Abstract
Biofilms are the main challenges in the treatment of common oral diseases such as caries, gingival and endodontic infection and periimplantitis. Oral plaque is the origin of microbes colonizing in the form of biofilms on hydroxyapatite (tooth) and titanium (dental implant) surfaces. In this study, hydroxyapatite (HA) and titanium (Ti) disks were introduced, and their surface morphology was both qualitatively and quantitatively analyzed by a scanning electron microscope (SEM) and atomic force microscope (AFM). The average roughness of Ti disks (77.6 ± 18.3 nm) was less than that of HA (146.1 ± 38.5 nm) (p < 0.05). Oral multispecies biofilms which were cultured on Ti and HA disks for 6 h and three weeks were visualized by SEM. We investigated the ability of two new antibiofilm peptides, DJK-5 and 1018, to induce killing of bacteria in oral multispecies biofilms on Ti and HA disks. A 6-h treatment by DJK-5 and 1018 (2 or 10 μg/mL) significantly reduced biomass of the multispecies biofilms on both Ti and HA disks. DJK-5 was able to kill more bacteria (40.4–75.9%) than 1018 (30.4–67.0%) on both surfaces (p < 0.05). DJK-5 also led to a more effective killing of microbes after a 3-min treatment of 3-day-old and 3-week-old biofilms on Ti and HA surfaces, compared to peptide 1018 and chlorhexidine (p < 0.05). No significant difference was found in the amount of biofilm killing between Ti and HA surfaces. Both peptide DJK-5 and 1018 may potentially be used as effective antibiofilm agents in clinical dentistry.
Keywords: Antimicrobial, Biofilm, Hydroxyapatite, Peptide 1018, Peptide DJK-5, Titanium
Graphical abstract
Highlights
-
•
Peptide DJK-5 showed strong antibiofilm activity in pre-formed oral biofilms on both Ti and HA disks.
-
•
The peptides DJK-5 killed similar biofilms on Ti and HA surfaces, despite the difference in surface roughness and morphology between HA and Ti.
1. Introduction
Most microorganisms in nature live in a biofilm state, as aggregates with a complex construction on different surfaces. In the oral cavity, biofilms on tooth surfaces (hydroxyapatite) form the “bacterial plaque”, which lead to caries [1], gingival infection and periodontitis [2]. Titanium has been widely employed as a dental implant material for several decades owing to its excellent properties of biocompatibility, low allergenicity, osseointegration, and resistance to corrosion [3,4]. Ti implants have high success in the oral cavity [5]. However, biofilm associated infections (including periimplant mucositis and periimplantitis) are the leading causes of Ti implant failures [6,7]. Once Ti implants or abutments are installed in the oral environment, bacteria start to immediately colonize the Ti interfaces [8] providing target for new planktonic bacteria to adhere, proliferate and develop into a complex biofilm structure.
The scaffolds of biofilms are composed of extracellular polymeric matrix [9] and DNA of microbial origin [10], which can protect bacteria in biofilms against disinfecting solutions [11]. Therefore, microbes in the biofilm are more resistant than planktonic bacteria to most antimicrobial agents [12,13]. Various antibiofilm substances and strategies have been explored and reported in the past decades. However, over time many bacteria have developed resistance to commonly used antibiotics [14]. Hence, more efficient antibiofilm agents need to be developed to overcome this challenge and ensure success in treating infections in the oral cavity.
Antimicrobial peptides (AMPs) have drawn researchers' attentions due to their promising antimicrobial effect on biofilms related to infections over the past several years. AMPs can be either natural or synthetic peptides with antimicrobial properties against most bacterial pathogens [15]. Most AMPs are positively charged amphipathic peptides which interact with the negative charged groups on the bacterial cell membrane destroying its integrity [16]. Recently, peptides DJK-5 and 1018 have been developed with a special broad-spectrum antibiofilm activity against both Gram-positive and Gram-negative bacteria. The two peptides prevent the intracellular ppGpp accumulation which plays a critical role in biofilm development [17,18].
Because artificial implants (titanium) and natural teeth (hydroxyapatite) are in a similar ecological environment (the oral cavity), biofilms on the tooth surface may have similar formations and compositions [19]. However, some research on implant abutment surfaces implies that biofilm activities on biofilm surfaces may have characteristics (such as material, roughness and so on) different from tooth surface biofilms [20]. Therefore, the purpose of this study was to 1) analyze the influence of the surface morphology of Ti and HA disks on the development of biofilms; 2) evaluate the antimicrobial effects of two new peptides, DJK-5 and 1018 against biofilms on both Ti and HA surfaces.
2. Materials and methods
2.1. Ti and HA disks preparation
Commercially available pure titanium disks (ASTM Grade 1) were processed from a rod into a disk with a diameter of 12 mm and a thickness of 2 mm. They were then wet-polished with silicon carbide abrasive papers in sequence (400, 800 and 1200 grit). Afterwards, ultrasonic bathing was executed in distilled water, acetone, 75% ethanol and distilled water.
HA disks (Clarkson Chromatography Products, Williamsport, PA, USA) were autoclave sterilized (121 °C for 20 min), while titanium (Ti) disks were sterilized by ultraviolet radiation overnight.
2.2. Surface characteristics by atomic force microscope (AFM) and scanning electron microscopy (SEM)
Ti and HA disk surfaces were detected and analyzed with the SPM-9600 AFM system (Shimadzu, Kyoto, Japan). The scanning was processed in air under ambient conditions with a silicon nitride tip of NSG01 (NT-MDT, Moscow, Russia) in phase mode and a scanning rate of 1 Hz. For both kinds of surfaces (Ti and HA), three samples of each were analyzed. Five areas of 10 μm × 10 μm were randomly chosen in each sample and scanned. A two-dimensional picture was captured and a three-dimensional reconstruction was obtained for each area. The mean roughness of Ti and HA disk surfaces was calculated by the Ra values of both materials.
