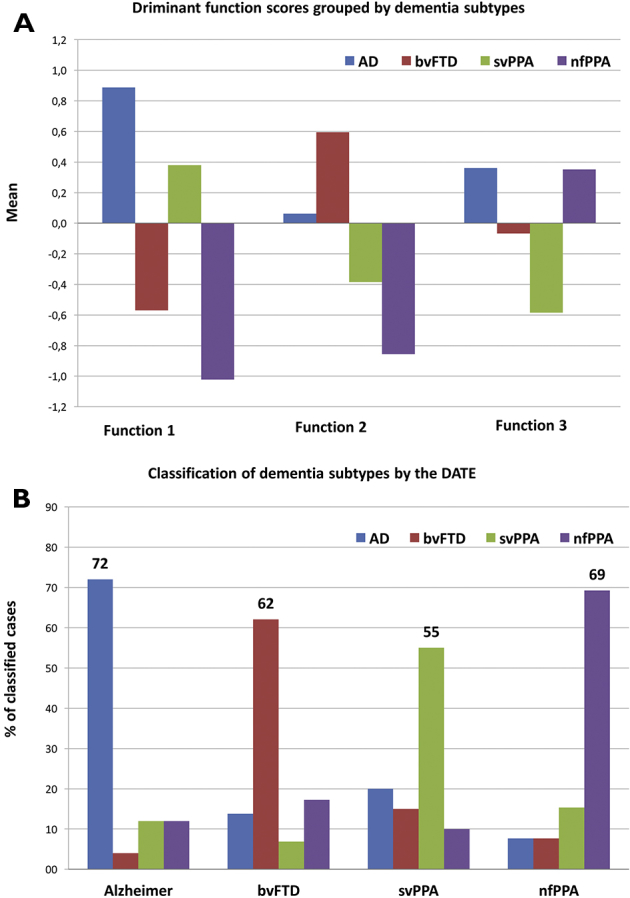
Results of discriminant function analysis of the DATE subdomains. (A) Displays group means of the discriminant functions based on the DATE subdomains. (B) Displays the results of the classification of cases into the different dementia groups using only the DATE discriminant functions as discriminating variables. Abbreviations: HC, healthy controls; AD, Alzheimer's disease; bvFTD, behavioral variant of frontotemporal dementia; svPPA, semantic variant of primary progressive aphasia; nfPPA, nonfluent primary progressive aphasia.
3.4. Associations between DATE subscales and cognitive screening tests
To confirm our hypothesis that praxis performance taps into specific cognitive abilities and that the disease-specific praxis-profiles reflect cognitive core symptoms of the different dementia subtypes, we computed rank correlations between the DATE subscales and neuropsychological background tests across the whole patient sample. Significant correlations were found between the VOSP “number location” subtest performance and subscales measuring limb apraxia: limb imitation (r = .258) and object pantomime (r = .240), indicating associations between visuospatial processing and these subscales. The language screening test LAST showed significant correlations with subscales that are highly dependent on linguistic-semantic skills: object pantomime (r = .277) and buccofacial emblems (r = .390). The Mini-Social Emotional Assessment Emotion Recognition Test significantly correlated with subscales for buccofacial apraxia: face imitation (r = .240) and buccofacial emblems (r = .345). For the Frontal Behavioral Inventory, no significant correlations were found, but there was a trend showing negative correlations with subscales depending on buccofacial abilities (i.e. face imitation, buccofacial emblems, and pseudowords), indicating that more behavioral dysfunction may be associated with less accurate face imitation. Table 3 summarizes results of these correlational analyses.
Table 3.
