Abstract
We report first case of pulmonary siderosis appearing as a consolidation upon radiological examination and being misdiagnosed as pneumonia. A 59-year-old man visited our hospital with a cough and sputum that had persisted for more than a month. He had undergone chest computed tomography (CT) after abnormal findings on chest X-ray at other hospitals. Based on the chest CT results, he was diagnosed with pneumonia. He was then administered antibiotics for 3 weeks, but there was no improvement. We identified the patient's occupational history first, and then performed bronchoalveolar lavage and chest CT-guided transthoracic lung biopsy. The obtained specimen showed alveolar, macrophage-containing, Prussian blue-positive iron particles. Based on the results, we diagnose/d the patient with pulmonary siderosis. We advised him to discontinue his job. He is currently undergoing observation, and has not shown any special symptoms.
Keywords: Pulmonary siderosis, Bronchoalveolar lavage, Transthoracic lung biopsy
1. Introduction
Pulmonary siderosis or welder's lung is an occupational lung disease that is usually observed after chronic exposure to iron dust. Pulmonary siderosis was first described in 1936 and occurs in about 7% of arc welders. Inhaled iron dust is deposited in intra-alveolar spaces, which leads to radiological changes and respiratory symptoms. The diagnosis of pulmonary siderosis is based on patient history of iron inhalation, radiological findings, and pathological findings of iron-laden macrophages in lung tissue or bronchoalveolar lavage fluid [1].
Recently, diagnostic imaging methods have been developed. Thus, based on these imaging developments, the diagnosis and treatment direction of the disease is determined. There are many reports that these demonstrate that imaging findings are helpful in diagnosing pulmonary siderosis [2,[7], [8], [9]]. Typical radiological findings of pulmonary siderosis are poorly-defined centrilobular micronodules, followed by branching linear structures, and ground-glass attenuation. However, rarely, atypical radiologic findings can appear, which may be misdiagnosed as other diseases.
2. Case report
A 59-year-old man who appeared healthy without any underlying disease, visited our hospital with a cough and sputum that had persisted for more than a month. The patient had undergone chest computed tomography (CT) after abnormal findings on chest X-ray at another hospital. Based on the chest CT results, he was diagnosed with pneumonia in the right upper lobe and was admitted to that hospital. He was treated with intravenous injections of third-generation cephalosporin antibiotics for 3 weeks. However, after the treatment, there was no improvement in the lesion. Hence, he was transferred to our hospital. He had a 40-pack-years smoking history and no family history. He also had a working history of iron welding in the preceding 5 months.
He was alert, and the following findings were noted in the physical examinations upon admission: blood pressure, 140/70 mmHg; pulse rate, 85 beats per minute; respiratory rate, 20 breaths per minute; and body temperature, 36.8 °C. He had a chronically ill appearance. His conjunctiva was not anemic and his sclera was not icteric. Clear breathing sounds were auscultated on both lung fields and his heartbeats were regular without murmur upon auscultation. The other examination results were also within normal limits. The following were the results of a laboratory examination: hemoglobin and hematocrit levels, normal; white blood cell count, 11,670/μL: differential count, 52.4% neutrophils, 31.7% lymphocytes, 12.5% monocytes, and 3.2% eosinophils; platelet count 292 × 109/L; C-reactive protein levels, slightly elevated at 32 mg/L (normal range 0–5 mg/L); serum ferritin levels, 151.4 μg/L (normal range 30–400 μg/L); serum transferrin saturation levels 31.1% (normal range 20–50% in males); and renal and liver function tests, normal. Arterial blood examination while breathing room air showed a blood pH of 7.44, a PaO2 of 71.0 mmHg, a PaCO2 of 36.7 mmHg, and HCO3 of 24.7 mM/L, with 96% oxygen saturation.
Pulmonary function studies revealed results within a normal range. Forced vital capacity (FVC) of 4.19 liters (96% of predicted) and forced expiratory volume-one second (FEV1) of 3.16 liters (102% of predicted) were observed. To examine the possible source of infection, he underwent recurrent sputum analysis. However, the bacterial, viral, fungal and acid fast bacilli cultures from sputum were all negative.
Three months before admission, he had undergone a chest X-ray at our hospital. At that time, his chest X-ray showed normal images. When he was admitted, his chest X-ray showed a pattern of consolidation in the right upper lung field (Fig. 1). A CT scan of the chest showed peribronchial distribution with peripheral segmental air-space consolidation in the right upper posterior segment, and small ill-defined nodules. It also showed centrilobular emphysema in both the upper and mid-lung zones. The air space consolidation on right upper lung field was increased compared with previous study results (Fig. 2).