The surface morphologies were also observed using SEM. Briefly, HA disks were coated with gold palladium sputter (Hummer VI; Technic Inc, Anaheim, CA, USA), and both Ti and HA disks were surveyed by SEM (Hitachi SU3500 VPSEM; Hitachi High-Technologies Canada Inc, Toronto, Canada) at 3 kV and at a magnification of 1000 × and 3000×.
2.3. Biofilm model
Multispecies biofilm was grown using subgingival plaque from the second upper molar from one healthy adult volunteer. The subgingival plaque was suspended in brain heart infusion broth (BHI) (Becton Dickinson, Sparks, MD, USA) and incubated anaerobically at 37 °C overnight. The present study was approved by the University of British Columbia Clinical Research Ethics committee review boards (certificate H12-02430) and written informed consent was obtained from the volunteer.
The dispersed plaque suspension was measured in a microplate reader (ELx808 Absorbance Reader, BioTek Instruments, Inc, Winooski, VT, USA) in 96-well plates with 150 μL per well at 405 nm, and an optical density (OD) of 0.1 was used as the standardized density of the bacterial solution.
2.4. Coating the Ti and HA disks
Fresh saliva was collected in polypropylene tubes (Corning, NY, USA) from a healthy volunteer at least 2 h after meals and filtered using sterilized 0.22 μm syringe filters (Pall corporation, Ann Arbor, MI, USA). Ti and HA disks were precoated with filter sterilized saliva for 4 h at room temperature before use and gently rinsed with phosphate buffer saline (PBS, pH = 7.0) (Sigma-Aldrich, St Louis, MO, USA).
2.5. SEM examination of biofilms at different stages of development
After biofilm growth on Ti and HA disks for 6 h (initial adhesion stage) and three weeks (mature biofilm stage), the specimens were washed with PBS for 5 min. Fixation was performed by adding 2.5% glutaraldehyde for 10 min and 1% osmium tetroxide for 1 h. The specimens were dehydrated by increasing concentrations of ethanol, dried by using a critical point drier (Samdri-795; Tousimis Research Corporation, Rockville, MD, USA), and sputter-coated with gold-palladium in a vacuum evaporator (Hummer VI; Technics West Inc, Anaheim, CA, USA). SEM observation was executed at 3 kV, under a low (1000 ×) and a high magnification (5000 × or 6000 ×).
2.6. Preparation of antimicrobial agents
Peptides 1018 and DJK-5 were synthesized by CPC Scientific (Sunnyvale, CA, USA) using solid-phase 9-fluorenylmethoxy carbonyl (Fmoc) chemistry and purified to a purity of >95% using reverse-phase high-performance liquid chromatography as previously described [21]. For the experiments the peptide was obtained from peptide stocks in deionized water.
Two percentages and 0.2% chlorhexidine digluconate (CHX) was freshly prepared by diluting from a 20% solution (Sigma Chemical Co.).
2.7. Long-term antibiofilm effect of the peptides on pre-formed biofilms on Ti and HA disks
For long term exposure experiment, 2 mL of the above mentioned plaque bacteria suspension was cultured anaerobically at 37 °C for 6 and 24 h or 7 days. Fresh BHI containing DJK-5 or 1018 peptide (2 or 10 μg/mL) was supplied to wells holding Ti and HA disks. Control groups for biofilms on both Ti and HA disks were only submerged BHI and sterilized water for the same times as described above. All samples were further incubated for 6 h.
2.8. Short-term antibiofilm effect of the peptides on pre-formed biofilms on Ti and HA surfaces
For short term exposure experiment (eradication), 0.2 mL of the above mentioned plaque bacteria suspension was added to 1.8 mL fresh BHI to saliva-coated Ti or HA disks and incubated anaerobically at 37 °C for either three days or three weeks. Fresh BHI was replaced once every week. At the end of incubation period, each sample was gently rinsed with 2 mL PBS in a well for 1 min and exposed to the peptide DJK-5 (2 and 10 μg/mL), 1018 (2 and 10 μg/mL) or CHX (0.2% and 2%) for 3 min.
2.9. Confocal laser scanning microscopy (CLSM) examination of biofilms on Ti and HA surfaces
Following the exposure to the above solutions, all specimens were rinsed gently in 0.85% physiological saline and then stained with a 1:1 mixture of SYTO 9 and propidium iodide (BacLight LIVE/DEAD Bacterial Viability kit, Molecular Probes, Eugene, OR) following the manufacturer's instructions. Images of the stained samples were taken by a CSLM (FV10i-LIV, Olympus, Canada) at 480/500 nm for SYTO 9 and 490/635 nm for propidium iodide, respectively. Five random areas were chosen from each specimen and scanned at a resolution of 512 × 512 pixels with 2 μm step size from the top to the bottom of the biofilm for each chosen area. Three samples were observed for each group and five randomly selected areas of each sample were scanned. Three-dimensional volume stacks were reconstructed with Imaris 7.2 software (Bitplane Inc., St Paul, MN, USA), and the total volume (red and green fluorescence) was measured. The proportion of dead bacteria was indicated by the proportion of red fluorescence of the total of green and red fluorescence.
2.10. Statistical analysis
Statistical analysis was done with SPSS 19.0 software (SPSS, Chicago, IL, USA). To determine the differences between the Ra values on Ti and HA disks, independent-samples T test was used. In other experiments, one-way ANOVA was implemented, and post hoc Fisher's LSD multiple comparison test was applied when necessary. The significance level was determined as p < 0.05.