Associations between the DATE praxis subscales and neuropsychological background tests
| Neuropsychological background test | Limb imitation | Object pantomime | Face imitation | Buccofacial emblems | Pseudowords |
|---|---|---|---|---|---|
| LAST | .177 | .277∗ | .145 | .390∗ | .176 |
| VOSP- number location | .258∗ | .240∗ | .005 | .067 | .120 |
| Mini-SEA- Emotion recognition | .143 | .138 | .240∗ | .345∗ | .211 |
| FBI | .146 | .156 | −.197 | −.087 | −.175 |
Abbreviations: LAST, Language Aphasia Screening Test; FBI, Frontal Behavioral Inventory; VOSP, Visual Object and Space Perception Battery; Mini-SEA, Mini Social Cognition and Emotional Assessment.
NOTE. All values are Kendall-Tau-b rank correlations for ordinal data.
Significant correlation at the P = .0025 level (Bonferroni correction for the number of comparisons).
4. Discussion
Praxis impairments have extensively been studied in stroke, and its assessment in early stages of neurodegenerative diseases has only recently been a subject of systematic research [2], [3], [30]. Previous studies suggested that apraxia is promising as a cognitive marker for AD versus bvFTD [6], [7]. We showed here that different neurodegenerative dementia subtypes display characteristic patterns of praxis impairment in early stages when directly contrasted using a single apraxia screening test. These praxis-profiles were effective differential diagnostic markers and showed close and coherent associations with already established and typical core cognitive deficits of AD, bvFTD, svPPA, and nfPPA.
4.1. The DATE as a versatile tool for clinical dementia subtype classification
Neuropsychological tests are designed to tap into dysfunction of specific neuroanatomical structures (e.g., episodic memory tests primarily quantify medial temporal lobe function) [31]. Clinicians, however, are often confronted with a large overlap between dementia subgroups regarding neuropsychological test performance [19], [20]. In light of diffuse neuronal loss common to neurodegenerative diseases irrespective of the underlying etiology, overlapping cognitive symptoms are the rule rather than the exception in clinical routine. “Profiles” of deficits reflecting foci of relative neuronal loss in certain brain regions (e.g., frontal lobe vs. parietal lobe) or in large-scale functional networks may better account for cognitive and clinical heterogeneity in dementia syndromes than performance in single cognitive domains [32], [33]. A detailed functional assessment of remote neuroanatomical structures is however time-consuming, and some tests are not applicable in certain patient groups (e.g., due to comprehension deficits in aphasic patients). Standardized brief tests that display characteristic patterns in different etiologies are sparse. In line with this, we found that no standard neuropsychological background screening test used here was able to reliably differentiate between all four diagnostic groups and HC simultaneously. In contrast, patient's performance across the DATE subscales varied systematically depending on the diagnosis. While there was overlap between dementia subtypes in single praxis domains, variance between groups extracted by multivariate discriminant function analysis revealed disease-specific patterns. Based on this between-group variance, a correct classification of 64.4% of patients into the correct diagnostic category (according to current clinical guidelines) was achieved, a proportion that is remarkable given that chance-level is at 25% with four diagnostic categories. For the differentiation between all clinical variants of FTLD combined versus AD, an even better diagnostic value of 78.2% correct classifications was reached. We interpret these findings as a proof-of-principle that disease-specific profiles in praxis performances can be effectively used for differential diagnosis across a range of early neurodegenerative diseases. Given that the DATE can be administered in less than 15 minutes, our results argue to include praxis assessment into the diagnostic workup for early neurodegenerative diseases.
4.2. Specific profiles of praxis impairments in each dementia syndrome
Previous studies have shown that praxis can be impaired early in AD and bvFTD with evidence for more severe impairment in limb praxis for AD and in buccofacial praxis for bvFTD [5], [34]. Only two studies have specifically explored apraxic deficits in patients with PPA, showing higher degrees of praxis deficits across all domains in patients with nfPPA compared with other subtypes [4], [35]. Because definitions of apraxia and methods of assessment differed profoundly between studies, results cannot be directly compared, and the relevance of such findings for differential diagnosis is unclear. To our knowledge, this is the first study to directly contrast results in a single standardized test for praxis abilities in clinically well-defined samples of the most frequent young-onset neurodegenerative dementia syndromes, AD, bvFTD, nfPPA, and svPPA.