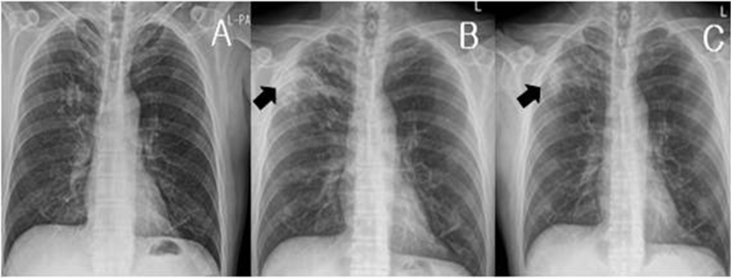
Fig. 1.
(A) Chest posterioranterior (PA) X-ray showed normal findings 3 months before admission. (B) Chest PA obtained at admission showed air space consolidation in the right upper lobe. (C) Two months after admission, the chest PA was performed in the outpatient clinic. The consolidation lesion observed in the right upper lobe was slightly improved.
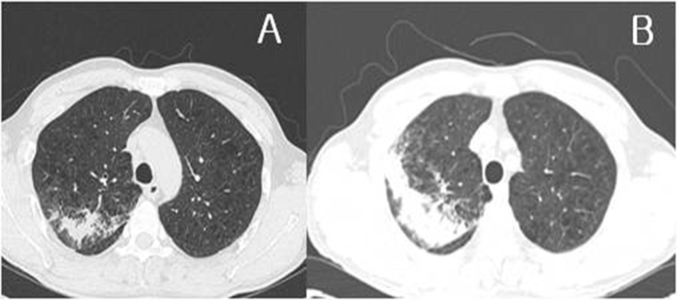
Fig. 2.
(A) Chest computed tomography (CT) images acquired three weeks before admission, showing patchy air space consolidation in the right upper lobe. (B) The chest CT was re-performed at admission. It showed peribronchial distribution with peripheral segmental air-space consolidation in the right upper posterior segment and small ill-defined nodules. The air space consolidation in the right upper lung field was increased compared with previous images.
Bronchoscopy was performed 3 days after admission and there was no endobronchial lesion observed. Bronchial washing was performed in the right upper lobe and bronchoalveolar lavage (BAL) was performed in the right middle lobe. Retrieved lavage fluid volume was 60ml (31%). Cytospin examination showed 4% neutrophils, 6% lymphocytes, 88% alveolar cells, and 2% eosinophils. Photomicrographic findings after bronchoalveolar lavage revealed many alveolar macrophage-containing iron particles. The intracellular iron particles were markedly positive upon Prussian blue staining (× 1000) (Fig. 3). Moreover, no malignant cells were observed. Acid-fast bacilli cultures and smears, tuberculosis detection PCR, bacterial detection Gram stain and cultures, and bronchoalveolar lavage fluid fungus cultures also presented negative results.
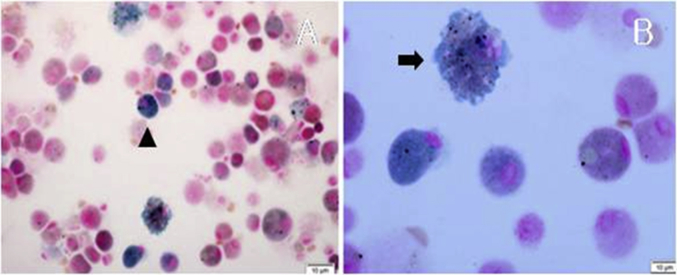
Fig. 3.
Photomicrograph after bronchoalveolar lavage shows numerous macrophages containing bright blue colored granular pigments in the cytoplasm. (Prussian blue stain, (A) ×400, (B) × 1000).
We did not measure the ferritin levels in bronchoalveolar lavage fluid. CT-guided trans thoracic needle biopsy was performed for histological confirmation. The biopsy specimen also showed alveolar, macrophage-containing, Prussian blue-positive iron particles in the alveolar space (Fig. 4). No evidence of vasculitis and malignancy was observed in the histological examination. Serum ANCA levels were also measured and confirmed to be negative. Based on the patient's occupational exposure history and test results, the diagnosis of pulmonary siderosis (welder's lungs) was confirmed.
Fig. 4.