3. Results and discussion
3.1. Surface characteristics of Ti and HA disks
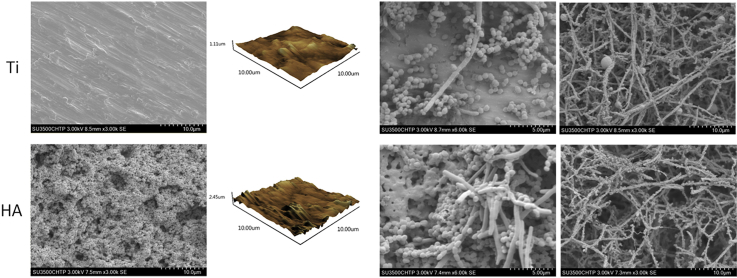
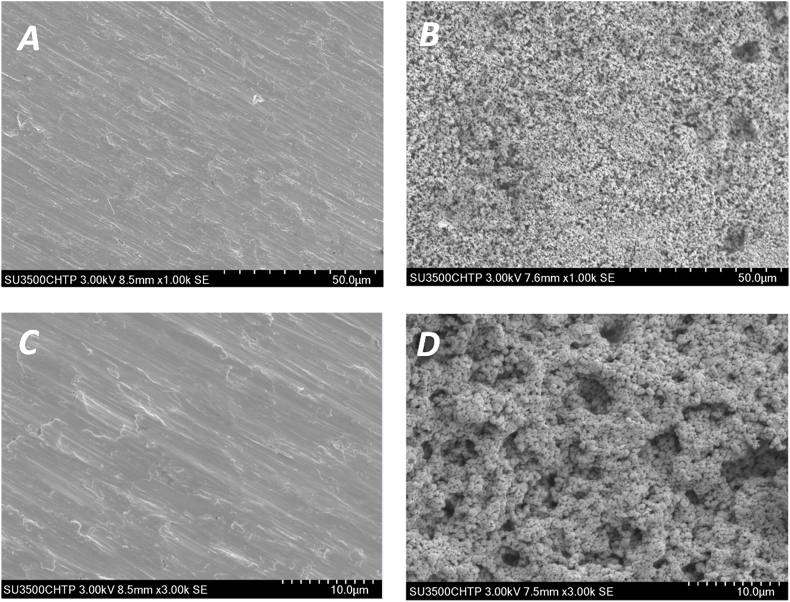
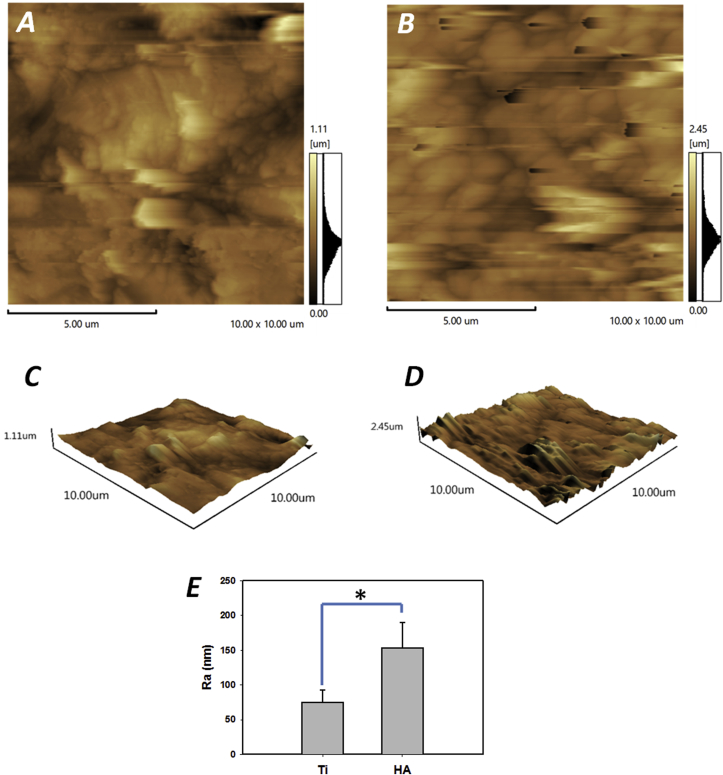
In this study, the surface morphology of the two different materials (Ti and HA) was qualitatively evaluated by AFM and SEM microscopy. Fig. 1 shows the surface morphologies of both Ti and HA using SEM observation. On Ti disks, the grind marks presented linear and covered the whole surface. The surface topography was relatively smooth without any obvious rises and falls or pores (Fig. 1A and C). HA disks had a more uneven surface composed of particulate matter and showed numerous pores of various sizes (Fig. 1B and D). The two and three dimensional images from AFM exhibited peaks and valleys on both Ti and HA surfaces. Ti surfaces had 1.1 ??m high peaks, while the peaks on HA were 2.45 ??m high (Fig. 2A and D). Roughness is one of the indicators of the characteristics of material surfaces. AFM is commonly employed to detect the surface morphology and measure the average roughness (Ra) of the material being examined. In this study, the mean Ra values were 77.6 ± 18.3 nm on Ti disks and 146.1 ± 38.5 nm on HA, respectively, showing a significant difference between the two (p < 0.05) (Fig. 2E). The degree of roughness can influence bacteria adhesion, making it perhaps the most relevant factor in surface properties that can interfere with bacterial and surface interaction [22]. Therefore, oral biofilms cultured on Ti and HA surfaces were examined further.
Fig. 1.
SEM images of Ti and HA surfaces without saliva coating and biofilm growth. (A) Ti surface under low magnification (1000 ×); (B) HA surface under low magnification (1000 ×); (C) Ti surface under high magnification (3000 ×); (D) HA surface under high magnification (3000 ×).
Fig. 2.
AFM images for Ti and HA surfaces. (A) Ti and (B) HA surfaces as 2D image; (C) Ti and (D) HA surfaces as 3D image (X, Y and Z scales are in μm); (E) Comparison of Ra values between Ti and HA, asterisk indicates significant differences between groups (p < 0.05).