In line with our previous work on the degree and profile of praxis impairment, patients with AD were impaired in limb imitation and to a lesser degree in object pantomime, whereas performance in buccofacial praxis domains was mostly unimpaired. Current evidence from structural neuroimaging points to a crucial dependence on left and medial parietal lobe integrity for limb imitation and to more ventrally temporal neural substrates for object pantomime [30], [36], [37]. Such results form the basis of the proposed dual-route model for gesture production [38]. Consistent with this, parietal and temporal cortices are early targets of neurodegeneration in AD and have been previously associated with visuospatial processing and object semantics, respectively, thus reflecting core features of cognitive and neural dysfunctioning in AD. In line with this, limb apraxia subscales as measured by the DATE were significantly correlated with performance in the VOSP 7, a cognitive test for visuospatial processing.
In contrast, patients with bvFTD showed major impairments across the buccofacial praxis subscales. Although the neural basis of buccofacial praxis abilities is far less clear, functional neuroimaging studies in healthy participants point to a crucial role of frontal, subcortical, and anterior temporal areas (including the insular) for decoding information from visually presented faces [39]. As both faces and speech convey information relevant for social conduct, deficits in buccofacial praxis may be related to core symptoms of bvFTD such as loss of empathy, thus reflecting core symptoms of this dementia subtype [10]. Although more evidence is needed here, our result of significant correlations between buccofacial praxis subscales and measures for social cognition (i.e., emotion recognition and behavioral abnormalities) support this hypothesis.
We also revealed disease-specific profiles of praxis impairment using the DATE in patients with the two language variants of FTLD. Similar to patients with bvFTD, patients with svPPA showed relatively little impairment in limb imitation and pseudoword repetition, domains that do not place high demands on semantic memory and foreknowledge. Instead, the praxis-profile of svPPA was characterized by pronounced and unique impairment regarding buccofacial emblems (e.g., “show me how you clear your throat”) and to a lesser degree regarding object pantomime. Correlations between these scales and the Language Aphasia Screening Test suggest that these impairments are mediated by deficits regarding stimulus-specific semantic memory. This interpretation also fits well with the neuroanatomical evidence that semantic knowledge is predominantly subserved by areas in the anterior (ventral) temporal lobe, the key target of neurodegeneration in svPPA [40].
Compatible with previous studies, patients with nfPPA showed the most pronounced praxis impairment from all dementia groups across all subscales but with a unique feature of apraxia of speech (pseudoword repetition) [4], [35]. Clinically, nfPPA is the group with the most severe and global language production and comprehension deficits, similar to the prototypic Broca's aphasia following left media infarction. Neuroanatomically, nfPPA is characterized by a large-scale damage to the left temporal and posterior frontal lobe [40]. Within the framework of the aforementioned dual-route model for gesture production, atrophy in these areas may ultimately impair both routes, the ventral “what” route, associated with the semantic components of gestures (e.g., object and manipulation knowledge) and the dorsal “how” route, crucial for online-processing during imitation and spatial decoding of novel stimuli [38].
5. Conclusion
Brief cognitive tests for standard neuropsychological domains (e.g., memory, language, and executive functions) often yield inconclusive results regarding clear differential diagnoses of clinical variants of FTLD and AD. In the case of PPA, a wide range of neuropsychological tests is invalid as they heavily rely on comprehension and language skills. The present study showed that distinctive praxis impairments can efficiently and validly be assessed across clinically heterogeneous neurodegenerative dementia syndromes, supporting clinicians in the differential diagnosis of AD and FTLD subtypes in early disease stages. Results also showed that standardized assessment of praxis domains effectively taps into core cognitive deficits of these dementia syndromes, validating previous hypotheses regarding the cognitive basis of differential praxis-profiles in dementia (i.e., limb apraxia associated with visuospatial impairment, buccofacial imitation associated with sociocognitive abilities, and pantomime/communicative gestures associated with semantic memory and language). The current results thus extend the evidence that apraxia is a multifaceted rather than a homogeneous cognitive disorder. Future studies should directly explore the structural and functional neural bases for praxis domains beyond the known brain-behavior links for limb imitation and object pantomime in early neurodegenerative diseases.