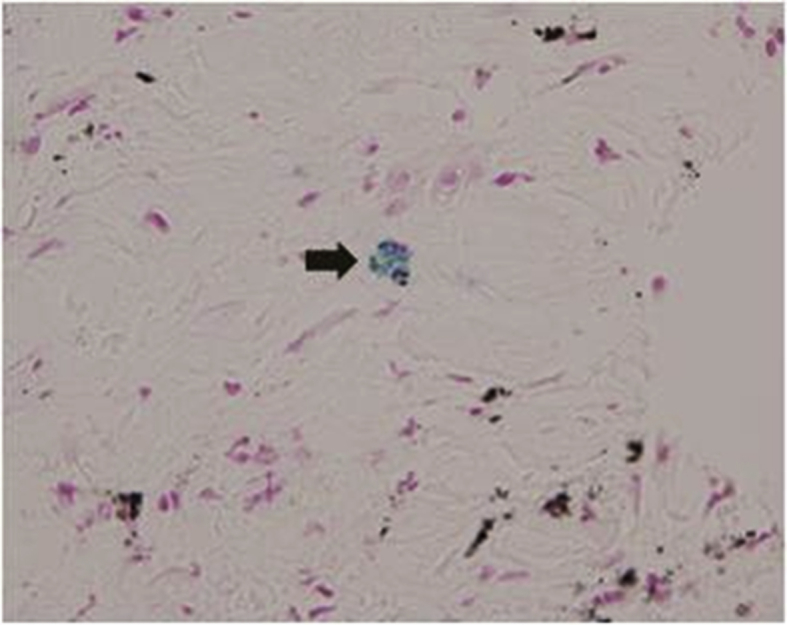
A computed tomography guided transthoracic needle biopsy specimen showed macrophage containing Prussian blue stain positive iron particles. (Prussian blue stain, ×400).
Subsequently, we advised him to discontinue his job and discharged him from the hospital. No specific treatment was administered and only medications to control symptoms were prescribed. One month later, we performed bronchoalveolar lavage again. Two months later, a chest X-ray was performed in the outpatient clinic. The consolidation lesion observed in the right upper lobe had slightly improved (Fig. 1). The patient is currently undergoing observation, and has not shown any special symptoms.
3. Discussion
Pulmonary siderosis is caused by the accumulation of iron oxide in macrophages within the lung. It is observed most commonly in workers exposed to metal fumes during welding and thus is known as welder's siderosis or arc-welder's pneumoconiosis [8]. Although it takes years of exposure for a patient to become symptomatic, rapid development of symptomatic disease within a year after exposure has been reported. In addition, high-intensity, brief exposure to iron dust can also result in future symptoms [10]. Since the original description of the condition in 1936, it has been generally assumed that the disease is benign. Some authors, however, have reported respiratory difficulties or histological findings of emphysema or pulmonary parenchymal fibrosis in welder's lung disease [2].
The diagnosis of pulmonary siderosis is made by identifying a patient's exposure history and, chest radiographic findings, and confirming iron accumulation by iron dyeing the bronchoalveolar lavage fluid or biopsy specimen. Recently, because of the development of radiological technology, radiological findings play an important role in the differential diagnosis of the disease. At the time of welding, the aspirated iron oxide is stored in alveolar macrophages and then transferred to the lung parenchyma. Subsequently, macrophages are distributed through the blood and peribronchial lymphatic vessels, and form small nodules. Because of these pathophysiological characteristics, typical CT findings of pulmonary siderosis may appear [4].
In 1995, Akira [3] reported that the most common CT findings, present in 15 of 21 arc welders studied, were ill-defined micronodules diffusely distributed in the lung. Emphysema was observed in 7 cases and was the predominant CT finding in 3 cases. A honeycomb pattern was found in 3 cases, presenting the predominant CT finding in all 3 cases. The CT appearance of this honeycomb pattern resembled that observed in usual interstitial pneumonia.
These chest CT findings are further supported by Han et al. [2] who reported that the most frequent thin-section CT findings in patients with welder's lung disease were poorly defined small centrilobular nodules, branching linear structures, and less commonly, extensive ground-glass attenuation without zonal predominance. All of these findings represent macrophage accumulation in the alveolar space. However, in addition to these typical imaging findings, pulmonary siderosis can appear in unusual forms. Other chest CT findings in welder's lungs included localized stable tuberculous lesions, bronchial dilatation or wall thickening, localized emphysema, non-calcified mediastinal lymphadenopathy, sub-segmental atelectasis, or tracheal dilatation [2].
In 2004, since the report of a case of pulmonary siderosis with typical imaging findings in Korea [1], Kinosita et al. reported a case of pulmonary siderosis suspected to be lung cancer. In that case, pulmonary siderosis showed a 3-cm sized pulmonary nodule in radiological findings, but was confirmed by histological examination. In 2011, there was a case of pulmonary siderosis with atypical imaging findings in Korea. In that case, chest CT showed a 1.3 × 1.5 - cm sized mass in the left upper lobe and multiple ill-defined irregular nodules in both lung fields. Thus, similarly, that pathologic lesion was misdiagnosed as metastatic lung cancer [5]. All the pulmonary siderosis cases with atypical imaging findings as described above included mass-like lesions or large nodules. However, in the present case, the patient's chest CT scan showed lung consolidation predominantly in the right upper lobe. This case is the first case of pulmonary siderosis being misdiagnosed as pneumonia.