3.2. Biofilms development on Ti and HA surfaces
Bacteria from tooth surface plaque are the causes of not only periodontal and endodontic diseases but also infections associated with dental implant failure [23]. In this study, plaque multispecies biofilms were cultured on both Ti and HA surfaces. SEM images of the Ti and HA disks (Fig. 3) showed the presence of multispecies biofilms, consisting of rods, and filaments as well as small clusters of cocci dispersed across the surface after 6 h of biofilm development. After three weeks, dividing cells and clusters of bacteria that were closely packed and interlaced by a well-developed inter-microbial matrix were observed in the biofilm. These characteristics are typical of natural structural network of mature biofilms (Fig. 3). There was no obvious difference between the structures and compositions of mature biofilms grown on Ti and HA disks.
Fig. 3.
SEM images of biofilms at different stages of development (6 h and 3 weeks) on Ti and HA disks.
Although there are numerous reports showing differences in biofilm formation on materials with different surface roughness, there is still a debate on how and to what extent the roughness of the material influence bacteria adhesion and biofilm maturation on surfaces [24,25]. In the present study, HA disks were made by compressing small particles to achieve a highly porous surface, while Ti disks were polished by silicon paper into a uniform and smooth surface.
3.3. Effect of antibiofilm peptides on biofilms grown on different surfaces
3.3.1. Effect on biofilms of long-term exposure by the peptides
Microorganisms colonizing a body site such as the root canal space are present either as free-floating single cells or cells attached to each other or to the surface (or both) in a biofilm. A central tenet of biofilm formation is its dynamic nature. Most current models depict biofilm formation as a linear process that commences when free-floating bacterial cells attach to a surface. This attachment is followed by growth into mature, structurally complex biofilms culminating in the dispersal of detached bacterial cells into the bulk fluid [9]. These various phases of microbial interactions with the surface appear to require the production of extracellular polymers that assist in initial adhesion, maintenance of biofilm structure, and detachment from matrix-enclosed aggregates. This is an important area of biofilm investigation because the phenotypic behavior of bacteria might be quite distinct during the different phases of biofilm formation [11]. The first 6 h is deemed a critical period for preventing early colonization of a Ti implant [26].
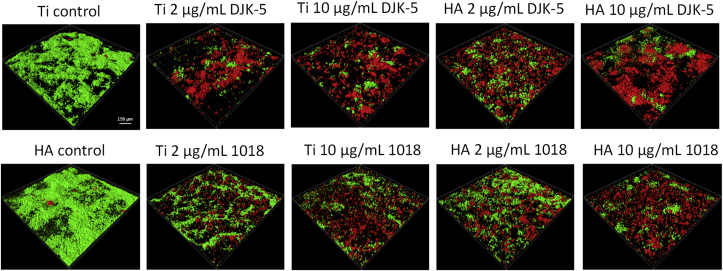
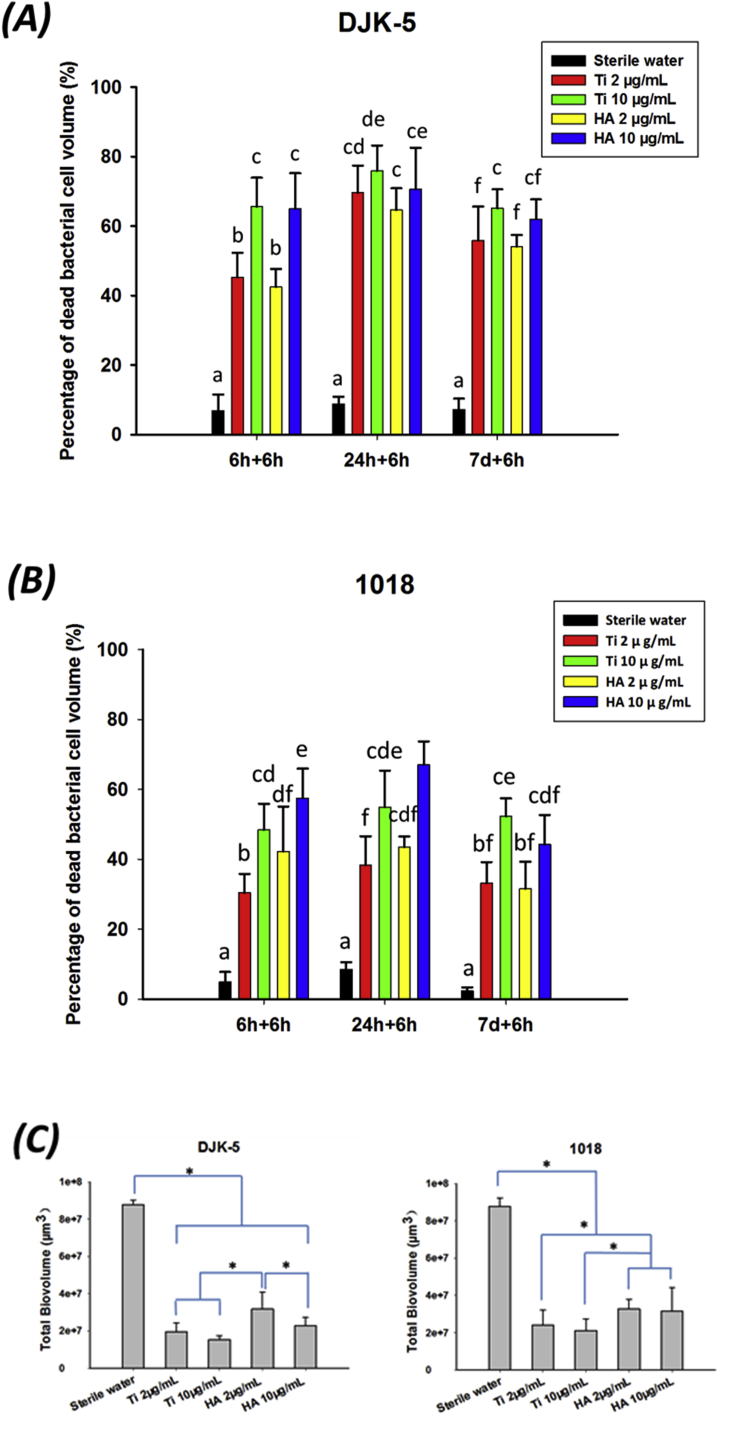
The peptide DJK-5 was superior to 1018 in killing biofilm bacteria in all instances (Fig. 4). In the long-term exposure test, significantly more microorganisms were killed at the higher concentration (10 μg/mL) of DJK-5 and 1018 than at the lower concentration (2 μg/mL) (Fig. 4; p < 0.05) on both Ti and HA disks, as also shown in previously studies [27,28]. Interestingly, exposure of pre-formed biofilms to DJK-5 after 24 h of biofilm growth showed a higher proportion of killed bacteria on both disks, compared to biofilms grown only for 6 h (Fig. 5A; p < 0.05). There was no significant difference in this respect after exposure to peptide 1018 between biofilms grown for 6 and 24 h and 7 days (Fig. 5B). However, the proportion of bacteria killed by peptide 1018 in biofilms grown for 6 and 24 h was higher on HA disks than on Ti disks (Fig. 5B; p < 0.05).