Research in Context.
-
1.
Systematic review: We searched PubMed/MEDLINE for literature on the prevalence of apraxia in neurodegenerative dementias. Apraxia is far less studied than other cognitive domains in dementia. While few studies on clinical feasibility and diagnostic accuracy of apraxia assessment exist for single diagnoses (e.g., Alzheimer's dementia), the applied methodology is rather heterogeneous. Relevant studies have been cited. We found no work regarding differential diagnoses between language and behavioral variants of frontotemporal lobar degeneration and Alzheimer's disease.
-
2.
Interpretation: Our findings show that the assessment of apraxia can effectively and efficiently help to discriminate early stages of dementia of the Alzheimer's dementia and frontotemporal lobar degeneration spectrum as each dementia subtype presents with distinctive praxis-profiles. These profiles show consistent associations with disease-typical standard neuropsychological domains which are more time consuming to assess.
-
3.
Future directions: Results strongly argue for the inclusion of praxis impairment into clinical criteria for Alzheimer's dementia and the three major clinical variants of frontotemporal lobar degeneration. Neural correlates of disease-specific praxis-profiles by structural magnetic resonance imaging may validate our findings.
Acknowledgments
The authors thank Hubertus Lohmann, Sabine Bruchmann, Jana Frommeyer, Lena Rösch, and Sewda Yavari for assistance and help in patient acquisition and neuropsychological assessments. The authors thank Lisa-Marie Rutter for patholinguistic input.
Funding: This research did not receive any specific grant from funding agencies in the public, commercial, or not for-profit sectors.
Authors' contribution: A.J. contributed to study concept and design, acquisition of data, analysis and interpretation of data, statistical analyses, drafting the manuscript, study coordination, and study supervision. S.R. contributed to acquisition of data, analysis and interpretation of data, statistical analyses, and drafting the manuscript. H.W. contributed to study supervision and reading of the manuscript for intellectual and medical content. S.G.M. contributed to study supervision and reading of the manuscript for intellectual and medical content. T.D. contributed to study coordination, supervision, and reading of the manuscript for intellectual and medical content.
Footnotes
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. A.J. and S.R. have no disclosures. H.W. receives honoraria for acting as a member of Scientific Advisory Boards and as consultant for Biogen, Evgen, MedDay Pharmaceuticals, Merck Serono, Novartis, Roche Pharma AG, and Sanofi-Genzyme, as well as speaker honoraria and travel support from Alexion, Biogen, Cognomed, F. Hoffmann-La Roche Ltd., Gemeinnützige Hertie-Stiftung, Merck Serono, Novartis, Roche Pharma AG, Sanofi-Genzyme, TEVA, and WebMD Global. H.W. is acting as a paid consultant for Abbvie, Actelion, Biogen, IGES, Novartis, Roche, Sanofi-Genzyme, and the Swiss Multiple Sclerosis Society. His research is funded by the German Ministry for Education and Research (BMBF), Deutsche Forschungsgesellschaft (DFG), Else Kröner Fresenius Foundation, Fresenius Foundation, Hertie Foundation, NRW Ministry of Education and Research, Interdisciplinary Center for Clinical Studies (IZKF) Muenster and RE Children's Foundation, Biogen GmbH, GlaxoSmithKline GmbH, Roche Pharma AG, and Sanofi-Genzyme. S.G.M. has received honoraria for lecturing, travel expenses for attending meetings and financial research support from Almirall, Bayer Health Care, Biogen, Diamed, Fresenius Medical Care, Genzyme, Merck Serono, Novartis, Novo Nordisk, ONO Pharma, Roche, Sanofi-Aventis, and Teva. T.D. has received speaker honoraria, consultancy fees, and travel expenses from Genzyme, Shire, Sanofi Aventis, Novartis, Actelion Pharmaceuticals and Amicus, research support from Genzyme, Shire, Amicus and Actelion Pharmaceuticals, and educational grants from Novartis, Roche and Biogen.