In this patient, we identified the history of exposure to iron dust first, and then performed bronchoalveolar lavage and lung biopsy. In the obtained specimen, we performed Prussian blue staining which is a common histopathology staining technique used to detect the presence of iron. There was a report demonstrating that serum ferritin and transferrin saturation levels were elevated in relation to exposure to iron dust. However, in this case, no elevation was observed [5].
Treatment of pulmonary siderosis is important to minimize the effects of exposure of iron dust. In addition to the respiratory symptoms, we can expect to improve the radiological findings. There was a case treated with bronchoalveolar lavage to remove iron dust accumulated in the lung, but it is not a universally used treatment. In this patient, after diagnosis of pulmonary siderosis, we advised him to discontinue welding and performed bronchoalveolar lavage twice more [6].
As in this case, pulmonary siderosis rarely presents as atypical imaging findings and it can be easily misdiagnosed as another disease because it appears as a common lesion, such as a consolidation. Therefore when diagnosing pulmonary diseases, it is important to know the patient's occupational history.
4. Summary
We report the first case of pulmonary siderosis appearing in the form of consolidation upon radiological examination and being misdiagnosed as pneumonia. We identified the patient's occupational history and confirmed pulmonary siderosis through bronchoalveolar lavage and computed tomography guided transthoracic lung biopsy.
Acknowledgements
This study was supported by a grant from Wonkwang University in 2017.
References
- 1.Kim Eun A., Bang ByoungUk, Kim Lucia. A case of pulmonary siderosis confirmed by bronchoalveolar lavage and transbronchial lung biopsy. Tuberc. Respir. Dis. 2004 Nov;57(5):476–479. [Google Scholar]
- 2.Han D.1, Goo J.M., Im J.G. Thin-section CT findings of arc-welders' pneumoconiosis. Kor. J. Radiol. 2000 Apr-Jun;1(2):79–83. doi: 10.3348/kjr.2000.1.2.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Akira M. Uncommon pneumoconioses: CT and pathologic findings. Radiology. 1995 Nov;197(2):403–409. doi: 10.1148/radiology.197.2.7480684. [DOI] [PubMed] [Google Scholar]
- 4.Kim Jung Ho, Park Yun Jung, Park Ki Hoon. A case of Welder's lung disease and concurred non-tuberculotic mycobacterial infection confirmed with thoracoscopic lung biopsy. Tuberc. Respir. Dis. 2012 Feb;72(2):223–227. [Google Scholar]
- 5.Koo So My, Park Sung Woo, Park Jong Sook. A case of pulmonary siderosis mimicking metastatic lung cancer. Tuberc. Respir. Dis. 2011 Jan;70(1):58–62. [Google Scholar]
- 6.Yamada G.1, Igarashi T., Sonoda H. Use of bronchopulmonary lavage for eliminating inhaled fume particles from a patient with arc welder's lung. Intern. Med. 1998 Nov;37(11):962–964. doi: 10.2169/internalmedicine.37.962. [DOI] [PubMed] [Google Scholar]
- 7.Flors Lucía, Domingo Maria L., Leiva-Salinas Carlos. Uncommon occupational lung diseases: high-resolution CT findings. Am. J. Roentgenol. 2010;194:W20–W26. doi: 10.2214/AJR.09.2593. [DOI] [PubMed] [Google Scholar]
- 8.Kim Kun-II., Choi Seok-Jin, Sohn Hae-Sook. High-resolution CT findings of welders' pneumoconiosis. J. Kor. Radiol. Soc. 1996;34(3):367–371. [Google Scholar]
- 9.Yoshii C.1, Matsuyama T., Takazawa A. Welder's pneumoconiosis: diagnostic usefulness of high-resolution computed tomography and ferritin determinations in bronchoalveolar lavage fluid. Intern. Med. 2002 Dec;41(12):1111–1117. doi: 10.2169/internalmedicine.41.1111. [DOI] [PubMed] [Google Scholar]
- 10.Khalid I.1, Khalid T.J., Jennings J.H. A welder with pneumosiderosis: a case report. Cases J. 2009 Apr 20;2:6639. doi: 10.1186/1757-1626-2-6639. [DOI] [PMC free article] [PubMed] [Google Scholar]