Fig. 4.
Confocal microscopy images of 24-h-old biofilms on Ti and HA surfaces treated with peptides DJK-5 and 1018 for 6 h. The bar indicates 150 μm.
Fig. 5.
Long-term antibiofilm effect of peptides on biofilms on different surfaces. (A) DJK-5 and (B) 1018, dead bacteria proportion as measured by viability staining and CLSM. Different lowercase letters in each treatment group indicate statistically significant difference (p < 0.05). (C) Biovolume of 24-h-old biofilm exposure to peptide for 6 h, asterisks indicate significant differences between groups (p < 0.05).
The growth of biofilm (24 h old) volume (biovolume) after exposure to peptides DJK-5 and 1018 slowed down by more than 5-fold, compared to untreated control group biofilms (Fig. 5C). DJK-5 always performed better than peptide 1018 in slowing down the biofilm grow of volume, which is consistent with results from previous studies [27,28]. At the higher concentration, the peptides caused a higher biovolume reduction than when used at lower concentration. The difference was particularly strong with peptide DJK-5. Interestingly, residual biofilm volume of biofilms on HA disks was higher than on Ti disks after exposure to both peptides (Fig. 5C; p < 0.05). This may be related to the lacunae of the roughness and morphology of HA surface. Sanchez et al. [29] compared a complex biofilm development in vitro on Ti, Zr (zirconium) and HA surfaces and they found that after 72 h the bacterial number by CFU was significantly higher on HA surface than on Ti and Zr ones, which was coherent with our present study. They concluded that although the formation and dynamics of biofilms on different materials were similar, the thickness and three-dimensional structure of biofilms were significantly different.
One limitation of this study is that only one type of Ti disk was used as substratum; in some studies, machined Ti (smooth surface) and sandblasted acid-etched Ti (rough surface) had both been studied on the biofilms formation [30,31]. However, there is few studies focused on whether type of material and surface morphology will act on antibacterial agent effect on biofilms on them. So, further study should evaluate the effect of peptides on biofilm grown on different implant surfaces.
In order for antimicrobial peptides to have good antimicrobial activity, it is important to be resistant to proteolytic degradation by proteases produced by bacteria or host cells at sites of infections [32]. The method to overcome this obstacle is to incorporate non-natural D-isomers into the peptide chain to form a mirror image of its original native L-peptide structure, which averts it from interacting with specific receptors, thus preventing proteolytic degradation [33]. DJK-5 is designed to be a D-enantiomeric protease-resistant peptide, to help keep its high quality antibiofilm efficacy in use. On the other hand, besides its antibiofilm activity [34], peptide 1018 can also act as an immunomodulator [[34], [35], [36], [37]]. Although its bactericidal action is not as strong as that of DJK-5, peptide 1018 can exert its function by regulating the host's immunologic system, which will synergy and increase its antibiofilm effectiveness [38].