References
- 1.Heilman K., Rothi L. Apraxia. In: Heilman K., Valenstein E., editors. Clinical Neuropsychology. Oxford University Press; New York, Oxford: 1993. pp. 141–164. [Google Scholar]
- 2.Goldenberg G. Elsevier Ltd; New York, Oxford: 2013. Apraxia - The Cognitive Side of Motor Control. [DOI] [PubMed] [Google Scholar]
- 3.Ahmed S., Baker I., Thompson S., Husain M., Butler C.R. Utility of testing for apraxia and associated features in dementia. J Neurol Neurosurg Psychiatr. 2016;87:1158–1162. doi: 10.1136/jnnp-2015-312945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rohrer J.D., Rossor M.N., Warren J.D. Apraxia in progressive nonfluent aphasia. J Neurol. 2010;257:569–574. doi: 10.1007/s00415-009-5371-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Johnen A., Tokaj A., Kirschner A., Wiendl H., Lueg G., Duning T., Lohmann H. Apraxia profile differentiates behavioural variant frontotemporal from Alzheimer's dementia in mild disease stages. J Neurol Neurosurg Psychiatr. 2015;86:809–815. doi: 10.1136/jnnp-2014-308773. [DOI] [PubMed] [Google Scholar]
- 6.Costa A., Bak T., Caffarra P., Caltagirone C., Ceccaldi M., Collette F. The need for harmonisation and innovation of neuropsychological assessment in neurodegenerative dementias in Europe: consensus document of the Joint Program for Neurodegenerative Diseases Working Group. Alzheimers Res Ther. 2017;9:27. doi: 10.1186/s13195-017-0254-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.American Psychiatric Association . American Psychiatric Pub; Washington, DC: 2013. Diagnostic and statistical manual of mental disorders, (DSM-5®) [Google Scholar]
- 8.Mioshi E., Hsieh S., Savage S., Hornberger M., Hodges J.R. Clinical staging and disease progression in frontotemporal dementia. Neurology. 2010;74:1591–1597. doi: 10.1212/WNL.0b013e3181e04070. [DOI] [PubMed] [Google Scholar]
- 9.Ramanan S., Bertoux M., Flanagan E., Irish M., Piguet O., Hodges J.R. Longitudinal executive function and episodic memory profiles in behavioral-variant frontotemporal dementia and Alzheimer's disease. J Int Neuropsychol Soc. 2017;23:34–43. doi: 10.1017/S1355617716000837. [DOI] [PubMed] [Google Scholar]
- 10.Rascovsky K., Hodges J.R., Knopman D., Mendez M.F., Kramer J.H., Neuhaus J. Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain. 2011;134:2456–2477. doi: 10.1093/brain/awr179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gorno-Tempini M.L., Hillis A.E., Weintraub S., Kertesz A., Mendez M., Cappa S.F. Classification of primary progressive aphasia and its variants. Neurology. 2011;76:1006–1014. doi: 10.1212/WNL.0b013e31821103e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vijverberg E.G.B., Dols A., Krudop W.A., Peters A., Kerssens C.J., van Berckel B.N. Diagnostic accuracy of the frontotemporal dementia consensus criteria in the late-onset frontal lobe syndrome. Dement Geriatr Cogn Disord. 2016;41:210–219. doi: 10.1159/000444849. [DOI] [PubMed] [Google Scholar]
- 13.Shinagawa S., Catindig J.A., Block N.R., Miller B.L., Rankin K.P. When a little knowledge can be dangerous: false-positive diagnosis of behavioral variant frontotemporal dementia among community clinicians. Dement Geriatr Cogn Disord. 2016;41:99–108. doi: 10.1159/000438454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harris J.M., Thompson J.C., Gall C., Richardson A.M., Neary D., du Plessis D. Do NIA-AA criteria distinguish Alzheimer's disease from frontotemporal dementia? Alzheimers Dement. 2015;11:207–215. doi: 10.1016/j.jalz.2014.04.516. [DOI] [PubMed] [Google Scholar]