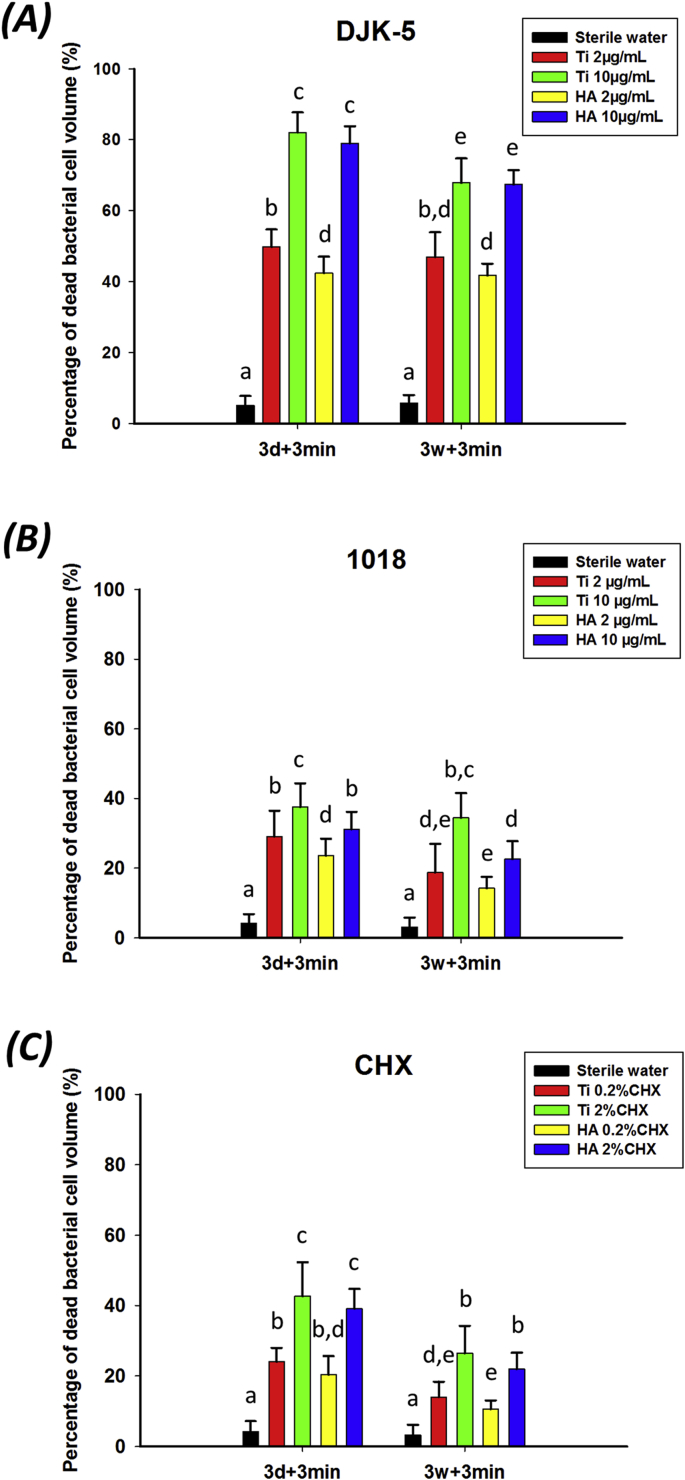
3.3.2. Effect on biofilms of short-term exposure by the peptides
In short-term exposures of pre-formed biofilms to the two peptides, the proportion of dead bacterial cells was significantly correlated with the peptide used, the concentration of the peptide, and the age of biofilms on both surfaces (Fig. 6). Peptide DJK-5 showed the best results in bacterial killing among all groups (p < 0.05). DJK-5 used for 3 min on 3-day-old and 3-week-old biofilms killed one to three times more biofilm bacteria than peptide 1018 (Fig. 6A and B; p < 0.05). As a positive control, 0.2% CHX showed the lowest antibiofilm effect, with only 20.3–24.1% of the bacteria killed in 3-day-old biofilms. Again, in high concentration the peptides killed more biofilm bacteria than in low concentration (p < 0.05). The three-week-old biofilms on both Ti and HA surface were more resistant to peptide 1018 and CHX than the three-day-old biofilms. This is consistent with earlier studies by Shen et al. [39] and Stojicic et al. [40] that involved other disinfecting agents and multispecies biofilms grown on collagen-coated hydroxyapatite disks. Those studies showed that mature, three-week-old biofilms were much more resistant than the younger biofilms. However, there was no significant difference between young and old biofilms exposed to DJK-5 in the present study (p > 0.05), in line with results in a previous study with DJK-5 [41].
Fig. 6.
Short-term antibiofilm effect of peptides on biofilms on different surfaces. (A) DJK-5, (B) 1018 and (C) CHX, dead bacteria proportion as measured by viability staining and CLSM. Different lowercase letters in each treatment group indicate statistically significant difference (p < 0.05).
No significant difference was found in the percentage of biofilm bacteria killed on Ti and HA surfaces, despite the difference in surface roughness and morphology between HA and Ti. The small difference of the surface characteristic between two surfaces, such as topography and even surface energy of materials may be attributed to the saliva coating [[42], [43], [44]]. Therefore, the differences in biofilm architecture and composition may be non-distinctive. It can also be speculated that the difference in roughness of the two surfaces does not play a big role. Quirynen and Bollen [45] found that 200 nm was the threshold of surface roughness, after which greater surface roughness can influence protein adsorption and bacterial adhesion. In the present study, the roughness of Ti (78 nm) and HA (146 nm) are both less than 200 nm, having little or no impact on the characteristics of multispecies biofilms on saliva coated Ti and HA disks.
4. Conclusion
In conclusion, peptide DJK-5 showed strong antibiofilm activity during long-term (6 h) and short-term (3 min) exposures on both Ti and HA disks. DJK-5 was more effective against oral biofilms than peptide 1018. No significant difference was found in the percentage of biofilm killed on Ti and HA surfaces, despite the difference in surface roughness and morphology between HA and Ti. Interestingly, residual biofilm volume of biofilms on HA disk was higher than on Ti disks after exposure to both peptides. Both peptides DJK-5 and 1018 may be promising agents for use in oral antibiofilm strategies in the future, including peri-implantitis.
Acknowledgments
This work was partly supported by National Natural Science Foundation of China (NSFC, No. 81301327 and No. 81641035), by Canada Foundation for Innovation (CFI: 32623).
Footnotes
Peer review under responsibility of KeAi Communications Co., Ltd.
Contributor Information
Jingzhi Ma, Email: majingzhi2002@163.com.
Ya Shen, Email: yashen@dentistry.ubc.ca.