- 15.Hornberger M., Piguet O., Graham A.J., Nestor P.J., Hodges J.R. How preserved is episodic memory in behavioral variant frontotemporal dementia? Neurology. 2010;74:472–479. doi: 10.1212/WNL.0b013e3181cef85d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.de Souza L.C., Chupin M., Bertoux M., Lehéricy S., Dubois B., Lamari F. Is hippocampal volume a good marker to differentiate Alzheimer's disease from frontotemporal dementia? J Alzheimers Dis. 2013;36:57–66. doi: 10.3233/JAD-122293. [DOI] [PubMed] [Google Scholar]
- 17.Osher J.E., Wicklund A.H., Rademaker A., Johnson N., Weintraub S. The mini-mental state examination in behavioral variant frontotemporal dementia and primary progressive aphasia. Am J Alzheimers Dis Other Demen. 2008;22:468–473. doi: 10.1177/1533317507307173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vigliecca N.S., Peñalva M.C., Molina S.C., Voos J.A., Vigliecca M.R. Is the Folstein's mini-mental test an aphasia test? Appl Neuropsychol Adult. 2012;19:221–228. doi: 10.1080/09084282.2011.643962. [DOI] [PubMed] [Google Scholar]
- 19.Reul S., Lohmann H., Wiendl H., Duning T., Johnen A. Can cognitive assessment really discriminate early stages of Alzheimer's and behavioural variant frontotemporal dementia at initial clinical presentation? Alzheimers Res Ther. 2017;9:1–12. doi: 10.1186/s13195-017-0287-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hutchinson A.D., Mathias J.L. Neuropsychological deficits in frontotemporal dementia and Alzheimer's disease: a meta-analytic review. J Neurol Neurosurg Psychiatr. 2007;78:917–928. doi: 10.1136/jnnp.2006.100669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McKhann G.M., Knopman D.S., Chertkow H., Hyman B.T., Jack C.R., Jr, Kawas C.H. The diagnosis of dementia due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7:263–269. doi: 10.1016/j.jalz.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sajjadi S.A., Patterson K., Arnold R.J., Watson P.C., Nestor P.J. Primary progressive aphasia A tale of two syndromes and the rest. Neurology. 2012;78:1670–1677. doi: 10.1212/WNL.0b013e3182574f79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Folstein M.F., Folstein S.E., McHugh P.R. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 24.Johnen A., Frommeyer J., Modes F., Wiendl H., Duning T., Lohmann H. Dementia Apraxia Test (DATE): a brief tool to differentiate behavioral variant frontotemporal dementia from Alzheimer's dementia based on apraxia profiles. J Alzheimers Dis. 2016;49:593–605. doi: 10.3233/JAD-150447. [DOI] [PubMed] [Google Scholar]
- 25.Benke T., Karner E., Delazer M. FAB-D: German version of the Frontal Assessment Battery. J Neurol. 2013;260:2066–2072. doi: 10.1007/s00415-013-6929-8. [DOI] [PubMed] [Google Scholar]
- 26.Flamand-Roze C., Falissard B., Roze E., Maintigneux L., Beziz J., Chacon A. Validation of a new language screening tool for patients with acute stroke: the language screening test (LAST) Stroke. 2011;42:1224–1229. doi: 10.1161/STROKEAHA.110.609503. [DOI] [PubMed] [Google Scholar]