References
- 1.Becker M.R., Paster B.J., Leys E.J. Molecular analysis of bacterial species associated with childhood caries. J. Clin. Microbiol. 2002;40:1001–1009. doi: 10.1128/JCM.40.3.1001-1009.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Socransky S.S., Smith C., Haffajee A.D. Subgingival microbial profiles in refractor periodontal disease. J. Clin. Periodontol. 2002;29:260–268. doi: 10.1034/j.1600-051x.2002.290313.x. [DOI] [PubMed] [Google Scholar]
- 3.Long M., Rack H.J. Titanium alloys in total joint replacement - a materials science perspective. Biomaterials. 1998;19:1621–1639. doi: 10.1016/s0142-9612(97)00146-4. [DOI] [PubMed] [Google Scholar]
- 4.Liu X.Y., Chu P.K., Ding C.X. Surface modifi cation of titanium, titanium alloys and related materials for biomedical applications. Mater. Sci. Eng. R. 2004;47:49–121. [Google Scholar]
- 5.Setzer F.C., Kim S. Comparison of long-term survival of implants and endodontically treated teeth. J. Dent. Res. 2014;93:19–26. doi: 10.1177/0022034513504782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mahato N., Wu X., Wang L. Management of peri-implantitis: a systematic review, 2010-2015. SpringerPlus. 2016;5:105. doi: 10.1186/s40064-016-1735-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Han A.F., James K.H., Rodrigues F.Pi. Bacterial adhesion mechanisms on dental implant surfaces and the influencing factors. Int. J. Adhesion Adhes. 2016;69:58–71. [Google Scholar]
- 8.Fürst M.M., Salvi G.E., Lang N.P., Persson G.R. Bacterial colonization immediately after installation on oral titanium implants. Clin. Oral Implants Res. 2007;18:501–508. doi: 10.1111/j.1600-0501.2007.01381.x. [DOI] [PubMed] [Google Scholar]
- 9.Flemming H.C., Neu T.R., Wozniak D.J. The EPS matrix: the “house of biofilm cells”. J. Bacteriol. 2007;189:7945–7947. doi: 10.1128/JB.00858-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mann E.E., Rice K.C., Boles B.R. Modulation of eDNA release and degradation affects Staphylococcus aureus biofilm maturation. PLoS One. 2009;4 doi: 10.1371/journal.pone.0005822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.De la Fuente-Núñez C., Reffuveille F., Fernández L., Hancock R.E.W. Bacterial biofilm development as a multicellular adaptation: antibiotic resistance and new therapeutic strategies. Curr. Opin. Microbiol. 2013;16:580–589. doi: 10.1016/j.mib.2013.06.013. [DOI] [PubMed] [Google Scholar]
- 12.Gilbert P., Das J., Foley I. Biofilm susceptibility to antimicrobials. Adv. Dent. Res. 1997;11:160–167. doi: 10.1177/08959374970110010701. [DOI] [PubMed] [Google Scholar]
- 13.Xu K.D., McFeters G.A., Stewart P.S. Biofilm resistance to antimicrobial agents. Microbiology. 2000;146:547–549. doi: 10.1099/00221287-146-3-547. [DOI] [PubMed] [Google Scholar]
- 14.Mansour S.C., de la Fuente- Núñez C., Hancock R.E.W. Peptide IDR-1018: modulating the immune system and targeting bacterial biofilms to treat antibiotic-resistant bacterial infections. J. Pept. Res. 2015;21:323–329. doi: 10.1002/psc.2708. [DOI] [PubMed] [Google Scholar]
- 15.Pletzer D., Hancock R.E.W. Antibiofilm peptides: potential as broad-spectrum agents. J. Bacteriol. 2016;198:2572–2578. doi: 10.1128/JB.00017-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.De la Fuente-Núñez C., Korolik V., Bains M. Inhibition of bacterial biofilm formation and swarming motility by a small synthetic cationic peptide. Antimicrob. Agents Chemother. 2012;56:2696–2704. doi: 10.1128/AAC.00064-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mansour S.C., Pletzer D., de la Fuente- Núñez C. Bacterial abscess formation is controlled by the stringent stress response and can Be targeted therapeutically. EBioMedicine. 2016;12:219–226. doi: 10.1016/j.ebiom.2016.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ribeiro S.M., de la Fuente-Núñez C., Baquir B. Antibiofilm peptides increase the susceptibility of carbapenemase-producing Klebsiella pneumoniae clinical isolates to beta-lactam antibiotics. Antimicrob. Agents Chemother. 2015;59:3906–3912. doi: 10.1128/AAC.00092-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lang N.P., Berglundh T. Periimplant diseases: where are we now?–Consensus of the seventh european workshop on periodontology. J. Clin. Periodontol. 2011;38:178–181. doi: 10.1111/j.1600-051X.2010.01674.x. [DOI] [PubMed] [Google Scholar]
- 20.Widodo A., Spratt D., Sousa V. An in vitro study on disinfection of titanium surfaces. Clin. Oral Implants Res. 2016;27:1227–1232. doi: 10.1111/clr.12733. [DOI] [PubMed] [Google Scholar]
- 21.de la Fuente-Núñez C., Reffuveille F., Mansour S.C. D-enantiomeric peptides that eradicate wild-type and multidrug-resistant biofilms and protect against lethal Pseudomonas aeruginosa infections. Chem. Biol. 2015;22:196–205. doi: 10.1016/j.chembiol.2015.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Teughels W., Van Assche N., Sliepen I., Quirynen M. Effect of material characteristics and/or surface topography on biofilm development. Clin. Oral Implants Res. 2006;17:68–81. doi: 10.1111/j.1600-0501.2006.01353.x. [DOI] [PubMed] [Google Scholar]
- 23.Jakubovics N.S., Kolenbrander P.E. The road to ruin: the formation of disease associated oral biofilms. Oral Dis. 2010;16:729–739. doi: 10.1111/j.1601-0825.2010.01701.x. [DOI] [PubMed] [Google Scholar]
- 24.Schmidlin P.R., Muller P., Attin T. Polyspecies biofilm formation on implant surfaces with different surface characteristics. J. Appl. Oral Sci. 2013;21:48–55. doi: 10.1590/1678-7757201302312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Al-Ahmada A., Wiedmann-Al-Ahmad M., FacklerIn A. In vivo study of the initial bacterial adhesion on different implant materials. Arch. Oral Biol. 2013;58:1139–1147. doi: 10.1016/j.archoralbio.2013.04.011. [DOI] [PubMed] [Google Scholar]
- 26.Emmerson M. A microbiologist's view of factors contributing to infection. New. Horiz. 1998;6:S3–S10. [PubMed] [Google Scholar]
- 27.Wang Z.J., de la Fuente-Núñez C., Shen Y. Treatment of oral multispecies biofilms by an anti-biofilm peptide. PLoS One. 2015;10 doi: 10.1371/journal.pone.0132512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang T., Wang Z.J., Hancock R.E.W., de la Fuente-Nunez C., Haapasalo M. Treatment of oral biofilms by a D-Enantiomeric peptide. PLoS One. 2016;11 doi: 10.1371/journal.pone.0166997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sanchez M.C., Llama-Palacios A., Fernández E. An in vitro biofilm model associated to dental implants: structural and quantitative analysis of in vitro biofilm formation on different dental implant surfaces. Dent. Mater. 2014;30:1161–1171. doi: 10.1016/j.dental.2014.07.008. [DOI] [PubMed] [Google Scholar]
- 30.Almaguer-Flores A., Olivares-Navarrete R., Wieland M. Influence of topography and hydrophilicity on initial oral biofilm formation on microstructured titanium surfaces in vitro. Clin. Oral Implants Res. 2012;23:301–307. doi: 10.1111/j.1600-0501.2011.02184.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pita P.P.C., Rodrigues J.A., Ota-Tsuzuki C. Oral streptococci biofilm formation on different implant surface topographies. BioMed Res. Int. P. 2015;2015:1–6. doi: 10.1155/2015/159625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee S.H., Kim S.J., Lee Y.S. De novo generation of short antimicrobial peptides with simple amino acid composition. Regul. Pept. 2011;166:36–41. doi: 10.1016/j.regpep.2010.08.010. [DOI] [PubMed] [Google Scholar]
- 33.Haney E.F., Hancock R.E.W. Peptide design for antimicrobial and immunomodulatory applications. Biopolymers. 2013;100:572–583. doi: 10.1002/bip.22250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.De la Fuente-Núñez C., Reffuveille F., Haney E.F., Straus S.K., Hancock R.E.W. Broad-spectrum anti-biofilm peptide that targets a cellular stress response. PLoS Pathog. 2014;10 doi: 10.1371/journal.ppat.1004152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wieczorek M., Jenssen H., Kindrachuk J. Structural studies of a peptide with immune modulating and direct antimicrobial activity. Chem. Biol. 2010;17:970–980. doi: 10.1016/j.chembiol.2010.07.007. [DOI] [PubMed] [Google Scholar]
- 36.Achtman A.H., Pilat S., Law C.W. Effective adjunctive therapy by an innate defense regulatory peptide in a pre-clinical model of severe malaria. Sci. Transl. Med. 2012;23 doi: 10.1126/scitranslmed.3003515. 135ra64. [DOI] [PubMed] [Google Scholar]
- 37.Rivas-Santiago B., Castañeda-Delgado J.E., Rivas Santiago C.E. Ability of innate defence regulator peptides IDR-1002, IDR-HH2 and IDR-1018 to protect against Mycobacterium tuberculosis infections in animal models. PLoS One. 2013;8 doi: 10.1371/journal.pone.0059119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pletzer D., Coleman S.R., Hancock R.E.W. Anti-biofilm peptides as a new weapon in antimicrobial warfare. Curr. Opin. Microbiol. 2016;33:35–40. doi: 10.1016/j.mib.2016.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shen Y., Stojicic S., Haapasalo M. Antimicrobial efficacy of chlorhexidine against bacteria in biofilms at different stages of development. J. Endod. 2011;37:657–661. doi: 10.1016/j.joen.2011.02.007. [DOI] [PubMed] [Google Scholar]
- 40.Stojicic S., Shen Y., Haapasalo M. Effect of the source of biofilm bacteria, level of biofilm maturation, and type of disinfecting agent on the susceptibility of biofilm bacteria to antibacterial agents. J. Endod. 2013;39:473–477. doi: 10.1016/j.joen.2012.11.024. [DOI] [PubMed] [Google Scholar]
- 41.Wang D., Shen Y., Ma J.Z., Hancock R.E.W., Haapasalo M. Antibiofilm effect of D-enantiomeric peptide alone and combined with EDTA in vitro. J. Endod. 2017;43:1862–1867. doi: 10.1016/j.joen.2017.06.037. [DOI] [PubMed] [Google Scholar]
- 42.Elter C., Heuer W., Demling A. Supra- and subgingival biofilm formation on implant abutments with different surface characteristics. Int. J. Oral Maxillofac. Implants. 2008;23:327–334. [PubMed] [Google Scholar]
- 43.van Brakel R., Cune M.S., van Winkelhoff A.J. Early bacterial colonization and soft tissue health around zirconia and titanium abutments: an in vivo study in man. Clin. Oral Implants Res. 2011;22:571–577. doi: 10.1111/j.1600-0501.2010.02005.x. [DOI] [PubMed] [Google Scholar]
- 44.Satou J., Fukunaga A., Morikawa A. Streptococcal adherence to uncoated and saliva-coated restoratives. J. Oral Rehabil. 1991;18:421–429. doi: 10.1111/j.1365-2842.1991.tb01687.x. [DOI] [PubMed] [Google Scholar]
- 45.Quirynen M., Bollen C.M. The influence of surface roughness and surface-free energy on supra- and subgingival plaque formation in man, A review of the literature. J. Clin. Periodontol. 1995;22:1–14. doi: 10.1111/j.1600-051x.1995.tb01765.x. [DOI] [PubMed] [Google Scholar]