- 27.Warrington E.K., James M. Thames Valley Test Company; Bury St Edmunds: 1991. The visual object and space perception battery. [Google Scholar]
- 28.Bertoux M., Delavest M., de Souza L.C., Funkiewiez A., Lépine J.P., Fossati P. Social Cognition and Emotional Assessment differentiates frontotemporal dementia from depression. J Neurol Neurosurg Psychiatr. 2012;83:411–416. doi: 10.1136/jnnp-2011-301849. [DOI] [PubMed] [Google Scholar]
- 29.Kertesz A., Davidson W., Fox H. Frontal behavioral inventory: diagnostic criteria for frontal lobe dementia. Can J Neurol Sci. 1997;24:29–36. doi: 10.1017/s0317167100021053. [DOI] [PubMed] [Google Scholar]
- 30.Johnen A., Brandstetter L., Kärgel C., Wiendl H., Lohmann H., Duning T. Shared neural correlates of limb apraxia in early stages of Alzheimer's dementia and behavioural variant frontotemporal dementia. Cortex. 2016;84:1–14. doi: 10.1016/j.cortex.2016.08.009. [DOI] [PubMed] [Google Scholar]
- 31.Witt J.-A., Coras R., Schramm J., Becker A.J., Elger C.E., Blümcke I. The overall pathological status of the left hippocampus determines preoperative verbal memory performance in left mesial temporal lobe epilepsy. Hippocampus. 2014;24:446–454. doi: 10.1002/hipo.22238. [DOI] [PubMed] [Google Scholar]
- 32.Zhou J., Seeley W.W. Network dysfunction in Alzheimer's disease and frontotemporal dementia: implications for psychiatry. Biol Psychiatry. 2014;75:565–573. doi: 10.1016/j.biopsych.2014.01.020. [DOI] [PubMed] [Google Scholar]
- 33.Bergeron D., Bensaïdane R., Jr., Laforce R. Untangling Alzheimer's disease clinicoanatomical heterogeneity through selective network vulnerability – An effort to understand a complex disease. Curr Alzheimer Res. 2016;13:589–596. doi: 10.2174/1567205013666151116125155. [DOI] [PubMed] [Google Scholar]
- 34.Lesourd M., Le Gall D., Baumard J., Croisile B., Jarry C., Osiurak F. Apraxia and Alzheimer's disease: review and perspectives. Neuropsychol Rev. 2013;23:234–256. doi: 10.1007/s11065-013-9235-4. [DOI] [PubMed] [Google Scholar]
- 35.Adeli A., Whitwell J.L., Duffy J.R., Strand E.A., Josephs K.A. Ideomotor apraxia in agrammatic and logopenic variants of primary progressive aphasia. J Neurol. 2013;260:1594–1600. doi: 10.1007/s00415-013-6839-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Buxbaum L.J., Shapiro A.D., Coslett H.B. Critical brain regions for tool-related and imitative actions: a componential analysis. Brain. 2014;137:1971–1985. doi: 10.1093/brain/awu111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hoeren M., Kümmerer D., Bormann T., Beume L., Ludwig V.M., Vry M.S. Neural bases of imitation and pantomime in acute stroke patients: distinct streams for praxis. Brain. 2014;137:2796–2810. doi: 10.1093/brain/awu203. [DOI] [PubMed] [Google Scholar]
- 38.Binkofski F., Buxbaum L.J. Two action systems in the human brain. Brain Lang. 2013;127:222–229. doi: 10.1016/j.bandl.2012.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Del A., Kotzalidis G.D., Rapinesi C., Janiri D., Aragona M., Puzella A. Neural functional correlates of empathic face processing. Neurosci Lett. 2017;655:68–75. doi: 10.1016/j.neulet.2017.06.058. [DOI] [PubMed] [Google Scholar]
- 40.Gordon E., Rohrer J.D., Fox N.C. Advances in neuroimaging in frontotemporal dementia. J Neurochem. 2016;138:193–210. doi: 10.1111/jnc.13656. [DOI] [PubMed] [Google Scholar]


